Abstract
Coxiella burnetii causes acute Q fever in humans and occasional chronic infections that typically manifest as endocarditis or hepatitis. Isolates associated with acute disease were found to be distinct from a group of chronic disease isolates by a variety of biochemical parameters and in a guinea pig fever model of acute disease, suggesting a difference in virulence potential. We compared antigenic polypeptides among C. burnetii isolates and found an immunodominant 28-kDa protein in acute group isolates but not in chronic group isolates (T. Ho, A. Hotta, G. Q. Zhang, S. V. Nguyen, M. Ogawa, T. Yamaguchi, H. Fukushi, and K. Hirai, Microbiol. Immunol. 42:81-85, 1998). In order to clone the adaA gene, the N-terminal amino acid sequence of adaA was determined and a 59-bp fragment was amplified from Nine Mile phase I DNA by PCR. The putative gene fragment was used to screen a lambda ZAP II genomic DNA library, and an open reading frame expressing a 28-kDa immunoreactive protein was identified. Sequence analysis predicted a gene encoding an ∼28-kDa mature protein with a typical signal sequence. The adaA (acute disease antigen A) gene was detected in acute group C. burnetii isolates but not identified in chronic group isolates by PCR and Southern blotting. A typical signal peptide was predicted in adaA, and specific antibody to adaA reacted with the purified membrane fraction of acute group isolates by Western blotting, suggesting that adaA is exposed on the outer surface of C. burnetii. adaA was overexpressed in pET23a as a fusion protein in Escherichia coli to develop anti-recombinant adaA (anti-radaA) specific antibody, which recognized a ∼28-kDa band in acute group isolates but not in chronic group isolates. In addition, immunoblotting indicates that radaA reacted with sera derived from animals infected with acute group isolates but did not react with sera from animals infected with chronic group isolates. These results support the idea that an adaA gene-targeted PCR assay and an radaA antigen-based serodiagnostic test may be useful for differential diagnosis of acute and chronic Q fever.
Coxiella burnetii is an obligate intracellular bacterium that causes acute and chronic forms of Q fever in humans. Acute Q fever is an influenza-like illness that usually is self-limiting and effectively treated by antibiotics (11). In contrast, chronic Q fever is a severe, sometimes fatal disease, and patients have responded poorly to various antibiotics (8, 20). Endocarditis is the most common chronic manifestation, while vascular infection, bone infection, and chronic hepatitis are also reported (21). Infection in most animals is mainly subclinical, but abortion and infertility are common manifestations in ruminants (2). Domestic animals, especially cattle, sheep, and goats, are important reservoirs of the agent responsible for infection of humans (7, 11).
C. burnetii has been isolated from various sources including milk, ticks, and humans with acute and chronic Q fever worldwide (2, 7, 8, 10). Previous studies have demonstrated that C. burnetii isolates originating from milk, ticks, and humans with acute Q fever differ in plasmid type (22), lipopolysaccharide profiles (3), and chromosomal DNA restriction endonuclease fragment patterns (5) from many isolates originating from chronic Q fever. The differences at the phenotypic and molecular levels between acute and chronic disease-associated isolates suggested that there may be a virulence potential characteristic of each group of isolates. Samuel et al. first reported that C. burnetii isolates associated with acute Q fever contained the QpH1 plasmid, while isolates associated with chronic Q fever possessed the QpRS plasmid or the plasmid sequences were integrated into the chromosome (22, 23). More recent studies of several C. burnetii isolates from Europe detected either the QpH1 plasmid-specific sequences (25, 26) or plasmid type QpDV (27) in both acute and chronic disease-associated isolates, suggesting that there was no specific gene(s) on plasmids responsible for a specific virulence phenotype. These data supported the notion that chronic disease could result from isolates associated with acute disease and might result from unique patient factors associated with immune status (25-27). However, no chronic disease-associated organisms have been isolated from acute Q fever patients. Therefore, it is quite possible that there are bacterial genetic factors responsible for acute disease. This hypothesis was supported in a study by Moos and Hackstadt (17) comparing virulence of a prototype isolate from each group in guinea pigs. The acute disease group prototype isolate (Nine Mile phase I RSA493) caused infection and fever when delivered intraperitoneally with less than 10 organisms, while the chronic disease group prototype isolate (Priscilla Q177) required at least 105 organisms to cause fever.
Our previous study identified a 28-kDa protein (P28) that was immunodominant in isolates originating from milk, ticks, and humans with acute Q fever but not immunogenic in isolates originating from chronic Q fever (6). This finding suggested that adaA could be associated with a pathogenic factor of acute Q fever. adaA may also have value as a marker to distinguish isolate groups. In order to clone and characterize the adaA-encoding gene, the N-terminal amino acid sequence of the protein was determined by protein sequencing. A 59-bp gene fragment was amplified from Nine Mile phase I DNA by PCR with one primer pair designed based on the N-terminal amino acid sequence and was used as a probe to screen a genomic library by Southern hybridization. The gene encoding P28 was cloned and sequenced. Outer membrane localization and antigenicity of adaA indicated that adaA may be a virulence factor related to acute Q fever, and the adaA gene may be a useful genetic marker for differentiation of isolates of C. burnetii.
MATERIALS AND METHODS
Bacterial strains, phage, and growth conditions.
Seventeen C. burnetii isolates from various clinical and geographical sources were used in this study. The original source, pathogenic characteristics, and genetic properties of these strains are summarized in Table 1. All the isolates were propagated in BGM or L929 cell cultures and purified as described elsewhere (7, 22). The bacteriophage lambda ZAP II (Stratagene, La Jolla, Calif.) was used as the vector for construction of the C. burnetii expression genomic DNA library. Escherichia coli XL-Blue MRF′ (Stratagene) was cultured in Luria broth (LB) with 12.5 μg of tetracycline/ml and used as the host strain for recombinant plasmids and bacteriophage lambda ZAP II.
TABLE 1.
Original source, pathogenic characteristics, genetic group, and plasmid type of C. burnetii strains
| Groupa | Plasmid typeb | Isolatec | Phase | Original source | Disease or type | Passaged |
|---|---|---|---|---|---|---|
| I | QpH1 | Nine Mile RSA493 | I | Montana, tick, 1935 | 307GP/1TC/1EP | |
| Turkey RSA333 | II | Turkey, human blood, 1948 | Acute | 31EP | ||
| African RSA334 (>e) | I | Central Africa, human blood, 1949 | Acute, Congolese red fever | 3HP/4EP | ||
| Giroud RSA431 (>e) | I | Central Africa, human blood, 1949 | Acute, Congolese red fever | 2GP/2EP | ||
| El Tayeb RSA342 | I | Egypt, tick, 1967 | 4GP/2EP | |||
| Panama RSA335 | I | Panama, chiggers, 1961 | 4EP | |||
| California 33 RSA329 | I | California, cow's milk, 1947 | Persistent | 6EP | ||
| Ohio 314 RSA270 | I | Ohio, cow's milk, 1956 | Persistent | 4EP | ||
| II | Henzerling RSA331 | II | Italy, human blood, 1945 | Acute | 36EP | |
| IV | QpRS | Priscilla | I | Montana, goat cotyledon, 1980 | Abortion | GP/2EP |
| KQ154 | I | Oregon, human heart valve, 1976 | Endocarditis | HV/2EP | ||
| V | NP | GQ212 | I | Nova Scotia, Canada, human heart valve, 1981 | Endocarditis | HV/2EP |
| SQ217 | I | Montana, human liver biopsy sample, 1981 | Hepatitis | BX/2EP | ||
| KoQ229 | I | Nova Scotia, Canada, human heart valve, 1982 | Endocarditis | HV/2EP | ||
| VI | QpDG | Dugway 7E22-57 | I | Utah, rodents, 1958 | 3EP | |
| —f | QpDV | MAN | I | French, human blood | Aortic aneurysm | ? |
| —f | ME | I | French, human heart valve | Endocarditis | ? |
As defined by restriction enzyme banding patterns (5).
Provided by Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, Hamilton, Mont. Reference strains were determined by complement block titration (M. G. Peacock, Rocky Mountain Laboratories).
Numbers indicate passage number; GP, guinea pig passage; TC, tissue culture; EP, egg passage; HP, hamster passage; HV, heart valve; BX, liver biopsy sample; ?, passage prior to receipt in authors' laboratory not known.
>, passage history variants.
—, MAN and ME were not classified (27).
Preparation of C. burnetii OMPs.
The outer membrane proteins (OMPs) of C. burnetii were extracted from purified C. burnetii Nine Mile based on the method described by Ohashi et al. (19). Briefly, purified organisms were suspended in 10 mM sodium phosphate buffer, pH 7.4, containing 1% Sarkosyl (Sigma, St. Louis, Mo.) and 50 μg each of DNase I and RNase A and incubated at 37°C for 30 min. EDTA at a final concentration of 15 mM was added to stop the nuclease reaction. The insoluble precipitates were obtained by centrifugation at 10,000 × g for 1 h, washed twice with 0.1% Sarkosyl-phosphate-buffered saline, and then resuspended in STE buffer (100 mM NaCl, 10 mM Tris-HCl, and 1 mM EDTA, pH 8.0) containing 1 mM phenylmethylsulfonyl fluoride (Sigma).
Analysis of the N-terminal amino acid sequences of adaA.
The OMPs of C. burnetii Nine Mile were separated by reversed discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to a polyvinylidene difluoride membrane as described elsewhere (19). The presence of adaA in the purified membrane fraction of C. burnetii Nine Mile was confirmed by immunoblotting as described previously (6). The portion of the polyvinylidene difluoride membrane containing adaA was excised and analyzed with the HP G1005A protein sequencing system (Takara Shuzo Co., Kyoto, Japan).
Preparation of DNA probe specific to the P28-encoding gene.
The N-terminal amino acid sequence of adaA was determined as ENRPILNTINYQQQVEKWVTTDSADVMVSVN. Based on the N-terminal amino acid sequence, a pair of primers, P28a (5′-ATHAAYTAYCARCARCARGT-3′) and P28b (5′-AGCATNACRTCNGC-3′), were designed and used to amplify a 59-bp fragment of the putative adaA gene. The expected 59-bp product was amplified from C. burnetii Nine Mile DNA by PCR with these primers. The nucleotide sequence of the 59-bp fragment was determined by the dideoxy nucleotide chain-termination method as described previously (29). Sequence analysis of the 59-bp fragment indicates that the deduced amino acid sequence is identical to the chemically determined N-terminal amino acid sequence of P28, suggesting that the 59-bp fragment is specific DNA of the adaA gene. Based on the determined nucleotide sequence, specific primers P28a1 (5′-ATTAATTATCAACAGCAGGTTG-3′) and P28b1 (5′-AGCATTACATCGGCAGAATCC-3′) were designed and used to amplify the 59-bp specific fragment of the adaA gene from C. burnetii Nine Mile DNA. The amplified 59-bp fragment was labeled by the random primer extension method with the digoxigenin DNA labeling kit (Roche Diagnostics K. K., Tokyo, Japan) and used as a DNA probe to screen the genomic DNA library of C. burnetii by Southern hybridization.
Construction and screening of genomic DNA library.
A lambda ZAP II genomic DNA library was constructed as described by Macellaro et al. (9) and screened by Southern hybridization with the adaA gene-specific probe. Briefly, the genomic library was plated on E. coli XL-Blue MRF′ to yield about 500 plaques per plate. Plates were incubated at 37°C until plaques were 1 mm in diameter. Plaques were transferred onto a nylon membrane (Amersham Pharmacia Biotech, Piscataway, N.J.) and were hybridized with the adaA gene-specific probe according to the protocol provided by the manufacturer (Roche Diagnostics K. K.). The positive plaques were detected by using the digoxigenin luminescent detection kit (Roche Diagnostics K. K.). In vivo excision of the pBluescript vector along with the inserted DNA of each positive clone was performed according to the protocol of the supplier of the lambda ZAP II cloning system.
Immunoblot analysis of adaA expression in E. coli.
E. coli containing the recombinant plasmid was cultured in LB supplemented with 4 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at 37°C overnight, and then cells were pelleted by centrifugation. The cell pellet was analyzed by SDS-PAGE and immunoblotting with rabbit anti-Nine Mile serum as described previously (30).
DNA sequence analysis.
Plasmid DNAs from positive clones that expressed immunoreactive protein were isolated and purified by using the FlexyPrep kit (Amersham Pharmacia Biotech). The nucleotide sequence was partially determined by the dideoxy nucleotide chain-termination method with the Thermo Sequenase Cy5.5 dye terminator cycle sequencing kit and SEQ4x4 personal sequencer system (Amersham Pharmacia Biotech). A BLAST search against the complete genomic sequence of Nine Mile phase I (24) was achieved to identify the complete nucleotide sequence of the cloned gene. The nucleotide sequence and the deduced amino acid sequence were analyzed by the GENETYX analyzing system (Software Development Co., Ltd., Tokyo, Japan).
Detection of the adaA gene from various isolates of C. burnetii by PCR.
A pair of primers, P28F and P28R, was designed based on the adaA gene sequence and used to amplify a 269-bp fragment (ranging from positions 369 to 637 in the open reading frame [ORF] region of the adaA gene) from DNAs of 17 isolates from various clinical and geographical sources. The sequences of the primers are as follows: P28F, 5′-AATAGATTCGCTCTCTCAAGCCG-3′, and P28R, 5′-TCACCGCTGTTTTTTCAGACG-3′. PCR was performed with 2.5 U of Taq DNA polymerase (Invitrogen, Carlsbad, Calif.) in 50 μl of reaction mixture containing 20 ng of genomic DNA, 0.2 μM (each) primer, and 200 μM (each) deoxynucleotide triphosphates in 10 mM Tris-HCl (pH 8.3)-50 mM KCl-2.5 mM MgCl2. The reactant was subjected to 35 cycles of 30 s at 94°C, 30 s at 53°C, and 1 min at 72°C in a DNA thermal cycler (PTC-0200 DNA Engine; MJ Research, Inc., Waltham, Mass.).
Southern blotting.
Restriction enzyme-digested DNAs from various clinical phenotypic isolates including two acute prototypic isolates (Nine Mile and Henzerling) and five chronic prototypic isolates (Priscilla, Q217, Q229, MAN, and ME) were tested by Southern blotting with the adaA gene-specific probe.
Expression and purification of the adaA fusion protein.
The 602-bp DNA fragment of the adaA gene was amplified from C. burnetii Nine Mile DNA by PCR with primers P28EF-P28ER, which were designed from the adaA gene sequence and included 602 bp of the ORF region without the signal peptide-encoding sequence. Primer P28EF (5′-TTCGCTGCCACCGGATCCTTC-3′) is the 5′ end of the adaA gene with an additional BamHI restriction site, and primer P28ER (5′-ATCAACTCGAGGTTTCTTCG-3′) is complementary to the 3′ end of the gene with a XhoI restriction site in the sequence. The amplified adaA gene fragment was digested with BamHI and XhoI, ligated to expression vector pET23a, and then transformed into E. coli BL21(DE3)LysS competent cells. Expression of T7-tagged (N-terminal) and His-tagged (C-terminal) recombinant adaA (radaA) was induced by 4 mM IPTG. radaA was purified by using a ProBond resin column (Invitrogen) under denaturing conditions.
Antiserum preparation and immunoblot analysis of adaA among various strains of C. burnetii.
The anti-adaA specific antibody was produced by immunization of BALB/c mice with purified radaA. Briefly, BALB/c mice (6 weeks old) were immunized with purified recombinant fusion protein in adjuvant (Titermax) three times at 14-day intervals. At each immunization, mice were subcutaneously injected with 50 μl of antigen (containing 20 μg of radaA) mixture with 50 μl of Titermax. After the third immunization, serum was collected and stored at −20°C.
The expression of adaA in various strains of C. burnetii was confirmed by immunoblotting with anti-adaA specific serum. SDS-PAGE and immunoblotting were performed as described elsewhere (31).
Reactivity of purified radaA with infection-derived sera.
The reactivity of radaA with sera from guinea pigs infected with various strains of C. burnetii was analyzed by immunoblotting. Guinea pig serum was collected at 4 weeks post-aerosol infection with 106 organisms of the Nine Mile phase I, Ohio, Q217, or Q229 strain and stored at −80°C until use. C. burnetii Nine Mile whole-cell lysate and purified rCom1, which is a protein common to all isolates tested (29, 30), were used as a control to confirm the presence of the antibodies to C. burnetii antigens in infection-derived sera. SDS-PAGE and immunoblotting were performed as described previously (31).
RESULTS
Cloning the adaA gene.
Immunoblotting identified an immunoreactive band at 28 kDa in the purified membrane fraction of C. burnetii Nine Mile but did not detect reactivity in the Q217 strain (Fig. 1). The result confirmed that the 28-kDa protein corresponds to the adaA previously noted (6). To identify the adaA gene, we determined the N-terminal 31 amino acids of a 28-kDa protein from C. burnetii Nine Mile. Based on the amino acid sequence, we designed several primer pairs and successfully amplified a 59-bp fragment from C. burnetii Nine Mile DNA by PCR. The 59-bp fragment was used as a DNA probe to screen a genomic library of C. burnetii Nine Mile DNA. Approximately 10,000 plaques were screened by Southern hybridization with the adaA gene-specific probe. Forty positive plaques were purified and compared for expression of immunoreactive proteins. Coomassie brilliant blue (CBB) staining on an SDS-polyacrylamide gel identified one clone, designated p110, expressed as an ∼24-kDa protein. Immunoblotting indicates that the protein expressed by clone p110 reacted with rabbit anti-Nine Mile serum (data not shown), suggesting that the clone p110-expressed recombinant protein is specific for C. burnetii.
FIG. 1.

Immunoblot analysis of purified OMPs of Nine Mile and Q217 strains with rabbit anti-Nine Mile hyperimmune serum. Lane 1, Nine Mile; lane 2, Q217.
Sequence analysis.
To determine the nucleotide sequence of the ORF encoding adaA, the purified recombinant plasmid from clone p110 was sequenced. The adaA gene-specific primers P28a1 and P28b1 were used in a sequence reaction to directly determine the nucleotide sequence of the adaA gene. The sequence of the p110 cloned insert was BLAST searched against the complete genome sequence of C. burnetii Nine Mile RSA493, allowing confirmation of the nucleotide sequence of the cloned gene (24). The nucleotide sequence (1,240 bp) from the cloned gene is shown in Fig. 2, which includes the flanking regions and the deduced amino acid sequence of P28. The adaA gene has a predicted ORF consisting of 684 bp, starting with an ATG codon at position 264 and ending with a TAG codon at position 947. The ORF is preceded by a putative ribosome-binding site, GGAGG, from 7 bp upstream of the ATG start codon. A predicted promoter sequence, TTGAAT-21 nt-TGTTAT, was found 36 bp upstream from the putative ribosome-binding site. The G+C content of the ORF coding region was 40%, which is similar to the value of C. burnetii total genomic G+C content (43%). The predicted mature P28 protein consists of 227 deduced amino acid residues and has a calculated molecular mass of 25,950 Da and a theoretical isoelectric point of 8.60. A 20-amino-acid signal peptide was also predicted for the N-terminal sequence of adaA. We also confirmed that the chemically determined N-terminal 31 amino acids of adaA were identical to the amino acid sequence of the mature protein deduced from the nucleotide sequence of the cloned adaA gene, which is the region immediately adjacent to a predicted signal peptide (Fig. 2). In addition, a BLAST search of GenBank with the deduced amino acid sequence of the adaA gene indicated that the ORF encoding adaA was identical to CBU0952, which was predicted by The Institute for Genomic Research gene annotation (24). These results suggest that the ORF identified in the p110 cloned gene sequence is a gene unique to C. burnetii and encodes adaA.
FIG. 2.
Nucleotide sequence of the adaA gene. The deduced amino acid sequence is shown beneath the nucleotide sequence of the ORF. A putative promoter (positions −35 and −10) is underlined (thin line). The predicted ribosome-binding site (RBS) is underlined (thick line). The 20-amino-acid sequence at the N-terminal end of adaA is predicted to be a signal peptide. The chemically determined N-terminal 31-amino-acid sequence was identical to the N-terminal end of the deduced amino acid sequence of adaA.
Detection of the adaA gene in various isolates of C. burnetii.
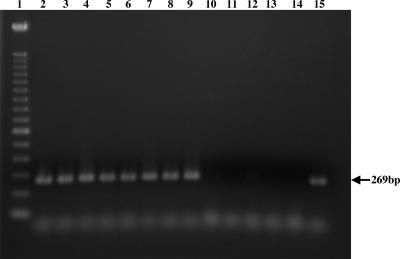
PCR and Southern blotting were used to test and confirm whether the adaA gene is unique for a subgroup of isolates. The PCR result indicated that the adaA gene-specific fragment was amplified from isolates originating from humans with acute Q fever, ticks, cattle, and rodents, but PCR did not amplify any product from isolates from goats or humans with chronic Q fever (Fig. 3). Southern blotting also indicated that the adaA gene-specific probe hybridized with one band with SalI-digested DNAs of Nine Mile and Henzerling strains associated with acute Q fever but did not hybridize with any band with SalI-digested DNAs of Priscilla, Q217, Q229, MAN, and ME strains, which have been linked to chronic Q fever (data not shown). These results suggest that the adaA gene is specific for C. burnetii isolates originating from humans with acute Q fever, ticks, cattle, and rodents.
FIG. 3.
Detection of the adaA gene from various isolates of C. burnetii by PCR with primers P28F-P28R. Shown is an ethidium bromide-stained agarose gel electrophoretogram of PCR-amplified products. Lane 1, molecular size markers (100-bp DNA ladder); lanes 2 to 9, isolates originating from ticks, milk, and humans with acute Q fever (Nine Mile, Ohio, California, El Tayeb, Africa, Panama, Turkey, and Giround, respectively); lanes 10 to 14, isolates originating from a goat and humans with chronic Q fever (Priscilla, KQ154, KoQ229, SQ217, and GQ212, respectively); lane 15, Dugway isolate.
Expression of adaA in E. coli and various isolates of C. burnetii.
The partial adaA protein of the Nine Mile strain was overexpressed as a fusion protein in pET23a. An IPTG-inducible fusion protein with a molecular mass of 28 kDa was detected in the adaA gene recombinant pET23a-transformed E. coli culture by CBB staining of the SDS-polyacrylamide gel and immunoblotting with a His-tagged specific monoclonal antibody (Fig. 4A and B, lanes 2 and 3). The expressed fusion protein was not detected in the negative control of pET23a-transformed E. coli culture (Fig. 4A and B, lanes 1). SDS-PAGE and immunoblotting also indicated that radaA was successfully purified from the adaA gene recombinant pET23a-transformed E. coli culture by using ProBond resin column (Fig. 4A and B, lanes 4). To confirm that P28 is expressed by acute disease isolates but not carried by chronic disease isolates, anti-radaA specific antibody was produced and used in immunoblotting with antigens of various strains of C. burnetii. Immunoblotting indicated that a ∼28-kDa reaction band was detected from acute-disease-associated isolates Nine Mile and Henzerling but not observed in chronic-disease-associated isolates Priscilla and Q217 (Fig. 4C). This result confirmed that our cloned adaA gene encodes adaA and that adaA is expressed by acute-disease-associated isolates but not carried by chronic-disease-associated isolates.
FIG. 4.
Expression of the adaA gene in the pET23a expression vector system. Expression of the T7-tagged (N-terminal) and His-tagged (C-terminal) fusion protein was monitored by CBB staining of uninduced and induced cultures by SDS-PAGE and immunoblotting with His-tag-specific monoclonal antibody. (A) CBB staining profile of the expressed and purified fusion adaA protein. Lane M, molecular size markers; lane 1, pET23a-transformed E. coli (negative control); lanes 2 and 3, adaA gene recombinant pET23a-transformed E. coli uninduced and induced cultures, respectively; lane 4, purified fusion radaA. (B) Immunoblot analysis of expressed and purified recombinant proteins with His-tag-specific monoclonal antibody. The samples shown in panel B are the same as in panel A. (C) Immunoblot analysis of adaA in various strains of C. burnetii with adaA-specific antiserum. Lanes 1 to 4, whole-cell lysate of Nine Mile, Henzerling, Priscilla, and Q217 strains, respectively. A ∼28-kDa reaction band was observed in acute disease isolates including the Nine Mile and Henzerling strains but not detected in Priscilla and Q217. Numbers at left are molecular masses in kilodaltons.
Reactivity of purified radaA with sera derived from infected animals.
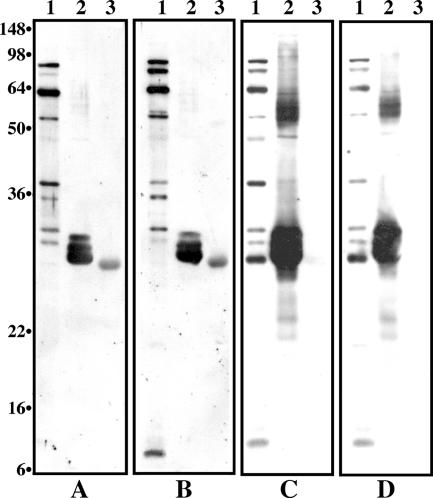
Figure 5 shows the immunoblots of the whole-cell antigen, rCom1, and radaA with sera derived from a guinea pig 4 weeks post-aerosol infection with 106 organisms of the acute or chronic prototypic strain of C. burnetii. All sera from guinea pigs aerosol infected with 106 organisms of various strains strongly reacted with Nine Mile whole-cell antigen at a wide range of molecular weights and with rCom1 at similar levels (Fig. 5, lanes 1 and 2). However, radaA reacted with sera from guinea pigs infected with isolates Nine Mile and Ohio (Fig. 5A and B, lanes 3) but not with sera from those infected with isolates Q217 and Q229 (Fig. 5C and D, lanes 3). These results indicate that anti-adaA specific antibody was present in sera derived from animals infected with the acute prototypic isolate but absent in sera from animals infected with the chronic prototypic isolate.
FIG. 5.
Immunoblots of whole-cell antigen, rCom1, and radaA with sera derived from guinea pigs 4 weeks post-aerosol infection with 106 organisms of either the acute or the chronic prototypic isolate of C. burnetii. Sera (diluted 1:500) came from guinea pigs infected with Nine Mile (A), Ohio (B), Q217 (C), and Q229 (D). The samples shown in panels A to D are the same. Lanes 1, Nine Mile whole-cell antigen; lanes 2, purified rCom1 protein; lanes 3, purified recombinant adaA protein. Numbers at left are molecular masses in kilodaltons.
DISCUSSION
Cloning and characterization of adaA demonstrated that this protein is specific for acute-Q-fever-related isolates but deleted in chronic-disease-associated isolates despite geographical source, suggesting that adaA may be a virulence factor involved in the pathogenesis of acute Q fever in humans.
The predicted adaA mature protein consists of 227 amino acids and has a predicted molecular mass of 25,950 Da. This is very close to the molecular size of native adaA expressed in C. burnetii but about 2 kDa larger than the expression product of the adaA gene in E. coli (data not shown). The 25-amino-acid signal peptide is predicted in the N-terminal sequence of adaA, which is probably cleaved from the mature protein when the adaA gene is expressed in E. coli. The chemically determined N-terminal and internal peptide (data not show) amino acid sequences of adaA were identical to the deduced amino acid sequence of the cloned adaA gene, confirming that the identified ORF encodes adaA. The cloned adaA gene recombinant pUC19 expressed radaA in E. coli DH5α cells without induction by IPTG (data not shown). A potential promoter sequence, TTGAAT-21 nt-TGTTAT, was identified in the adaA gene sequence, suggesting that the adaA gene was expressed in E. coli by using the endogenous promoter. A BLAST search of GenBank with either the nucleotide sequence or the deduced amino acid sequence for the adaA gene did not identify significant DNA or amino acid homologies, suggesting that adaA is unique to C. burnetii.
OMPs of gram-negative bacteria are employed in several important roles in the host-parasite interaction and relate to both pathogenesis and protective immunity. Due to the difficulties in cultivation and purification of C. burnetii, only a limited group of OMP-encoding genes have been characterized (4, 16, 28). Candidates for OMPs include the QpH1 plasmid-specific gene cbhE′ for a 42-kDa surface protein (15) and the QpRS plasmid-specific gene cbbE′ for a 55-kDa surface protein (12-14), which have been speculated to be virulence related and associated with acute or chronic Q fever in humans. However, recent investigations of several European isolates suggested that there were no specific genes on plasmids responsible for acute or chronic Q fever (25-27) and supported the notion that host factors may play a key role in the development of chronic Q fever. It remains unknown whether there are specific genes on the chromosome responsible for acute or chronic Q fever. Isolates from acute disease are distinct from chronic-disease-associated isolates at the molecular level (3, 5, 22) and in a guinea pig fever model of acute disease (17; K. Russell, unpublished data), suggesting different virulence potentials for groups of isolates of C. burnetii. In this study, we identified a novel ∼28-kDa membrane-associated protein and demonstrated that adaA is expressed in acute group isolates but not carried by chronic group isolates, suggesting that adaA may be a virulence factor related to acute Q fever. Immunoblotting with purified radaA antigen recognized anti-adaA specific antibody from sera derived from animals infected with acute group isolates but not from sera from animals infected with chronic group isolates, suggesting that adaA is an important antigen in acute disease. Since there has been no suitable animal model developed to represent the manifestation of chronic Q fever and because there is a lack of genetic tools for C. burnetii, it is not possible to directly test whether a specific gene is related to acute or chronic disease. Recently, SCID mice have been used as a model highly sensitive to lethal challenge by an acute-disease-associated isolate of C. burnetii (1), and preliminary comparison in this model shows dramatic differences in disease from isolates which do not carry adaA (M. Andoh, unpublished data). Further studies to test whether the adaA gene can be delivered on a stable plasmid to a adaA-negative isolate may allow its role in virulence to be determined.
Since prompt antibiotic therapy could lead to a better prognosis for individual patients with chronic Q fever, developing a diagnostic method for rapid differential diagnosis of acute and chronic Q fever could be very important for control of chronic disease. Recently, based on point mutations unique to isolate groups, com1 and icd genes have been used as genetic markers to distinguish acute and chronic isolates (18, 29). However, comparison of nucleotide sequences of com1 and icd genes among isolates indicates that they are highly conserved between acute and chronic isolates, except for these few point mutations (18, 29). The finding that the adaA protein and the adaA gene are unique to acute group isolates can be used for development of radaA antigen-based serodiagnostic methods and/or an adaA gene-targeted PCR assay for differential diagnosis of acute and chronic Q fever in clinical samples. We have designed primers based on the nucleotide sequence of the adaA gene and used them to amplify products from DNA of various strains of C. burnetii. Amplicon products were amplified from DNA templates of isolates originating from humans with acute Q fever, ticks, cattle, and rodents but not from isolates originating from humans with chronic Q fever, suggesting that PCR for the adaA gene can be used for differentiation of acute- and chronic-disease-associated isolates. In addition, immunoblotting indicated that radaA reacted with sera derived from animals infected with acute group isolates but was not recognized by sera derived from animals infected with chronic group isolates, suggesting that an radaA antigen-based serodiagnostic test may be useful for differential diagnosis of acute and chronic Q fever in human sera. Further studies will evaluate the usefulness of an adaA gene-targeted PCR assay and an radaA antigen-based enzyme-linked immunosorbent assay for differential diagnosis of acute and chronic Q fever in clinical samples from acute and chronic Q fever patients.
Acknowledgments
This study was supported by Public Health Service grants AI37744 (J.E.S.), AI448191 (J.E.S.), and AI057156 (J.E.S.) from the National Institute of Allergy and Infectious Diseases and a Health Sciences Research Grant-in-Aid for Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health and Welfare of Japan.
Editor: D. L. Burns
REFERENCES
- 1.Andoh, M., T. Naganawa, A. Hotta, T. Yamaguchi, H. Fukushi, T. Masegi, and K. Hirai. 2003. SCID mouse model for lethal Q fever. Infect. Immun. 71:4717-4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baca, O. G., and D. Paretsky. 1983. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol. Rev. 47:127-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hackstadt, T., M. G. Peacock, P. J. Hitchcock, and R. L. Cole. 1985. Lipopolysaccharide variation in Coxiella burnetti: intrastrain heterogeneity in structure and antigenicity. Infect. Immun. 48:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendrix, L. R., L. P. Mallavia, and J. E. Samuel. 1993. Cloning and sequencing of Coxiella burnetii outer membrane protein gene com1. Infect. Immun. 61:470-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendrix, L. R., J. E. Samuel, and L. P. Mallavia. 1991. Differentiation of Coxiella burnetii isolates by analysis of restriction-endonuclease-digested DNA separated by SDS-PAGE. J. Gen. Microbiol. 137:269-276. [DOI] [PubMed] [Google Scholar]
- 6.Ho, T., A. Hotta, G. Q. Zhang, S. V. Nguyen, M. Ogawa, T. Yamaguchi, H. Fukushi, and K. Hirai. 1998. Antigenic characteristics of polypeptides of Coxiella burnetii isolates. Microbiol. Immunol. 42:81-85. [DOI] [PubMed] [Google Scholar]
- 7.Ho, T., K. K. Htwe, N. Yamasaki, G. Q. Zhang, M. Ogawa, T. Yamaguchi, H. Fukushi, and K. Hirai. 1995. Isolation of Coxiella burnetii from dairy cattle and ticks, and some characteristics of the isolates in Japan. Microbiol. Immunol. 39:663-671. [DOI] [PubMed] [Google Scholar]
- 8.Kimbrough, R. C., R. A. Ormsbee, M. Peacock, R. Rogers, R. W. Bennetts, J. Raff, A. Krause, and C. Gardner. 1979. Q fever endocarditis in the United States. Ann. Intern. Med. 91:400-402. [DOI] [PubMed] [Google Scholar]
- 9.Macellaro, A., E. Tujulin, K. Hjalmarsson, and L. Norlander. 1998. Identification of a 71-kilodalton surface-associated Hsp70 homologue in Coxiella burnetii. Infect. Immun. 66:5882-5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrie, T. J. 1990. Acute Q fever, p. 125-160. In T. J. Marrie (ed.), Q fever, vol. 1. The disease. CRC Press, Boca Raton, Fla.
- 11.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 12:518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minnick, M. F., R. A. Heinzen, R. Douthart, L. P. Mallavia, and M. E. Frazier. 1990. Analysis of QpRS-specific sequences from Coxiella burnetii. Ann. N. Y. Acad. Sci. 590:514-522. [DOI] [PubMed] [Google Scholar]
- 13.Minnick, M. F., R. A. Heinzen, M. E. Frazier, and L. P. Mallavia. 1990. Characterization and expression of the cbbE′ gene of Coxiella burnetii. J. Gen. Microbiol. 136:1099-1107. [DOI] [PubMed] [Google Scholar]
- 14.Minnick, M. F., R. A. Heinzen, D. K. Reschke, M. E. Frazier, and L. P. Mallavia. 1991. A plasmid-encoded surface protein found in chronic-disease isolates of Coxiella burnetii. Infect. Immun. 59:4735-4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minnick, M. F., C. L. Small, M. E. Frazier, and L. P. Mallavia. 1991. Analysis of the cbhE′ plasmid gene from acute disease-causing isolates of Coxiella burnetii. Gene 103:113-118. [DOI] [PubMed] [Google Scholar]
- 16.Mo, Y. Y., N. P. Cianciotto, and L. P. Mallavia. 1995. Molecular cloning of a Coxiella burnetii gene encoding a macrophage infectivity potentiator (Mip) analogue. Microbiology 141:2861-2871. [DOI] [PubMed] [Google Scholar]
- 17.Moos, A., and T. Hackstadt. 1987. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect. Immun. 55:1144-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen, S. V., and K. Hirai. 1999. Differentiation of Coxiella burnetii isolates by sequence determination and PCR-restriction fragment length polymorphism analysis of isocitrate dehydrogenase gene. FEMS Microbiol. Lett. 180:249-254. [DOI] [PubMed] [Google Scholar]
- 19.Ohashi, N., N. Zhi, Y. Zhang, and Y. Rikihisa. 1998. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raoult, D. 1993. Treatment of Q fever. Antimicrob. Agents Chemother. 37:1733-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raoult, D., A. Raza, and T. J. Marrie. 1990. Q fever endocarditis and other forms of chronic Q fever, p. 179-200. In T. J. Marrie (ed.), Q fever, vol. 1. The disease. CRC Press, Boca Raton, Fla.
- 22.Samuel, J. E., M. E. Frazier, and L. P. Mallavia. 1985. Correlation of plasmid type and disease caused by Coxiella burnetii. Infect. Immun. 49:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuel, J. E., M. E. Frazier, and L. P. Mallavia. 1988. Stability of plasmid sequences in an acute Q-fever strain of Coxiella burnetii. J. Gen. Microbiol. 134:1795-1805. [DOI] [PubMed] [Google Scholar]
- 24.Seshadri, R., I. T. Paulsen, J. A. Eisen, T. D. Read, K. E. Nelson, W. C. Nelson, N. L. Ward, H. Tettelin, T. M. Davidsen, M. J. Beanan, R. T. Deboy, S. C. Daugherty, L. M. Brinkac, R. Madupu, R. J. Dodson, H. M. Khouri, K. H. Lee, H. A. Carty, D. Scanlan, R. A. Heinzen, H. A. Thompson, J. E. Samuel, C. M. Fraser, and J. F. Heidelberg. 2003. Complete genome sequence of the Q-fever pathogen Coxiella burnetii. Proc. Natl. Acad. Sci. USA 100:5455-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein, A., and D. Raoult. 1993. Lack of pathotype specific gene in human Coxiella burnetii isolates. Microb. Pathog. 15:177-185. [DOI] [PubMed] [Google Scholar]
- 26.Thiele, D., and H. Willems. 1994. Is plasmid based differentiation of Coxiella burnetii in ‘acute’ and ‘chronic’ isolates still valid? Eur. J. Epidemiol. 10:427-434. [DOI] [PubMed] [Google Scholar]
- 27.Valkova, D., and J. Kazar. 1995. A new plasmid (QpDV) common to Coxiella burnetii isolates associated with acute and chronic Q fever. FEMS Microbiol. Lett. 125:275-280. [DOI] [PubMed] [Google Scholar]
- 28.Varghees, S., K. Kiss, G. Frans, O. Braha, and J. E. Samuel. 2002. Cloning and porin activity of the major outer membrane protein P1 from Coxiella burnetii. Infect. Immun. 70:6741-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, G. Q., T. Ho, T. Yamagushi, H. Fukushi, and K. Hirai. 1997. Differentiation of Coxiella burnetii by sequence analysis of the gene (com1) encoding a 27 kDa outer membrane protein. Microbiol. Immunol. 41:871-877. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, G. Q., A. Hotta, T. Ho, T. Yamagushi, H. Fukushi, and K. Hirai. 1998. Evaluation of a recombinant 27-kDa outer membrane protein of Coxiella burnetii as an immunodiagnostic reagent. Microbiol. Immunol. 42:423-428. [DOI] [PubMed] [Google Scholar]
- 31.Zhang, G. Q., K. Kiss, R. Seshadri, L. Hendrix, and J. Samuel. 2004. Identification and cloning of immunodominant antigens of Coxiella burnetii. Infect. Immun. 72:844-852. [DOI] [PMC free article] [PubMed] [Google Scholar]