Abstract
Persistent Helicobacter felis infection in (C57BL/6 × 129SvEv)F1 mice induces chronic gastritis. Expression of inducible nitric oxide synthase (iNOS) is upregulated in response to Helicobacter infection. In this study, 20 10-week-old iNOS−/− mice and 20 wild-type [(C57BL/6 × 129SvEv)F1] mice were infected with H. felis by oral gavage and were assessed histologically and serologically at 32 weeks postinfection. Equal numbers of uninfected controls were sham inoculated. The mice were scored for severity of gastric inflammation, hyperplasia, glandular atrophy, and mucous metaplasia in the corpus and for the level of helicobacter colonization. The immunoglobulin G1 (IgG1), IgG2a, and IgG2c antibody responses to H. felis were determined. As a secondary measure, serum cholesterol levels were assessed. iNOS−/− mice have a propensity for increased serum cholesterol, and although controversial, several human epidemiologic studies have demonstrated an association between Helicobacter infection and several risk factors for cardiovascular disease, including elevated serum cholesterol. Nevertheless, no differences in serum cholesterol levels were observed between the H. felis-infected and -uninfected iNOS−/− mice in this study. The uninfected animals had minimal to no gastric pathology. The gastric pathology scores for the infected animals were reduced significantly in the iNOS-deficient mice relative to those for the wild-type mice (all P < 0.01). Helicobacter-infected iNOS−/− mice had chronic lymphoid infiltration and negligible to mild glandular atrophy and mucous metaplasia in the fundic mucosa, while H. felis-infected wild-type mice had severe atrophic and metaplastic mucosal changes. The atrophic gastritis in the infected wild-type mice, particularly the female mice, was also accompanied by greater granulocytic infiltration, antral hyperplasia, and diminished antral colonization, unlike that in the infected iNOS−/− mice. iNOS−/− mice developed significantly lower Th1-associated IgG2c antibody responses to H. felis (P < 0.0003); the Th2-associated IgG1 responses were similar (P = 0.09), suggesting a greater effect of the iNOS defect on Th1 responses. H. felis colonization was significantly greater in the iNOS-deficient mice. These findings are indicative of an impaired Th1 component of the H. felis-induced inflammatory response when the influence of iNOS is removed.
Helicobacter felis is a gram-negative helical bacterium that persistently colonizes the gastric mucosa of experimentally infected C57BL/6 mice, resulting in chronic inflammation and, given sufficient time, gastric adenocarcinoma (14, 38, 39, 42). As in human Helicobacter pylori infection, it has been recognized for some time that the destruction of gastric tissue caused by chronic inflammation characterized by a predominantly Th1-type immune response may contribute to the carcinogenic effects of H. felis infection in mice (2, 8, 16, 20, 25, 31, 35). Recently, nitric oxide (NO) has been studied as a component of the microbially induced inflammatory process as both an effector and regulatory molecule of the immune response. When highly expressed, particularly by inducible nitric oxide synthase (iNOS), NO has been shown to be an important cytotoxic molecule in a variety of infectious and inflammatory diseases. In addition, elevated levels of NO affect the types of cytokines expressed in tissues through the action of NO as a signaling molecule and its regulation of transcription factors (4, 17, 22, 47). H. pylori has been shown to stimulate iNOS production in humans and in mice, both in vitro (e.g., in gastric epithelial cell lines) and in vivo (34). Although NO is a very small molecule, it has wide-ranging and sometimes contradictory biological effects. For example, NO inhibits the expression of genes for Th1-type cytokines such as interleukin-1, gamma interferon, and tumor necrosis factor gamma (TNF-γ). Conversely, it has also been shown that these same cytokines are decreased when NO is inhibited (1).
Several epidemiological and clinical studies have suggested an association between H. pylori seropositivity and cardiovascular disease (10, 27, 44, 45). In a meta-analysis of 18 epidemiological studies which measured H. pylori serum antibody titers and risk factors for cardiovascular disease in a large number (>10,000) of patients, a weak association was found between H. pylori infection and cardiovascular disease (7).
Other studies have reported elevated serum cholesterol levels and modified lipid profiles associated with H. pylori infection (3, 23, 41). A prior study from this laboratory demonstrated that iNOS-deficient (iNOS−/−) mice have elevated serum cholesterol levels and concomitant atherosclerotic lesions when they are fed a basal synthetic diet (19).
To isolate the effect of inhibiting elevated concentrations of NO in vivo, we infected wild-type (WT) and iNOS−/− mice with H. felis, correlated the damage to gastric tissue attributable to chronic Helicobacter-induced inflammation with H. felis colonization levels, and characterized the corresponding IgG isotype responses associated with the balance between Th1 and Th2 host responses. Also, given our previous observations that iNOS−/− mice are predisposed to developing high cholesterol levels and the debate surrounding H. pylori infection as a risk factor for cardiovascular disease, we measured serum cholesterol levels in infected and uninfected WT and iNOS−/− mice.
MATERIALS AND METHODS
Animals.
Forty specific-pathogen-free (C57BL/6 × 129SvEv)F1 (B6129F1) mice (Taconic Inc., Germantown, N.Y.) were used as WT controls for 40 iNOS−/− mice bred on a B6129 mixed genetic background. The mice were free of viral antibodies, ecto- and endoparasites, and selected murine bacterial pathogens, including Helicobacter spp. The animals, which were all fed a normal rodent chow diet (RMH 3000; Purina, St. Louis, Mo.), were divided into equal groups by genotype, gender, and infection status. Eight groups of 10 mice each were inoculated by oral gavage three times at 2-day intervals with either 108 H. felis cells in 0.2 ml of medium or sterile medium alone at 6 to 8 weeks of age. Mice were necropsied at 32 weeks postinfection, serum was collected, and tissues were fixed in 10% buffered formalin for histopathology. The Massachusetts Institute of Technology is an animal facility accredited with the Association for Assessment and Accreditation of Laboratory Animal Care International, and its Committee on Animal Care approved all procedures.
Culture of H. felis.
H. felis was grown in brucella broth containing 5% fetal calf serum under microaerophilic conditions (80% N-10% C-10% H) on a shaker (120 rpm) overnight or until the culture appeared turbid. The culture was checked by Gram staining and phase microscopy to confirm that it was pure and that the bacteria had normal motility and morphology. The bacteria were then centrifuged at 6,000 × g for 20 min, the supernatant was discarded, and the pellet was resuspended in brucella broth to achieve an optical density at 660 nm of 1.0 as determined by spectrophotometry.
Evaluation of serum antibody responses to H. felis.
Serum collected at 32 weeks postinfection was evaluated by enzyme-linked immunosorbent assay for serum IgG1, IgG2a (Th1-promoted isotype in 129SvEv mice), and IgG2c (Th1-pomoted isotype in C57BL/6 mice) using an outer membrane antigen preparation of H. felis as previously described (13, 26). Immulon II plates (Thermo Labsystems, Franklin, Mass.) were coated with antigen overnight at 4°C at a concentration of 10 μg/ml, and sera were diluted to a ratio of 1:100. Biotinylated secondary antibodies included monoclonal antimouse antibodies produced by clones A85-1, R19-157, and 5.7 (Pharmingen, San Diego, Calif.) for detecting IgG1, IgG2a, and IgG2c, respectively. Incubation with extravidin peroxidase (Sigma, St. Louis, Mo.) was followed by treatment with ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) for color development. The development of optical density at 405 nm was recorded by an enzyme-linked immunosorbent assay plate reader (MR7000; Dynatech Laboratories, Inc., Chantilly, Va.).
Histopathology.
Formalin-fixed tissues were embedded in paraffin, cut into 4-mm-thick sections, and stained with hematoxylin and eosin for an assessment of morphology. A board-certified veterinary pathologist blind to sample identity evaluated stomach tissues for histopathologic changes. Warthin-Starry staining was used to visualize H. felis. Lesions were scored on the basis of size and frequency on a scale of severity from 0 to 4. Scores for gastric H. felis colonization were assigned based on the relative frequency of colonized glands in the stomach by use of Warthin-Starry silver-stained sections as follows: 0, no Warthin-Starry stain-positive organisms observed; 1, low numbers in a few glands per field; 2, low numbers in most glands; 3, moderate numbers in most glands; 4, densely packed glands with higher numbers of H. felis cells.
Serum cholesterol measurement.
Immediately following euthanasia of the mice with CO2, blood was collected by cardiac puncture from each mouse and analyzed for cholesterol concentration by an outside laboratory using Cholesterol/HP (Boehringer Mannheim, Indianapolis, Ind.). This assay comprises a series of enzymatic reactions that hydrolyze cholesterol esters, oxidize free cholesterol, and ultimately, form an o-quinone imine dye, which is measured photometrically as an indicator of cholesterol concentration.
Statistical analyses.
An analysis of variance was performed for serum cholesterol and each of the dependent histopathology variables to assess the concurrent impact of genotype, infection status, and gender on the pathogenesis of H. felis-induced disease. The Mann-Whitney nonparametric test was performed to compare the effects of individual independent variables on the pathology scores, and unpaired t tests were used to compare the cholesterol levels among the groups. Linear regression was used to analyze the antibody isotype responses to H. felis in infected mice, with genotype and gender included as independent variables in the analysis. Results were considered significant at P values of <0.05.
RESULTS
Evaluation of serum antibody responses to H. felis.
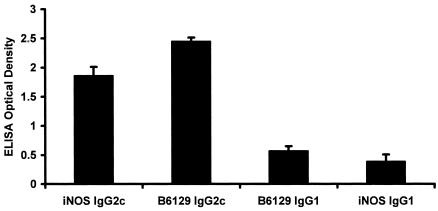
Analysis of infected mice demonstrated that the Th1-associated C57BL/6 antibody phenotype (IgG2c) predominated in both WT [(C57BL/6 × 129SvEv)F1] and iNOS−/− mice (data not shown). There was no gender effect on the IgG1 or IgG2c antibody responses to H. felis (P = 0.96 and 0.99, respectively). WT mice had a more robust IgG2c response to H. felis than iNOS−/− mice (P < 0.0003), but their IgG1 responses were similar (P = 0.09) (Fig. 1).
FIG. 1.
Mean values + standard errors for serum IgG2c and IgG1 responses of iNOS−/− and B6129F1 mice to H. felis at 32 weeks postinfection. iNOS−/− mice developed significantly lower Th1-associated IgG2c antibody responses to H. felis (P < 0.0003); the Th2-associated IgG1 responses of the two types of mice were similar (P = 0.09), suggesting a greater effect of the iNOS defect on Th1-associated responses.
Pathology.
H. felis infection resulted in significant gastric inflammation, epithelial hyperplasia, metaplasia, and glandular atrophy (P < 0.003), with the most-severe lesions noted in female WT mice, not WT males (Fig. 2 and 3; Table 1). Lesions tended to be only mild to moderate in severity in male and female iNOS−/− mice, and less-severe disease was associated with higher levels of H. felis colonization in iNOS−/− mice than in WT mice (Fig. 4).
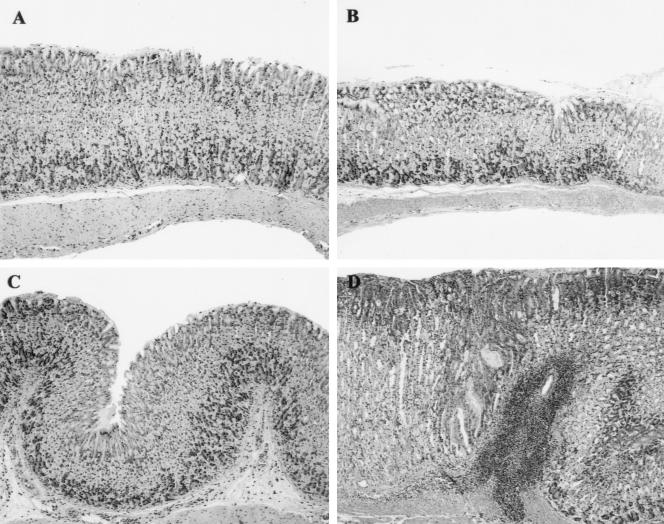
FIG. 2.
Stomach tissue sections from control and H. felis-infected iNOS−/− and WT males. (A) Stomach tissue from an iNOS−/− uninfected male control mouse. There is minimal leukocytic infiltration of the deep mucosa, and the glandular epithelium is normal. (B) Stomach tissue from a WT uninfected male mouse. There is no significant leukocytic infiltrate or mucosal alteration. (C) Stomach tissue from an iNOS−/− male mouse. In response to H. felis infection, there is minimal infiltration of the superficial and deep fundic mucosa and underlying submucosa with a small number of mononuclear cells. Alteration of the mucosal epithelium is limited to minimal hyperplasia of the foveolar epithelium. (D) Stomach tissue from a WT male mouse. In response to H. felis infection, the fundic mucosa has moderate lymphoplasmacytic and granulocytic inflammation centered chiefly in the submucosa, accompanied by moderate hyperplasia of the foveolar epithelium and concurrent atrophy of the parietal and chief cells. Hematoxylin and eosin staining. Magnification, ×10.
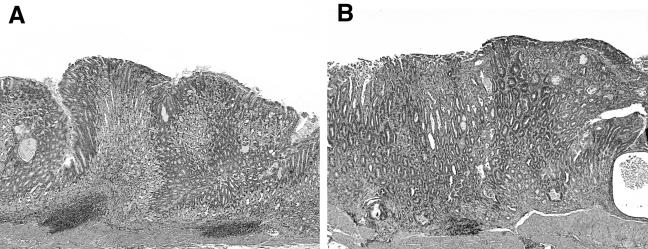
FIG. 3.
Tissue sections from H. felis-infected WT male and female mice illustrating the influence of gender on the severity of disease. WT females consistently had more-severe pathology than did males. (A) Stomach tissue from a WT male mouse. In response to H. felis infection, the fundic mucosa has moderate lymphoplasmacytic and granulocytic inflammation centered chiefly in the submucosa, accompanied by moderate hyperplasia of the foveolar epithelium and concurrent atrophy of the parietal and chief cells. (B) Stomach tissue from a WT female mouse. In response to H. felis infection, there is moderate to marked inflammation of the fundic mucosa and submucosa. The inflammation is characterized by moderate to marked accumulation of lymphoplasmacytic and granulocytic foci in the submucosa and extensive mixed inflammatory infiltration of the mucosa, including marked intraepithelial leukocytic infiltration. The glandular epithelium has marked foveolar hyperplasia and mucous cell metaplasia, with concurrent loss of parietal and chief cells. Hematoxylin and eosin staining. Magnification, ×10.
TABLE 1.
Gastric lesions and H. felis colonization levels in WT (B6129F1) and iNOS−/− micea
| Genotype | Infection | Sexg (no.) | Score for finding inh:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Corpus
|
Antrum
|
||||||||
| Inflammation | Hyperplasia | Metaplasia | Atrophy | Inflammation | Hyperplasia | H. felis colonization | |||
| B6129F1 | None (control) | M (10)b | 0.25 (0-2) | 0 (0-3) | 0 (0-4) | 0 (0-3) | 1 (0-1) | 1 (0-1) | 0 (0) |
| F (10)b | 0.75 (0-1) | 0 (0-1) | 1 (0-3) | 0.5 (0-1) | 1 (1-3) | 1 (0-2) | 0 (0) | ||
| iNOS−/− | None (control) | M (11)b | 0.5 (0-3.5) | 1 (0-2) | 0 (0-2) | 0 (0-4) | 0 (0-4) | 1 (0-4) | 0 (0) |
| F (6) | 0.75 (0.5-1) | 0.5 (0-1) | 0 (0-1) | 0 (0) | 1 (0-1) | 1 (0-1) | 0 (0) | ||
| B6129F1 | H. felis | M (10) | 2.25 (0.5-4)e | 2 (1-4)c,e | 1.5 (0-4)d,e | 1 (0-4)c | 2 (1-2)c | 3 (1-4) | 4 (0-4)d,f |
| F (9) | 3.5 (2-3.5)e | 4 (1-4)e | 4 (0-4)e | 4 (1-4)e | 3 (1-3)e | 3 (2-4) | 0 (0-4)e | ||
| iNOS−/− | H. felis | M (9) | 1 (0.5-2.5) | 1 (1-2) | 0 (0-2) | 1 (0-2) | 2 (1-2) | 2 (1-3) | 4 (4)d |
| F (8) | 2 (0.5-3) | 2 (0-4) | 0 (0-1) | 2 (0-2) | 2 (0-2) | 2 (1-3) | 4 (0-4) | ||
See Results for specific P values.
This control group included one mouse with a high score in at least one parameter.
Significant influence of gender at a P of <0.05.
Trend for influence of gender at a P of <0.10.
Significant influence of genotype (iNOS−/− vs WT) at a P of <0.05.
Trend for influence of genotype (iNOS−/− vs WT) at a P of <0.10.
M, male; F, female.
Values presented are median scores (with ranges in parentheses). See Materials and Methods for a description of the scoring technique.
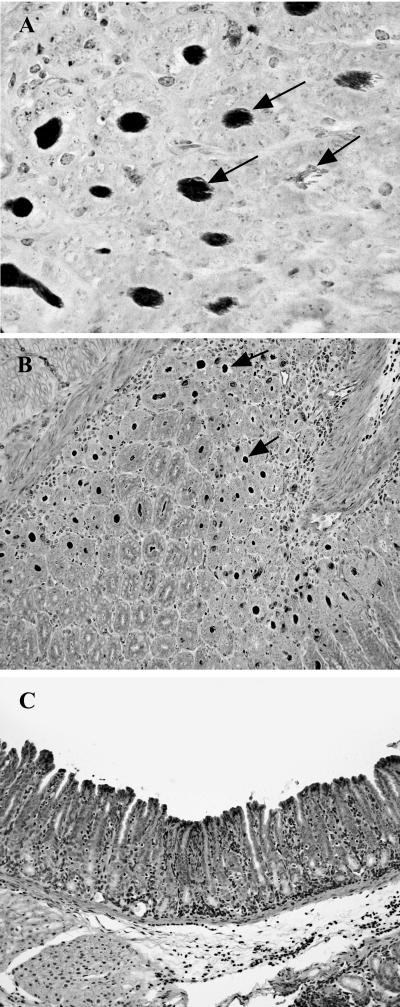
FIG. 4.
(A) H. felis cells (arrows) filling the gastric crypts of an iNOS−/− mouse. Warthin-Starry staining. Magnification, ×60. (B) Numerous H. felis cells (arrows) are evident in the stomach crypts of an infected iNOS−/− mouse. Magnification, ×20. (C) Fundus of H. felis-infected WT mouse with few discernible organisms evident by Warthin-Starry staining contrasts with the depiction in panel B. Magnification, ×20.
Female H. felis-infected WT mice developed more-severe epithelial hyperplasia (P < 0.03) and glandular atrophy (P < 0.02) in the corpus than did infected male WT mice. There was a trend toward more-severe corpus gastritis (P = 0.06) and metaplasia (P = 0.09) in infected female WT mice than in WT males. With respect to the antrum, H. felis-infected female WT mice developed more-severe inflammation than did males (P < 0.008), but male and female WT mice developed similar degrees of moderate to severe epithelial hyperplasia (P = 0.83). The milder lesions in male WT mice were associated with a trend toward higher colonization levels of H. felis in male WT mice than in female WT mice (P = 0.06).
The potential influence of gender on the severity of lesions noted in the corpus or antrum and on colonization levels of H. felis in infected WT mice was not observed in infected iNOS−/− mice. Infected male and female iNOS−/− mice developed similar low-grade lesions of all types, including corpus gastritis (P = 0.27), epithelial hyperplasia (P = 0.81), metaplasia (P = 0.74), glandular atrophy (P = 0.81), antral gastritis (P = 0.96), and hyperplasia of the antral epithelium (P = 0.45). H. felis colonization levels were similar for male and female iNOS−/− mice (P = 0.09) (Table 1).
Female H. felis-infected WT mice developed more-severe corpus gastritis (P < 0.02), epithelial hyperplasia (P < 0.03), metaplasia (P < 0.003), and glandular atrophy (P < 0.003) than did infected female iNOS−/− mice. Antral gastritis of lesser severity in female iNOS−/− mice (P < 0.01) along with a trend toward similarly severe epithelial hyperplasia in the antra of female iNOS−/− and WT mice (P = 0.05) were associated with higher colonization levels of H. felis in female iNOS−/− mice (P < 0.04). Although the lesions observed in infected male WT mice were less severe than those in WT females, corpus gastritis (P < 0.05), epithelial hyperplasia (P < 0.04), and metaplasia (P < 0.02) were more severe in H. felis-infected male WT mice than in infected male iNOS−/− mice. Milder lesions in male iNOS−/− mice were associated with a trend toward higher H. felis colonization levels in these mice than in male WT mice (P = 0.07) (Table 1).
Serum cholesterol.
Although no atherosclerotic lesions were observed in the present study (data not shown), serum cholesterol levels were significantly higher in the iNOS−/− mice than in the WT controls (Table 2), a result which is consistent with our previous study (19). Elevated cholesterol levels were influenced by genotype (P < 0.0007; the levels for iNOS−/− mice were higher than those for WT mice) and gender (P < 0.002; the levels for males were higher than those for females). However, no effect due to H. felis infection status was observed (P = 0.30).
TABLE 2.
Mean serum cholesterol levels in WT (B6129F1) and iNOS−/− micea
| Genotype | Infection | Sexf (no.) | Cholesterol (mg/dl) |
|---|---|---|---|
| WT | None (control) | M (10) | 138.9b,d |
| F (10) | 114.0b | ||
| iNOS−/− | None (control) | M (11) | 169.1c,d |
| F (6) | 129.5c | ||
| WT | H. felis | M (10) | 145.4b |
| F (9) | 118.6b,e | ||
| iNOS−/− | H. felis | M (10) | 168.6 |
| F (8) | 156.5e |
Serum cholesterol levels were not influenced by infection status.
Significant influence of gender at a P of <0.05.
Trend for influence of gender at a P of <0.10.
Significant influence of genotype at a P of <0.05.
Trend for influence of genotype at a P of <0.10.
M, male; F, female.
DISCUSSION
The present study investigated the role of iNOS in the immune response and subsequent pathology of mice chronically infected with H. felis. Prior studies established that NO may have either protective or detrimental effects, depending on a variety of factors (9, 15, 18, 21, 28, 29, 37, 39, 43, 49). Importantly, iNOS−/− mice of the same strain and from the same vendor have been previously characterized as to their levels of nitrate or nitrite (NOx) in plasma and expression of myeloperoxidase in gastric tissue when colonized with H. pylori (28). In each case, WT control and WT H. pylori-infected mice had statistically higher levels of NOx in plasma and had increased myeloperoxidase activity in the gastric mucosa than iNOS−/− mice (28).
The relationship between gastric Helicobacter infection and a Th1-type immune response has been well established for both humans and murine models of Helicobacter-induced disease (2, 8, 16, 20, 25, 31, 35, 46). In their sera, infected humans and mice develop significant IgG2 levels in response to Helicobacter, which is indicative of a Th1 response and is supported by the predominance of Th1-associated cytokines in affected tissues (9, 18, 20, 21).
In addition to determining the predominance of Th1 cytokines, previous studies determined that Helicobacter infection leads to upregulation of iNOS, which has been implicated in DNA damage, apoptosis, carcinogenesis, and the cytotoxicity observed in Helicobacter infections (15, 21, 28, 31). Previous studies have shown that patients infected with H. pylori have higher levels of both oxidized and nitrated proteins and increased iNOS expression than uninfected humans do (24, 40).
Results from this study suggest that iNOS is at least partially responsible for the polarized Th1 proinflammatory response typically associated with gastric Helicobacter infection in WT mice bred on a C57BL/6 background. In the iNOS−/− mice, the Th1-associated IgG2c response was significantly reduced relative to that of the WT mice, supporting the observation that the H. felis-induced inflammatory response was lower in iNOS−/− mice than in infected WT mice. Moreover, the pathology, including premalignant lesions such as gastric atrophy, observed in the gastric tissue of the infected WT mice was much less severe in the H. felis-infected iNOS−/− mice. Finally, the level of H. felis colonization in the stomachs of the iNOS−/− mice was much greater than that in the WT mouse stomachs. This constellation of changes in the iNOS−/− mice is consistent with those encountered in two other examples of inhibited Helicobacter-associated Th1 immune response. C57BL/6 mice coinfected with H. felis and the enteric helminth Heligmosomoides polygyrus developed less-severe gastric atrophy than did C57BL mice infected with H. felis only. Similarly, H. felis-infected p53+/− hemizygous mice had less-severe gastric pathology than their H. felis-infected WT counterparts. Also p53+/− and coinfected mice had higher levels of H. felis colonization than in WT mice or, in the later case, the mice infected with H. felis only (11, 14, 48).
A partial explanation for the significantly less gastric atrophy, i.e., loss of parietal cells, in infected H. felis iNOS−/− mice compared to that in infected WT mice was provided in a study of TNF-α-induced apoptosis of parietal cells (32). In this in vitro model, TNF-α-stimulated apoptosis in rat parietal cells was triggered by iNOS. These data are also consistent with the gastric pathology associated with a Th2 response noted in Heligomosomoides polygyrus- and H. felis- coinfected mice, in contrast to a Th1-related pathology in mice infected with H. felis alone (11). In the parasitized mice infected with H. felis alone, the gastritis was attenuated, resulting in less gastric atrophy (parietal cell and chief cell loss) and intestinal metaplasia than that in the coinfected mice. Correspondingly, gastric tissue from these dually infected mice had significantly less TNF-α expression and, by inference, less iNOS produced in the gastric mucosa than did the H. felis-infected mice, which developed more severe atrophy and intestinal metaplasia (11). These results are mirrored by a recent study using iNOS−/− C57BL/129 male mice (similar to the mice in this study) infected with the SS strain of H. pylori (28). The authors noted that, while inflammation did not differ significantly between WT and iNOS−/− mice, epithelial cell apoptosis induced by H. pylori was attenuated in the iNOS−/− mice at 32 weeks postinfection. Unfortunately, details regarding gastric atrophy were not discussed. In addition, the iNOS−/− mice had higher levels of H. pylori colonization, although not to a statistically significant degree, a result similar to our finding that iNOS−/− mice infected with H. felis had higher colonization levels than infected WT mice (28). Also of interest is a recent report of H. pylori-infected iNOS mice that supports our findings (30). The authors reported that 1 year after infection with H. pylori in combination with oral administration of nitrosourea, there was a significantly (P < 0.05) lower incidence of gastric adenocarcinoma in iNOS−/− mice than in the WT controls treated with the same agents.
A significant elevation in serum cholesterol levels was observed in iNOS−/− mice relative to WT mice, consistent with results from our previous study (19). In contrast to our findings in the previous study, however, there were no corresponding lesions of cardiovascular disease. We attribute this inconsistency in atheroma development to the differing fat content of the diets used in the two studies. In our earlier study, mice were fed a basal synthetic diet which contained 22% fat, whereas in this study the mice were maintained on regular rodent chow, which contained 14% fat.
Infection-dependent differences in serum choloesterol levels were not observed between the H. felis-infected iNOS−/− and WT mice. This outcome reinforces the emerging evidence that gastric Helicobacter infection most likely does not exacerbate or accelerate atherosclerotic lesion development (6, 33, 36).
Our findings indicate that iNOS is a key component in sustaining the Th1-type immune response observed in persistent gastric Helicobacter infections. When iNOS is absent, a Th1-like response is inhibited, as evidenced by the reduced gastric pathology, reduced IgG2c responses, and increased levels of gastric colonization in the iNOS−/− mice relative to those in the WT mice. H. felis infection induced gastric inflammation in both the WT and iNOS−/− mice; however, the iNOS gene deletion in the iNOS−/− mice was associated with less tissue damage previously associated with the host response to gastric Helicobacter infection than that in the WT mice. In WT mice, gastritis was more severe in females than in males, consistent with the H. felis-induced gastritis previously described for (C57BL/6 × 129SvEv)F1 mice (12). A female gender effect has been recently described for H. felis-infected C57BL/6 mice (5). This effect contrasts with a male gender effect reported for H. pylori-induced cancer in INS/GAS mice bred on an FVB background (13), supporting the potential role for interaction between sex hormones and host genetics in response to chronic bacterial gastritis.
Furthermore, no evidence for the purported extragastric cardiovascular effects of Helicobacter infection was observed in this study, although iNOS−/− mice did have elevated cholesterol levels as previously reported. In conclusion, the targeted deletion of the iNOS gene appears to play a protective role against the progression of premalignant gastric lesions in Helicobacter-infected mice. This model should prove useful in dissecting the role of NO in the tumorigenicity of chronic H. pylori infection in humans.
Acknowledgments
This work was supported by NIH grants R01 AI 37750 (J.G.F.), P01 CA26731 (J.G.F.), T32 RR07036 (J.G.F.), and P30 ES02109.
Editor: D. L. Burns
REFERENCES
- 1.Bauer, H., T. Jung, D. Tsikas, D. O. Stichtenoth, J. C. Frolich, and C. Neumann. 1997. Nitric oxide inhibits the secretion of T-helper 1- and T-helper 2-associated cytokines in activated human T cells. Immunology 90:205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchard, T. G., and S. J. Czinn. 1998. Immunological determinants that may affect the Helicobacter pylori cancer risk. Aliment. Pharmacol. Ther. 12(Suppl. 1):83-90. [DOI] [PubMed] [Google Scholar]
- 3.Chimienti, G., F. Russo, B. L. Lamanuzzi, M. Nardulli, C. Messa, A. Di Leo, M. Correale, V. Giannuzzi, and G. Pepe. 2003. Helicobacter pylori is associated with modified lipid profile: impact on lipoprotein(a). Clin. Biochem. 36:359-365. [DOI] [PubMed] [Google Scholar]
- 4.Coleman, J. W. 2001. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 1:1397-1406. [DOI] [PubMed] [Google Scholar]
- 5.Court, M., P. A. Robinson, M. F. Dixon, A. H. Jeremy, and J. E. Crabtree. 2003. The effect of gender on Helicobacter felis-mediated gastritis, epithelial cell proliferation, and apoptosis in the mouse model. J. Pathol. 201:303-311. [DOI] [PubMed] [Google Scholar]
- 6.Danesh, J., J. Koreth, L. Youngman, R. Collins, J. R. Arnold, Y. Balarajan, J. McGee, and D. Roskell. 1999. Is Helicobacter pylori a factor in coronary atherosclerosis? J. Clin. Microbiol. 37:1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danesh, J., and R. Peto. 1998. Risk factors for coronary heart disease and infection with Helicobacter pylori: meta-analysis of 18 studies. BMJ 316:1130-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst, P. 1999. The role of inflammation in the pathogenesis of gastric cancer. Aliment. Pharmacol. Ther. 13(Suppl. 1):13-18. [DOI] [PubMed] [Google Scholar]
- 9.Felley, C. P., B. Pignatelli, G. D. Van Melle, J. E. Crabtree, M. Stolte, J. Diezi, I. Corthesy-Theulaz, P. Michetti, B. Bancel, L. M. Patricot, H. Ohshima, and E. Felley-Bosco. 2002. Oxidative stress in gastric mucosa of asymptomatic humans infected with Helicobacter pylori: effect of bacterial eradication. Helicobacter 7:342-348. [DOI] [PubMed] [Google Scholar]
- 10.Folsom, A. R., F. J. Nieto, P. Sorlie, L. E. Chambless, D. Y. Graham, et al. 1998. Helicobacter pylori seropositivity and coronary heart disease incidence. Circulation 98:845-850. [DOI] [PubMed] [Google Scholar]
- 11.Fox, J. G., P. Beck, C. A. Dangler, M. T. Whary, T. C. Wang, H. N. Shi, and C. Nagler-Anderson. 2000. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 6:536-542. [DOI] [PubMed] [Google Scholar]
- 12.Fox, J. G., X. Li, R. J. Cahill, K. Andrutis, A. K. Rustgi, R. Odze, and T. C. Wang. 1996. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology 110:155-166. [DOI] [PubMed] [Google Scholar]
- 13.Fox, J. G., A. B. Rogers, M. Ihrig, N. S. Taylor, M. T. Whary, G. Dockray, A. Varro, and T. C. Wang. 2003. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res. 63:942-950. [PubMed] [Google Scholar]
- 14.Fox, J. G., B. J. Sheppard, C. A. Dangler, M. T. Whary, M. Ihrig, and T. C. Wang. 2002. Germ-line p53-targeted disruption inhibits helicobacter-induced premalignant lesions and invasive gastric carcinoma through down-regulation of Th1 proinflammatory responses. Cancer Res. 62:696-702. [PubMed] [Google Scholar]
- 15.Goto, T., K. Haruma, Y. Kitadai, M. Ito, M. Yoshihara, K. Sumii, N. Hayakawa, and G. Kajiyama. 1999. Enhanced expression of inducible nitric oxide synthase and nitrotyrosine in gastric mucosa of gastric cancer patients. Clin. Cancer Res. 5:1411-1415. [PubMed] [Google Scholar]
- 16.Guiney, D. G., P. Hasegawa, and S. P. Cole. 2003. Helicobacter pylori preferentially induces interleukin 12 (IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect. Immun. 71:4163-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holan, V., M. Krulova, A. Zajicova, and J. Pindjakova. 2002. Nitric oxide as a regulatory and effector molecule in the immune system. Mol. Immunol. 38:989-995. [DOI] [PubMed] [Google Scholar]
- 18.Holck, S., A. Norgaard, M. Bennedsen, H. Permin, S. Norn, and L. P. Andersen. 2003. Gastric mucosal cytokine responses in Helicobacter pylori-infected patients with gastritis and peptic ulcers. Association with inflammatory parameters and bacteria load. FEMS Immunol. Med. Microbiol. 36:175-180. [DOI] [PubMed] [Google Scholar]
- 19.Ihrig, M., C. A. Dangler, and J. G. Fox. 2001. Mice lacking inducible nitric oxide synthase develop spontaneous hypercholesterolaemia and aortic atheromas. Atherosclerosis 156:103-107. [DOI] [PubMed] [Google Scholar]
- 20.Ismail, H. F., P. Fick, J. Zhang, R. G. Lynch, and D. J. Berg. 2003. Depletion of neutrophils in IL-10(−/−) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J. Immunol. 170:3782-3789. [DOI] [PubMed] [Google Scholar]
- 21.Kaise, M., J. Miwa, K. Iihara, N. Suzuki, Y. Oda, and Y. Ohta. 2003. Helicobacter pylori stimulates inducible nitric oxide synthase in diverse topographical patterns in various gastroduodenal disorders. Dig. Dis. Sci. 48:636-643. [DOI] [PubMed] [Google Scholar]
- 22.Kroncke, K. D., K. Fehsel, C. Suschek, and V. Kolb-Bachofen. 2001. Inducible nitric oxide synthase-derived nitric oxide in gene regulation, cell death and cell survival. Int. Immunopharmacol. 1:1407-1420. [DOI] [PubMed] [Google Scholar]
- 23.Laurila, A., A. Bloigu, S. Nayha, J. Hassi, M. Leinonen, and P. Saikku. 1999. Association of Helicobacter pylori infection with elevated serum lipids. Atherosclerosis 142:207-210. [DOI] [PubMed] [Google Scholar]
- 24.Li, C. Q., B. Pignatelli, and H. Ohshima. 2001. Increased oxidative and nitrative stress in human stomach associated with cagA+ Helicobacter pylori infection and inflammation. Dig. Dis. Sci. 46:836-844. [DOI] [PubMed] [Google Scholar]
- 25.Li, H., C. Stoicov, X. Cai, T. C. Wang, and J. Houghton. 2003. Helicobacter and gastric cancer disease mechanisms: host response and disease susceptibility. Curr. Gastroenterol. Rep. 5:459-467. [DOI] [PubMed] [Google Scholar]
- 26.Martin, R. M., J. L. Brady, and A. M. Lew. 1998. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 212:187-192. [DOI] [PubMed] [Google Scholar]
- 27.Mendall, M. A., P. M. Goggin, N. Molineaux, J. Levy, T. Toosy, D. Strachan, A. J. Camm, and T. C. Northfield. 1994. Relation of Helicobacter pylori infection and coronary heart disease. Br. Heart J. 71:437-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazawa, M., H. Suzuki, T. Masaoka, A. Kai, M. Suematsu, H. Nagata, S. Miura, and H. Ishii. 2003. Suppressed apoptosis in the inflamed gastric mucosa of Helicobacter pylori-colonized iNOS-knockout mice. Free Radic. Biol. Med. 34:1621-1630. [DOI] [PubMed] [Google Scholar]
- 29.Nagata, K., H. Yu, M. Nishikawa, M. Kashiba, A. Nakamura, E. F. Sato, T. Tamura, and M. Inoue. 1998. Helicobacter pylori generates superoxide radicals and modulates nitric oxide metabolism. J. Biol. Chem. 273:14071-14073. [DOI] [PubMed] [Google Scholar]
- 30.Nam, K. T., S. Y. Oh, B. Ahn, Y. B. Kim, D. D. Jang, K. H. Yang, K. B. Hahm, and D. Y. Kim. 2004. Decreased Helicobacter pylori associated gastric carcinogenesis in mice lacking inducible nitric oxide synthase. Gut 53:1250-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nardone, G. 2003. Review article: molecular basis of gastric carcinogenesis. Aliment. Pharmacol. Ther. 17(Suppl. 2):75-81. [DOI] [PubMed] [Google Scholar]
- 32.Neu, B., A. J. Puschmann, A. Mayerhofer, P. Hutzler, J. Grossmann, F. Lippl, W. Schepp, and C. Prinz. 2003. TNF-alpha induces apoptosis of parietal cells. Biochem. Pharmacol. 65:1755-1760. [DOI] [PubMed] [Google Scholar]
- 33.Neureiter, D., P. Heuschmann, S. Stintzing, P. Kolominsky-Rabas, L. Barbera, A. Jung, M. Ocker, M. Maass, G. Faller, and T. Kirchner. 2003. Detection of Chlamydia pneumoniae but not of Helicobacter pylori in symptomatic atherosclerotic carotids associated with enhanced serum antibodies, inflammation and apoptosis rate. Atherosclerosis 168:153-162. [DOI] [PubMed] [Google Scholar]
- 34.Obonyo, M., D. G. Guiney, J. Fierer, and S. P. Cole. 2003. Interactions between inducible nitric oxide and other inflammatory mediators during Helicobacter pylori infection. Helicobacter 8:495-502. [DOI] [PubMed] [Google Scholar]
- 35.Obonyo, M., D. G. Guiney, J. Harwood, J. Fierer, and S. P. Cole. 2002. Role of gamma interferon in Helicobacter pylori induction of inflammatory mediators during murine infection. Infect. Immun. 70:3295-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilotto, A., F. Rumor, M. Franceschi, G. Leandro, R. Novello, G. Soffiati, M. Scagnelli, F. Di Mario, and G. Valerio. 1999. Lack of association between Helicobacter pylori infection and extracardiac atherosclerosis in dyspeptic elderly subjects. Age Ageing 28:367-371. [DOI] [PubMed] [Google Scholar]
- 37.Rajnakova, A., P. M. Goh, S. T. Chan, S. S. Ngoi, A. Alponat, and S. Moochhala. 1997. Expression of differential nitric oxide synthase isoforms in human normal gastric mucosa and gastric cancer tissue. Carcinogenesis 18:1841-1845. [DOI] [PubMed] [Google Scholar]
- 38.Rogers, A. B., and J. G. Fox. 2004. Inflammation and Cancer. I. Rodent models of infectious gastrointestinal and liver cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 286:361-366. [DOI] [PubMed] [Google Scholar]
- 39.Roth, K. A., S. B. Kapadia, S. M. Martin, and R. G. Lorenz. 1999. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J. Immunol. 163:1490-1497. [PubMed] [Google Scholar]
- 40.Sakaguchi, A. A., S. Miura, T. Takeuchi, R. Hokari, M. Mizumori, H. Yoshida, H. Higuchi, M. Mori, H. Kimura, H. Suzuki, and H. Ishii. 1999. Increased expression of inducible nitric oxide synthase and peroxynitrite in Helicobacter pylori gastric ulcer. Free Radic. Biol. Med. 27:781-789. [DOI] [PubMed] [Google Scholar]
- 41.Scharnagl, H., M. Kist, A. B. Grawitz, W. Koenig, H. Wieland, and W. Marz. 2004. Effect of Helicobacter pylori eradication on high-density lipoprotein cholesterol. Am. J. Cardiol. 93:219-220. [DOI] [PubMed] [Google Scholar]
- 42.Schreiber, S., M. Stüben, C. Josenhans, P. Scheid, and S. Suerbaum. 1999. In vivo distribution of Helicobacter felis in the gastric mucus of the mouse: experimental method and results. Infect. Immun. 67:5151-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Son, H. J., J. C. Rhee, D. I. Park, Y. H. Kim, P. L. Rhee, K. C. Koh, S. W. Paik, K. W. Choi, and J. J. Kim. 2001. Inducible nitric oxide synthase expression in gastroduodenal diseases infected with Helicobacter pylori. Helicobacter 6:37-43. [DOI] [PubMed] [Google Scholar]
- 44.Strachan, D. P., M. A. Mendall, D. Carrington, B. K. Butland, J. W. Yarnell, P. M. Sweetnam, and P. C. Elwood. 1998. Relation of Helicobacter pylori infection to 13-year mortality and incident ischemic heart disease in the Caerphilly prospective heart disease study. Circulation 98:1286-1290. [DOI] [PubMed] [Google Scholar]
- 45.Wald, N. J., M. R. Law, J. K. Morris, and A. M. Bagnall. 1997. Helicobacter pylori infection and mortality from ischaemic heart disease: negative result from a large, prospective study. BMJ 315:1199-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker, M. M., and J. E. Crabtree. 1998. Helicobacter pylori infection and the pathogenesis of duodenal ulceration. Ann. N. Y. Acad. Sci. 859:96-111. [DOI] [PubMed] [Google Scholar]
- 47.Wang, S., W. Wang, R. A. Wesley, and R. L. Danner. 1999. A Sp1 binding site of the tumor necrosis factor alpha promoter functions as a nitric oxide response element. J. Biol. Chem. 274:33190-33193. [DOI] [PubMed] [Google Scholar]
- 48.Whary, M. T., and J. G. Fox. 2004. Th1-mediated pathology in mouse models of human disease is ameliorated by concurrent Th2 responses to parasite antigens. Curr. Top. Med. Chem. 4:531-538. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, X. J., J. H. Thompson, E. E. Mannick, P. Correa, and M. J. Miller. 1998. Localization of inducible nitric oxide synthase mRNA in inflamed gastrointestinal mucosa by in situ reverse transcriptase-polymerase chain reaction. Nitric Oxide 2:187-192. [DOI] [PubMed] [Google Scholar]