Abstract
Shigella flexneri causes human dysentery after invading the cells of the colonic epithelium. The best-studied effectors of Shigella entry into colonocytes are the invasion plasmid antigens IpaC and IpaB. These proteins are exported via a type III secretion system (TTSS) to form a pore in the host membrane that may allow the translocation of other effectors into the host cytoplasm. TTSS-mediated secretion of IpaD is also required for translocation pore formation, bacterial invasion, and virulence, but the mechanistic role of this protein is unclear. IpaD is also known to be involved in controlling Ipa protein secretion, but here it is shown that this activity can be separated from its requirement for cellular invasion. Amino acids 40 to 120 of IpaD are not essential for IpaD-dependent invasion; however, deletions in this region still lead to constitutive IpaB/IpaC secretion. Meanwhile, a central deletion causes only a partial loss of control of Ipa secretion but completely eliminates IpaD's invasion function, indicating that IpaD's role in invasion is not a direct outcome of its ability to control Ipa secretion. As shigellae expressing ipaD N-terminal deletion mutations have reduced contact-mediated hemolysis activity and are less efficient at introducing IpaB and IpaC into erythrocyte membranes, it is possible that IpaD is responsible for insertion of IpaB/IpaC pores into target cell membranes. While efficient insertion of IpaB/IpaC pores is needed for optimal invasion efficiency, it may be especially important for Ipa-dependent membrane disruption and thus for efficient vacuolar escape and intercellular spread.
Shigella flexneri is a facultative intracellular bacterial pathogen responsible for shigellosis, a mild to severe gastrointestinal syndrome with worldwide occurrence (11). Invasion of colonic epithelial cells is a required step in the pathogenesis of shigellosis, as these cells provide the primary site for pathogen multiplication, and it has been shown that mutations rendering the bacterium noninvasive eliminate its virulence potential (11, 35). The protein effectors of enterocyte invasion by Shigella invasion are the invasion plasmid antigens (Ipa proteins); however, invasion is also dependent upon the production of Ipa-specific chaperones and a type III secretion system (TTSS) composed of the Mxi/Spa secretion apparatus or secreton (4). TTSS-dependent integration of the hydrophobic proteins IpaB and IpaC into the host cell plasma membrane following pathogen contact is essential for triggering the actin reorganization that precedes S. flexneri entry into enterocytes (3, 36). The globular and hydrophilic protein IpaD is also secreted by the Shigella TTSS and is required for bacterial invasion of host cells; however, little is known of the role IpaD plays in Shigella invasion of human colonic epithelial cells (17, 22, 38).
Prior to pathogen contact with a host cell, IpaB and IpaC are stored in the Shigella cytoplasm, where they independently associate with the chaperone IpgC, which prevents their premature association (23) and may queue them for proper secretion (2). Following their secretion, IpaB and IpaC form a pore-like complex (3) within the host cell membrane to trigger bacterial uptake by macropinocytosis (3, 7, 12, 20, 23) and to serve in the translocation of other effectors (e.g., IpaA and IpgD) into the target cell cytoplasm (4). Both IpaB and IpaC have been implicated in directly triggering signal transduction cascades in target cells that lead to apoptosis in macrophages (6) and cytoskeletal rearrangements in macrophages (16) and epithelial cells (18, 20, 35, 36). Insertion of the IpaB-IpaC translocon into a target cell membrane requires bacterial contact, but this insertion event appears to be largely cell type independent as all mammalian cell types thus far tested in vitro can be invaded by shigellae, including neuronally derived cells (J. C. Osiecki and W. D. Picking, unpublished results). Likewise, S. flexneri inserts the IpaB-IpaC translocon into red blood cells (RBC), where many of the characteristics of these pores were first described (3). Because of this, contact-dependent osmotic lysis of RBC has provided a valuable tool for monitoring the formation and function of the S. flexneri translocon (31).
No direct effector activity has ever been specifically associated with IpaD; however, this protein is required for the invasion and contact-mediated hemolysis activities of S. flexneri (22). Terajima and coworkers have described experiments in which all three essential Ipa effectors (IpaB, IpaC, and IpaD) must be preincubated with HeLa cells to induce the uptake of noninvasive Escherichia coli K-12 (34) and IpaD may be required, with IpaB and IpaC, for Ipa complex association with α5β1 integrins in vitro (39). In another report, IpaD has been described as being a regulator of Ipa protein secretion as a nonpolar ipaD null mutant constitutively secretes the remaining Ipa proteins, as well as approximately 15 other proteins (21). IpaD has also been found in a complex with IpaB within bacterial membrane fractions and was therefore postulated to serve as part of a “plug” that prevents excessive and/or premature secretion of IpaB and IpaC prior to contact with a target eukaryotic cell (21). It is possible that parts of IpaD may be exposed on the surface of S. flexneri as part of this role (38). It was also found that an ipaD nonpolar mutant strain was incapable of inserting IpaB and IpaC into host membranes, but this effect could not be dissociated from the deregulated secretion of IpaB and IpaC that this mutant also displayed (3, 21). Thus, the relevance of constitutive Ipa protein secretion to the loss of Shigella invasiveness has yet to be tested. Comparisons between the Ipa-dependent invasion mechanism of shigellae and the Salmonella invasion protein (Sip)-dependent invasion of eukaryotic cells by Salmonella enterica suggest a similar role for SipD, which has some sequence similarity to IpaD (15). However, the effect of SipD-dependent control of effector secretion on Salmonella invasion has yet to be clearly described.
While type III secretion has come to be known as a shared property of many gram-negative pathogens of animals and plants, the mechanistic subtleties by which the different TTSSs insert translocation proteins (or, in the case of shigellae, translocation-effector proteins) into the host cytoplasmic membrane continues to evade description (4, 14). One of the mysterious features of Shigella invasion of eukaryotic cells is the requirement for IpaD, a protein whose role in pathogen uptake appears to be linked to its ability to prevent IpaB and IpaC secretion. In this report, the role of IpaD in the interaction of shigellae with target cells is explored in detail.
MATERIALS AND METHODS
Materials.
S. flexneri strain M90T was provided by E. V. Oaks (Walter Reed Army Institute for Research) and grown at 37°C on Trypticase soy agar containing 0.025% Congo red. S. flexneri mutant strains SF621 and SF622 (nonpolar ipaC and ipaD mutants, respectively) were from P. J. Sansonetti (Institut Pasteur) (22). Specific Ipa protein monoclonal antibodies, rabbit antisera, and the baby hamster kidney (BHK) cell line were from E. V. Oaks (37, 38). S. flexneri mxiD (1) mutant BS612 and mxiM mutant BS547 were provided by A. T. Maurelli (Uniformed Services University of the Health Sciences) (33). Plasmid pKLP103 was provided by B. D. Jones (University of Iowa) for the expression of sipD, the Salmonella homologue of ipaD, in S. flexneri SF622 (28). Plasmid pWPsfc (for ipaC expression) was described in previous work (30). Henle 407 cells (ATCC CCL6) were propagated as monolayers in Eagle's modified minimal essential medium (MEM; Fisher Scientific, St. Louis, Mo.) supplemented with 10% newborn calf serum (Life Technologies, Gaithersburg, Md.) and grown in 5% CO2. PCR SuperMix High Fidelity, and oligonucleotides were from Invitrogen (Carlsbad, Calif.). Clonables 2X Ligation Premix and competent E. coli NovaBlue were from Novagen (Madison, Wis.). Sheep RBC were from Colorado Serum Co. (Denver, Colo.) or TCS Biosciences (Buckingham, United Kingdom). Polyethylene glycol (PEG) of all sizes was from Fluka (Buch SG, Switzerland). All other chemicals were reagent grade.
Generation of ipaD deletion mutations for expression in S. flexneri SF622.
The plasmids used in this work are listed in Table 1. Expression plasmid pWPsf4 is a pUC18 derivative that has an NdeI site inserted so that translation starts with the Met encoded by the NdeI site (12). The ipaD gene was subcloned from pET15b into the NdeI/BamHI sites of pWPsf4 as previously described for other constructs (30). The resulting plasmid, pWPsf4D, was introduced into S. flexneri ipaD mutant strain SF622 by electroporation. All ipaD deletion mutations were made by inverse PCR with pWPsf4D as the template and primers composed of GAGAGA, an XhoI or BamHI restriction site, and 18 nucleotides flanking the region to be deleted. The desired linear plasmid was amplified by PCR, digested with BamHI or XhoI, intramolecularly ligated, and transformed into E. coli NovaBlue. The resulting plasmid was introduced into S. flexneri SF622 by electroporation. Ampicillin selection ensured the presence of the recombinant plasmid while kanamycin resistance and/or Congo red binding ensured the presence of the S. flexneri virulence plasmid.
TABLE 1.
Plasmids used in this study
| Plasmid | Protein encoded | Reference |
|---|---|---|
| pWPsf4 | None | 12a |
| pWPsf4D | IpaD | This work |
| pWPsf4D6 | IpaDΔ1-20 | This work |
| pWPsf4D910 | IpaDΔ41-80 | This work |
| pWPsf4D1112 | IpaDΔ81-120 | This work |
| pWPsf4D1314 | IpaDΔ121-160 | This work |
| pWPsf4D1516 | IpaDΔ161-180 | This work |
| pWPsf4D1718 | IpaDΔ201-240 | This work |
| pWPsf4D1920 | IpaDΔ241-280 | This work |
| pWPsf4D2122 | IpaDΔ281-320 | This work |
| pWPsf4D23E | IpaDΔ321-332 | This work |
| pWPsf4D5E | IpaDΔ328-332 | This work |
| pWPsf4Dcys | IpaDSC321AS | This work |
| pWPsfc | IpaC | 30 |
| pKPL103 | SipD | 28 |
pWPsf4 was derived from pUC18 for specific expression of ipa genes in S. flexneri as described by Harrington et al. (12).
To prepare IpaDSC321AS, the substitution of AlaSer for SerCys at 321, a 5′ primer containing GAGAGA, an NheI site (encoding AlaSer), and 18 bases 5′ of codon 321 and a 3′ primer containing GAGAGA, an NheI site, and 18 bases 3′ of codon 322 were used with pWPsf4D as the template in an inverse PCR. The product was digested, intramolecularly ligated, and used to transform E. coli NovaBlue. The resulting plasmid was introduced into S. flexneri SF622 by electroporation. Ampicillin selection ensured the presence of the recombinant plasmid, while kanamycin resistance and/or Congo red binding ensured the presence of the S. flexneri virulence plasmid.
Assay of bacterial entry into cultured epithelial cells.
S. flexneri invasion of Henle 407 cells was monitored with a gentamicin protection assay as previously described (27, 30). Henle 407 cells were seeded into 24-well plates and grown overnight to form preconfluent monolayers. SF622 harboring the desired plasmid was grown in Trypticase soy broth (TSB) containing 100 μg of ampicillin per ml and 50 μg of kanamycin per ml to an A600 of 0.4 to 0.6. The bacteria were diluted into serum-free MEM containing 0.45% glucose, centrifuged onto the surface of semiconfluent Henle 407 monolayers at a multiplicity of infection of 10 to 100, and incubated with the cells for 30 min at 37°C. Free bacteria were removed by aspiration, and the monolayers were washed with MEM containing 5% calf serum and 50 μg of gentamicin per ml. The cells were incubated in the final gentamicin wash for 1 h to kill adherent but noninternalized bacteria and then rinsed with MEM-0.45% glucose. The cells were lysed by overlaying them with 250 μl of 0.5% agarose in water. The solidified agarose was then overlaid with 0.5% agar containing 2× Luria-Bertani medium. After overnight incubation at 37°C, internalized bacteria formed subsurface colonies that were readily counted. Alternatively, the host cells were lysed following a 30-min invasion with a 20-min gentamicin incubation, and dilution plating was used to quantify the number of viable, internalized bacteria. A shorter gentamicin wash was used with this assay so that enumeration of bacteria that had invaded could be done with minimal interference caused by bacterial intracellular multiplication.
As an additional measure of invasion, plaque assays were performed as described by Oaks and colleagues (26). These allowed assessment of both quantitative and qualitative aspects of invasion—particularly intercellular spread, which could be influenced by altered vacuolar escape. Briefly, early log phase bacteria were diluted and added to preconfluent BHK cell monolayers, which were then incubated at 37°C in a humidified 5% CO2 environment for 90 min. An overlay composed of high-glucose Dulbecco modified Eagle medium with 5% calf serum, 20 μg of gentamicin per ml, and 0.5% SeaKem ME agarose was added to the monolayers, which were further incubated for 48 h. A second overlay composed of high-glucose Dulbecco modified Eagle medium with 20 μg of gentamicin per ml, 0.5% SeaKem ME agarose, and 0.01% neutral red was added. The plaques were then monitored for size after an additional 24 h of incubation.
Measurement of Ipa protein secretion by S. flexneri.
Ipa protein secretion was measured by enzyme-linked immunosorbent assay (ELISA) as previously described (12). Briefly, bacteria were grown to an A600 of 0.5 to 0.8 and 10 ml of the culture was centrifuged for 10 min at 4,500 × g. The bacterial pellet was resuspended in 400 μl of 0.1 M carbonate, pH 9, and the cells were lysed by sonication (15 s, 40% power) to release cytoplasmic IpaD or IpaC. The culture supernatant was the source of secreted IpaD or IpaC. Each antigen source was diluted 1:3 with carbonate buffer, and 100 μl was used to coat the wells of a polystyrene 96-well microtiter plate overnight in preparation for a standard ELISA. IpaC or IpaD in the bacterial cytoplasm was detected by preparing cell lysates, separating the cell-associated proteins by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and monitoring for the presence of IpaC or IpaD by immunoblot analysis with anti-IpaC or anti-IpaD monoclonal or polyclonal antibodies, respectively. Constitutive secretion of IpaB, IpaC, and IpaD was determined by precipitating the proteins present in the overnight culture supernatants of bacteria grown in TSB and separating the total proteins from equal amounts of bacteria by SDS-PAGE, followed by Coomassie blue staining. Relative amounts of IpaB and IpaC present in culture supernatant samples were determined by densitometric scans of Coomassie-stained gels with the Alpha Innotech (San Leandro, Calif.) Chemi-Imager system. Relative amounts of IpaD in culture supernatants were also assessed by immunoblot analysis with anti-IpaD polyclonal antibodies.
Measurement of contact-mediated hemolysis.
Contact-mediated hemolysis was measured as described by Sansonetti's group (3, 31). Briefly, S. flexneri was grown overnight on Trypticase soy agar-Congo red plates and a single red colony was used to inoculate TSB. The bacteria were grown to mid-log phase, and the cells were collected by centrifugation and resuspended in phosphate-buffered saline (PBS) at 1/40 of the original volume. Sheep RBC were washed and resuspended in PBS at a concentration of 1010/ml. RBC and bacteria (50 μl each) were added to wells of microtiter plates, and the plates were centrifuged at 2,200 × g for 15 min at 20°C. The plates were then incubated at 37°C for 2 h. The cells were resuspended by adding 90 μl of cold PBS, and the plates were centrifuged again at 2,200 × g and 15°C for 15 min. The supernatant fraction (100 μl) was transferred to a second microtiter plate, and the A545 of the released hemoglobin was measured with a microtiter plate reader. The negative control for hemolysis (to establish a baseline value) was PBS, and the positive control (to give 100% hemolysis) was water, which resulted in complete release of hemoglobin because of osmotic lysis of the RBC.
Osmoprotection of RBC.
To determine the size of IpaB/IpaC pores inserted into erythrocyte membranes, osmoprotection experiments were carried out as previously described (3). RBC that had been incubated with bacteria were resuspended in PBS or in PBS containing 60 mM PEG400, PEG600, PEG1000, PEG1500, PEG2000, or PEG3000 and incubated at 37°C. The samples were cooled, resuspended, and immediately recentrifuged. The amount of hemolysis was then measured by spectrophotometric determination of the amount of released hemoglobin as described above.
Measurement of association of IpaB and IpaC with RBC membranes after hemolysis.
After scaled-up hemolysis reactions, 800 μl of water was added to each sample to lyse all of the RBC that might have remained intact so that membrane-associated proteins could be determined, as described previously (3). Briefly, the lysed RBC were centrifuged to remove bacteria and 2 ml of the supernatant was removed and made to 2.4 ml with TBS. This sample was then made to 46% sucrose by adding 7 ml of 62% sucrose in TBS containing protease inhibitors. These samples were overlaid with 2 ml of 44% sucrose and then 25% sucrose, both in TBS. The step gradients were centrifuged at 15,000 × g for 16 h at 4°C. The material at the 44 to 25% sucrose interface was collected, diluted with TBS, and concentrated by centrifugation at 450,000 × g for 20 min at 4°C. The pellet was resuspended in SDS sample buffer, and equal amounts of total protein were separated by SDS-PAGE. IpaB or IpaC was then detected by immunoblot analysis with anti-IpaB or anti-IpaC antibodies. Protein concentrations were determined by the method of Bradford (5).
RESULTS
IpaD possesses distinct functional domains.
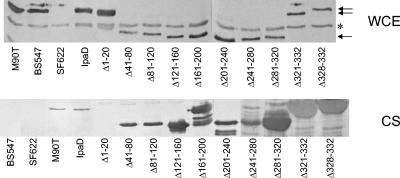
To explore the functional relevance of IpaD in S. flexneri invasion, a series of deletion mutations spanning nearly the entire length of ipaD was generated and each was inserted into pWPsf4 (Table 1). These plasmids were introduced into S. flexneri ipaD mutant SF622. All ipaD mutants were expressed at roughly equal levels, as determined by immunoblot analysis of whole-cell extracts (Fig. 1). Next, the ability of the mutants to restore invasion-related functions was monitored. The data, summarized in Table 2, suggest a distinct functional organization for IpaD. As expected on the basis of available information on other TTSS substrates (12, 14), deletion of the first 20 amino acids of IpaD gives rise to a protein that does not appear to be secreted by the TTSS and does not restore invasiveness or contact-mediated hemolysis activity to SF622 (Table 2) but is readily detected in bacterial cell lysates by immunoblot analysis (Fig. 1). It is likely that a general signal for IpaD secretion via the TTSS is encoded within the first 15 amino acids of the protein because these IpaD residues are sufficient to restore secretion and invasion function to an IpaC derivative that has had its first 20 amino acids removed (12). No other deletions that are downstream of the first 40 amino acids have a negative effect on IpaD secretion (Table 2; Fig. 1); however, in order to distance the deletions introduced here from the identified IpaD secretion signal, it was decided to begin the 40-amino-acid deletions at residue 40. In ongoing experiments, it has been noticed that deletion of residues 31 to 40 has little or no impact on IpaD function while deletion of residues 21 to 30 may influence IpaD synthesis or stability (A. Olive, K. Flentie, and W. L. Picking, unpublished results).
FIG. 1.
The IpaD deletion mutations are synthesized in S. flexneri SF622, and all but IpaDΔ1-20 are secreted into the bacterial culture supernatant (CS). Overnight cultures were used to prepare culture supernatants and whole-cell extracts (WCE). The presence or absence of IpaD was then determined by immunoblot analysis. The antibodies used were a mixture of rabbit antisera (numbers 18, 20, and 23) generated against three different IpaD-derived peptides composed of amino acids 55 to 70, 102 to 115, and 280 to 295 (38) that were derived from different regions of the protein so that they could recognize all of the deletion mutations described here. The negative control for production of IpaD was S. flexneri SF622 harboring pWPsf4 (no ipaD insert), and the negative control for secretion was S. flexneri BS547 (lacking a functional TTSS). Positive controls for synthesis and secretion were wild-type S. flexneri M90T and SF622 harboring pWPsf4D (for expressing a wild-type copy of ipaD). The relative amount of IpaD either synthesized or secreted was roughly estimated on the basis of the intensity and size of the resulting IpaD bands. A nonspecific band present in the whole-cell extracts (indicated by the asterisk) provides a crude control for protein loading, while equal volumes of culture supernatant were loaded onto the culture supernatant lanes. The bands corresponding to IpaDΔ321-332 and IpaDΔ328-332 migrate slower than the other IpaD deletion mutant proteins because they have only 12 and 5 amino acids deleted, respectively (arrows). The complete experiment was performed in toto twice (with most parts being performed at least three times) with identical results.
TABLE 2.
Abilities of IpaD deletion mutations to restore invasiveness and contact-mediated hemolysis activity to S. flexneri SF622
| Straina | Relative invasionb (%) | Contact-mediated hemolysis (%)c | IpaD secretiond |
|---|---|---|---|
| SF622 (ipaD) | 0.1 ± 0.2e | 0.0 ± 0e | − |
| M90T | 123 ± 12 | 100 ± 5 | + |
| BS547 (mxiM) | 0.0 ± 0e | 0.0 ± 0e | − |
| IpaD | 100 ± 16 | 100 ± 0.1 | + |
| IpaDΔ1-20 | 0.0 ± 0e | 0.0 ± 0e | − |
| IpaDΔ41-80 | 95.0 ± 15 | 47 ± 5f | + |
| IpaDΔ81-120 | 101.0 ± 11 | 32 ± 2f | + |
| IpaDΔ121-160 | 1.0 ± 1e | 0.0 ± 0e | + |
| IpaDΔ161-180 | 3.0 ± 0.2e | 0.0 ± 0e | + |
| IpaDΔ201-240 | 1.0 ± 0.4e | 0.0 ± 0e | + |
| IpaDΔ241-280 | 3.0 ± 0.2e | 0.0 ± 0e | + |
| IpaDΔ281-320 | 0.1 ± 0.2e | 0.0 ± 0e | + |
| IpaDΔ321-332 | 3.0 ± 0.1e | 0.0 ± 0e | ++ |
| IpaDΔ328-332 | 0.1 ± 0.1e | 0.0 ± 0e | ++ |
| IpaDSC321AS | 147 ± 10 | 100 ± 0.1 | ++ |
| SipD | 0.0 ± 0e | 0.0 ± 0e | Not done |
Strains that have IpaD or an IpaD deletion mutation listed are actually S. flexneri SF622 (a nonpolar ipaD mutant) expressing the gene for that particular IpaD derivative.
Restoration of invasion is presented relative to invasion by SF622 transformed with pWPsf4D±the standard deviation (n = 3). The data shown are from one of three representative experiments.
Contact-mediated hemolysis is presented as a percentage of complete hemolysis, such as that which occurs upon the addition of water (n = 3 from one of five representative experiments).
IpaD secretion was measured by a semiquantitative ELISA system (n = 5) with overnight culture supernatants (also see Fig. 1). The antibodies used here were a mixture of rabbit antisera (numbers 18, 20, and 23) generated against three different IpaD-derived peptides composed of amino acids 55 to 70, 102 to 115, and 280 to 295 (38) that were derived from different regions of the protein so that they could recognize all of the deletion mutations described here. Whole bacterial lysates were also used to determine whether the IpaD derivatives described here were similarly synthesized (Fig. 1). −, no secretion; +, normal secretion; ++, increased secretion.
P < 0.001 by a paired Student t test.
P < 0.05 by a paired Student t test.
When different 40-amino-acid deletions were introduced beginning at residue 41, it was found that amino acids 41 to 80 (IpaDΔ41-80) and 81 to 120 (IpaDΔ81-120) could be removed with no negative effect on IpaD-dependent invasion function, as determined by a gentamicin protection assay that uses a Luria-Bertani agar overlay, followed by counting of subsurface bacterial colonies (Table 2). However, expression of these ipaD deletion mutations in SF622 did result in a decrease in the efficiency with which shigellae lysed RBC (Table 2). Beyond amino acid 120, all of the deletions introduced into IpaD eliminate its ability to restore invasion and contact-mediated hemolysis functions to Shigella strain SF622 (Table 2). Even additional small deletions introduced at or near the IpaD C terminus, as seen for IpaDΔ328-332, eliminate its ability to restore invasiveness and contact-mediated hemolysis activity to SF622 (Table 2). Interestingly, these C-terminal mutations result in the secretion of large amounts of IpaD (Fig. 1), while steady-state levels of IpaD within the bacteria apparently remain the same as in wild-type bacteria (Fig. 1). Moreover, the wild-type ipaD gene restores efficient invasion to SF622, and ipaD possessing a substitution mutation that eliminates a cysteine residue near the C terminus, which has been observed to lead to dimerization of recombinant IpaD in vitro, restored invasion activity to a higher level than did wild-type IpaD (Table 2).
Interestingly, when bacterial invasion of cultured cells was quantified by lysing the host Henle 407 cells following a 20-min gentamicin wash and counting the released bacteria by dilution plating, shigellae expressing ipaDΔ41-80 and ipaDΔ81-120 both appeared to invade at lower levels than shigellae expressing ipaD (41 and 21%, respectively, ± 10%; n = 3). These invasion data more closely parallel the contact-mediated hemolysis data given in Table 2. To allow determination of whether the expression of ipaDΔ41-80 or ipaDΔ81-120 influences the ability of shigellae to spread to adjacent cells, invasion activity was determined by a plaque assay with BHK cells used as host cells (28). This assay also indicated that the invasion activity of SF622 cells expressing ipaDΔ41-80 and ipaDΔ81-120 was reduced (Table 3), but to a lesser extent than when it was measured by releasing the intracellular bacteria (see above). However, it was interesting that while the sizes of the plaques formed by the ipaDΔ41-80 mutant were indistinguishable from those formed by SF622 expressing wild-type ipaD and by wild-type strain M90T, those formed by the ipaDΔ81-120 mutant were approximately 25% smaller than those produced by SF622 expressing either wild-type ipaD or ipaDΔ41-80 and nearly 30% smaller than those produced by M90T (Table 2). These data suggest that the lower level of contact-mediated hemolysis observed for SF622 expressing ipaDΔ81-120, but not ipaDΔ41-80, has an impact on the intercellular spread of this bacterium, as might be expected if it were experiencing difficulty in vacuolar escape either during the initial invasion event or during spread to adjacent cells. Interestingly, none of the deletion mutations tested had a dominant negative effect when introduced into wild-type shigellae (data not shown), suggesting that their possible interactions with other proteins do not interfere with the function of the wild-type IpaD protein already made by these cells.
TABLE 3.
Plaque size on BHK cell monolayers for S. flexneri SF622 expressing ipaD, ipaDΔ41-80, or ipaDΔ81-120
| Strain | Relative invasion (%)a | Plaque size (mm)b |
|---|---|---|
| None | 0.0 ± 0 | NAc |
| M90T | 115 ± 7 | 1.28 ± 0.23 |
| BS547 (mxiM) | 0.0 ± 0 | NA |
| ipaD | 100 ± 10 | 1.21 ± 0.51 |
| ipaDΔ41-80 | 68 ± 17d | 1.18 ± 0.15 |
| ipaDΔ81-120 | 48 ± 13d | 0.91 ± 0.08d |
Invasion is shown relative to that of SF622 expressing wild-type ipaD and with a multiplicity of infection of 1 in six-well tissue culture plates. The data shown are averages of three measurements from a single representative experiment.
Plaque size was determined on day 4 following a 3-day incubation of bacteria with BHK cells and including a 1-day incubation in the presence of neutral red (average ± standard deviation obtained with ≥15 randomly selected plaques in each of two independent experiments).
NA, not applicable because the complete absence of invasion did not give rise to observable plaques.
P ≤ 0.06 by the paired Student t test.
All deletions introduced into IpaD resulted in increased secretion of IpaC.
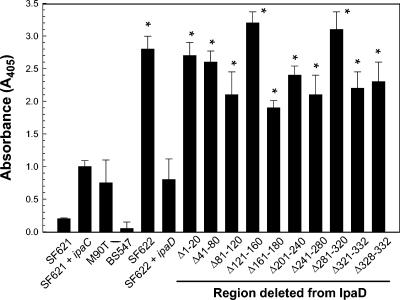
Because the only role for IpaD described to date that relates to Shigella invasion of target cells is its ability to control the secretion of IpaB and IpaC (21), each of the mutations described in Table 2 was tested for the ability to prevent the constitutive secretion of IpaC that occurs in S. flexneri ipaD mutant SF622. SF622 harboring the different ipaD-containing pWPsf4 constructs was grown overnight in TSB, and the resulting culture supernatants were tested for the relative levels of IpaC present by ELISA (Fig. 2). As expected, the absence of IpaD gave rise to increased levels of IpaC detected in culture supernatants, and this increase in IpaC secretion could be eliminated when SF622 was transformed with pWPsf4 expressing wild-type ipaD (Fig. 2). Interestingly, all of the ipaD deletion mutants gave rise to an apparent IpaC-constitutive secretion phenotype, even those that restored invasiveness and a substantial portion of the contact-mediated hemolysis activity to SF622 (Table 2). In each case, the amount of IpaC made in these mutants was largely unchanged as detected by ELISA and immunoblot analysis with whole-cell lysates (data not shown), indicating that altered expression of ipaC was probably not influencing the invasion data.
FIG. 2.
Secretion of IpaC is increased in S. flexneri expressing mutant forms of ipaD. An ELISA was used to allow initial detection of IpaC quantities in the culture supernatants of S. flexneri strain SF622 expressing ipaD harboring different deletions. The primary antibody used here was a mouse monoclonal antibody that recognizes an epitope precisely mapped to the central hydrophilic region of IpaC (37). All analyses were performed in triplicate and are presented as an average ± standard deviation (n = 5) from one of two nearly equal experiments in which the upper limit with respect to linearity for the IpaC protein is approached but not exceeded. On the basis of rough standard curves with purified recombinant IpaC, all of the values observed for the detection of IpaC are within the linear range of detection by this ELISA system, although the higher IpaC values are near the upper range of linear detection. The asterisks indicate P values (Student t test) of ≤0.10.
Constitutive secretion of IpaC and IpaB by SF622 expressing different ipaD deletion mutations.
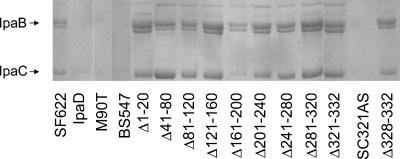
Although the 40-amino-acid deletions near the IpaD N terminus do not eliminate invasion and contact-mediated hemolysis, they do lead to a marked increase in IpaC secretion, as determined by ELISA analysis (Fig. 2). This increase in the secretion of IpaC appears to be nearly equal to that seen in the ipaD null mutant. To examine this more carefully, SF622 expressing the different ipaD deletion mutations were grown overnight and the proteins present in equal amounts of culture supernatant were trichloroacetic acid precipitated and separated by SDS-PAGE. After Coomassie staining, the resulting gels corroborate the data obtained by ELISA analysis by showing that all of the deletions introduced into IpaD result in the constitutive secretion of both IpaB and IpaC (Fig. 3). In fact, the magnitude of this constitutive secretion event appears more pronounced when determined by SDS-PAGE (Fig. 3) than it does when determined by ELISA (Fig. 2). Once again, even the IpaD mutants capable of restoring efficient invasiveness and a substantial amount of contact-mediated hemolysis activity to SF622 allow constitutive secretion of IpaB and IpaC (Fig. 3). These data show that the IpaB/IpaC constitutive-secretion phenotype observed in SF622 bacteria is not responsible per se for their inability to invade cultured cells. These data again indicate that IpaD's role in Shigella invasion is more complex than simply controlling Ipa protein secretion. Interestingly, when a 20-amino-acid deletion is introduced near the middle of IpaD (e.g., IpaDΔ161-180), much of the control of IpaB and IpaC secretion is restored; however, this does not correspond to any level of restoration of either invasion or contact-mediated hemolysis activity (Fig. 3 and Table 2). This finding also implies that IpaD's ability to control Ipa protein secretion alone does not explain the importance of IpaD in Shigella invasion of enterocytes.
FIG. 3.
SDS-PAGE analysis of IpaB and IpaC secreted into culture supernatants by SF622 expressing different ipaD deletion mutations. The strains of S. flexneri shown were grown overnight in TSB, and the proteins present in culture supernatants were trichloroacetic acid precipitated, separated on SDS-10% polyacrylamide gels, and stained with Coomassie blue. The level of secretion of IpaB and IpaC is compared to that for SF622 harboring pWPsf4 without an ipaC insert (designated SF622) and SF622 harboring pWPsf4D for expressing wild-type ipaD (IpaD). Wild-type S. flexneri M90T and S. flexneri BS547 (mxiM mutant lacking a functional TTSS) were also included as controls. The latter of these was performed in an experiment separate from the one with all of the deletion mutations but with SF622, IpaD, and M90T. The overall experiment was performed three times with identical results.
A subtle but possibly significant observation related to the constitutive secretion of Ipa proteins allowed by the different IpaD deletion mutations is that the ratio of IpaB and IpaC found in culture supernatants appears to vary somewhat depending upon the location of the deletion introduced into IpaD. For IpaDΔ41-80 and IpaDΔ81-120, the amount of IpaC in the Shigella culture supernatants appears to exceed the amount of IpaB on the basis of Coomassie blue staining (Fig. 3). However, this trend seems to reverse itself as the mutations are introduced nearer to the C terminus of IpaD (Fig. 3). These differences in the ratio of IpaC to IpaB are subtle, but they are reproducible and can be quantified by densitometric analysis. The ratio of IpaB to IpaC, on the basis of densitometry, is 0.7 to 0.8 for the IpaDΔ41-80 and IpaDΔ81-120 mutants but increases to 1.3 to 1.8 for the remainder of the deletion mutants (and the parent ipaD mutant SF622) described in Fig. 3. When one considers the relative sizes of IpaB and IpaC (approximately 62 versus 42 kDa, respectively), the change in ratio between the two becomes even more striking. Assuming equivalent Coomassie staining, the estimated IpaB/IpaC molar ratio is 0.5 for the IpaDΔ41-80 and IpaDΔ81-120 mutants, while it is 0.9 to 1.22 for the other IpaD deletion mutants. The low level of IpaB and IpaC secretion by SF622 expressing wild-type ipaD prevented making such measurements for this Shigella strain. These findings may implicate IpaD not only in controlling overall Ipa secretion but also in potentially fine-tuning the hierarchy of the relative rates of IpaB and IpaC export.
IpaD does not control the size of the IpaB/IpaC pore inserted into target cell membranes.
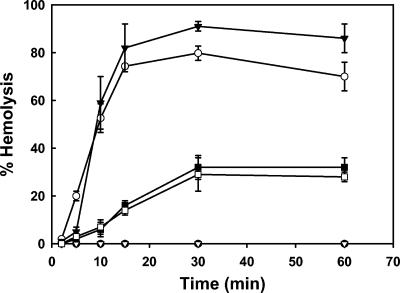
Since there is a reduction in contact-mediated hemolysis activity for SF622 synthesizing IpaDΔ41-80 or IpaDΔ81-120 relative to wild-type IpaD, it was deemed important to determine whether the mutant forms of IpaD were causing a change in the presentation of the IpaB/IpaC complex to RBC. To accomplish this, a time course for contact-mediated hemolysis by S. flexneri M90T and SF622 expressing ipaD, ipaDΔ41-80, or ipaDΔ81-120 was established (Fig. 4). In these experiments, the time required to reach the half-maximal level of contact-mediated hemolysis by a particular Shigella strain (defined here as t1/2) was determined for SF622 expressing wild-type ipaD and SF622 expressing the ipaD deletion mutations still capable of allowing hemolysis. As shown in Fig. 4, each mutant form of IpaD resulted in a slowed kinetics of contact-mediated hemolysis (the t1/2 for M90T and SF622 synthesizing wild-type IpaD is ≤9 min, and the t1/2 for the IpaDΔ41-80 and IpaDΔ81-120 mutants is about 17 min), which could, in part, explain the reduced amount of total hemolysis observed at the end of the assay. However, at this point we could not exclude the possibility that the mutant forms of IpaD resulted in an altered form of the IpaB/IpaC complex inserted into the RBC membranes.
FIG. 4.
Kinetics of contact-mediated hemolysis by S. flexneri. A typical contact-mediated hemolysis reaction was performed, and the extent of hemoglobin release was monitored as a function of time (up to 60 min). The percentage of hemolysis was determined for wild-type S. flexneri M90T (filled triangles) or S. flexneri SF622 expressing ipaD (open circles), ipaDΔ41-80 (closed squares), or ipaDΔ81-120 (open squares). Negative controls included S. flexneri SF622 harboring pWPsf4 (no ipaD insert; filled circles) and S. flexneri BS547 (defective TTSS; open triangles). Complete hemolysis was determined by the addition of water. The values shown are the average of triplicates (± the standard deviation) and are from a representative experiment performed three times.
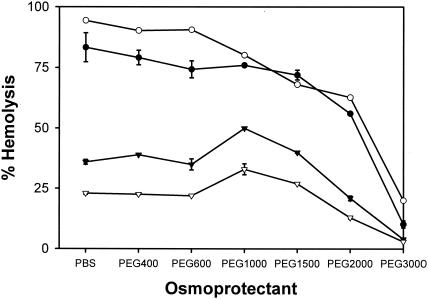
Introduction of Ipa pores into RBC membranes leads to lysis that is detected by measuring the release of hemoglobin following osmotic shock (3, 31). It is possible to prevent lysis by adding molecules that are large enough to be unable to pass through the pore. These molecules counter the increased internal pressure generated within the erythrocytes following pore insertion (3), thereby serving as osmotic protection agents. For Ipa pores introduced by wild-type S. flexneri or by SF622 expressing wild-type ipaD, molecules larger than those of PEG2000 result in significant (greater than 50%) levels of protection against hemolysis (Fig. 5). These data have been shown to correspond to a pore size of about 2.5 to 3.0 nm (3). When these experiments were analyzed as previously described (3) to determine pore size, the IpaDΔ41-80 and IpaDΔ81-120 mutant strains gave rise to 2.80- and 2.90-nm pores, respectively, while the wild-type strain generated a 2.85-nm pore. These values are all within the range established previously (3), i.e., 2.6 ± 0.4 nm, and argue strongly that the N-terminal IpaD mutations do not affect pore size.
FIG. 5.
Influence of different-sized osmoprotectants on the ability for S. flexneri expressing different forms of ipaD to lyse RBC. A typical contact-mediated hemolysis reaction was performed in the presence of different osmoprotectants with wild-type S. flexneri M90T (open circles) or S. flexneri SF622 expressing ipaD (filled circles), ipaDΔ41-80 (filled triangles), or ipaDΔ81-120 (open triangles). Hemolysis in the absence of osmoprotectant is indicated by PBS, and all of the values shown are relative to complete hemolysis, such as that which occurs with the addition of water. The values shown are an average of triplicates ± the standard deviation (n = 3) from a single experiment performed three times.
IpaB and IpaC associate with erythrocyte membranes less efficiently in the presence of IpaDΔ41-80 and IpaDΔ80-120.
To more carefully examine the reason why N-terminal IpaD mutations give rise to reduced contact-mediated hemolysis, whole erythrocyte membranes were prepared following a typical hemolysis assay and the amount of IpaB and IpaC associated with those membranes was determined by immunoblot analysis. As shown in Fig. 6, S. flexneri M90T (wild type) and SF622 harboring a plasmid for the expression of wild-type ipaD insert nearly equal amounts of IpaB and IpaC into the RBC membranes. In contrast, SF622 expressing either ipaDΔ41-80 or ipaDΔ81-120 inserts IpaB and IpaC much less efficiently (Fig. 6). In fact, it is estimated that there is less than a 1/10 of the amount of IpaB and IpaC introduced into the RBC by the ipaDΔ41-80 and ipaDΔ81-120 mutants than there is by either M90T or SF622 expressing wild-type ipaD. No IpaD was found associated with the RBC membranes in any of these experiments (data not shown). The amount of total proteins in the RBC membrane fraction is the same in each case, as determined by the method of Bradford (5). Taken together, the data suggest that the invasion deficiency caused by elimination of ipaD is due to an inability of IpaB and IpaC to be presented to target cells and is not explained solely by constitutive secretion of IpaB and IpaC. Indeed, the constitutive-secretion phenotype appears to have a relatively minor effect on invasion efficiency, as indicated by the IpaDΔ41-80 and IpaDΔ81-120 phenotypes. Moreover, while the constitutive secretion of these proteins alone could solely account for the reduction in contact-mediated hemolysis, this is not supported by data from SF622 expressing ipaDΔ161-180, which secretes at levels more closely resembling those of SF622 expressing ipaD but is still completely noninvasive and completely incapable of contact-mediated hemolysis (Table 2).
FIG. 6.
IpaD controls the efficiency of IpaB and IpaC insertion into erythrocyte membranes. The amount of IpaB and IpaC present in prepared RBC membranes was determined by immunoblot analysis with anti-IpaB and anti-IpaC antibodies. M90T (lane 1) and SF622 expressing ipaD (lane 2) inserted similar amounts of these proteins into the membranes. In contrast, SF622 expressing ipaDΔ41-80 or ipaDΔ81-120 inserted much lower levels of IpaB and IpaC into the membranes (lanes 3 and 4, respectively). SF622 not producing any IpaD failed to insert detectable amounts of IpaB or IpaC into these membranes (lane 6), as did the mxiD TTSS apparatus mutant (lane 5) (1, 33). This experiment was performed three times with similar results.
DISCUSSION
Until now, it has been generally accepted that IpaD's primary function in Shigella invasion is to associate with IpaB within the context of the Mxi/Spa type III secreton to form a plug that prevents or controls the release of the remaining Shigella effectors prior to pathogen contact with a host cell (21). Following activation of the Shigella TTSS, IpaB and IpaC form a translocon pore in the host cell membrane (3, 4). As an integral component of the translocon (3), IpaC may directly initiate the cytoskeletal rearrangements responsible for Shigella entry (18, 31, 36). Similarly, IpaB triggers apoptosis in macrophages (6) and may mediate host cell responses via interactions with CD44 (35). In contrast, IpaD is required for invasion, but it has not been implicated in triggering any specific events in host cells. Recently, it was proposed that IpaD may be involved in delivering IpaB into target cell membranes to initiate formation of the membrane-imbedded Ipa translocon, which then allows the passage of early effectors (IpaA, IpgD) into the host cytoplasm while directly generating the signals needed for initial subversion of the host cytoskeleton (3, 35). This hypothesis assigns a direct role to IpaD in translocon formation and would explain why ipaD mutants are completely unable to invade cultured cells or lyse RBC. IpaD's ability to direct IpaB insertion into RBC membranes in the absence of IpaC would explain why ipaC mutants retain measurable background levels (∼10%) of hemolysis activity but are totally incapable of entering cultured cells (4). Meanwhile, ipaB mutants completely lack contact-mediated hemolysis activity, suggesting that IpaD is not capable of directly inserting IpaC into RBC membranes (4). However, the latter observations could also be explained by the loss of secretion control in ipaD and ipaB mutants, which could potentially cause depletion of the IpaB/IpaC stores within the bacterial cytoplasm, thereby preventing it from being available for insertion into RBC membranes (21).
This study represents the first detailed investigation of IpaD's potential role in the invasion of target cells. Deletion of the immediate N terminus of IpaD (amino acids 1 to 20) eliminates its export via the Shigella TTSS, its ability to control IpaB and IpaC secretion, and its ability to restore invasiveness and contact-mediated hemolysis activities to S. flexneri SF622. This suggests that IpaD's invasion function occurs after it has entered or exited the Mxi/Spa secreton. This is consistent with the original hypothesis that IpaD's major role in host cell invasion is to serve as an external plug within the secreton. Other deletions near the IpaD N terminus had no adverse effects on IpaD secretion and only partial effects on the restoration of invasiveness and contact-mediated hemolysis to SF622. However, these mutations still eliminated IpaD's ability to control IpaB and IpaC secretion. All deletions downstream of amino acid 120 completely eliminated IpaD's ability to restore invasiveness, contact-mediated hemolysis, and control of IpaB/IpaC secretion to SF622. Taken together, these data show that IpaD's essential role in target cell invasion can be uncoupled from its ability to plug Ipa secretion. We find it intriguing that that nearly all of the 20- to 40-amino-acid deletions made in IpaD led to deregulated Ipa secretion, and we therefore propose that secretion regulation involves sensing the folding of the entire protein.
Of particular interest with respect to IpaD's role in hemolysis and cell invasion is that IpaDΔ41-80 and IpaDΔ81-120 restore a significant, albeit reduced, level of invasion and contact-mediated hemolysis activity to SF622. The reduction in contact-mediated hemolysis activity for SF622 expressing ipaDΔ41-80 or ipaDΔ81-120 could be accounted for by a reduction in the number of IpaB/IpaC molecules or pores inserted into the target cell membrane. However, the kinetics of RBC lysis by SF622 expressing ipaDΔ41-80 or ipaDΔ81-120 suggest that these mutants give rise not only to fewer translocons being inserted into target cells but also to a delay in reaching maximal translocon insertion. This reduced number of translocons would still be largely capable of triggering host cell signal transduction events to allow invasion, but the reduced number of IpaB-IpaC pores inserted into the host cell membrane would lead to a reduced rate of pathogen escape into the host cell cytoplasm, slowing intracellular growth and intercellular spread. This is supported by the plaque assay data showing that SF622 expressing ipaDΔ81-120 produces plaques on BHK cell monolayers that are smaller than those produced by SF622 expressing wild-type ipaD, although this does not appear to be the case for SF622 expressing ipaDΔ41-80, perhaps simply because it has a slightly higher pore insertion rate. Taken together, the accumulated invasion data suggest that IpaDΔ41-80 and IpaDΔ81-120 lead to reduced invasion efficiency for SF622, where the lowest level of invasion efficiency was obtained when quantifying the number of bacteria released following lysis of the host cells. However, this observation could be influenced by differences in the intracellular growth rates of SF622 expressing the different forms of ipaD during the invasion and gentamicin wash steps (possibly a result of different rates of vacuolar escape).
Although there is no sequence homology between IpaD and Yersinia LcrV, these two proteins have important functional homology with respect to controlling translocon component secretion, effector secretion, and the formation of the resulting translocon channel (9, 19, 24, 25, 32). Likewise, LcrV has been detected on the Yersinia cell surface prior to Yop secretion (29) as IpaD has been reported to be partially exposed on the surface of S. flexneri cells (21, 38). It is interesting that the residues of IpaD that were reported to be surface exposed by Turbyfill and colleagues (spanning amino acids 30 to 122) (38) are nearly identical to those that can be deleted from IpaD to reduce its ability to restore invasiveness and contact-mediated hemolysis activity to SF622. Furthermore, the atomic structure of LcrV was recently solved (8) and found to contain a long central coiled-coil motif, which is readily identified within the predicted secondary structure of PcrV/IpaD/SipD with available algorithms. Unfortunately, a great deal also remains to be learned about the function of LcrV in Yersinia interactions with host cells.
It was recently shown that when PcrV of Pseudomonas aeruginosa is used to replace Yersinia LcrV, the resulting YopB/YopD translocon pore has a slightly reduced inner diameter (13), indicating that the size of the translocon pore can be specifically regulated. When a similar swap experiment was carried out with Salmonella SipD, which has significant sequence homology with IpaD (15), SipD was found to be incapable of replacing IpaD for Shigella entry, contact-mediated hemolysis, or control of IpaB and IpaC secretion (W. L. Picking and M. A. Baxter, unpublished results). On the basis of the observations described here with different IpaD deletion mutants, it seems likely that even if sipD expression in shigellae resulted in altered Ipa pore size or number, it should still result in some degree of Shigella uptake. Therefore, it appears that, unlike for PcrV and LcrV, IpaD and SipD are not functionally interchangeable.
After performing osmoprotection experiments, it was determined that IpaDΔ41-80 and IpaDΔ81-120 lead to the insertion of IpaB/IpaC pores that are identical in size to those formed in the presence of wild-type IpaD. However, the total amount of IpaB and IpaC that is inserted into RBC membranes by these IpaD mutants is greatly reduced with respect to that seen with wild-type S. flexneri or SF622 expressing full-length ipaD. While it is possible that depletion of cytoplasmic stores of IpaB and IpaC could be responsible for the reduced efficiency of Ipa translocon insertion into target cell membranes, this does not turn out to be the case (K. Komoriya et al., unpublished data). On the basis of the data presented here, it is more likely that IpaD has a role in controlling the efficiency with which IpaB and IpaC are physically inserted into target cell membranes. This is similar to the recent findings of Goure and colleagues, who found that P. aeruginosa PcrV has an important role in the formation of the PopB/PopD translocation pore in target cells (10).
The data presented here implicate IpaD as having an important role in targeting the Shigella effectors IpaB and IpaC to the plasma membrane of target cells for translocon formation. An additional IpaD function is control of IpaB and IpaC secretion; however, this particular activity does not appear to be essential for cellular invasion, at least in vitro. Instead, control of secretion may be an energy-conserving function that ensures maximal invasion efficiency in the human colon, where regulation of the later stages of infection is critical. It is also likely that efficient insertion of IpaB-IpaC pores is needed for maximum disruption of the phagosome immediately following entry. What remains to be determined is how IpaD exerts its function. Whether IpaD ever physically associates with the IpaB/IpaC complex is not clear; however, it is evident that IpaD has a role in the invasion of intestinal epithelial cells that extends well beyond its ability to control Ipa protein secretion.
Acknowledgments
This work was supported by PHS grants AI034428 and RR017708 and the University of Kansas Research Development Fund. W.D.P., A.B., and H.N. were supported by the Guy Newton Research Fund Senior Research Fellowship and a PHS Career Transition Grant from the NIH (K22 AI01847).
Technical assistance from A. Olive, M. Froelich, K. Flentie, and M. Buechner is gratefully acknowledged, as is assistance from E. V. Oaks (WRAIR) in developing the plaque assay used in this study.
Editor: J. T. Barbieri
REFERENCES
- 1.Allaoui, A., P. J. Sansonetti, and C. Parsot. 1993. MxiD, an outer membrane protein necessary for the secretion of the Shigella flexneri Ipa invasins. Mol. Microbiol. 7:59-68. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, J. C. Q., and C. Hughes. 2000. From flagellum assembly to virulence: the extended family of type III export chaperones. Trends Microbiol. 8:202-204. [DOI] [PubMed] [Google Scholar]
- 3.Blocker, A., P. Gounon, E. Larquet, K. Niebuhr, V. Cabiaux, C. Parsot, and P. Sansonetti. 1999. The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blocker, A., K. Komoriya, and S-I. Aizawa. 2003. Type III secretion systems and bacterial flagella: insights into their function from structural similarities. Proc. Natl. Acad. Sci. USA 100:3027-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 7:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Y., M. R. Smith, K. Thirumalai, and A. Zychlinsky. 1996. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 15:3853-3860. [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, R., M. E. Marquart, D. Lucius, and W. D. Picking. 1998. Protein-protein interactions in the assembly of Shigella flexneri invasion plasmid antigens IpaB and IpaC into protein complexes. Biochim. Biophys. Acta 1429:45-56. [DOI] [PubMed] [Google Scholar]
- 8.Derewenda, U., A. Mateja, Y. Devedjiev, K. M. Routzahn, A. G. Evdokimov, Z. S. Derewenda, and D. S. Waugh. 2004. The structure of Yersinia pestis V-antigen, an essential virulence factor and mediator of immunity against plague. Structure (Cambridge) 12:301-306. [DOI] [PubMed] [Google Scholar]
- 9.Fields, K. A., M. L. Nilles, C. Cowan, and S. C. Straley. 1999. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect. Immun. 67:5395-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goure, J., A. Pastor, E. Faudry, J. Chabert, A. Dessen, and I. Attree. 2004. The V antigen of Pseudomonas aeruginosa is required for assembly of the functional PopB/PopD translocation pore in host cell membranes. Infect. Immun. 72:4741-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hale, T. L. 1991. Genetic basis of virulence in Shigella species. Microbiol. Rev. 55:206-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington, A. T., P. D. Hearn, W. L. Picking, J. R. Barker, A. Wessel, and W. D. Picking. 2003. Structural characterization of the N terminus of IpaC from Shigella flexneri. Infect. Immun. 71:1255-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmstrom, A., J. Olsson, P. Cherepanov, E. Maier, R. Nordfelth, J. Pettersson, R. Benz, H. Wolf-Watz, and A. Forsberg. 2001. LcrV is a channel size-determining component of the Yop effector translocon of Yersinia. Mol. Microbiol. 39:620-632. [DOI] [PubMed] [Google Scholar]
- 14.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaniga, K., D. Trollinger, and J. E. Galan. 1995. Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J. Bacteriol. 177:7078-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwae, A., S. Yoshida, K. Tamano, H. Mimuro, T. Suzuki, and C. Sasakawa. 2001. Shigella invasion of macrophage requires the insertion of IpaC into the host plasma membrane: functional analysis of IpaC. J. Biol. Chem. 276:32230-32239. [DOI] [PubMed] [Google Scholar]
- 17.Marquart, M. E., W. L. Picking, and W. D. Picking. 1995. Structural analysis of invasion plasmid antigen D (IpaD) from Shigella flexneri. Biochem. Biophys. Res. Commun. 214:963-970. [DOI] [PubMed] [Google Scholar]
- 18.Marquart, M. E., W. L. Picking, and W. D. Picking. 1996. Soluble invasion plasmid antigen C (IpaC) from Shigella flexneri elicits epithelial cell responses related to pathogen invasion. Infect. Immun. 64:4182-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matson, J. S., and M. L. Nilles. 2001. LcrG-LcrV interaction is required for control of Yops secretion in Yersinia pestis. J. Bacteriol. 183:5082-5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menard, R., M. C. Prevost, P. Gounon, P. Sansonetti, and C. Dehio. 1996. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc. Natl. Acad. Sci. USA 93:1254-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menard, R., P. Sansonetti, and C. Parsot. 1994. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 13:5293-5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menard, R., P. Sansonetti, C. Parsot, and T. Vasselon. 1994. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell 79:515-525. [DOI] [PubMed] [Google Scholar]
- 24.Nilles, M. L., K. A., Fields, and S. C. Straley. 1998. The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J. Bacteriol. 180:3410-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilles, M. L., A. W. Williams, E. Skrzypek, and S. C. Straley. 1997. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J. Bacteriol. 179:1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oaks, E. V., M. E. Wingfield, and S. B. Formal. 1985. Plaque formation by virulent Shigella flexneri. Infect. Immun. 48:124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osiecki, J. C., J. Barker, W. L. Picking, A. B. Serfis, E. Berring, S. Shah, A. Harrington, and W. D. Picking. 2001. IpaC from Shigella and SipC from Salmonella possess similar biochemical properties but are functionally distinct. Mol. Microbiol. 42:469-481. [DOI] [PubMed] [Google Scholar]
- 28.Penheiter, K. L., N. Mathur, D. Giles, T. Fahlen, and B. D. Jones. 1997. Non-invasive Salmonella typhimurium mutants are avirulent because of an inability to enter and destroy M cells of ileal Peyer's patches. Mol. Microbiol. 24:697-709. [DOI] [PubMed] [Google Scholar]
- 29.Pettersson, J., A. Holmstrom, J. Hill, S. Leary, E. Frithz-Lindsten, A. von Euler-Matell, E. Carlsson, R. Titball, A. Forsberg, and H. Wolf-Watz. 1999. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32:961-976. [DOI] [PubMed] [Google Scholar]
- 30.Picking, W. L., L. Coye, J. C. Osiecki, A. Barnoski Serfis, E. Schaper, and W. D. Picking. 2001. Identification of functional regions within invasion plasmid antigen C (IpaC) of Shigella flexneri. Mol. Microbiol. 39:100-111. [DOI] [PubMed] [Google Scholar]
- 31.Sansonetti, P. J., A. Ryter, P. Clerc, A. T. Maurelli, and J. Mounier. 1986. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect. Immun. 51:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarker, M. R., C. Neyt, I. Stainier, and G. R. Cornelis. 1998. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J. Bacteriol. 180:1207-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuch, R., and A. T. Maurelli. 2001. MxiM and MxiJ, base elements of the Mxi-Spa type III secretion system of Shigella, interact with and stabilize the MxiD secretin in the cell envelope. J. Bacteriol. 183:6991-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terajima, J., E. Moriishi, T. Kurata, and H. Watanabe. 1999. Preincubation of recombinant Ipa proteins of Shigella sonnei promotes entry of non-invasive Escherichia coli into HeLa cells. Microb. Pathog. 27:223-230. [DOI] [PubMed] [Google Scholar]
- 35.Tran Van Nhieu, G., R. Bourdet-Sicard, G. Dumenil, A. Blocker, and P. J. Sansonetti. 2000. Bacterial signals and cell responses during Shigella entry into epithelial cells. Cell. Microbiol. 2:187-193. [DOI] [PubMed] [Google Scholar]
- 36.Tran Van Nhieu, G., E. Caron, A. Hall, and P. J. Sansonetti. 1999. IpaC induces actin polymerization and filopodia formation during Shigella entry into epithelial cells. EMBO J. 18:3249-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turbyfill, K. R., S. W. Joseph, and E. V. Oaks. 1995. Recognition of three epitopic regions on invasion plasmid antigen C by immune sera of rhesus monkeys infected with Shigella flexneri 2a. Infect. Immun. 63:3927-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turbyfill, K. R., J. A. Mertz, C. P. Mallett, and E. V. Oaks. 1998. Identification of epitope and surface-exposed domains of Shigella flexneri invasion plasmid antigen D (IpaD). Infect. Immun. 66:1999-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watarai, M., S. Funato, and C. Sasakawa. 1996. Interaction of Ipa proteins of Shigella flexneri with α5β1 integrin promotes entry of the bacteria into mammalian cells. J. Exp. Med. 183:991-999. [DOI] [PMC free article] [PubMed] [Google Scholar]