Abstract
The capsule of N. meningitidis serogroup B, (α2→8)-linked polysialic acid and the capsules of other meningococcal serogroups and of other gram-negative bacterial pathogens are anchored in the outer membrane through a 1,2-diacylglycerol moiety. Previous work on the meningococcal cps complex in Escherichia coli K-12 indicated that deletion of genes designated lipA and lipB caused intracellular accumulation of hyperelongated capsule polymers lacking the phospholipid substitution. To better understand the role of lip and lipB in capsule expression in a meningococcal background, the location, sequence, and relationship to related bacterial capsule genes were defined and specific mutations in lipA and lipB were generated in the serogroup B meningococcal strain NMB. The lipA and lipB genes are located on the 3′ end of the ctr operon and are most likely transcribed independently. Inactivation of lipA, lipB, and both resulted in the same total levels of capsular polymer production as in the parental controls; however, these mutants were as sensitive as an unencapsulated mutant to killing by normal human serum. Immunogold electron microscopy and flow cytometric analyses revealed intracellular inclusions of capsular polymers in lipA, lipB, and lipA lipB mutants. Capsular polymers purified from lipA, lipB, and lipA lipB mutants were lipidated. The phospholipid anchor was shown by gas chromatography-mass spectroscopy analysis to be a phosphodiester-linked 1,2-dipalmitoyl (C16:0) glycerol moiety and was identical in structure to that found on the wild-type meningococcal capsule polymers. Thus, lipA and lipB do not encode proteins responsible for diacylglycerophosphatidic acid substitution of the meningococcal capsule polymer; rather, they are required for proper translocation and surface expression of the lipidated polymer.
Neisseria meningitidis is an encapsulated gram-negative bacterium and the cause of epidemic bacterial meningitis and fulminant sepsis worldwide. Meningococcal strains are classified into serogroups based on structural differences of the capsule. Thirteen capsular serogroups of N. meningitidis have been identified, of which five (A, B, C, Y, and W-135) cause most of the invasive meningococcal disease (38). Large epidemics occur each year in the “meningitis belt” of sub-Saharan Africa, mainly due to serogroup A meningococci (18). Serogroup B, C, and Y N. meningitidis strains are associated with sporadic disease, case clusters, and outbreaks seen in the United States, Canada, New Zealand, South America, and Europe (52). Worldwide outbreaks of serogroup W-135 have occurred recently due to spread by pilgrims during the Hajj (46).
The capsule expressed by N. meningitidis is categorized as a group II capsule based on the similar chemical and physical properties of capsular polymers (3, 37, 54). With the exception of the capsule expressed by serogroup A (25), which is composed of (α1→6)-linked N-acetylmannosamine-1-phosphate, capsules expressed by each of the other four major invasive meningococcal serogroups, B, C, Y, and W135, contain sialic acid. Serogroups Y and W-135 are composed of alternating units of d-glucose and d-galactose and sialic acid, respectively (2). The capsular polysaccharides of serogroups B and C are composed entirely of sialic acid in an (α2→8) or an (α2→9) linkage (24).
The genetic organization and sequence of the meningococcal capsule locus have homology to the capsule gene complexes of other pathogenic bacteria expressing group II capsules, such as Escherichia coli K1 and K5 and Haemophilus influenzae (3, 37, 54). Sequence analyses of capsule gene clusters from bacteria expressing group II capsules have revealed a genetic organization that consists of three functional regions. Regions 1 and 3 are conserved between organisms expressing the group II capsule and, in most cases, flank region 2, which encodes species- and serotype-specific biosynthesis genes (3, 37, 54). As an example, region 3 of E. coli K1 encodes kpsMT, which assemble to form an ABC transporter, while region 1 contains an operon (kpsFEDUCS) composed of genes involved in various capsule modification and transport steps (3) (Fig. 1).
FIG. 1.
Genetic organization of the E. coli K1 capsule locus and the N. meningitidis capsule gene complex (cps). The genes responsible for capsule polymer biosynthesis (dotted arrows, synABCD and siaABCD), capsule transport (hatched arrows, ctrABCD), putative phospholipid substitution (black arrows, lip and lipB), and the E. coli K1 homologues of each are shown. The locations of lipA and lipB within the cps complex in the serogroup B strain NMB were similar to those of the published serogroup B MC58 meningococcal genome sequence (47) and other sequenced meningococcal genomes.
The genes for meningococcal capsule biosynthesis are located on a ≈24-kb meningococcal virulence island known as the capsule gene complex (cps) (Fig. 1) (14). The capsule biosynthesis genes synABCD (siaABCD, region A) are transcribed divergently from a shared 134-bp promoter region as the transport genes, ctrABCD (region C) (43, 51). In meningococci, the genes lipA and lipB (region B) are separate from the syn and ctr operons and encode proteins proposed to be involved in the substitution of a diacylglycerophosphatidic acid group at the reducing end of the capsular polymer (13). The ctrABCD operon and lipA and lipB are highly conserved among meningococcal serogroups, whereas the capsule polymerase genes located within each capsule biosynthesis cluster are serogroup specific (11, 45).
The capsular polymers of all meningococcal serogroups are also believed to be covalently linked to diglyceride moieties through phosphodiester linkages (16). Analysis of purified meningococcal polysaccharides of serogroups A, B, and C has revealed the presence of a covalently attached lipid moiety, 1,2-diacylglycerol, at the polymer reducing end, with dipalmitoyl (C16:0) glycerol being the major lipid component (≈85%) of capsule and the rest being distearoyl (C18:0) glycerol (16). Newly synthesized capsule polymers are thought to require this phospholipid substitution for translocation across the inner membrane and for proper anchoring of the polymer in the outer leaflet of the outer membrane (13). A cloned cps complex of group B meningococci in E. coli K-12 that contained a deletion of the region encoding lipA and lipB resulted in the intracellular accumulation of hyperelongated (α2→8)-linked capsule polymers lacking a phospholipid substitution (13). Thus, the genes were named and the gene products of lipA and lipB are proposed to be involved in the diacylglycerophosphatidic acid substitution of the capsule polymers prior to transport to the extracellular surface. However, mutations of kpsC and kpsS, the lipA and lipB homologues in K1 E. coli, which also expresses an (α2→8)-linked polysialic acid capsule, result in the intracellular accumulation of lipid-modified polysaccharides (9). This suggests that KpsC and KpsS are not required for the phospholipid modification and that lipidation is not sufficient for translocation of K1 capsule polymers (9).
The conflicting reports on the requirement of LipA and LipB homologues for generating the phospholipid substitution in group II capsule producers led us to better characterize the exact functions of meningococcal LipA and LipB. We found that mutation of lipA, lipB, and lipA and lipB in meningococci resulted in intracellular accumulation of the meningococcal capsule polymers that are identical to the wild-type capsule (i.e., are substituted with diacylglycerol moieties). Thus, LipA and LipB are not lipidation enzymes; rather, they are involved in the proper translocation and surface expression of capsule polymers. We propose renaming LipA and LipB CtrE and CtrF, respectively, to reflect their roles in capsule translocation, not lipidation.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, and media.
The strains and plasmids used in this study are listed in Table 1. Meningococcal strain NMB (CDC 8201085) is a serogroup B N. meningitidis strain originally isolated from the cerebrospinal fluid of a patient with meningococcal meningitis in Pennsylvania in 1982. Meningococcal strain M7 is a nonencapsulated mutant derivative of NMB that contains a single truncated Tn916 insertion within synA (41). All meningococcal strains were grown on GC base agar (Difco Laboratories) supplemented with 0.4% glucose and 0.68 mM Fe(NO3)3 at 37°C with 3.5% CO2. Meningococcal mutants with kanamycin selection were grown on brain heart infusion base agar (BHI; Becton Dickinson) containing 1.25% fetal bovine serum (Gibco-BRL). Liquid cultures were grown in GC broth with the same supplements and 0.43% NaHCO3 at 37°C.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| N. meningitidis | ||
| NMB | B:2b:P1.2,5:L2 (CDC 8201085) | 41 |
| M7 | synA::Tn916, unencapsulated derivative of NMB | 41 |
| NMB16 | NMB with chromosomal lipA::Ω(Sp) mutation | This study |
| NMB17 | NMB with chromosomal lipB::Ω(Sp) mutation | This study |
| NMB18 | NMB with chromosomal lipA::aphA-3 mutation | This study |
| NMB19 | NMB with chromosomal lipB::aphA-3 mutation | This study |
| NMB20 | NMB with chromosomal ΔlipA::Ω(Sp) mutation | This study |
| NMB21 | NMB with chromosomal ΔlipA::aphA-3 mutation | This study |
| NMB1718 | NMB with chromosomal lipA::aphA-3 and lipB::Ω(Sp) double mutations | This study |
| NMB1624 | lipA::Ω(Sp) mutant carrying pCAS24 | This study |
| NMB1824 | lipA::aphA-3 mutant carrying pCAS24 | This study |
| NMB17289 | lipB::Ω(Sp) mutant carrying pYT289 | This study |
| NMB19289 | lipB::aphA-3 mutant carrying pYT289 | This study |
| E. coli | ||
| DH5α | Cloning strain | New England Biolabs |
| Plasmids | ||
| pCR2.1 | TA cloning vector | Invitrogen |
| pHP45Ω | Source of Ω(Sp) cassette | 32 |
| pUC18k | Source of aphA-3 cassette | 27 |
| pYT250 | Shuttle vector | 50 |
| pCAS14 | pCR2.1 containing the cloned internal fragment of lipA PCR amplified with primers JD39 and JD42 | This study |
| pCAS15 | pCR2.1 containing the cloned internal fragment of lipB PCR amplified with primers JD43 and JD46 | This study |
| pCAS16 | pCAS14 with polar Ω cassette (SmaI) inserted into the BsiWI site of lipA | This study |
| pCAS17 | pCAS15 with polar Ω cassette (SmaI) inserted into the NcoI site of lipB | This study |
| pCAS18 | pCAS14 with nonpolar aphA-3 cassette (EcoRI-BamHI digested and Klenow blunted) inserted into the HincII site of lipA | This study |
| pCAS19 | pCAS15 with nonpolar aphA-3 cassette (SacI-HincII digested and Klenow blunted) inserted into the HincII site of lipB | This study |
| pCAS20 | pCAS14 with 750-bp deletion (BbsI-HincII) of lipA and polar Ω cassette insertion | This study |
| pCAS21 | pCAS14 with 750-bp deletion (BbsI-HincII) of lipA and aphA-3 cassette insertion | This study |
| pCAS22 | pCR2.1 containing the 5′ region and full length of lipA amplified with primers CAS23 and JD44 | This study |
| pCAS23 | pCR2.1 containing the 5′ region and full length of lipB amplified with primers JD41 and CAS9 | This study |
| pCAS24 | pYT250 shuttle vector containing EcoRI fragment of pCAS22 | This study |
| pYT289 | pYT250 shuttle vector containing EcoRI fragment of pCAS23 | This study |
E. coli strains were grown in Luria-Bertani (LB) broth (Bethesda Research Laboratories) at 37°C with appropriate antibiotic selection. Antibiotics were used in the following concentrations for meningococci: tetracycline, 5 μg/ml; spectinomycin, 60 μg/ml; kanamycin, 80 μg/ml; and erythromycin, 3 μg/ml. Antibiotics used for E. coli were as follows: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; spectinomycin, 100 μg/ml; and erythromycin, 300 μg/ml. Monoclonal antibodies against the meningococcal serogroup B capsule (2-2-B) and the serogroup C capsule (4-2-C) are generous gifts of Wendell Zollinger (Walter Reed Army Institute of Research). Primers CAS10 (5′-CAAACACCGCGCAAGAAATCG-3′), CAS11 (5′-CACACATTATTGACAACTTAAGGG-3′), and JD45 (5′-TCACCATCAACAGCACCAGCG-3′) were used to determine the genetic organization of lipA and lipB in relation to other capsule transport genes.
Construction of lipA, lipB, and lipA lipB mutants.
An internal coding region was amplified by PCR with primer pairs JD39 (5′-AAATACAACCACGCGCCCGAACTT-3′) and JD42 (5′-GCGCGGGATGGGGAGCAGAT-3′) for lipA and JD43 (5′-GCGTATGCTTGAAAGTTCAACC-3′) and JD46 (5′-CGCTGGTGCTGTTGATGGTGA-3′) for lipB with NMB chromosomal DNA as a template. The resulting PCR fragments were cloned into pCR2.1 (Invitrogen), yielding pCAS14 and pCAS15, respectively. A spectinomycin-resistant Ω (Sp) cassette flanked by strong transcriptional and translational terminators was obtained from pHP45Ω (32).
To generate a potentially polar mutation, a SmaI fragment containing the entire Ω (Sp) cassette was inserted into the BsiWI site of pCAS14 (yielding pCAS16) and the NcoI site of pCAS15 (yielding pCAS17), both of which had been treated with Klenow DNA polymerase to produce blunt ends. The nonpolar aphA-3 kanamycin resistance cassette was excised from pUK18K (27) with EcoRI and BamHI for insertion in lipA and with SacI and HincII for insertion in lipB. The excised aphA-3 cassettes were treated with Klenow DNA polymerase to produce blunt-ended fragments. For the nonpolar insertions, the aphA-3 cassettes were inserted into the unique HincII site in pCAS14 (yielding pCAS18) and the unique HincII site in pCAS15 (yielding pCAS19) with the 3′ end of the cassette in-frame to the downstream coding sequence.
LipA contains 704 residues and was disrupted by either polar or nonpolar insertion at amino acid 540 and amino acid 503, respectively. LipB is a protein of 419 residues and was similarly disrupted at amino acid 172 and amino acid 135. Putative transformants for the Ω and aphA-3 insertion constructs were confirmed by colony PCR that yielded larger PCR products with the expected additional cassette length. Additional sequencing analysis of the nonpolar construct with the primer 3′ of the cassette was performed to confirm in-frame insertion of the aphA-3 cassette with the downstream sequence. In addition, since the polar and nonpolar cassettes used to disrupt LipA were inserted closer to the C terminus of the protein, it was possible that the translated 5′ gene fragment was able to fold into a partially functional LipA protein. To verify that LipA was completely disrupted, a 750-bp internal lipA fragment was excised from pCAS14 with BbsI and HincII digestion before insertion of the polar SmaI-Ω cassette or the nonpolar aphA-3 cassette, yielding pCAS20 and pCAS21, respectively.
The plasmid extracted from the confirmed transformants was linearized by ScaI and used to transform (19) meningococcal serogroup B strain NMB with selection for antibiotic resistance. Putative meningococcal transformants were confirmed for the acquisition of the cassettes via double-crossover homologous recombination events by colony PCR that established the linkage between the lipA and lipB loci and inserted cassettes with primers specific for the chromosome and cassette, respectively. Furthermore, colony PCR with chromosomal specific primers outside the cloned fragments gave PCR products of the sizes expected for insertion of the cassettes.
Sequence analysis of the PCR product further confirmed acquisition of the cassettes (data not shown). Meningococcal mutants with mutations in both lipA and lipB (lipA::aphA-3/lipB::Ω) were constructed by transformation of the meningococcal lipB::Ω mutant with the nonpolar lipA mutation with selection for spectinomycin- and kanamycin-resistant transformants. Several transformants for each mutant were selected from multiple independent transformation experiments to confirm the reproducibility of the phenotypes.
Complementation of lipA lipB mutants.
Primers CAS23 (5′-GAAGTATTCGACGTGGCCG-3′) and JD44 (5′-GGTTGAACTTTCAAGCATACGC-3′) were used to amplify a 2,547-bp DNA fragment that contains sequence 333 bp upstream of the lipA start codon and the entire lipA coding sequence. PCR amplification with primers JD41 (5′-ATCTGCTCCCCATCCCGCGC-3′) and CAS9 (5′-GAGGGCTTTGTAAAAATATTGCTCGGC-3′) yielded a 1,938-bp PCR product that encompasses 475 bp of upstream sequence and the lipB coding sequence. The PCR products were cloned into pCR2.1 via TA cloning (Invitrogen) to give pCAS22 and pCAS23, respectively. The inserts were released by EcoRI digestion and made blunt ended with Klenow treatments. These DNA fragments were subsequently cloned into the EcoRV site of the meningococcal shuttle vector pYT250 (erythromycin-resistant) to yield pCAS24 (lipA) and pYT289 (lipB), respectively. The plasmids were methylated with HaeIII methylase (New England Biolabs) according to the manufacturer's protocol and were then used to transform the corresponding lipA and lipB mutants. Kanamycin-resistant-erythromycin-resistant and spectinomycin-resistant-erythromycin-resistant transformants were saved as aphA-3 and Ω insertion mutants, respectively. PCR analyses confirmed the presence of the original insertional mutation at the chromosomal locus with chromosome-specific primers, and PCR amplification with vector-specific primers confirmed the presence of an intact copy of the complemented gene.
Capsule ELISA.
Serogroup B capsule-specific immunoglobulin M monoclonal antibody 2-2-B was used in the whole-cell enzyme-linked immunosorbent assay (ELISA) as previously described (44). The monoclonal antibodies in the whole-cell ELISA experiments detected total capsule produced. The bacteria are dried on the ELISA plate overnight, resulting in bacterial lysis and access to intracellular compartments. The lysate obtained by repeated freeze-thawing of bacteria gave similar results. The dilution factors of samples used in the ELISA have been predetermined with both the wild-type strain and the mutants with checkerboard dilution experiments at various cell dilution and antibody dilution factors. The comparison between the wild-type strain and the mutants was performed within the linear range at various low and high response levels, and all gave similar results.
Serum bactericidal assay.
A microdilution serum bactericidal assay was performed as described previously (20).
Hydrophobic interaction chromatography.
A protocol described by Karlyshev et al. (21) was applied to determine the surface hydrophobicity of meningococcal strains. Disposable plastic columns packed with 2 ml (bed volume) of octyl- Sepharose CL-4B resin (Pharmacia) were washed with 10 ml of 0.2 M ammonium sulfate in 10 mM sodium phosphate buffer, pH 6.8 (buffer A). Overnight plate cultures were suspended in phosphate-buffered saline (PBS) to obtain an optical density at 600 nm of 10. A 100-μl aliquot of cells was gently pipetted onto the surface of the column, and the columns were then washed with 5 ml of buffer A. As a loading control, a 100-μl aliquot of cells was added directly to 5 ml of buffer A. The absorbances were determined at an optical density at 600 nm. The results were expressed as the percentage of cells released from the columns in the flowthrough divided by that of the loading control.
Flow cytometry.
Meningococci grown to early stationary phase were collected by centrifugation at 10,000 × g for 3 min and suspended in PBS. Meningococci were permeabilized with 70% ethanol treatment in order to detect intracellular capsular polysaccharides, whereas cells suspended in PBS without ethanol were treated as samples of intact meningococci. Fixed samples were obtained by incubating aliquots equal to 108 cells/ml with 70% ethanol for 5 min, while equivalent aliquots in PBS without ethanol fixation were treated as unfixed cells. Cells were washed with 0.5 ml of PBSB buffer (0.5% [wt/vol] bovine serum albumin in PBS) once before the incubation with 100 μl of either monoclonal antibody 2-2-B or 4-2-C (1:1,000 dilution) for 1 h at 37°C. After washing with 0.5 ml of PBSB buffer, the cells were further incubated with a 1:100 dilution of R-phycoerythrin-conjugated goat F(ab)2 anti-mouse immunoglobulin G (Molecular Probes, Eugene, Oreg.) for 1 h at 37°C. The cell pellets obtained after centrifugation were resuspended in 1 ml of PBS and analyzed for fluorescence labeling with a FACScalibur flow cytometer (Becton Dickinson).
A fluorescent nucleic acid-binding dye, SYTOX Green, a dye with positive charges and thus excluded from living cells, was used to assess the integrity of the plasma membranes of the organisms (23, 39). Internalization and binding of SYTOX Green stain by nucleic acids resulted in a greater than 500-fold enhancement in fluorescence emission, and the fluorescence signal from membrane-compromised meningococci labeled with SYTOX Green stain was typically >10-fold brighter than that from intact organisms. Aliquots of similarly treated cells were also mixed with an equal volume of 5 μM SYTOX Green (Molecular Probes) prepared in PBS from a 5 mM stock solution in dimethyl sulfoxide to assess the membrane integrity of the bacteria by fluorescence-activated cell sorting analysis (39).
Electron microscopy.
Both overnight plate-grown cultures and stationary-phase broth cultures were used for electron microscopy analysis. For whole-cell immunogold labeling, nickel 200-mesh grids coated with Formvar and carbon were floated on droplets of cell suspension in PBS. Excess liquid was blotted off, and then the cells were fixed with 0.05% glutaldehyde for 15 min followed by a 5-min incubation with 50 mM glycine. The grids were blocked with a blocking solution containing 5% acylated bovine serum albumin, 5% normal goat serum, and 0.1% gelatin in PBS for 30 min and washed six times with buffer PBSBc (0.1% acylated bovine serum albumin in PBS) before incubation with a 1:25 dilution of the serogroup B-specific monoclonal antibody 2-2-B for 30 min. Following another six washes the grids were incubated for 30 min with a solution containing 6-nm-gold-conjugated mouse anti-immunoglobulin G/M Fab (1:20 dilution). The grids were further washed with PBSBc buffer and water and then processed for negative staining with 1% ammonium molybdate.
For section electron microscopy, thin sections of Epon-embedded samples on grids were etched with an NaOH-methanol solution, followed by a 2-min treatment with 1% metaperiodate. The grids were incubated with 50 mM glycine in PBS for 15 min, followed by a 1-h incubation in the blocking solution. Incubation with the primary monoclonal antibody (2-2-B, 1:25 dilution) was done overnight at 4°C and with the secondary antibody (6-nm-gold-conjugated goat anti-mouse immunoglobulin G/M, 1:20) for 2 h at room temperature. The grids were further treated with 2.5% glutaraldehyde in PBS and stained with lead citrate and uranyl acetate. The grids were viewed on a Hitachi H-7500 transmission electron microscope.
Capsular polysaccharide purification.
Capsular polysaccharide was purified from each strain according to the protocols of Gotschlich (15), modified as follows. Three liters of culture was vigorously aerated in supplemented GC broth for 16 h. Ten percent Cetavlon (hexadecyltrimethyl ammonium bromide) was added to a final concentration of 1.0%, wt/vol. Cetavlon results in bacterial lysis and the release of capsular polysaccharide regardless of cell localization. The precipitate and bacterial debris were collected by centrifugation (11,000 × g for 15 min) and then resuspended in 50 ml of distilled water. To dissociate the polysaccharide-Cetavlon complex, 1 volume of 2 M CaCl2 was added, and the mixture was allowed to stir for 1 h. Absolute ethanol was added to a final concentration of 25% (vol/vol) to precipitate nucleic acids. After 2 h, the precipitated nucleic acids and the bacterial debris were removed by centrifugation (25,000 × g for 20 min). The ethanol concentration of the supernatant was raised to 80%, vol/vol, to precipitate polysaccharide. This polysaccharide was collected by centrifugation (2,000 × g for 10 min), washed three times with absolute ethanol to remove CaCl2 and Cetavlon, three times with acetone, and twice with diethyl ether (centrifuging at 13,000 × g for 10 min between washes), and dried in a vacuum. Contaminating proteins were then removed by cold phenol extraction at neutral pH. The phenol-extracted aqueous phase was dialyzed against 0.1 M CaCl2 for 48 h and then centrifuged for 5 h at 100,000 × g to sediment contaminating lipooligosaccharide. To the supernatant solution, 3 volumes of ethanol were added and the polysaccharide precipitate was collected by centrifugation. The precipitate was then washed with ethanol and acetone and dried under vacuum.
Treatment with phospholipases.
Treatment of the purified capsule with phospholipases disrupts the phosphodiester linkage and releases the phospholipid from the polysaccharide. Phospholipases A2 (from bee venom), C (from Clostridium perfringens, type I), and D (from Streptomyces chromofuscus) were purchased from Sigma Chemical Co. and suspended in 50 mM Tris-HCl, pH 8.0, at concentrations of 1 U/μl, 0.2 U/μl, and 5 U/μl, respectively. Purified capsular polysaccharides (5 μg) were incubated with 1 U of each phospholipase in 50 mM Tris-HCl, pH 8.0, at 30°C for 1 h and then at 37°C for 16 h. The capsular polysaccharides were incubated under the same conditions without phospholipases as control samples.
Electrophoresis and staining.
The Tris-borate-buffered polyacrylamide gel electrophoresis (PAGE) gel system (TBE-PAGE) previously described by Pelkonen et al. (31) and deoxycholic acid (DOC)-containing polyacrylamide gel electrophoresis with the Laemmli buffer system (DOC-PAGE) (33) were used for analysis of purified capsular polysaccharides. Phospholipase-treated and untreated samples of purified capsule (2.5 to 5 μg) were mixed with 0.1 volume of sample buffer according to the protocol. A solution containing trypan blue, xylene cyanol, and bromphenol blue was used as a marker for polymer length, corresponding to 200, 52, and 19 sialic acid residues, respectively (31).
DOC-PAGE was also performed on capsular polysaccharide preparations. Capsular polysaccharide preparations of 2.5 to 5 μg were mixed 1:1 with sample buffer containing 1.0% DOC, 0.1 M Tris-glycine buffer, and 20% glycerol. The samples were then analyzed on an 18% separating gel containing 0.9% DOC with a 4% stacking gel. Electrophoresis was performed with Tris-glycine buffer containing 0.25% DOC. The capsular polysaccharide separated by TBE-PAGE and DOC-PAGE analysis was then detected by Alcian blue staining (0.005% Alcian blue in 40% ethanol-5% acetic acid) followed by silver staining (49).
Characterization of the lipid component of meningococcal capsular polysaccharides.
We extracted 20 mg of each meningococcal capsular polysaccharide three times with ethanol-water (9:1) to remove any contaminating phospholipids. These extracts were pooled, evaporated to dryness, methanolyzed with 1 M methanolic HCl (80°C, 4 h), trimethysilylated, and analyzed by gas chromatography-mass spectroscopy (GC-MS). No significant levels of phospholipids were found in these extracts. The resulting capsular polysaccharides were then treated with 1% acetic acid (80°C, 4 h) (7, 28), lyophilized, and extracted three times with ethanol-water (9:1), and dried. The phospholipids released from the capsular polysaccharide by this mild acid treatment were extracted by the ethanol-water procedure. These ethanol-water extracts were characterized first by MS analysis with matrix-assisted laser desorption ionization-time of flight (MALDI-TOF). To verify the presence of dipalmitoylphosphotidic acid, each sample was incubated with 48% aqueous HF (0°C, 1 h), and transferred to saturated NaHCO3 solution. After cessation of gas evolution, 2 ml of chloroform-methanol (2:1) was added and vortexed. The chloroform phase was separated, washed with water three times, dried with anhydrous Na2SO4, and evaporated to dryness. One portion of each sample was trimethylsilylated and analyzed directly by GC-MS. A second portion of each chloroform extract was methanolyzed (1 M methanolic HCl, 80°C, 4 h) and then trimethylsilylated, followed by GC-MS analysis.
RESULTS
Location and characterization of lipA and lipB in the serogroup B meningococcal strain NMB.
In the genome sequences of the serogroup B strain MC58 (47) and the serogroup A strain Z2491 (29), the open reading frame of lipA (NMB0082 and NMA0186, respectively) is 2,112 bp and encodes a protein of 704 amino acids. The open reading frame of lipB (NMB0083 and NMA0185, respectively), which is 3′ from lipA and is 1,257 bp, encodes a protein of 419 amino acids. Sequencing of the lipA and lipB genes of the serogroup B strain NMB revealed 99% identity to that of the published MC58 and Z2491 sequences, confirming that the lipA and lipB genes are highly conserved. However, the previously reported LipA sequence from serogroup B strain B1940 (13) is 492 amino acids, shorter than those predicted from the two genomes and the NMB sequence.
Analysis of the B1940 sequence revealed frameshifts which caused a truncation of ≈180 amino acids at the N terminus and ≈30 amino acids in the C terminus of the lipA coding sequence. Furthermore, the genetic locations of lipA and lipB in NMB, MC58, and Z2491 are also different from that reported in strain B1940 (Fig. 1) (13). In strain B1940, lipA and lipB and the transport gene operon (ctrABCD) flank the serogroup-specific biosynthesis gene cassette (Fig. 1), an organization similar to that reported in many of the bacterial species expressing group II capsules (37). However, in the published genomes of MC58 and Z2491, lipA and lipB are located on the 3′ end of the ctr operon.
PCR analysis was performed to confirm the location of lipA and lipB within the capsule complex of meningococcal strain NMB. Primer CAS11 was made within the 5′ end of gltS, a gene downstream of lipB in the published serogroup B strain MC58 sequence (47) (Fig. 1), and primer CAS10 was designed within the 3′ end of dnaJ, a gene located at the opposite end of the MC58 capsule complex (Fig. 1). The C-terminal coding sequence of lipB was amplified by PCR from NMB chromosomal DNA with primer JD45, located within lipB at the 3′ end, and either primer CAS11 or CAS10. A PCR product of approximately 2 kb was obtained with primers JD45 and CAS11, but not JD45 and CAS10, that corresponds to the expected size based on the MC58 genome (data not shown). The PCR product was further verified to contain the 3′ coding region of lipB and the published downstream sequence of the serogroup B meningococcal strain MC58 by direct sequencing analysis. These results confirmed that lipA and lipB are located on the 3′ end of the ctr operon (Fig. 1). Furthermore, the recently released genome sequence of a serogroup C strain, FAM18 (www.sanger.ac.uk), also indicated the same genomic location of lipA and lipB as found in the other two genomes and in NMB.
lipA and lipB are separated by a 136-bp intergenic region. Reverse transcription-PCR performed with RNA isolated from the wild-type strain NMB confirmed the presence of independent lipA and lipB mRNAs, while no lipA-lipB cotranscript could be detected (data not shown). However, the absence of a cotranscript could result from processing of the larger lipAB transcript. Independent transcription of lipA and lipB was further supported by the complementation experiments (see below).
There is very limited sequence homology between the two proteins, and no other homologues are found in the meningococcal genomes. In addition, neither LipA nor LipB contains any conserved domains or homology to proteins known to be involved in lipid or phospholipid synthesis. LipA and LipB show homology to KpsC and KpsS of E. coli strain K1 (62% and 58% similarity, respectively) and K5 (62% and 56% similarity, respectively), HcsA and HcsB (68% and 67% similarity, respectively) of Haemophilus influenzae (40), PhyA and PhyB (67% and 68% similarity, respectively) of Pasteurella multocida (4, 8), and WcbA and WcbO (52% and 49% similarity, respectively) of Burkholderia pseudomallei (34). These genes are predicted to be involved in the expression of group II capsules.
Meningococcal lipA, lipB, and lipA lipB mutants produced nearly wild-type levels of total capsular polysaccharide yet were sensitive to the complement-mediated killing of normal human serum.
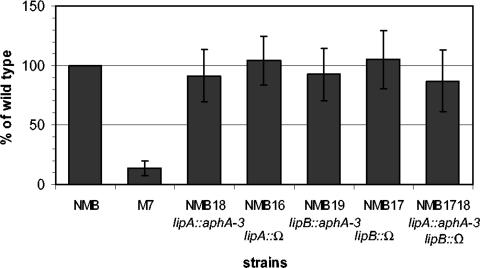
Whole-cell ELISAs, which detect total capsular polysaccharides, were performed with an anti-serogroup B capsular monoclonal antibody. As shown in Fig. 2, lipA, lipB, and lipA lipB meningococcal mutants produced levels of capsular polysaccharide comparable to that of the wild-type strain. Independent transformants selected from multiple transformations were assayed, and levels of capsular polysaccharide similar to that of the wild type were seen in all lipA and lipB mutants (data not shown). The phenotype of the lipA deletion and insertion mutants reproduced those of the lipA insertion mutants (data not shown). In addition, bacteria collected from broth cultures at both the mid-log and stationary phases showed similar levels of capsular polysaccharide production (data not shown), indicating that there is no significant difference in the rate of capsule biosynthesis between the mutants and the wild-type parent strain.
FIG. 2.
Serogroup B meningococcal capsule-specific whole-cell ELISA. The OD405 readings of the serogroup B wild-type strain NMB were used as 100% for normalizing the OD405 of the NMB18 (lipA::aphA-3), NMB16 (lipA::Ω), NMB19 (lipB::aphA-3), NMB17 (lipB::Ω), and the NMB1718 (lipA::aphA-3, lipB::Ω) mutants. The nonencapsulated mutant M7 (synA::Tn916) was included as a negative control (n ≥ 4).
The resistance of encapsulated meningococcal strains to the complement-mediated killing of normal human serum has been well documented (20). No detectable killing was observed at 10% normal human serum for the encapsulated wild-type strain NMB, whereas the unencapsulated mutant (synA::Tn916, designated M7) is completely killed (20). Based on the observation that strains with mutations in lipA and/or lipB produced capsular polysaccharide at levels comparable to that of the wild type, we anticipated that these mutants would be resistant to normal human serum. However, as shown in Fig. 3, the nonpolar lipA (NMB18), lipB (NMB19), and lipA lipB double (NMB1718) mutants were rapidly and completely killed by 10% normal human serum over 30 min, as was the nonencapsulated M7 mutant. The bactericidal activity of human serum against M7 and the lipA, lipB, and lipA lipB mutants was due to complement-mediated killing, as all strains survived in heat-inactivated (56°C for 30 min) serum (data not shown). Thus, meningococcal capsular polysaccharides produced by the lipA, lipB, and lipA lipB mutants did not confer protection to meningococci against the complement-mediated bactericidal activity of human sera.
FIG. 3.
Killing of serogroup B meningococci by normal human serum (NHS). Serum bactericidal assays were performed with the parent strain, the mutants, and the corresponding complemented mutant strains with 10% normal human serum. The solid lines represent the parent and the mutants, while the dotted lines represent the complemented mutant strains. ♦, wild-type strain NMB (solid line); □, nonpolar lipA mutant NMB18 (solid line) and complemented strain NMB1824 (dotted line); ▴, lipA::Ω mutant NMB16 (solid line) and complemented strain NMB1624 (dotted line); and *, nonpolar lipB mutant NMB19 (solid line) and complemented strain NMB19289 (dotted line).
Resistance to normal human serum of lipA and lipB mutants was restored by complementation.
To ensure that the serum sensitivity of the mutants was due to the defect in the lipA and lipB gene products, complementation experiments were conducted. DNA products, which contain 300- to 500-bp sequences upstream of the start codon and the entire coding sequence of lipA and lipB, were obtained by PCR amplification. The PCR products were cloned into meningococcal shuttle vector pYT250 as described in Materials and Methods to yield pCAS24 (lipA) and pYT289 (lipB), respectively. These plasmids were introduced into the corresponding Ω and aphA-3 insertion lipA and lipB mutants via transformation, and the complemented strains were subjected to the serum bactericidal assays.
As shown in Fig. 3, resistance to killing by 10% normal human serum was restored in all complemented strains. Capsular polysaccharide production was not different in all the complemented strains compared to the corresponding parental mutants (data not shown). These data demonstrated that the serum-sensitive phenotype was caused by the mutations in lipA and lipB. Interestingly, the lipB mutation was complemented by pYT289, which carries lipB under the control of the lipB upstream sequence, suggesting the presence of an independent promoter within the intergenic region of lipA and lipB, although the presence of a promoter within the vector sequence has not been excluded. In addition, providing lipA alone (pCAS24) reversed the serum-sensitive phenotype of a lipA mutant containing a strongly polar cassette within lipA. Taken together with the reverse transcription-PCR results, these data indicated that lipA and lipB are likely transcribed independently.
Capsular polysaccharide produced by lipA, lipB, and lipA lipB mutants was not surface expressed.
The observation that lipA lipB mutants produce wild-type levels of capsular polysaccharide but are sensitive to killing by normal human serum suggested that the capsular polymers produced in the mutants may either be localized intracellularly or surface expressed in a defective fashion. As polysialic acid polymers are highly negatively charged, capsule expressed on the surface of the meningococcus will display a decreased hydrophobic surface compared to unencapsulated meningococci.
Surface hydrophobicity was examined by hydrophobic interaction chromatography as described in Materials and Methods. For the encapsulated wild-type strain NMB, the percentage of cells not retained on the column was 52.1% ± 9.0%; while for the unencapsulated strain M7 it was 1.6% ± 1.0%, indicating that the surface of nonencapsulated meningococci was considerably more hydrophobic and associated with the octyl-Sepharose resins through hydrophobic interactions. The lipA, lipB, and lipA lipB mutants all had surface properties not significantly different (Student's t test) from that of the unencapsulated M7 strain (5.0% ± 4.0%, 5.0% ± 2.0%, and 2.2% ± 1.0%, respectively), indicating very little capsular polysaccharide present on the meningococcal surface. These data suggested an intracellular localization of capsular polysaccharide in these mutants, which is consistent with the results of the serum bactericidal assays.
lipA lipB mutants accumulate intracellular capsular polysaccharide, as demonstrated by fluorescence-activated cell sorting analysis and immunogold electron microscopy.
To further assess whether capsule polymers were surface localized in lipA and lipB mutants, flow cytometry (see Materials and Methods) was performed with the wild-type strain NMB and the lipA lipB mutants with the serogroup B capsule-specific monoclonal antibody 2-2-B. A monoclonal antibody, 4-2-C, that specifically recognizes the serogroup C α2→9-linked sialic acid capsule was used as a negative control. As shown in Fig. 4A, with SYTOX Green staining (23, 39), PBS-suspended “intact meningococci” of both the wild type and the lipA lipB dual mutant contained a small population of meningococci that were compromised in membrane integrity, while meningococci permeabilized with 70% ethanol demonstrated a single highly fluorescent population. With the serogroup B capsule-specific antibody 2-2-B, the wild-type serogroup B strain NMB was intensively labeled. As anticipated, labeling with the group C capsule-specific antibody yielded fluorescence intensity similar to that of the unencapsulated M7 mutant (Fig. 4B), and no significant additional labeling by antibody 4-2-C was detected with the permeabilized wild-type serogroup B meningococci (Fig. 4C). These results confirmed the labeling specificity of the flow cytometry analysis and that capsular polysaccharide on the wild-type serogroup B strain was surface expressed.
FIG. 4.
Fluorescence-activated cell sorting analysis of capsular polysaccharide expression by serogroup B N. meningitidis. All strains were grown in GC broth, collected at early stationary phase, and processed for the fluorescence-activated cell sorting analysis as described in Materials and Methods. Panel A, SYTOX Green-stained samples of unfixed NMB (thick black line), unfixed NMB1718 (thick gray line), fixed NMB (thin black line), and fixed NMB1718 (thin gray line). Meningococci suspended in PBS without fixing (panel B) or after fixing with 70% ethanol (panel C) were incubated with either serogroup B capsule-specific monoclonal antibody (2-2-B) or serogroup C capsule-specific antibody (4-2-C) followed by R-phycoerythrin-conjugated secondary antibodies. (B and C) Solid light gray line, wild-type strain NMB with 2-2-B; solid gray line, NMB1718 mutant with 2-2-B; thick black line, capsule-deficient strain M7 with 2-2-B; thin black line, strain NMB with 4-2-C; dotted line, M7 with 4-2-C; dashed line, NMB1718 with 4-2-C.
The lipA lipB dual NMB mutant gave a labeling intensity with the serogroup B-specific antibody similar to that of the nonencapsulated M7 mutant (Fig. 4B). A small population of cells showed high fluorescence intensity, likely from the fraction of cells with compromised membranes. However, fluorescence intensity similar to that of the wild-type strain NMB was demonstrated after lipA lipB mutant meningococci were permeabilized with ethanol (comparing the gray line in Fig. 4B to that in 4C). The detection of serogroup B capsular polysaccharide in permeabilized lipA lipB meningococci was not due to nonspecific labeling, as no such increase was seen with the serogroup C monoclonal antibody. Thus, the capsular polysaccharide produced in the lipA lipB mutant appeared to be localized in an intracellular compartment. Similar results were obtained with the single lipA and lipB mutants (data not shown).
The intracellular location of capsule in the lipA lipB mutant was further demonstrated by immunogold labeling electron microscopy with the 2-2-B monoclonal antibody. Both whole cells and cell sections were examined. As shown in Fig. 5, surface labeling of the antibody was demonstrated in meningococci of the serogroup B wild-type strain NMB but not the capsule-deficient mutant M7 or the lipA lipB dual mutant (panel A versus panels B and C). Cell cross sections subjected to immunogold staining revealed electron-translucent areas inside lipA lipB mutant meningococci that were labeled with gold particles (Fig. 5, panel F). Various forms of electron-transparent areas similar to those seen in the lipA lipB mutant have been observed in many capsule transport-deficient mutants of E. coli K1 and K5 strains (5, 9, 12, 22). A significant percentage (≈50%) of the lipA lipB meningococci contain such electron-transparent areas. Additionally, immunogold labeling was only detected in the electron-translucent zones of the lipA lipB mutant, not within similar zones infrequently seen in the wild-type serogroup B meningococci, indicating that this labeling phenomenon is not due to electron microscopy fixation artifacts. Thus, capsule polymers appear to be sequestered within meningococci with mutations in lip and lipB in a manner similar to that of insoluble material sequestered in inclusion bodies.
FIG. 5.
Electron photomicrographs of immunogold-labeled intact serogroup B N. meningitidis (A to C) and meningococcal cell sections (D to F). Panels A and D show the wild-type parental strain NMB, B and E show the nonencapsulated mutant M7, and C and F are the NMB1718 (lipA lipB) mutant. Labeling was performed with the serogroup B capsule-specific monoclonal antibody as the primary antibody, and the 6-nm-gold-conjugated anti-immunoglobulin G/M antibody was used as the secondary antibody. Bars, 250 nm.
Capsular polysaccharide produced by lipA, lipB, and lipA lipB mutants was similar in structure and contained diacylglycerophosphatidic acid substitutions identical to those of the wild-type strain.
To determine the structure of the capsular polysaccharide expressed by the lipA lipB mutants, the capsules of NMB (wild type), NMB18 (lipA::aphA-3), NMB19 (lipB::aphA-3), and NMB1718 (lipA::aphA-3/lipB::Ω) were purified from overnight cultures by precipitation with the cationic detergent cetyltrimethylammonium bromide (Cetavlon) (15, 17). Similar amounts of purified capsule were recovered from overnight cultures of the wild type and the lipA, lipB, and lipA lipB mutants, indicating no significant difference in the level of capsule production, which was consistent with the ELISA data.
Purified capsular polysaccharide from the wild-type strain NMB and the lipA lipB mutant were treated with phospholipases A2, C, and D, and the phospholipase-treated and untreated polysaccharides were separated on TBE-PAGE (data not shown) and DOC-PAGE (Fig. 6). The presence of diacylglycerophosphatidic acid on capsular polymers causes the formation of larger micelles that migrate more slowly during electrophoresis than polymers that are not lipidated. As shown in Fig. 6, phospholipase C but not A2 or D hydrolyzed the phosphodiester bond, and the release of the lipid moiety from the capsule polymers separated the polymers by size (9, 13, 21). The inability of phospholipase D to release the lipid is most likely due to the structural difference between sialic acid capsule and choline immediately adjacent to the diacylphosphatidyl acid cleavage site. Both phospholipases C and A2 act within the diacylphosphatidyl acid moiety and thus are less perceptive to distant structural variation; however, phospholipase A2 did not completely remove the fatty acid moiety.
FIG. 6.
Phospholipase treatment of capsular polysaccharides purified from serogroup B strain NMB (lanes 1 to 7) and the NMB1718 (lipA lipB) mutant (lanes 8 to 14). Lanes 1 and 8, no phospholipase; lanes 2 and 9, phospholipase A2; lanes 3 and 10, phospholipase C; lanes 4 and 11, phospholipase D; lanes 5 and 12, A2 and C; lanes 6 and 13, C and D; lanes 7 and 14, A2 and C and D. The enzymatic activity of each phospholipase is indicated with phosphatidylcholine as a substrate and compared to the possible activity on the diacylphosphatidyl acid linkage of the sialic acid capsule (16). T, trypan blue; X, xylene cyanol; B, bromophenol blue.
Comparison of the nonlipidated polymers resolved by DOC-PAGE (lane 3 versus lane 10 in Fig. 6) revealed no discernible difference in capsule polymer length between the lipA lipB mutant and the wild-type parent, suggesting that neither LipA nor LipB appeared to influence the number of repeating sialic acid residues incorporated into the individual capsule polymer. These data indicated that the capsular polysaccharides from NMB and the lipA lipB mutants were indistinguishable from each other at the resolution level of DOC-PAGE and are linked to diacylglycerophosphatidic acids.
Isolation and characterization of the lipid component of meningococcal capsular polysaccharides.
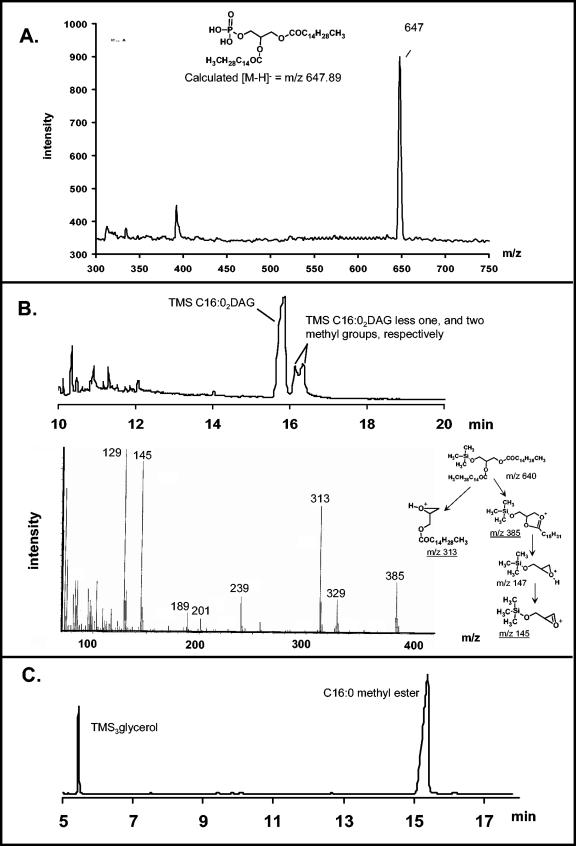
To further determine the structure of the phospholipid moiety, capsular polysaccharides purified from the wild-type parent and the lipA, lipB, and the lipA lipB mutants were analyzed (Fig. 7 and 8). After extraction of the capsular polysaccharides with 9:1 ethanol-water, no phospholipids were found in these further-purified capsular polysaccharide samples. Glycosidic phosphates are labile to mild acid hydrolysis (7, 28), and therefore, a single major phospholipid was released by mild acid hydrolysis of the glycosidic bond between the capsular polysaccharide and the phosphate group of the phospholipid. Each extract from the wild-type parent and the lipA, lipB, and lipA lipB mutants contained a predominant molecule with an [M-H]− ion of m/z 647 (Fig. 8A), which was consistent with dipalmitoyl (C16:0) phosphotidic acid.
FIG. 7.
Schematic diagram showing the method used to obtain the lipid anchor of the various capsular polysaccharide samples. The products shown in the boxes were analyzed as follows: (a) MALDI-TOF-MS analysis, (b and c) GC-MS analysis. The results of these analyses are shown in Fig. 8.
FIG. 8.
Analysis of the lipid anchor of the capsular polysaccharide from N. meningitidis. (A) Negative-ion MALDI-TOF-MS spectrum of dipalmitoyl glycerol phosphate that was liberated from the capsular polysaccharide by mild acid hydrolysis. The structure and calculated [M-H]− ion at m/z 647.89 are also shown in panel A. (B) GC-MS profile (top) of the Me3Si-1,2-di-O-palmitoyl glycerol derivatives obtained from HF treatment of the samples. The mass spectrum of this derivative is shown (bottom), and several of the major ions can be accounted for as described by Gotschlich et al. (16). (C) The GC profile of the components liberated from the samples by acid-catalyzed methanolysis and trimethylsilylation.
To verify the presence of dipalmitoyl (C16:0) phosphotidic acid, each sample was further treated with HF to remove the phosphate group, extracted with chloroform-methanol (2:1), and divided into two portions. One portion of each sample was trimethylsilylated and analyzed by GC-MS. This analysis revealed fragment ions at m/z 385 and 145 that are characteristic of Me3Si-O-1,2-dipalmitoyl glycerol (16) in the preparations from the wild-type parent and from each mutant (Fig. 8B). Ions characteristic of Me3Si-O-1,3-dipalmitoyl glycerol, e.g., m/z 371, were absent (16). A second portion of each chloroform extract was methanolyzed, trimethylsilylated, and analyzed by GC-MS. The results were the same for each mutant and revealed that each extract consisted of glycerol and palmitic acid (C16:0) (Fig. 8C).
In summary, MALDI-TOF-MS and GC-MS demonstrated that dipalmitoyl (C16:0) phosphotidic acid was released from the capsular polysaccharide of the serogroup B N. meningitidis parent and the lipA, lipB, and lipA lipB mutants, supporting the conclusion that each of these capsules is glycosidically linked to the phosphate of dipalmitoylphosphotidic acid. No significant amount of C18:0 species was detected in the capsule preparations from the parent and its mutants, in comparison to a 10 to 20% level of distearoyl glycerol observed previously (16). Thus, the structural characterization of the phospholipid moiety of meningococcal capsule polymers was consistent with the structures previously reported by Gotschlich et al. (16) and confirmed that LipA and LipB were not lipidation enzymes responsible for the dipalmitoylphosphotidic linkage.
DISCUSSION
The role of LipA and LipB in meningococcal capsule expression and the role of the KpsC and KpsS (LipA and LipB homologues, respectively) in E. coli K1 and K5 capsule expression (5, 9, 13) have been controversial. These two families of proteins have been proposed to be involved in the phospholipid substitution of capsule polymers, polymer assembly, transport of the mature polymers, or anchoring of the polysaccharide at the cell surface. In this report, meningococcal LipA and LipB were found to be critical for transport of the mature lipidated polymers to the meningococcal cell surface and not involved in phospholipid substitution or polymer assembly.
E. coli K5 strains have a capsule gene organization similar to that of K1 E. coli but express a structurally distinct 4-β-GlcA-[1→4]-α-GlcNAc-1→ capsule where GlcA is glucuronic acid and GlcNAc is N-acetylglucosamine (30, 37, 53). In addition, 3-deoxy-manno-2-octulosonic acid has been found in the K5 but not the K1 capsule as a linker between the phosphatidic acid moiety and the capsular polymer (54). With an E. coli K-12 strain carrying the complete K5 kps capsule gene cluster on a plasmid, Bronner et al. (5) reported that a kpsS insertion mutation and a kpsC deletion mutation caused cytoplasmic sequestration and accumulation of capsule polysaccharide lacking the phosphatidic-3-deoxy-manno-2-octulosonic acid substitution (5, 36). Thus, KpsC and KpsS were proposed to be involved in the assembly of the phosphatidic-3-deoxy-manno-2-octulosonic acid modification of capsule (37). However, hybrid K1-K-12 E. coli strains with Tn10 insertional mutation in either kpsC or kpsS accumulate lipidated capsule polymers of unaltered polymer length (9). Both reports utilized the K-12 genetic background and an immunoelectrophoresis method for lipidation determination.
In a study of meningococcal LipA and LipB, E. coli K-12 strains containing a cloned meningococcal cps complex with mutations in lipA and/or lipB were found to accumulate nonlipidated capsule polymers of increased polymer length (13). The lack of lipidation in capsular polymers was concluded from a slightly different migration pattern on a TBE-PAGE between samples before and after phospholipase treatment (13). However, with biochemical, chemical, and spectroscopic characterization, we found that the 1,2-dipalmitoylglycerol linkage, previously identified on meningococcal capsule polymers (16), was present on the capsular polysaccharide expressed by lipA, lipB, and lipA lipB meningococcal mutants. Meningococci containing these mutations continue to produce nearly wild-type levels of lipidated, fully formed, immunologically reactive capsular polysaccharide. The mature capsule polymers were found in intracellular inclusions and were not properly transported and inserted into the outer membrane. In addition, there was no discernible change in the length of the capsule polymers expressed by these meningococcal mutants. These data suggest that the loss of lipidation which led to the designation of meningococcal lipA and lipB (13) may have been due to the heterologous genetic background of the original study. The data also suggest that LipA/KpsC and LipB/KpsS are not required for diacylglycerophosphatidic acid modification of either the meningococcal or K1 capsule polymers. Further, the diacylglycerophosphatidic acid anchor per se, hypothesized to be an export motif (37, 54), does not provide sufficient signaling for translocation of capsule polymers to the cell surface in N. meningitidis. Additional signaling events or proper presentations of the polymer substrates, which are absent without the LipA and LipB proteins, are necessary for the proper surface translocation of capsular polymers.
In both K1 and K5 E. coli, kpsC and kpsS are the last two genes of a six-gene operon. In contrast, meningococcal lipA and lipB form an independent unit (Fig. 1), an organization similar to that found in E. coli K10 (10) and Campylobacter jejuni (21). Furthermore, meningococcal lipA and lipB are separated from other capsular genes by five genes unrelated to capsule expression and do not flank the capsule biosynthesis genes, a unique arrangement among many group II capsule-expressing bacteria. This organization in N. meningitidis may reflect DNA rearrangements and recombination events after the acquisition of capsule clusters through horizontal gene transfer.
The lack of secretion signals and transmembrane domains within the predicted amino acid sequences of proteins belonging to the LipA/KpsC and LipB/KpsS families suggests that these proteins are localized in the cytoplasm. Through fractionation and Western blot analysis, the LipA homologue KpsC was detected in both the membrane and cytoplasmic fractions of an E. coli K5-K-12 hybrid strain, while the LipB homologue KpsS was only detectable in the membrane fraction (36). KpsC and KpsS have been shown to individually associate with the membrane independently of each other, and this membrane association is not dependent on the ABC transporter proteins KpsM and KpsT (36). Interestingly, a mutation in either kpsC or kpsS results in the disappearance of membrane localization of two K5 capsule biosynthetic proteins (36). Furthermore, in vitro assays with membrane preparations of mutants with a defective kpsS or kpsC but an otherwise intact K5 capsule gene cluster yield diminished capsular polysaccharide synthesis (6). These findings have led to the hypothesis (36) that KpsC and KpsS are not only engaged in capsule translocation, but also play a role in the formation of the biosynthesis protein complex. The disruption of this hetero-oligomeric complex could explain the loss of K5 capsule lipidation in the K5-K-12 kpsC kpsS mutant background. Capsular polysaccharide purified from the meningococcal lipA lipB mutants was identical to that of the wild-type parent, supporting the translocation hypothesis and suggesting that the analogous biosynthetic complex in meningococci may remain assembled in the absence of the LipA and LipB proteins.
Since the lipA lipB mutants assembled and lipidated capsular polymers but were unable to transport them outside the cytoplasm, LipA and LipB may form a “delivery bridge” connecting the biosynthesis and transport complex or be involved in the initial stage of the capsule polymer translocation process and part of the transport complexes formed by CtrA to CtrD proteins. As the capsule translocation apparatus has to accommodate the highly negatively charged capsule polymers as well as the hydrophobic lipid moiety through the presumptive channel formed by the outer membrane porin CtrA and the ABC-type inner membrane protein CtrC, one can imagine that the lipid component of the capsule will have to be shielded from the hydrophilic environment during the transport process. LipA and LipB may thus function as chaperones necessary for shuffling lipidated capsule polymers from the biosynthesis protein complex to the ABC capsule transport machinery assembled by the Ctr proteins.
The enzymes responsible for the dipalmitoylphosphotidic substitution of the meningococcal capsular polymers are currently unknown. They appear to be cytoplasmically localized, as the accumulated polymers are already substituted with lipids within cytoplasm. In capsular polysaccharide elongation of serogroup B meningococci (26) and K1 E. coli (48), but not in the K5 system (12), undecaprenol phosphate may act as an intermediate carrier. The growing polymer is either polymerized directly on undecaprenol phosphate, or the sialic-undecaprenol intermediate is transferred to an unidentified acceptor molecule, where polymerization occurs (26, 48). Both hypotheses require a final step of diacylglycerophosphatidic acid substitution prior to capsule translocation. Interestingly, it has been shown that the enteric common antigen in Salmonella enterica serovar Typhimurium also links to a diacylglycerol moiety through a phosphodiester bond (35), although the gene encoding the enzyme is as yet unknown.
The phenotypes of the nonpolar mutations in lipA and lipB and the dual mutation in both lipA and lipB were identical. These data suggest that the functions of the two proteins are not redundant, but both are required for translocation of capsule polymers to the meningococcal surface. Interaction between E. coli KpsC and KpsS has not been demonstrated (5, 9, 13, 36). Attempts by us to detect the putative interaction between LipA and LipB with a bacterially based two-hybrid system (BacterioMatch II two-hybrid system; Stratagene) were unsuccessful (data not shown). These data suggest that lipA/kpsC and lipB/kpsS may function sequentially in the overall capsule transport process. We propose that lipA and lipB be renamed ctrE and ctrF, respectively, to correlate with their roles in capsule transport and to be consistent with the nomenclature of transport genes, ctrABCD, in meningococci. Furthermore, lipA and lipB are generally used for lipoic acid synthetase (NMB1216) and lipoate-protein ligase (NMB1217), respectively, which are present in the N. meningitidis genomes.
In contrast to wild-type meningococci, meningococci with mutations in lipA (ctrE), lipB (ctrF), and lipA and lipB are not protected from complement-mediated killing by human serum. Thus, LipA (CtrE) and LipB (CtrF) are required for the expression of a biologically functional capsule and are necessary for surface expression of a properly anchored capsule polymer. A signature-tagged mutagenesis study of N. meningitidis with the infant rat model identified lipB as one of the genes required to permit meningococcal bacteremia (42); and a similar signature-tagged mutagenesis study of uropathogenic E. coli (1) identified that a kpsC mutant was attenuated in survival within the murine urinary tract. Given the importance of meningococcal capsules in pathogenesis and the requirement of LipA (CtrE) and LipB (CtrF) for surface expression of meningococcal capsular polysaccharides, interference with the function of these proteins is a strategy in the treatment of invasive meningococcal disease and possibly other encapsulated gram-negative bacteria.
Acknowledgments
This work was supported by Public Health Service grants AI-33517 and AI-40247 from the National Institutes of Health to D.S.S.
We thank Hong Yi at the Neurology Microscopy Facility, Emory University, for help in the electron microscopy analysis. We are grateful to Yoon Kim Miller, Corie Noble, and Larry Martin for technical assistance and Lane Pucko for administrative assistance.
Editor: J. N. Weiser
REFERENCES
- 1.Bahrani-Mougeot, F. K., E. L. Buckles, C. V. Lockatell, J. R. Hebel, D. E. Johnson, C. M. Tang, and M. S. Donnenberg. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45:1079-1093. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee, A. K., H. J. Jennings, C. P. Kenny, A. Martin, and I. C. Smith. 1976. Structural determination of the polysaccharide antigens of Neisseria meningitidis serogroups Y, W-135, and BO1. Can. J. Biochem. 54:1-8. [DOI] [PubMed] [Google Scholar]
- 3.Bliss, J. M., and R. P. Silver. 1996. Coating the surface: a model for expression of capsular polysialic acid in Escherichia coli K1. Mol. Microbiol. 21:221-231. [DOI] [PubMed] [Google Scholar]
- 4.Boyce, J. D., J. Y. Chung, and B. Adler. 2000. Genetic organisation of the capsule biosynthetic locus of Pasteurella multocida M1404 (B:2). Vet. Microbiol. 72:121-134. [DOI] [PubMed] [Google Scholar]
- 5.Bronner, D., V. Sieberth, C. Pazzani, I. S. Roberts, G. J. Boulnois, B. Jann, and K. Jann. 1993. Expression of the capsular K5 polysaccharide of Escherichia coli: biochemical and electron microscopic analyses of mutants with defects in region 1 of the K5 gene cluster. J. Bacteriol. 175:5984-5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronner, D., V. Sieberth, C. Pazzani, A. Smith, G. Boulnois, I. Roberts, B. Jann, and K. Jann. 1993. Synthesis of the K5 (group II) capsular polysaccharide in transport-deficient recombinant Escherichia coli. FEMS Microbiol. Lett. 113:279-284. [DOI] [PubMed] [Google Scholar]
- 7.Caroff, M., A. Tacken, and L. Szabo. 1988. Detergent-accelerated hydrolysis of bacterial endotoxins and determination of the anomeric configuration of the glycosyl phosphate present in the “isolated lipid A” fragment of the Bordetella pertussis endotoxin. Carbohydr. Res. 175:273-282. [DOI] [PubMed] [Google Scholar]
- 8.Chung, J. Y., Y. Zhang, and B. Adler. 1998. The capsule biosynthetic locus of Pasteurella multocida A:1. FEMS Microbiol. Lett. 166:289-296. [DOI] [PubMed] [Google Scholar]
- 9.Cieslewicz, M., and E. Vimr. 1996. Thermoregulation of kpsF, the first region 1 gene in the kps locus for polysialic acid biosynthesis in Escherichia coli K1. J. Bacteriol. 178:3212-3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke, B. R., R. Pearce, and I. S. Roberts. 1999. Genetic organization of the Escherichia coli K10 capsule gene cluster: identification and characterization of two conserved regions in group III capsule gene clusters encoding polysaccharide transport functions. J. Bacteriol. 181:2279-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claus, H., U. Vogel, M. Muhlenhoff, R. Gerardy-Schahn, and M. Frosch. 1997. Molecular divergence of the sia locus in different serogroups of Neisseria meningitidis expressing polysialic acid capsules. Mol. Gen. Genet. 257:28-34. [DOI] [PubMed] [Google Scholar]
- 12.Finke, A., D. Bronner, A. V. Nikolaev, B. Jann, and K. Jann. 1991. Biosynthesis of the Escherichia coli K5 polysaccharide, a representative of group II capsular polysaccharides: polymerization in vitro and characterization of the product. J. Bacteriol. 173:4088-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frosch, M., and A. Muller. 1993. Phospholipid substitution of capsular polysaccharides and mechanisms of capsule formation in Neisseria meningitidis. Mol. Microbiol. 8:483-493. [DOI] [PubMed] [Google Scholar]
- 14.Frosch, M., C. Weisgerber, and T. F. Meyer. 1989. Molecular characterization and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc. Natl. Acad. Sci. USA 86:1669-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gotschlich, E. C. 1975. Development of polysaccharide vaccines for the prevention of meningococcal diseases. Monogr. Allergy 9:245-258. [PubMed] [Google Scholar]
- 16.Gotschlich, E. C., B. A. Fraser, O. Nishimura, J. B. Robbins, and T. Y. Liu. 1981. Lipid on capsular polysaccharides of gram-negative bacteria. J. Biol. Chem. 256:8915-8921. [PubMed] [Google Scholar]
- 17.Gotschlich, E. C., T. Y. Liu, and M. S. Artenstein. 1969. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J. Exp. Med. 129:1349-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart, C. A., and L. E. Cuevas. 1997. Meningococcal disease in Africa. Ann. Trop. Med. Parasitol. 91:777-785. [DOI] [PubMed] [Google Scholar]
- 19.Janik, A., E. Juni, and G. A. Heym. 1976. Genetic Transformation as a tool for detection of Neisseria gonorrhoeae. J. Clin. Microbiol. 4:71-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahler, C. M., L. E. Martin, G. C. Shih, M. M. Rahman, R. W. Carlson, and D. S. Stephens. 1998. The (α2→8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect. Immun. 66:5939-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlyshev, A. V., D. Linton, N. A. Gregson, A. J. Lastovica, and B. W. Wren. 2000. Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for Penner serotype specificity. Mol. Microbiol. 35:529-541. [DOI] [PubMed] [Google Scholar]
- 22.Kroncke, K. D., G. Boulnois, I. Roberts, D. Bitter-Suermann, J. R. Golecki, B. Jann, and K. Jann. 1990. Expression of the Escherichia coli K5 capsular antigen: immunoelectron microscopic and biochemical studies with recombinant E. coli. J. Bacteriol. 172:1085-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langsrud, S., and G. Sundheim. 1996. Flow cytometry for rapid assessment of viability after exposure to a quaternary ammonium compound. J. Appl. Bacteriol. 81:411-418. [DOI] [PubMed] [Google Scholar]
- 24.Liu, T. Y., E. C. Gotschlich, F. T. Dunne, and E. K. Jonssen. 1971. Studies on the meningococcal polysaccharides. II. Composition and chemical properties of the group B and group C polysaccharide. J. Biol. Chem. 246:4703-4712. [PubMed] [Google Scholar]
- 25.Liu, T. Y., E. C. Gotschlich, E. K. Jonssen, and J. R. Wysocki. 1971. Studies on the meningococcal polysaccharides. I. Composition and chemical properties of the group A polysaccharide. J. Biol. Chem. 246:2849-2858. [PubMed] [Google Scholar]
- 26.Masson, L., and B. E. Holbein. 1985. Role of lipid intermediate(s) in the synthesis of serogroup B Neisseria meningitidis capsular polysaccharide. J. Bacteriol. 161:861-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Brien, P. J. 1964. The synthesis of N-acetyl-alpha-and N-acetyl-beta-d-glucosamine I-phosphates (2-acetamido-2-deoxy-alpha- and beta-d-glucose I-phosphates). Biochim. Biophys. Acta 86:628-634. [DOI] [PubMed] [Google Scholar]
- 29.Parkhill, J., M. Achtman, K. D. James, S. D. Bentley, C. Churcher, S. R. Klee, G. Morelli, D. Basham, D. Brown, T. Chillingworth, R. M. Davies, P. Davis, K. Devlin, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, S. Leather, S. Moule, K. Mungall, M. A. Quail, M.-A. Rajandream, K. M. Rutherford, M. Simmonds, J. Skelton, S. Whitehead, B. G. Spratt, and B. G. Barrell.2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502-506. [DOI] [PubMed] [Google Scholar]
- 30.Pazzani, C., C. Rosenow, G. J. Boulnois, D. Bronner, K. Jann, and I. S. Roberts. 1993. Molecular analysis of region 1 of the Escherichia coli K5 antigen gene cluster: a region encoding proteins involved in cell surface expression of capsular polysaccharide. J. Bacteriol. 175:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelkonen, S., J. Hayrinen, and J. Finne. 1988. Polyacrylamide gel electrophoresis of the capsular polysaccharides of Escherichia coli K1 and other bacteria. J. Bacteriol. 170:2646-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 33.Rahman, M. M., J. Guard-Petter, K. Asokan, and R. W. Carlson. 1997. The structure of the capsular polysaccharide from a swarming strain of pathogenic Proteus vulgaris. Carbohydr. Res. 301:213-220. [DOI] [PubMed] [Google Scholar]
- 34.Reckseidler, S. L., D. DeShazer, P. A. Sokol, and D. E. Woods. 2001. Detection of bacterial virulence genes by subtractive hybridization: identification of capsular polysaccharide of Burkholderia pseudomallei as a major virulence determinant. Infect. Immun. 69:34-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rick, P. D., G. L. Hubbard, M. Kitaoka, H. Nagaki, T. Kinoshita, S. Dowd, V. Simplaceanu, and C. Ho. 1998. Characterization of the lipid-carrier involved in the synthesis of enterobacterial common antigen (ECA) and identification of a novel phosphoglyceride in a mutant of Salmonella typhimurium defective in ECA synthesis. Glycobiology 8:557-567. [DOI] [PubMed] [Google Scholar]
- 36.Rigg, G. P., B. Barrett, and I. S. Roberts. 1998. The localization of KpsC, S and T, and KfiA, C and D proteins involved in the biosynthesis of the Escherichia coli K5 capsular polysaccharide: evidence for a membrane-bound complex. Microbiology 144:2905-2914. [DOI] [PubMed] [Google Scholar]
- 37.Roberts, I. S. 1996. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu. Rev. Microbiol. 50:285-315. [DOI] [PubMed] [Google Scholar]
- 38.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, T. Popovic, and J. M. Hughes. 2001. Meningococcal disease: recent progress and future challenges. N. Engl. J. Med. 344:1378-1388. [DOI] [PubMed] [Google Scholar]
- 39.Roth, B. L., M. Poot, S. T. Yue, and P. J. Millard. 1997. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl. Environ. Microbiol. 63:2421-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satola, S. W., P. L. Schirmer, and M. M. Farley. 2003. Complete sequence of the cap locus of Haemophilus influenzae serotype b and nonencapsulated b capsule-negative variants. Infect. Immun. 71:3639-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stephens, D. S., J. S. Swartley, S. Kathariou, and S. A. Morse. 1991. Insertion of Tn916 in Neisseria meningitidis resulting in loss of group B capsular polysaccharide. Infect. Immun. 59:4097-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun, Y. H., S. Bakshi, R. Chalmers, and C. M. Tang. 2000. Functional genomics of Neisseria meningitidis pathogenesis. Nat. Med. 6:1269-1273. [DOI] [PubMed] [Google Scholar]
- 43.Swartley, J. S., J. H. Ahn, L. J. Liu, C. M. Kahler, and D. S. Stephens. 1996. Expression of sialic acid and polysialic acid in serogroup B Neisseria meningitidis: divergent transcription of biosynthesis and transport operons through a common promoter region. J. Bacteriol. 178:4052-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swartley, J. S., L. J. Liu, Y. K. Miller, L. E. Martin, S. Edupuganti, and D. S. Stephens. 1998. Characterization of the gene cassette required for biosynthesis of the (α1→6)-linked N-acetyl-d-mannosamine-1-phosphate capsule of serogroup A Neisseria meningitidis. J. Bacteriol. 180:1533-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swartley, J. S., A. A. Marfin, S. Edupuganti, L. J. Liu, P. Cieslak, B. Perkins, J. D. Wenger, and D. S. Stephens. 1997. Capsule switching of Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 94:271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taha, M. K., M. Achtman, J. M. Alonso, B. Greenwood, M. Ramsay, A. Fox, S. Gray, and E. Kaczmarski. 2000. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet 356:2159. [DOI] [PubMed] [Google Scholar]
- 47.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 48.Troy, F. A., I. K. Vijay, and N. Tesche. 1975. Role of undecaprenyl phosphate in synthesis of polymers containing sialic acid in Escherichia coli. J. Biol. Chem. 250:156-163. [PubMed] [Google Scholar]
- 49.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 50.Tzeng, Y. L., A. Datta, C. Strole, V. S. Kolli, M. R. Birck, W. P. Taylor, R. W. Carlson, R. W. Woodard, and D. S. Stephens. 2002. KpsF is the arabinose-5-phosphate isomerase required for 3-deoxy-d-manno-octulosonic acid biosynthesis and for both lipooligosaccharide assembly and capsular polysaccharide expression in Neisseria meningitidis. J. Biol. Chem. 277:24103-24113. [DOI] [PubMed] [Google Scholar]
- 51.Tzeng, Y. L., J. S. Swartley, Y. K. Miller, R. E. Nisbet, L. J. Liu, J. H. Ahn, and D. S. Stephens. 2001. Transcriptional regulation of divergent capsule biosynthesis and transport operon promoters in serogroup B Neisseria meningitidis. Infect. Immun. 69:2502-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Deuren, M., P. Brandtzaeg, and J. W. van der Meer. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 13:144-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vann, W. F., M. A. Schmidt, B. Jann, and K. Jann. 1981. The structure of the capsular polysaccharide (K5 antigen) of urinary-tract-infective Escherichia coli 010:K5:H4. A polymer similar to desulfo-heparin. Eur. J. Biochem. 116:359-364. [DOI] [PubMed] [Google Scholar]
- 54.Whitfield, C., and I. S. Roberts. 1999. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 31:1307-1319. [DOI] [PubMed] [Google Scholar]