Abstract
The V antigen (LcrV) of the plague bacterium Yersinia pestis is a potent protective antigen that is under development as a vaccine component for humans. LcrV is multifunctional. On the bacterial surface it mediates delivery of a set of toxins called Yops into host cells, and as a released protein it can cause production of the immunosuppressive cytokine interleukin-10 (IL-10) and can inhibit chemotaxis of polymorphonuclear neutrophils. It is not known how these mechanisms of LcrV operate, what their relative importance is, when they function during plague, and which are critical to protection by antibody. This study investigated several of these issues. C57BL/6 mice, mice unable to express IL-10, or mice with the macrophage lineage eliminated were treated with a protective anti-LcrV antibody or a nonprotective antibody against YopM and infected intravenously by Y. pestis KIM5 or a strain that lacked the genes encoding all six effector Yops. Viable bacterial numbers were determined at various times. The data indicated that Yops were necessary for Yersinia growth after the bacteria had seeded liver and spleen. Anti-LcrV antibody prevented this growth, even in IL-10−/− mice, demonstrating that one protective mechanism for anti-LcrV antibody is independent of IL-10. Anti-LcrV antibody had no effect on persistence in organs of Y. pestis lacking effector Yops, even though the yersiniae could strongly express LcrV, suggesting that Yops are necessary for building sufficient bacterial numbers to produce enough LcrV for its immunosuppressive effects. In vitro assays showed that anti-LcrV antibody could partially block delivery of Yops and downstream effects of Yops in infected macrophage-like J774A.1 cells. However, cells of the macrophage lineage were found to be dispensable for protection by anti-LcrV antibody in spleen, although they contributed to protection in liver. Taken together, the data support the hypothesis that one protective effect of the antibody is to block delivery of Yops to host cells and prevent early bacterial growth. The findings also identified the macrophage lineage as one host cell type that mediates protection.
The causative agent of plague, Yersinia pestis, is viewed as a facultative extracellular pathogen. It is able to survive and grow within macrophages (13, 48) and is invasive for epithelioid cells (17), but it possesses at least two properties designed to keep the bacteria extracellularly located, a protein fibrillar capsule called F1 and a protein called V antigen or LcrV. Antibodies against these two proteins protect against plague (26), and plague vaccines presently under development are based on F1 and LcrV and confer potent protection against both bubonic and pneumonic plague in mice, guinea pigs, and nonhuman primates (51). Anti-LcrV and anti-F1 antibodies also are potentially useful for postexposure prophylaxis against plague (1, 51).
LcrV is produced by all three human-pathogenic yersiniae, Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica. It is a multifunctional virulence protein encoded on a ca. 70-kb plasmid that also encodes a set of toxins called Yops and the Ysc type III secretion system (15, 16). LcrV was recognized from early studies on plague virulence to confer resistance to phagocytosis for Y. pestis (7). It is now recognized that this effect is due to the role of LcrV as part of the Ysc injection mechanism that delivers Yops into host cells upon bacterial contact (15, 16) (Fig. 1A). The Yops must be injected to have effect, and once within the host cell cytoplasm, they derange cellular signaling and cytoskeletal functions. There are six so-called effector Yops with known pathogenic effects; four of these act synergistically to incapacitate the actin cytoskeleton and are responsible for resistance to phagocytosis (15, 30). LcrV also has a regulatory function within the bacterial cell, where it binds and inactivates the Ysc gate protein LcrG that permits full Yop secretion activity (16) (Fig. 1A). LcrV is released into the medium during contact with host cells in vitro and into tissues during infection of animals (46) (Fig. 1A). In addition, it has been found to enter epithelioid cells by a contact-dependent mechanism (termed VCAT) that is distinct from the Ysc (21) (Fig. 1A), but the consequence of this entry is not yet known. Purified LcrV has been shown to be immunosuppressive by eliciting the production of interleukin-10 (IL-10) in vivo (38), and this is believed to be an important effect of the LcrV that is released by the yersiniae during contact with host cells. This activity of LcrV has been demonstrated in vitro with a monocyte/macrophage cell line (44), but in vivo this effect potentially could involve multiple cell types that produce IL-10 (36). Pure LcrV also has been shown to inhibit chemotaxis of polymorphonuclear neutrophils (PMNs) into sponges (56). This effect may contribute to a key histopathological feature of experimental plague, whereby lesions that form in liver and spleen have an initial acute inflammatory character followed by decomposition of PMNs and little further influx of cells (37, 52). Subsequently, cell-poor lesions spread over the entire liver and spleen. However, if the mice are immunized actively or passively against LcrV, waves of inflammatory cells migrate into sites of infection, protective granulomas develop, and the bacteria are cleared (37). The detailed mechanisms of all of these effects of LcrV, their timing during the course of infection, and their relative importance in pathogenesis of plague are not known.
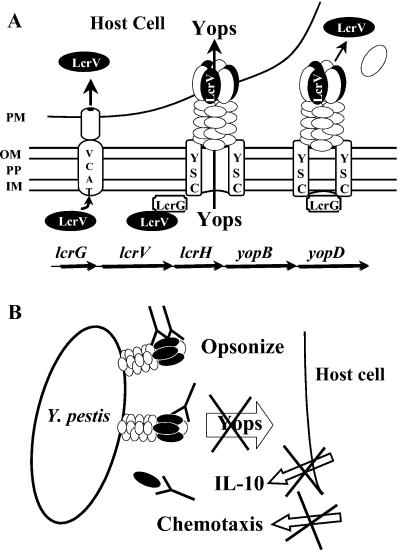
FIG. 1.
Locations and activities of LcrV and ways that anti-LcrV antibody might protect. (A) The bottom line of arrows depicts genes in the Y. pestis ysc delivery operon that encodes LcrV. LcrG is a gate protein for the Ysc that functions in the bacterial cytoplasm to control secretion activity. YopB and YopD, along with LcrV, participate in pore formation and Yops delivery to host cells. LcrH is a specific chaperone for YopB and YopD. The center of the panels shows that, upon contact with the plasma membrane (PM) of a host cell, LcrV acts in the bacterial cytoplasm to bind LcrG and activate the Ysc for secretion. It also is part of a putative complex with YopB and YopD that functions to create a pore in the PM and is essential for Yops delivery. The right-hand side of the panel shows that LcrV and probably also YopB and YopD are released into the medium during Yops delivery to a host cell. The figure depicts this release occurring from non-contact-activated Ysc units, but that has not been proven. The left-hand portion shows that LcrV also has been shown to be delivered into host cells by a contact-dependent mechanism termed VCAT, which has not yet been characterized. Abbreviations: OM, outer membrane of Y. pestis; PP, periplasm; IM, inner bacterial membrane. (B) Three ways that anti-LcrV antibody might decrease virulence of Y. pestis, all dependent upon surface accessibility of LcrV.
Anti-LcrV antibody potentially could neutralize any effect of surface-accessible LcrV (Fig. 1B). In vitro, two of these effects have been shown to be blocked by protective anti-LcrV antibodies. Anti-LcrV antibody can interfere with LcrV-mediated delivery of Yops, thereby promoting phagocytosis and blocking cytoskeletal derangement and apoptosis in macrophage-like cells infected with Yersinia (40, 43, 55). Anti-LcrV antibody also can block the ability of free LcrV to inhibit chemotaxis of PMNs into sponges (56). However, it is not known how important these potential protective mechanisms are during the course of plague. Because LcrV derivatives and anti-LcrV antibodies are being developed for prevention and prophylaxis against plague in humans, it is important to understand better when this protein functions during the course of the infection, how LcrV mediates its pathogenic effects, and what antibody against LcrV does and does not do to inactivate the multiple properties of LcrV.
This study investigated when anti-LcrV antibody protects during systemic plague in mice, how important Yops are to protection, and the role of cells of the macrophage lineage in mediating the protection. The data revealed that one protective mechanism of anti-LcrV antibody is to prevent Yops-dependent bacterial growth after the yersiniae have seeded liver and spleen. Macrophages were found to be important mediators of protection in liver but not in spleen.
MATERIALS AND METHODS
Bacteria and their cultivation.
Y. pestis KIM5 was obtained from R. R. Brubaker (KIM10 or substrain D27 in his nomenclature; Michigan State University). It is virulent from an intravenous (i.v.) route of infection but not from peripheral routes due to its chromosomal Δpgm mutation (53). In certain experiments, a multiple-Yop mutant (MYM) of Y. pestis KIM5 (Y. pestis KIM5-3004 [Y. pestis KIM5-MYM]) or of Y. pestis KIM8 (Y. pestis KIM8-3003.12 [Y. pestis KIM8-MYM]) was used. The MYM strains have in-frame deletions of all six genes for effector Yops but an otherwise intact Ysc. They were created by an allelic exchange method previously described, employing the suicide plasmid pLD55 (34), Escherichia coli SY327 (λpir) (35), and E. coli DH5α (Life Technologies, Grand Island, N.Y.) (60). The deletions were as follows: ΔyopM, −2 bp from ATG through TAG; ΔyopH, −3 bp from ATG through 53 bp past TAA; ΔyopE, −26 bp from ATG through 22 bp past TGA; ΔyopT, ATG through CTG (L306), last 51 bp intact to preserve sycT; ΔypkA/yopJ, −14 bp from ATG of ypkA through 78 bp past TAA of yopJ. Y. pestis KIM8 lacks the surface protease Pla that degrades Yops (47) that are secreted into the medium, and this strain and its MYM derivative were used in the one experiment that analyzed LcrV and Yops secreted into defined medium. E. coli strains were grown at 37°C in Luria-Bertani broth (33) or on Luria-Bertani agar appropriately supplemented with antibiotics. In general, yersiniae were grown for at least six generations in exponential phase in heart infusion broth (HIB; Difco Laboratories, Detroit, Mich.) containing 2.5 mM CaCl2. For the majority of experiments, they were washed once with phosphate-buffered saline (PBS; 135 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) by centrifugation and diluted into RPMI 1640 containing HEPES and l-glutamine (RPMI; Life Technologies) at the final desired concentration of bacteria.
Most of the experiments employed yersiniae grown at 26°C in HIB plus CaCl2 and then exposed to 37°C for only 2 h. These were prepared by incubating 1-ml amounts of Y. pestis grown at 26°C in wells of a 6-well cluster dish in RPMI in a CO2 incubator. At infection time, the yersiniae were recovered with scraping and pipetting to remove any bacteria adherent to the dishes. Yersiniae given this incubation for 2 h at 37°C in RPMI following pregrowth at 26°C have previously been shown to have LcrV on their surface (22), and the growth condition will be termed 26/37°C. For yersiniae pregrown only at 26°C, the yersiniae pregrown at 26°C in HIB plus CaCl2 were washed once with PBS and diluted in room temperature (RT) RPMI 1640 or PBS. For pregrowth of Y. pestis at 37°C, cultures were initiated at 26°C in HIB plus CaCl2 for ca. 5 h and then shifted to 37°C and grown for at least six generations in exponential phase before being washed in PBS and diluted in warm RPMI or PBS. Viable bacterial numbers recovered from mouse organs were determined as CFU after serially diluting the suspensions in PBS, plating on tryptose blood agar plates (Difco), and incubating the plates for 2 days at 30°C.
In an experiment to detect expression and secretion of LcrV and YopE in vitro, yersiniae were grown in the defined medium TMH with or without 2.5 mM CaCl2 essentially as described previously (49). Briefly, the bacteria were grown to ca. six generations in exponential phase at 26°C. They were then diluted to an A620 of 0.1, and growth was continued at 26°C for 2 h. The temperature was then shifted to 37°C, and growth was continued for 4 h. The bacteria were sedimented and solubilized in 6× electrophoresis sample buffer. The top half of the culture supernatant was recovered and trichloroacetic acid precipitated. Equivalent amounts of cells and supernatant were analyzed on immunoblots prepared from 12% (wt/vol) sodium dodecyl sulfate (SDS) polyacrylamide gels. Blots were probed with a mixture of the rabbit polyclonal anti-LcrV antibody used in protection experiments and antibody against YopE (a kind gift of Greg Plano, University of Miami Medical School) or with anti-YopD (57) or anti-YopH (60) antibody. Bands were visualized by treatment with alkaline phosphatase (AP)-conjugated goat anti-rabbit immunoglobulin G (IgG) (Sigma) and development with nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP; Sigma).
F1 and LcrV expression.
Y. pestis grown as described above at 26°C, 26/37°C, and 37°C were characterized for their total expression of LcrV and membrane-associated capsular protein F1. For LcrV, bacteria were sedimented and solubilized in 6× electrophoresis sample buffer, and proteins were resolved on 12.5% (wt/vol) SDS polyacrylamide gels. Blots were probed with anti-LcrV antibody and developed as described above. For membrane-associated F1, the bacteria were broken by two passages through a French press at 20,000 lb/in2, unbroken cells were removed by low-speed centrifugation, and total membranes were recovered by centrifugation. The total membrane pellet was solubilized in 6× electrophoresis buffer, and the proteins were resolved on 15% (wt/vol) SDS polyacrylamide gels. Blots were probed with monoclonal antibody YPF1 against F1 (Research Diagnostics, Inc., Flanders, N.J.), detected with AP-conjugated goat anti-mouse IgG (Sigma), and developed as described above. Surface expression of F1 on intact yersiniae was tested by immunofluorescence essentially as previously described for LcrV (21) but using a 1:2,000 dilution of YPF1, blocking with 10% fetal bovine serum (FBS; Life Technologies Inc.) plus 1% donkey serum (Sigma), and staining with Oregon Green-conjugated goat anti-mouse IgG (Molecular Probes, Inc., Eugene, Oreg.). Fluorescence was analyzed at 515 to 565 nm (fluorescein isothiocyanate [FITC] filter). Images were obtained with a Nikon Eclipse E800 microscope fitted with a Plan Fluor Phase 100× objective (Nikon USA via Fryer Co., Inc., Cincinnati, Ohio) and a Photometrics CoolSNAP cf CCD camera (Image Processing, Inc., North Reading, Mass.). Images were acquired and processed by using MetaMorph software (Universal Imaging Corp., Downingtown, Pa.).
Antibody preparation.
The protective polyclonal rabbit anti-LcrV IgG and nonprotective polyclonal rabbit anti-YopM IgG were described previously and were purified by using protein A-conjugated beads as previously described (21, 39). The concentration of antibody was determined by bicinchoninic acid protein assay (Pierce Chemical Co., Rockford, Ill.), and dilutions were made in PBS.
Infection of mice; assays for protection and virulence.
Unless otherwise stated, mice were females 5 to 8 weeks old. When only C57BL/6 mice were used in an experiment, they were obtained from the National Cancer Institute. IL-10 knockout mice B6.129P2-Il10tm1Cgn/J and control C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, Maine). Three experiments employed newly developed transgenic macrophage Fas-induced apoptosis (Mafia) mice on a C57BL/6 background (5). As previously described, these mice were bred and confirmed by PCR to be homozygous for the transgene before use in experiments (5). Mice were between 5 and 8 weeks old and both sexes were used, distributed equally among treatment groups. Cells of the macrophage lineage can be eliminated for these mice by treatment with the dimerizer drug AP20187 (gift of Ariad Pharmaceuticals, Inc., Cambridge, Mass.). Seven days prior to infection, the elimination regimen was initiated by i.v. injection of 10 mg of dimerizer drug or mock injection solution (water containing 4% [vol/vol] ethanol, 10% [wt/vol] polyethylene glycol 400, and 1.7% [vol/vol] Tween-20)/kg of body weight. Injection was done within 30 min of dilution of dimerizer into the injection solution, and the volume, adjusted by body weight, averaged 100 μl per mouse. The mice received daily injections of drug or mock solution for 5 days, and their weights were recorded daily to document the weight loss that correlates with effectiveness of the drug treatment (5). In one experiment we also verified depletion of macrophage-related cells by histochemical staining for green fluorescent protein (GFP) expressed by the transgene construct in cells of the macrophage lineage (5). Following treatment with dimerizer or injection solution, the mice were allowed to rest for 2 days before being infected with Y. pestis.
In two experiments, C57BL/6 mice were treated with the neutralizing rat anti-mouse IL-10 monoclonal antibody JES5-2A5 or the neutralizing rat anti-mouse IL-4 monoclonal antibody 11B11 (BD Biosciences Pharmingen, San Diego, Calif.) before being infected with Y. pestis KIM5. The mice received the antibody in two doses of 200 μg given at 18 and 1 h before infection (for a total of 400 μg of antibody). At 18 h before infection they also received a separate intraperitoneal (i.p.) injection of 200 μg of anti-LcrV or anti-YopM antibody.
T. assay protection against Y. pestis, mice were given anti-LcrV or anti-YopM i.p. before being infected with Y. pestis. A preliminary set of tests established the dose of anti-LcrV antibody and the time of its administration to achieve maximal protection with the minimum amount of antibody (data not shown). The resulting protocol was to give a single 100-μg dose of anti-LcrV or anti-YopM i.p. ca. 18 h prior to infection; this procedure was followed in all experiments unless otherwise indicated. The mice were infected i.v. in the retro-orbital plexus with 0.1 ml of Y. pestis in PBS. Groups of three mice each were humanely killed at various times postinfection, their spleens were recovered and dissociated individually in water or PBS by using a Stomacher 80 Lab Blender (Tekmar Co., Cincinnati, Ohio), and dilutions of each resulting lysed cell suspension were plated for CFU. In one experiment, duplicate groups of mice were infected i.v. in the retro-orbital plexus or in the tail vein.
To establish useful bacterial doses and minimize mouse usage, we determined the 50% lethal infectious dose (LD50) of Y. pestis for mice given anti-LcrV or anti-YopM. Groups of four C57BL/6 or four B6.129P2-Il10tm1Cgn/J mice were treated i.p. with antibody, and 18 h later they were infected i.v. with 10-fold dilutions of Y. pestis in PBS, the dilutions being chosen to bracket an anticipated LD50 dose. The mice were observed for 14 days for signs of illness, recovery, and mortality. The LD50 was calculated by the method of Reed and Muench (41).
Tests of significant differences in data sets from animal experiments employed the unpaired student's t test.
Growth and infection of mammalian cells.
Cultures of J774A.1 macrophage-like cells (American Type Culture Collection, Manassas, Va.) were initiated at 105 cells per well in 6-well cluster dishes containing 2 ml of RPMI plus 10% FBS per well. Duplicate wells were allowed for each treatment or infection. When microscopic analysis was to be performed, the wells contained glass coverslips that could be removed individually and two coverslips were allowed per treatment. The dishes were incubated at 37°C with 5% CO2 for 2 to 3 days to achieve near confluency (5 × 105 to 9 × 105 cells per well) on the day of the experiment. Thirty minutes before infection, the medium was removed and the cells were washed twice with warm PBS. In some experiments, the J774A.1 cells were pretreated with 500 μg of human gamma globulin (HgG; Sigma)/ml to prevent nonspecific binding of antibody to the cells. Just before infection, the bacterial suspension containing anti-LcrV or anti-YopM antibody was supplemented with HgG at 50 μg/ml. The PBS/HgG on the mammalian cells was then removed, and 2 ml of bacterial suspension was added per well. This blocking treatment did not affect experimental outcome. Unless otherwise indicated, the multiplicity of infection was 10. Infection was initiated by centrifugation for 5 min at 200 × g at room temperature, and then the plates were incubated for 2 h for assessment of apoptosis by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay or 4 h for measurement of Yops delivery by immunoblot and assay of apoptosis by cleavage of poly(ADP-ribose polymerase) (PARP).
Assays for Yops delivery.
Yops delivery during infection of cells in culture was measured by detecting the Yops directly in the mammalian cell cytosol by immunoblot or indirectly through the known effects of Yops: cytotoxicity (retracting of cellular processes and rounding up) due to the combined effects of YopH, YopE, YopT, and YpkA or apoptosis due to YopJ measured by TUNEL assay or immunoblot to detect PARP cleavage. For immunoblot assays, wells received 1 ml of (300 μg/ml) trypsin (giving a final volume 100 μg/ml) and then a protease inhibitor such as Pefabloc (Boehringer Mannheim, Indianapolis, Ind.) at 900 μg/ml or a mixture of 30 μg of each (final concentration) of Pefabloc, aprotinin, and leupeptin (Boehringer Mannheim)/ml. Medium, soluble cytoplasmic, and debris fractions were then obtained in the presence of protease inhibitors as previously described (21). The debris containing yersiniae, membranes, and large organelles was discarded. Other fractions were TCA precipitated, and equivalent amounts were resolved by SDS-12% polyacrylamide gel electrophoresis (PAGE), transferred to Immobilon P (Millipore Inc., Billerica, Mass.), and probed with a mouse anti-YopH antibody and a rabbit antibody to YopE as previously described (21). Blots were developed as described above or (as shown in Fig. 7) were treated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Sigma) and developed by enhanced chemiluminescence (ECL; SuperSignal West Pico chemiluminescent substrate from Pierce Chemical Co.). PARP was detected with a rabbit anti-PARP antibody from Cell Signaling Technology, Inc. (Beverly, Mass.), followed by ECL.
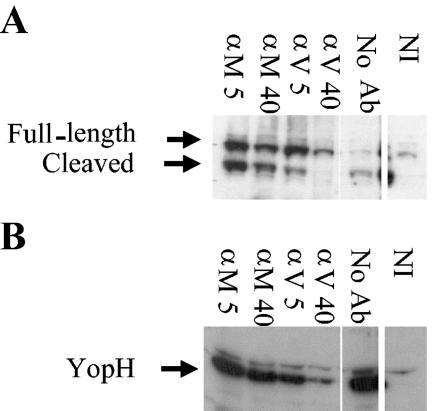
FIG. 7.
Anti-LcrV antibody significantly but incompletely inhibits delivery of Yops to J774A.1 cells infected by Y. pestis KIM5. J774A.1 cells were infected at a multiplicity of infection of 10 with 26/37°C-grown Y. pestis KIM5 in the presence of anti-YopM (αM) or anti-LcrV (αV) antibody. After 4 h, cleavage of the caspase substrate PARP was assayed to measure the ability of anti-LcrV antibody to inhibit delivery of YopJ, which activates the apoptotic cascade (A). (B) Delivery of YopH to the cytosol of J774A.1 cells was measured by immunoblot. Labels: 5, 5 μg of antibody/ml; 40, 40 μg of antibody/ml; No Ab, cells were infected in the absence of antibody; NI, noninfected J774A.1 cells. The illustrated experiment is representative of five experiments done on different days for YopH delivery. PARP cleavage was assayed twice; other downstream effects of caspase activation (TUNEL, trypan-blue exclusion) were measured in other experiments.
Cytotoxicity was assessed by cellular morphology in phase-contrast micrographic images. The TUNEL assay was performed with the In Situ Cell Death Detection kit (Roche Diagnostics Corp.) according to the manufacturer's instructions. Fluorescence was analyzed at 515 to 555 nm (FITC filter). Images were obtained as described above. As a corroborative assay, J774A.1 cells in suspension were infected in the presence of anti-LcrV or anti-YopM antibody in polypropylene tubes and tested periodically for exclusion of the dye trypan blue (present at 0.04% [wt/vol] in PBS; Invitrogen Corp., Carlsbad, Calif.). Numbers of trypan blue-positive (staining) and -negative cells were determined by counting samples in a hemacytometer.
RESULTS
Virulence of 26/37°C Y. pestis KIM5 in the presence of protective and nonprotective antibody.
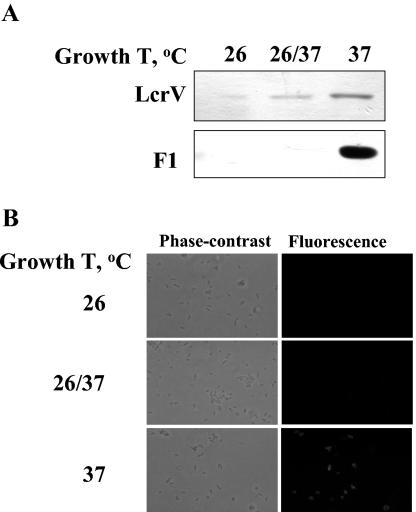
The goal of this study was to identify activities of LcrV that are blocked by anti-LcrV antibody, determine when during infection these activities are important for pathogenesis, and better understand host properties important for passive protection. We focused on events that take place early after infection. In all of the experiments, C57BL/6 mice were treated with a protective rabbit antibody against LcrV or a nonprotective rabbit antibody against YopM (21, 39) and then infected with Y. pestis KIM5. The yersiniae were grown at 26°C followed by a 2-h static incubation at 37°C, because bacteria treated this way were previously shown to have LcrV on their surface (21). Yersiniae grown this way expressed very little capsule. The F1 protein was barely detectable by immunoblot (Fig. 2A) and was not detectable on the bacterial surface by immunofluorescence (Fig. 2B), consistent with earlier findings (6, 8, 9, 13). Such yersiniae were not compromised in virulence, because F1 is not a virulence determinant for any route of infection in mice (7, 23, 59). Accordingly, by minimizing the coating of the bacteria with capsular fibrils and exposing LcrV, this growth condition might enhance the detection of early effects during infection that are due to the presence of LcrV on the bacterial surface.
FIG. 2.
Expression of LcrV and the F1 capsular protein by Y. pestis KIM5. (A) The yersiniae were grown with shaking in exponential phase in HIB at 26 or 37°C or at 26°C followed by a 2-h static incubation in RPMI in a CO2 incubator at 37°C (26/37). Bacterial cells were solubilized in electrophoresis sample buffer and analyzed by immunoblot for expression of LcrV and the capsular protein F1. (B) Yersiniae grown as described above were analyzed for surface expression of F1 by immunofluorescence.
To know the range of bacterial doses over which the antibody treatment would be protective, we carried out protection LD50 assays. These tests also determined if the virulence of the bacteria was different after the 26/37°C treatment compared to that after the more commonly used growth condition of 26°C. For 26/37°C-grown Y. pestis, anti-LcrV antibody afforded protection of C57BL/6 mice against bacterial doses as high as 6 × 105 (Table 1). All doses tested in mice given anti-YopM antibody were lethal (data not shown); we know from other studies that the LD50 for Y. pestis KIM5 in non-antibody-treated mice is less than 10 (unpublished data). The mean times to death (MTD) for mice treated with anti-LcrV or anti-YopM antibody were the same (3.5 ± 0.5 day versus 3.2 ± 0.5 day, respectively; based on pooled data for two experiments using doses of 2.4 × 106 and 1.6 × 106 26/37°C Y. pestis). Interestingly, the yersiniae grown at 26/37°C were more virulent than 26°C-grown Y. pestis, as reflected in a lower LD50 dose in the presence of anti-LcrV antibody. Based on these findings, we chose a dose of 4 × 105 to 8 × 105 yersiniae for experiments with C57BL/6 mice to be near the upper limit and hence best reveal any loss of protection due to experimental treatments. A high dose also would permit us to follow the time course of clearance of viable bacteria for several time points after infection.
TABLE 1.
LD50 of Y. pestis KIM5 in the presence of anti-LcrV or anti-YopM antibody in C57BL/6 and IL-10−/− mice
| Mouse strain | Growth condition(s) | Anti-LcrVa | Anti-YopMa |
|---|---|---|---|
| C57BL/6 | 26°C | 1 × 106 ± 1 × 106 (n = 2)b | |
| 26/37°C | 4 × 105 ± 2 × 105 (n = 3) | ||
| B6.129P2-Il10tm1Cgn/J | 26°C | 2 × 106 ± 0.5 × 106 (n = 2) | 6 × 105 (n = 1) |
| 26/37°C | 1 × 106 ± 1 × 106 (n = 2) | 4 × 105 ± 0.5 × 105 (n = 2) |
Groups of 4 mice were given a single dose of 100 μg of antibody the day before infection with Y. pestis KIM5 grown as indicated. Values are averages ± standard deviations or range from three or two replicate experiments, respectively. The number of experiments (n) is given in parentheses.
A third experiment gave an LD50 of >106.
Anti-LcrV antibody can protect in IL-10−/− mice.
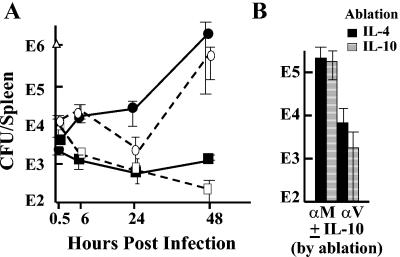
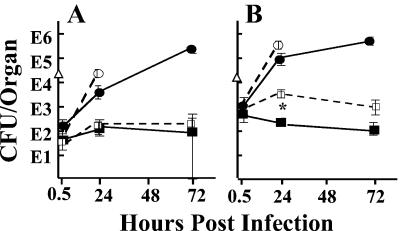
We wanted to test the importance of inhibition of Yop delivery in the absence of LcrV's effect on IL-10 production, and accordingly we made tests for protection by anti-LcrV antibody in transgenic mice lacking a functional gene for IL-10 and in C57BL/6 mice given anti-IL-10 antibody to neutralize IL-10. Because IL-10−/− C57BL/6 mice are highly resistant to Y. enterocolitica O:8 (44), we first tested whether this was the case for Y. pestis. Table 1 shows that IL-10−/− mice given the nonprotective anti-YopM antibody were remarkably resistant to lethality from experimental plague, although their MTD was not significantly different from that for similarly treated C57BL/6 mice (4.6 ± 1.8 days; based on pooled data for two experiments with doses of 2.4 × 106 and 1.6 × 106 26/37°C Y. pestis). Nonetheless, a dose of ca. 106 Y. pestis would be predicted to be lethal, and delivery of Yops might be crucial for lethality. To make the test, we gave the lowest dose that would be lethal for both C57BL/6 and B6.129P2-Il10tm1Cgn/J mice in the absence of anti-LcrV antibody (ca. 106 per mouse; Fig. 3A). In both IL-10−/− and control mice anti-LcrV antibody enabled infected mice to control bacterial growth in the spleen. We also tested C57BL/6 mice for which IL-10 was abolished by a neutralizing anti-IL-10 antibody and control mice that received similar dosing with a neutralizing anti-IL-4 antibody by measuring CFU in spleens at 24 h postinfection (hpi) (Fig. 3B). In this test, anti-LcrV antibody again protected equally well in both sets of mice. Moreover, anti-LcrV-treated IL-10−/− mice given doses slightly above the LD50 (2.4 × 106 and 1.6 × 106 26/37°C Y. pestis) had significantly longer MTD than anti-LcrV-treated C57BL/6 mice given the same doses: 6.8 ± 0.5 days (P < 0.0001). These findings show that a function of LcrV that is independent of IL-10 production plays a critical role in virulence. We hypothesize that this function is the delivery of Yops to host cells.
FIG. 3.
Anti-LcrV can protect in IL-10−/− mice. (A) Transgenic mice unable to express IL-10 or control C57BL/6 mice were infected with Y. pestis KIM5 (in the dose plotted at time zero as a triangle). At various times postinfection, viable bacterial numbers were determined in spleens of three mice per antibody treatment. Circles, mice were treated with anti-YopM antibody; squares, mice were treated with anti-LcrV antibody. Solid symbols and lines, C57BL/6 mice; open symbols and dashed lines, IL-10−/− mice. (B) C57BL/6 mice were given a neutralizing antibody for mouse IL-10 or IL-4 18 and 1 h before infection. At 18 h before infection they also received a 200-μg dose of anti-LcrV (αV) or anti-YopM (αM) antibody. Viable bacterial numbers (CFU) in spleens of 3 mice per treatment were determined at 24 hpi. Solid bars indicate mice given the anti-IL-4 control antibody; striped bars indicate mice given anti-IL-10 antibody. In both panels, one of two similar experiments is depicted; error bars are ±1 standard deviation from the mean.
Anti-LcrV antibody does not protect very early in infection.
For both C57BL/6 and IL-10−/− mice, the bacterial numbers recovered in liver and spleen at 30 min postinfection were two to three orders of magnitude lower than the input dose (Fig. 3A and data not shown). The retro-orbital site for i.v. inoculation was not responsible for this drop, because essentially identical results were obtained when mice were infected in the tail vein (data not shown). Treatment of liver with collagenase to improve the disruption of connective tissue did not result in greater numbers of CFU recovered (data not shown), suggesting that the tissue homogenization method used in this study did not limit bacterial recovery. Regardless of the mechanism for the low bacterial recovery (see Discussion), the numbers of yersiniae that were recovered at the 30-min time point in spleens were very similar for mice treated with anti-LcrV and anti-YopM antibody (Fig. 3A). This suggested that anti-LcrV antibody has little effect on this initial clearance or sequestering process. To test this hypothesis, we compared the viable numbers of Y. pestis in mice given antibody at 6 h after infection with a set of mice treated with antibody as usual, at 18 h before infection. Figure 4 shows that the infection dynamics were the same in the two cases, supporting the conclusion that the major protective effect of anti-LcrV antibody was not in preventing initial bacterial clearance but at a subsequent phase of infection.
FIG. 4.
Test for role of anti-LcrV antibody in initial bacterial clearance. C57BL/6 mice were treated with anti-YopM antibody at 18 h before infection (circles) or with anti-LcrV antibody (squares) at 18 h before (solid symbols) or 6 h after (open symbols) infection and infected at time zero with Y. pestis KIM5 grown at 26/37°C. At various times postinfection, viable bacterial numbers (CFU) were determined in spleens (A) or livers (B) from 3 mice per antibody treatment. The actual bacterial dose given to the mice is plotted at time zero (triangles). The plates contained less than 30 CFU for spleen samples from anti-LcrV-treated mice at 72 hpi (denoted by the arrow in panel A). One of two similar experiments is depicted; error bars are ±1 standard deviation. (B) The data at 72 h for pre- and postinfection treatment with anti-LcrV antibody differed (P = 0.0005 [*]), but it is not known if this difference is biologically significant.
Effect of anti-LcrV antibody in the absence of effector Yops.
We had found that anti-LcrV antibody has an IL-10-independent protective effect but that IL-10−/− mice were highly resistant to plague. This suggests that modulation of IL-10 expression by free LcrV could be an important or even the major way that LcrV subverts host defenses. Accordingly, we wanted to determine if there is a protective effect of anti-LcrV antibody that is independent of effector Yops. If free LcrV causes IL-10 production with resulting immunosuppression, and if protection by anti-LcrV antibody works at least in part by neutralizing this role of LcrV, then a strain lacking all six effector Yops might persist in animals longer in the presence of nonprotective anti-YopM antibody than when our protective anti-LcrV antibody is present. This experiment built on the design of one by Une and Brubaker (54), where Y. pestis, Y. pseudotuberculosis, or Y. enterocolitica lacking the Lcr virulence plasmid that encodes LcrV, Yops, and the Ysc type III secretion system was cleared more slowly from mice given an LcrV-containing extract from Y. pseudotuberculosis than in mice given an extract lacking LcrV (adsorbed with anti-LcrV antibody to remove LcrV or prepared from Y. pseudotuberculosis lacking the virulence plasmid). In the present case, the hypothesis being tested was that our protective anti-LcrV antibody could promote clearance by neutralizing LcrV produced in vivo in the absence of effector Yops.
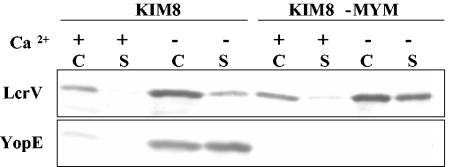
The test was made by infecting antibody-treated C57BL/6 mice with Y. pestis KIM5-MYM (multiple-Yop mutant), which has in-frame deletions removing the genes for the six effector Yops. This strain still has a functional LcrV-encoding Yops delivery operon: if YopM was expressed in trans from a plasmid, the MYM Y. pestis could deliver YopM to HeLa cells (T. Myers-Morales and S. C. Straley, unpublished data). It was crucial for this experiment that the MYM strain of Y. pestis still secreted LcrV at least as well as the parent strain. To test this, Y. pestis strains KIM8 and KIM8-MYM were grown in defined medium under conditions that promote (no added Ca2+) or inhibit (2.5 mM CaCl2) expression and secretion of LcrV and Yops. The KIM8 Y. pestis strains were used in this test, because they lacked the pPCP1 plasmid and hence the surface Pla protease present in Y. pestis KIM5 that degrades extracellular Yops (47). Otherwise, they were the same as the strains to be tested in mice. The MYM strain in fact expressed and secreted LcrV more strongly than did the parent strain (Fig. 5). This was also true for YopD, which is a type III secretion system component that, like LcrV, is part of the delivery mechanism for Yops and is encoded in the same operon as LcrV (Fig. 1A; data not shown). The MYM strain, of course, did not express YopE (Fig. 5) or other effector Yops (data not shown).
FIG. 5.
Expression and secretion of LcrV by Y. pestis KIM8 and Y. pestis KIM8-MYM. Growth was initiated at 26°C in defined medium containing (+) or lacking (−) 2.5 mM CaCl2; the temperature was shifted to 37°C, and growth was continued for 4 h. Whole cells (C) and culture supernatants (S) were recovered and analyzed by immunoblot probed with a mixture of antibodies against LcrV and YopE.
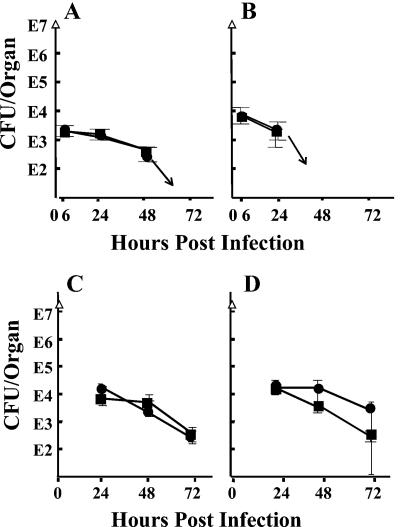
We chose a high bacterial dose for this experiment to allow for loss of the Yops-mediated antihost effects absent in the MYM strain. Indeed, 10- to 100-fold fewer bacteria seeded liver and spleen, respectively, by 6 hpi than when the infecting strain possessed effector Yops (compare Fig. 6 to Fig. 3 and 4). This indicated indirectly that Yops do play a role very early in infection, even though anti-LcrV has no effect at this time: effector Yops allow more yersiniae to seed liver and spleen (see Discussion). During the entire experiment, the mice treated with anti-LcrV or anti-YopM antibody showed no significant difference in viable numbers of Y. pestis KIM5-MYM, suggesting that there was no Yops-independent protective effect by anti-LcrV antibody. One experiment of the same design compared outcomes in C57BL/6 and IL-10−/− mice to see if there would be a greater protective effect of anti-LcrV antibody when IL-10 could be expressed than in mice unable to make this cytokine. The data for the two sets of mice were identical up to 24 hpi (data not shown). Longer times could not be examined, because the IL-10−/− mice died by day 2 postinfection.
FIG. 6.
Test for protection by anti-LcrV antibody in the absence of effector Yops. C57BL/6 mice were treated with anti-YopM (circles) or anti-LcrV (squares) antibody and infected with Y. pestis strain KIM5-MYM, which is unable to express any of the six effector Yops. At various times postinfection, viable numbers (CFU) were determined in spleens (A) and livers (B) of 3 mice per treatment. The plates contained less than 30 colonies at 72 h for spleen and after 48 h for liver (denoted by arrows). In a modified protocol to eliminate possible limitation by the amount of anti-LcrV antibody given the mice, the preimmunizing dose of anti-LcrV and anti-YopM antibody was 500 μg; on days 1 (6 hpi) and 2 (30 hpi), the mice received 200-μg doses of antibody. CFU are shown for spleens (C) and livers (D). The doses given the mice are plotted at time zero (triangles). Panels A and B represent one of two similar experiments. The modified protocol of panels C and D was done once. Error bars are ±1 standard deviation.
We were concerned that the somewhat stronger expression of LcrV by Y. pestis KIM5-MYM might have titrated the single bolus of anti-LcrV antibody in these experiments with large numbers of input yersiniae. To overcome this possible limitation, mice were given 500 μg of antibody instead of 100 μg at 18 h before infection and then were given additional treatments of 200 μg at 6 and 30 hpi. However, there still was no significant difference in bacterial clearance between mice treated with anti-LcrV antibody and those that received the nonprotective anti-YopM antibody (Fig. 6C and D). These data demonstrate that in the absence of Yops expression, anti-LcrV antibody does not exhibit its protective effect.
Anti-LcrV antibody partially inhibits Yop delivery to the cytosol of J774A.1 cells.
Macrophages are believed to be key host cells that encounter Y. pestis when the bacteria seed liver and spleen, hence this cell potentially is important for delivery of Yops and as a site of action of anti-LcrV antibody. Accordingly, we wanted to test whether our protective antibody would block delivery of Yops and prevent deleterious effects of Yops in a model system where the yersiniae infect macrophage-like J774A.1 cells in culture. Figure 7A shows an assay for protection by anti-LcrV against a downstream effect of YopJ delivery, cleavage of the caspase substrate PARP. Anti-LcrV at 40 μg/ml, which is the estimated peak concentration in mice treated with this antibody in protection experiments, caused essentially complete inhibition of PARP cleavage. Anti-YopM antibody had little effect. Two other tests for downstream apoptotic effects of YopJ, trypan-blue exclusion and TUNEL assay, similarly showed a strong inhibitory effect of anti-LcrV, but not anti-YopM, antibody (data not shown). However, the effects of Yops on the actin cytoskeleton were not completely blocked, although they were delayed (data not shown). When delivery of Yops was measured more directly by immunoblot of samples of the cytoplasm from the infected J774A.1 cells, a significant but incomplete inhibitory effect was seen when anti-LcrV antibody was present at 40 μg/ml (Fig. 7B for YopH; data not shown for YopE). In eight independent experiments, we never saw complete blockage of Yop delivery, despite the large amount of anti-LcrV antibody that had been present with the bacteria starting at 30 min prior to infection. In two of the experiments, there was essentially no inhibition of Yop delivery by anti-LcrV antibody, and in a third experiment, there was a small inhibitory effect. These findings show that anti-LcrV antibody can inhibit Yop delivery by Y. pestis KIM5 to J774A.1 cells but that the inhibition is not complete. The larger effect of anti-LcrV antibody on apoptosis due to YopJ may reflect the need for delivery of a large amount of Yops to cause apoptosis, as speculated by Welkos et al. (55): the partial inhibition of Yops delivery due to anti-LcrV antibody in our experiments was sufficient to prevent the build-up of enough YopJ to cause apoptosis during the time frame of our assay.
Role of macrophages in protection by anti-LcrV antibody.
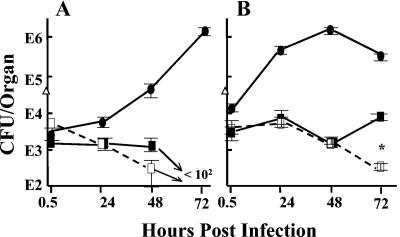
We next tested the importance of macrophages as mediators of protection by anti-LcrV antibody to determine if our in vitro data showing some inhibition of Yops delivery by anti-LcrV antibody correlated with any loss of protection in vivo. We exploited a newly created transgenic mouse to determine the relative contribution of monocytes/macrophages to the protection in vivo. The macrophage Fas-induced apoptosis (Mafia) mouse expresses a modified Fas from the macrophage-specific c-fms promoter and hence only in cells of the macrophage lineage (5). The transgene permits the dimerization of Fas by the dimerizer drug AP20187, with resulting induction of apoptosis and depletion of macrophage-related cells (e.g., macrophages, monocytes, dendritic cells, and Langerhans cells) by 70 to 90% from all tested body compartments except brain. (Tested compartments included peritoneum, spleen, blood, bone marrow, and skin; we confirmed depletion in liver and spleen [data not shown].) Accordingly, Mafia mice were pretreated with the dimerizer for 5 days (5) or were mock treated with the drug injection solution. On the sixth day mice were given anti-LcrV or anti-YopM antibody, and the next day they were infected with 26/37°C-grown Y. pestis KIM5. By 24 h there were at least 10-fold more yersiniae recovered from livers of drug-treated than from mock-treated mice that received anti-LcrV antibody (Fig. 8B). The bacterial numbers began to decline slowly over the next 2 days, but a difference persisted between the two mouse groups (Fig. 8B and data not shown). These results indicate that macrophages make a major contribution to protection in liver but that another cell type also contributes, and in the absence of macrophages this cell(s) can control bacterial numbers. In spleen, both the drug-treated and mock-treated mice given anti-LcrV antibody showed similar numbers of yersiniae up to 48 hpi, and anti-LcrV antibody prevented significant net growth (Fig. 8A and data not shown). Meanwhile, yersiniae in livers and spleens of mice treated with anti-YopM antibody grew similarly whether the mice were mock or dimerizer treated. (The dimerizer-treated mice given anti-YopM antibody did not survive to days 2 postinfection in the experiment illustrated; however, in a repetition of the experiment in which viable numbers were analyzed on days 1, 2, and 3 postinfection, they did survive to day 3 and were practically identical to the mock-treated mice in viable numbers recovered from livers and spleens on all days.) These findings indicate that in spleen, macrophages make little contribution to the anti-LcrV-related restraining of bacterial growth, and therefore a cell(s) other than the macrophage can mediate the protective effect of anti-LcrV antibody.
FIG. 8.
Test for the contribution of macrophages to protection by anti-LcrV antibody. Transgenic macrophage Fas-induced apoptosis (Mafia) mice were depleted of macrophage-related cells by treatment with dimerizer drug AP20187 (dashed lines and open symbols) or were given mock treatment with injection solution (solid lines and symbols) before being treated with anti-YopM (circles) or anti-LcrV (squares) antibody and infected as described earlier with Y. pestis KIM5 (dose plotted as triangles at time zero). At various time points postinfection, viable bacterial numbers (CFU) were determined in spleens (A) and livers (B) of groups of 3 mice per treatment. One of three similar experiments is illustrated. Error bars represent ±1 standard deviation. (B) Data at 24 h for mock- and drug-treated mice given anti-LcrV antibody were different at P < 0.0001 (*).
DISCUSSION
In this study we examined the mechanism of protection against plague by a protective rabbit polyclonal anti-LcrV antibody. The yersiniae were grown so as to display LcrV on their surface but not have much surface-exposed capsular fibril F1. This condition might model the state of the yersiniae 2 h after delivery by fleabite. Interestingly, 26/37°C-grown Y. pestis was increased in virulence for C57BL/6 mice by severalfold compared to that of yersiniae grown at 26°C. Previous studies have documented that Y. pestis grown 18 h at 37°C is ca. 20-fold more virulent (12) and initiates growth more rapidly in guinea pigs than Y. pestis grown in vitro at 26°C (24, 27). This is thought to underlie the explosive development of pneumonic plague, in which the bacteria directly transmitted person to person would be fully adapted for growth in a mammalian host (12). Part of this adaptation may include the induction of the Ysc type III secretion system and deployment of LcrV on the bacterial surface.
In our animal model of systemic plague, we found to our surprise that anti-LcrV antibody has no effect during the first several hours of infection: antibody could be administered at 6 hpi with the same outcome as pretreatment at 18 before infection. We know from previous work (5) that after retro-orbital i.v. injection, yersiniae are detectable in blood at 15 min postinfection and are seeding liver and spleen, a time course that is consistent with findings for an intravenously challenged guinea pig model (27). In the present study, the bacterial numbers recovered in organs showed an initial drop compared to the amount of input CFU (e.g., by 15- to 200-fold in spleens of mice treated with anti-YopM). Much of this drop may be due to the tight adherence or invasion of the yersiniae in tissue that was excluded when dilutions were prepared for plating. Recent experiments analyzing the distribution of yersiniae in tissues have found that 44% ± 1% of the injected bacteria can be recovered at 30 min postinfection in liver and spleen if the whole organs, including all tissue debris, are spread onto large plates (A. Philipovskiy and S. C. Straley, unpublished). The absence of any effect of anti-LcrV antibody on the initial fate of either the MYM or parent strain is perplexing. Either there is no Yops delivery during this time, or Yops delivery during the first few minutes of infection is ineffective in preventing bacterial killing or sequestration, and hence inhibition by anti-LcrV antibody is irrelevant. We favor the latter speculation: Y. pestis KIM5 is a Δpgm strain and is reduced in virulence over a wild-type Y. pestis strain by at least 10-fold (49, 53). It lacks 107 kb of chromosomal DNA encoding multiple putative regulators, transporters, and enzymes for metabolism and amino acid interconversion in addition to the well-known Ybt-mediated iron acquisition and Hms biofilm systems (4, 18, 31). These may have important roles in initial bacterial colonization, and the consequences of their absence may be exacerbated in the MYM strain for reasons not yet understood.
An early clearance phenomenon was seen previously in a guinea pig model for fully virulent Y. pestis grown at 26°C but not for virulent Y. pestis grown in vivo or in vitro at 37°C (24, 27). The identities of the host cells responsible for the clearance effect and for the ultimate site of seeding of the bacteria in liver and spleen have been a long-standing unknown aspect of the biology of plague. The early investigators felt that the bacteria were not being cleared into cells in the vasculature, because during the first 30 min following i.v. infection in guinea pigs the bacteria were cleared from kidneys and lungs (which would provide extensive vascular surface), while they were simultaneously appearing in liver and spleen (27). It was hypothesized that fixed macrophages in liver and spleen were the likely early niche for Y. pestis in liver and spleen (27, 28), although this was never proven. In the present study, the experiments with Mafia mice given the nonprotective anti-YopM antibody showed no significant difference in numbers of yersiniae between macrophage-depleted and mock-treated mice in either liver or spleen beginning at 30 min postinfection. This indicates that cells of the macrophage lineage make little contribution to the initial reduction in numbers of Y. pestis and shows that these cells also are not essential for the subsequent growth of Y. pestis in mice. However, macrophages did provide 90% of the growth restriction mediated by anti-LcrV antibody in liver. The remaining 10% was independent of macrophages, and macrophages were not essential, or were redundant, for the restriction of bacterial growth in spleen, at least for the first three days postinfection. Whether macrophages and related cells exert a protective effect in spleen later in infection is not yet known.
Cells that likely play an important role in controlling Y. pestis growth in spleen as well as in liver are polymorphonuclear neutrophils. Mafia mice treated with dimerizer are not depleted for PMNs and even show slightly elevated numbers of these cells (5). Accordingly, PMNs are a good candidate for the major host mediator of passive protection in spleen in Mafia mice as well as control mice and likely exert a major effect in liver also, as PMNs move to foci of infection in large numbers within hours, where they contribute to the inflammatory cytokine milieu in addition to phagocytosing and killing bacteria (10-12, 20). The Yersinia field has up to now mainly focused on macrophages as key host cells that are modulated by Yops, but the few studies that have been made with PMNs point to their importance and to a role for Yops in disarming these cells (2, 14, 25, 43). We are presently investigating their importance in plague.
This study demonstrated an IL-10-independent protective effect of anti-LcrV antibody, which we believe came from inhibition of LcrV's function in delivery of effector Yops to host cells. In the absence of effector Yops (when C57BL/6 mice were infected by Y. pestis KIM5-MYM), there was no net bacterial growth, whereas when the yop genes were functional, an IL-10-independent protection against bacterial growth by anti-LcrV antibody could be demonstrated (when IL-10−/− mice were infected by a high dose of Y. pestis KIM5). We hypothesize that in our experiments, a small fraction of the yersiniae escaped killing, seeded liver and spleen, and adapted fully for growth. This growth may have depended on inhibition of innate defenses by Yops (15), and the delivery of sufficient Yops for this effect was inhibited by anti-LcrV antibody. This is consistent with previous studies corroborated by our in vitro data, showing that protective anti-LcrV antibody inhibits Yops-dependent toxicity in mammalian cells infected with Y. pestis (40, 55) and with conclusions in the older literature that LcrV is necessary for survival and growth of yersiniae in vivo (28, 29).
Our findings did not critically test for possible roles of anti-LcrV antibody in blocking effects of free LcrV. For example, anti-LcrV antibody could potentially block the ability of free LcrV to inhibit chemotaxis of PMNs (56) and thereby promote bacterial clearance from organs. However, anti-LcrV antibody did not hasten clearance of Y. pestis KIM5-MYM, a strain that is capable of producing abundant free LcrV. It may be that this antichemotactic effect of LcrV requires a large concentration of LcrV to accumulate and that this does not happen with Y. pestis KIM5-MYM, which is incapable of growing to develop foci containing high bacterial numbers. Likewise, this may explain why anti-LcrV antibody had no detectable clearance effect for Y. pestis KIM5-MYM, whereas treatment of mice with 500 μg of an LcrV-containing extract of Y. pseudotuberculosis retarded clearance of Lcr mutant Y. pestis KIM lacking the virulence plasmid that encodes LcrV, Yops, and the type III secretion system (54).
We found that IL-10−/− C57BL/6 mice were remarkably resistant to plague, consistent with findings for Y. enterocolitica (44); high bacterial numbers were required to demonstrate the IL-10-independent protective effect of anti-LcrV antibody. IL-10 is a pleiotropic immunosuppressive cytokine with a homeostatic role to prevent overexpression of proinflammatory cytokines and reactive nitrogen intermediates and return the immune system to balance after an inflammatory assault has dissipated (19, 36, 58). It has a multitude of effects on the innate immune system and is critical for controlling early events thought to occur during plague, e.g., it inhibits macrophage activation, indirectly inhibits gamma interferon production by NK cells, and inhibits phagocytic and bactericidal activity of PMNs (32, 36). It is important as a mediator of Th2 immune responses and as a regulator of B-cell development, and mice lacking it are strongly polarized toward a Th1 phenotype as a result of uncontrolled production of Th1-promoting cytokines, such as IL-12 and gamma interferon (42). Our findings demonstrate how effective the Th1 response can be in combating plague. The ability of LcrV to stimulate IL-10 expression inappropriately and downregulate the Th1 response could be immensely important in the pathogenesis of plague (3, 38, 44). This downregulation effect requires functional TLR2. Significantly, mice lacking TLR2 allow much less growth of Y. enterocolitica than wild-type mice (45). Because TLR2−/− mice contrast with IL-10−/− mice in that they do not have gross distortions of their immune systems (50), this finding suggests all the more that immune modulation by free LcrV could be important. However, our test with Y. pestis KIM5-MYM did not support the hypothesis that anti-LcrV antibody specifically counteracts any property of free LcrV, again perhaps because the bacteria need to grow to sufficient numbers to make enough LcrV for a significant inhibitory effect.
We speculate that through modulation of host innate defenses, Yops are critical for the initiation of bacterial growth in organs and the development of concentrations of yersiniae that release sufficient amounts of free LcrV to induce effective levels of IL-10. This is in substantial agreement with an idea proposed by Brubaker (3). In this model, anti-LcrV antibody can block delivery of Yops sufficiently to prevent the crucial early growth of yersiniae that is necessary for immunosuppression. Accordingly, our findings provide further support for the development of an assay based on inhibition of Yops effects, such as cell killing by anti-LcrV antibody as an in vitro correlate of protection by subunit vaccines containing LcrV (55).
In conclusion, the present findings have provided insight into the time course of effects of anti-LcrV antibody during plague, identified one protective mechanism, and revealed that more than one host cell type is responsible for mediating protection. Future studies will be required to test the importance of neutralization of free LcrV to protection against plague and to identify all host cells that mediate protection.
Acknowledgments
This study was supported by Public Health Service (NIAID) grant AI21017.
We gratefully acknowledge skilled technical assistance from Martin Ward in construction of Y. pestis KIM5-MYM and from Michael Gray for talented assistance with some of the animal experiments.
Editor: D. L. Burns
REFERENCES
- 1.Anderson, G. W., Jr., P. L. Worsham, C. R. Bolt, G. P. Andrews, S. L. Welkos, A. M. Friedlander, and J. P. Burans. 1997. Protection of mice from fatal bubonic and pneumonic plague by passive immunization with monoclonal antibodies against the F1 protein of Yersinia pestis. Am. J. Trop. Med. Hyg. 56:471-473. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, K., K.-E. Magnusson, M. Majeed, O. Stendahl, and M. Fällman. 1999. Yersinia pseudotuberculosis-induced calcium signaling in neutrophils is blocked by the virulence effector YopH. Infect. Immun. 67:2567-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brubaker, R. R. 2003. Interleukin-10 and inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 71:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchreiser, C., C. Rusniok, L. Frangeul, E. Couve, A. Billault, F. Kunst, E. Carniel, and P. Glaser. 1999. The 102-kilobase pgm locus of Yersinia pestis: sequence analysis and comparison of selected regions among different Yersinia pestis and Yersinia pseudotuberculosis strains. Infect. Immun. 67:4851-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett, S. H., E. J. Kerschen, J. Zhang, L. Zeng, S. C. Straley, A. M. Kaplan, and D. A. Cohen. 2004. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J. Leuk. Biol. 75:612-623. [DOI] [PubMed] [Google Scholar]
- 6.Burrows, T. W. 1955. The basis for virulence for mice of Pasteurella pestis, p. 152-175. In J. W. Howie and A. J. O'Hea (ed.), Mechanisms of microbial pathogenicity. Fifth symposium of the Society for General Microbiology. Cambridge University Press, Cambridge, United Kingdom.
- 7.Burrows, T. W. 1957. Virulence of Pasteurella pestis. Nature 179:1246-1247. [DOI] [PubMed] [Google Scholar]
- 8.Burrows, T. W., and G. A. Bacon. 1956. The basis of virulence in Pasteurella pestis: the development of resistance to phagocytosis in vitro. Br. J. Exp. Pathol. 37:286-299. [PMC free article] [PubMed] [Google Scholar]
- 9.Burrows, T. W., and G. A. Bacon. 1956. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br. J. Exp. Pathol. 37:481-493. [PMC free article] [PubMed] [Google Scholar]
- 10.Cassatella, M. A. 1995. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today 16:21-26. [DOI] [PubMed] [Google Scholar]
- 11.Cassatella, M. A., L. Meda, S. Gasperini, A. D'Andrea, Z. Ma, and G. Trinchieri. 1995. Interleukin-12 production by human polymorphonuclear leukocytes. Eur. J. Immunol. 25:1-5. [DOI] [PubMed] [Google Scholar]
- 12.Cavanaugh, D. C., and J. E. Williams. 1980. Plague: some ecological interrelationships, p. 245-256. In R. Traub and H. Starcke (ed.), Fleas. Proceedings of the International Conference on Fleas. A. A. Balkema, Rotterdam, The Netherlands.
- 13.Cavanaugh, D. C., and R. Randall. 1959. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J. Immunol. 83:348-363. [PubMed] [Google Scholar]
- 14.Conlan, J. W. 1997. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect. Immun. 65:630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M.-P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowan, C., H. A. Jones, Y. H. Kaya, R. D. Perry, and S. C. Straley. 2000. Invasion of epithelial cells by Yersinia pestis: evidence for a Y. pestis-specific invasin. Infect. Immun. 68:4523-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng, W., V. Burland, G. Plunkett III, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. Infect. Immun. 184:4601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding, Y., D. Chen, A. Tarcsafalvi, R. Su, L. Qin, and J. S. Bromberg. 2003. Suppressor of cytokine signaling 1 inhibits IL-10-mediated immune responses. J. Immunol. 170:1383-1391. [DOI] [PubMed] [Google Scholar]
- 20.Ethuin, F., B. Gérard, J. E. Benna, A. Boutten, M.-A. Gougeret-Pocidalo, L. Jacob, and S. Chollet-Martin. 2004. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab. Investig. 28:1-9. [DOI] [PubMed] [Google Scholar]
- 21.Fields, K. F., and S. C. Straley. 1999. LcrV of Yersinia pestis enters infected eukaryotic cells by a virulence plasmid-independent mechanism. Infect. Immun. 67:4801-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fields, K. A., M. L. Nilles, C. Cowan, and S. C. Straley. 1999. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect. Immun. 67:5395-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedlander, A. M., S. L. Welkos, P. L. Worsham, G. P. Andrews, D. G. Heath, G. W. Anderson, M. L. M. Pitt, J. Estep, and K. Davis. 1995. The relationship between virulence and immunity as revealed in recent studies of the F1 capsule of Yersinia pestis. Clin. Infect. Dis. 21(Suppl. 2):S178—S181. [DOI] [PubMed] [Google Scholar]
- 24.Fukui, G. M., W. D. Lawton, W. A. Janssen, and M. J. Surgalla. 1957. Response of guinea pig lungs to in vivo and in vitro cultures of Pasteurella pestis. J. Infect. Dis. 100:103-107. [DOI] [PubMed] [Google Scholar]
- 25.Grosdent, N., I. Maridonneau-Parini, M. P. Sory, and G. R. Cornelis. 2002. Role of Yops and adhesins in resistance of Yersiniaenterocolitica to phagocytosis. Infect. Immun. 70:4165-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill, J., C. Copse, S. Leary, A. J. Stagg, E. D. Williamson, and R. W. Titball. 2003. Synergistic protection of mice against plague with monoclonal antibodies specific for the F1 and V antigens of Yersinia pestis. Infect. Immun. 71:2234-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssen, W. A., G. M. Fukui, and M. J. Surgalla. 1958. A study of the fate of Pasteurella pestis following intracardial injection into guinea pigs. J. Infect. Dis. 103:183-187. [DOI] [PubMed] [Google Scholar]
- 28.Janssen, W. A., and M. J. Surgalla. 1969. Plague bacillus: survival within host phagocytes. Science 163:950-952. [DOI] [PubMed] [Google Scholar]
- 29.Janssen, W. A., W. D. Lawton, G. M. Fukui, and M. J. Surgalla. 1963. The pathogenesis of plague. 1. A study of the correlation between virulence and relative phagocytosis resistance of some strains of Pasteurella pestis. J. Infect. Dis. 113:139-143. [DOI] [PubMed] [Google Scholar]
- 30.Kerschen, E. J., D. A. Cohen, A. M. Kaplan, and S. C. Straley. 2004. The plague virulence protein YopM targets the innate immune response by causing a global depletion of NK cells. Infect. Immun. 72:4589-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirillina, O., J. D. Fetherston, A. G. Bobrov, J. Abney, and R. D. Perry. 2004. HmsP, a putative phosphodiesterase, and HmsT, a putative diguanylate cyclase, control Hms-dependent biofilm formation in Yersinia pestis. Mol. Microbiol. 54:75. [DOI] [PubMed]
- 32.Laichalk, L. L., J. M. Danforth, and T. J. Standiford. 1996. Interleukin-10 inhibits neutrophil phagocytic and bactericidal activity. FEMS Immunol. Med. Microbiol. 15:181-187. [DOI] [PubMed] [Google Scholar]
- 33.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 34.Metcalf, W. W., W. Jiang, L. L. Daniels, S.-K. Kim, A. Haldimann, and B. L. Wanner. 1996. Conditionally replicative and conjugative plasmids carrying LacZα for cloning, mutagenesis, and allelic replacement in bacteria. Plasmid 35:1-13. [DOI] [PubMed] [Google Scholar]
- 35.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 37.Nakajima, R., V. Motin, and R. R. Brubaker. 1995. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 63:3021-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nedialkov, Y. A., V. Motin, and R. R. Brubaker. 1997. Resistance to lipopolysaccharide mediated by the Yersinia pestis V antigen-polyhistidine fusion peptide: amplification of interleukin-10. Infect. Immun. 65:1196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nemeth, J., and S. C. Straley. 1997. Effect of Yersinia pestis YopM on experimental plague. Infect. Immun. 65:924-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettersson, J., A. Hölmstrom, A. J. Hill, S. Leary, E. Frithz-Lindsten, A. von Euler-Matell, E. Carlsson, R. Titball, Å. Forsberg, and H. Wolf-Watz. 1999. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32:961-976. [DOI] [PubMed] [Google Scholar]
- 41.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 42.Rennick, D. M., M. M. Fort, and N. J. Davidson. 1997. Studies with IL-10−/− mice: an overview. J. Leukoc. Biol. 61:389-396. [DOI] [PubMed] [Google Scholar]
- 43.Roggenkamp, A., L. Leitritz, A. Sing, V. A. J. Kempf, K. Baus, and J. Heesemann. 1999. Anti-recombinant V antigen serum promotes uptake of Yersinia enterocolitica serotype 08 by macrophages. Med. Microbiol. Immunol. 188:151-159. [DOI] [PubMed] [Google Scholar]
- 44.Sing, A., A. Roggenkamp, A. M. Geiger, and J. Heesemann. 2002. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10 deficient mice. J. Immunol. 168:1315-1321. [DOI] [PubMed] [Google Scholar]
- 45.Sing, A., D. Rost, N. Tvardovskaia, A. Roggenkamp, A. Wiedemann, C. J. Kirschning, M. Apfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith, H., J. Keppie, E. C. Cocking, and K. Witt. 1960. The chemical basis of the virulence of Pasteurella pestis. 1. The isolation and the aggressive properties of Past. pestis and its products from infected guinea pigs. Br. J. Exp. Pathol. 41:452-459. [PMC free article] [PubMed] [Google Scholar]
- 47.Sodeinde, O., A. K. Sample, R. R. Brubaker, and J. D. Goguen. 1988. Plasminogen activator/coagulase of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect. Immun. 56:2749-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Straley, S. C., and P. A. Harmon. 1984. Growth in mouse peritoneal macrophages of Yersinia pestis lacking established virulence determinants. Infect. Immun. 45:649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Straley, S. C., and W. S. Bowmer. 1986. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect. Immun. 51:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takeda, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 51.Titball, R. W., and E. D. Williamson. 2001. Vaccination against bubonic and pneumonic plague. Vaccine 19:4175-4184. [DOI] [PubMed] [Google Scholar]
- 52.Une, T., R. Nakajima, and R. R. Brubaker. 1986. Roles of V antigen in promoting virulence in Yersiniae. Contrib. Microbiol. Immunol. 9:179-185. [PubMed] [Google Scholar]
- 53.Une, T., and R. R. Brubaker. 1984. In vivo comparison of avirulent Vwa− and Pgm− or Pstr phenotypes of yersiniae. Infect. Immun. 43:895-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Une, T., and R. R. Brubaker. 1984. Roles of V antigen in promoting virulence and immunity in yersiniae. J. Immunol. 133:2226-2230. [PubMed] [Google Scholar]
- 55.Weeks, S., J. Hill, A. Friedlander, and S. Welkos. 2002. Anti-V antigen antibody protects macrophages from Yersinia pestis-induced cell death and promotes phagocytosis. Microb. Pathogen. 32:227-237. [DOI] [PubMed] [Google Scholar]
- 56.Welkos, S., A. Friedlander, D. McDowell, J. Weeks, and S. Tobery. 1998. V antigen of Yersinia pestis inhibits neutrophil chemotaxis. Microb. Pathol. 24:185-196. [DOI] [PubMed] [Google Scholar]
- 57.Williams, A. W., and S. C. Straley. 1998. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J. Bacteriol. 189:350-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams, L., L. Bradley, A. Smith, and B. Foxwell. 2004. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J. Immunol. 172:567-576. [DOI] [PubMed] [Google Scholar]
- 59.Worsham, P. L., M. P. Stein, and S. L. Welkos. 1995. Construction of defined F1 negative mutants of virulent Yersinia pestis. Contrib. Microbiol. Immunol. 13:325-328. [PubMed] [Google Scholar]
- 60.Wulff-Strobel, C. R., A. W. Williams, and S. C. Straley. 2002. LcrQ and SycH function together at the Ysc type III secretion system in Yersinia pestis to impose a hierarchy of secretion. Mol. Microbiol. 43:411-423. [DOI] [PubMed] [Google Scholar]