Abstract
We have previously identified a chlamydial protein, chlamydial protease/proteasome-like activity factor (CPAF), for degrading host transcription factors in cells infected with the human chlamydial species Chlamydia trachomatis or Chlamydia pneumoniae. We now report that functional CPAF was also produced during infection with the species Chlamydia muridarum, Chlamydia psittaci, and Chlamydia caviae, which primarily infect nonhuman hosts.
Chlamydiae, a family of obligate intracellular bacterial pathogens that must replicate in cytoplasmic vacuoles of eukaryotic cells (11), consist of multiple species with a diverse range of tissue tropism and disease processes (8), including the human pathogens Chlamydia trachomatis (16) and Chlamydia pneumoniae (1, 2, 14, 15, 22) and the animal pathogens Chlamydia muridarum (formerly known as C. trachomatis mouse pneumonitis agent, designated MoPn) (3, 17, 18), Chlamydia caviae (also known as GPIC) (13), and Chlamydia psittaci 6BC (24, 25). Despite the profound difference in host range, the chlamydial species display a remarkable similarity in their genome sequences (20, 21, 23) and possess a profoundly conserved intracellular growth cycle with distinct biphasic stages (11). To complete their obligate intracellular replication, chlamydial species may have to protect the infected cells from host immune recognition and effector mechanisms. Both C. trachomatis and C. pneumoniae have been shown to possess various strategies for evading host defense (4, 9, 10, 12, 19, 26-29) and to produce a 70-kDa protease, chlamydial protease/proteasome-like activity factor (CPAF), for degrading host transcription factors, such as RFX5, required for major histocompatibility complex antigen expression (9, 27, 29). Interestingly, the activation of the 70-kDa CPAF is regulated via the processing of the inactive full-length CPAF into a 29-kDa N-terminal and a 35-kDa C-terminal fragment for forming functional intramolecular heterodimers (5, 6, 27). The goal of the present study is to assess whether CPAF is a functionally conserved protein in all chlamydial species.
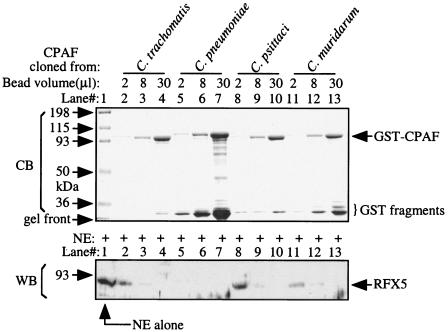
The available chlamydial genome sequences have revealed that CPAF is encoded not only by the human chlamydial organisms C. trachomatis serovar D (23) and various C. pneumoniae strains (20) but also by the animal chlamydial species C. muridarum (20) and C. caviae (21). We further sequenced the CPAF genes carried by the C. trachomatis L2 serovar and C. psittaci 6BC strain by using primers derived from C. trachomatis serovar D and C. caviae GPIC CPAFs, respectively (data not shown). The oligonucleotide primers were synthesized with an automated ABI 3900 synthesizer, and the DNA sequencing was done with an automated ABI 3100 genetic analyzer via a service from a core facility at the University of Texas Health Science Center. An alignment analysis of the deduced CPAF amino acid sequences from nine different chlamydial strains, representing five major chlamydial species, has revealed that although the intraspecies identity is as high as 99%, the interspecies identity is as low as 46% (Table 1). A CPAF homologue was recently identified in the genome of a Parachlamydia sp. UWE25 strain isolated from an amoeba, and the alignment score between UWE25 and other chlamydial CPAFs is ∼30% (Table 1). We next expressed CPAF from five different chlamydial species as glutathione S-transferase (GST) fusion proteins by using a pGEX6p-2 vector system (5) and compared the GST-CPAF fusion proteins for their abilities to degrade the human transcription factor RFX5 in a cell-free degradation assay (Fig. 1). A rabbit anti-RFX5 (Rockland Immunochemicals Inc., Gilbertsville, Pa.) was used to detect the residual RFX5 on a Western blot (29). Due to the difficulty in obtaining UWE25 genomic DNA, we did not analyze the UWE25 CPAF in the present study. The GST-CPAF fusion proteins from all species were properly expressed and purified (Fig. 1, top panel), and all of the GST-CPAF fusion proteins, regardless of species, displayed a significant RFX5 degradation activity (Fig. 1, bottom panel). This observation has not only confirmed our previous observations that CPAF is active when expressed as GST fusion proteins in Escherichia coli due to a processing-triggered activation to a portion of the GST fusion proteins (5), but also more importantly, it has demonstrated that CPAF molecules encoded by different chlamydial species possess a similar proteolytic property.
TABLE 1.
Alignment scores of CPAF amino acid sequences from five different chlamydia and one parachlamydia species
| Serovar or isolate | Alignment score for a
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
C. trachomatis
|
C. muridarum
|
C. pneumoniae
|
C. psittaci
|
C. caviae
|
Parachlamydia sp.
|
|||||
| D | L2 | MoPn | CWL029 | AR39 | TW183 | J138 | 6BC | GPIC | UWE25 | |
| D | 100 | 99 | 82 | 46 | 46 | 46 | 46 | 54 | 54 | 30 |
| L2 | 100 | 82 | 46 | 46 | 46 | 46 | 54 | 54 | 30 | |
| MoPn | 100 | 46 | 46 | 46 | 46 | 54 | 54 | 32 | ||
| CWL029 | 100 | 99 | 99 | 99 | 54 | 54 | 31 | |||
| AR39 | 100 | 99 | 99 | 54 | 54 | 31 | ||||
| TW183 | 100 | 99 | 54 | 54 | 31 | |||||
| J138 | 100 | 54 | 54 | 31 | ||||||
| 6BC | 100 | 99 | 31 | |||||||
| GPIC | 100 | 31 | ||||||||
| UWE25 | 100 | |||||||||
Note that the CPAF sequences from C. trachomatis serovar D (accession, version number NP_220380.1, GI:15605594); C. muridarum MoPn (AAF39117.1, GI:7190287); C. pneumoniae CWL029 (NP_225210.1, GI:15618924), AR39 (NP_445376.1, GI:16752010), TW183 (AAP98983.1, GI:33236896), and J138 (NP_301071.1, GI:15836547); C. caviae GPIC (AAP05486.1, GI:29834851); and Parachlamydia sp. UWE25 (YP_007915, GI:46446550) were obtained from the NCBI Entrez protein database (http://www.ncbi.nlm.nih.gov/entrez/viewer/fcgi?db=protein). However, the C. trachomatis serovar L2 and C. psittaci 6BC CPAF sequences were determined in our own lab using primers derived from C. trachomatis serovar D and C. caviae GPIC, respectively. The derived amino acid sequences were analyzed with the ClustalW software at the website http://www.ebi.ac.uk/clustalw/. The alignment scores (percent identical amino acids) were assigned between the 10 CPAF amino acid sequences after pairwise alignment analyses with minimal gaps.
FIG. 1.
Degradation of RFX5 by the recombinant CPAF proteins from different chlamydia species. The GST-CPAF fusion proteins expressed in bacteria were purified with the glutathione-conjugated beads. After extensive washing, the bead-bound fusion protein samples indicated at the top of the figure were loaded onto a sodium dodecyl sulfate gel, each with different quantities of bead volume, as indicated in the figure. The gel was stained with a Commassie blue dye (CB) to visualize protein bands (top panel). A parallel set of the bead-bound CPAF fusion proteins described above was used to mix with a nuclear extract (NE) containing RFX5 for measuring the ability of the CPAF fusion proteins to digest RFX5 in a cell-free degradation assay. The residual RFX5 was detected by Western blotting (WB) with an RFX5-specific antibody (bottom panel).
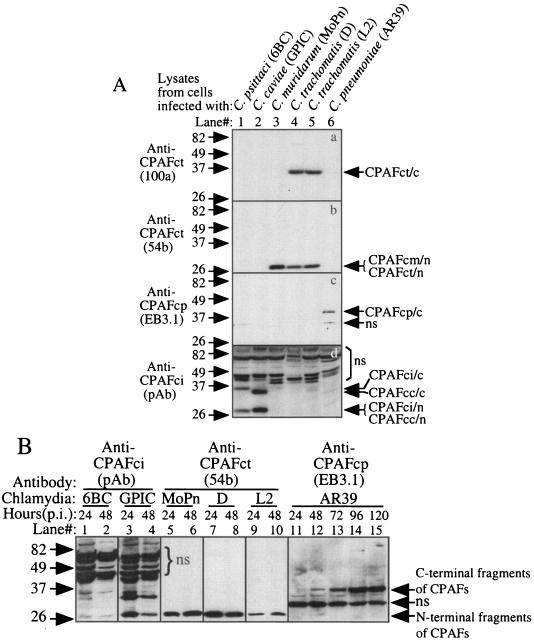
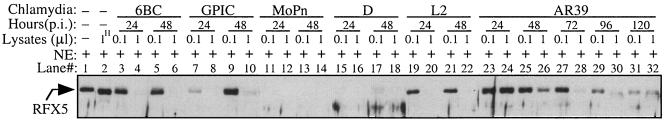
We further evaluated whether CPAF is expressed by all of these chlamydial species during infection (Fig. 2). A Western blot was used to monitor CPAF expression as described previously (27, 29). The infected cells were lysed with an MLB buffer (consisting of 25 mM HEPES, 150 mM NaCl, 1% Igepal, 10 mM MgCl2, 1 mM EDTA, 10% glycerol, 1 mM Na3VO3, 1 mM phenylmethylsulfonyl fluoride, 60 μM leupeptin, and 0.1% aprotinin), and each lane was loaded with the lysates from ∼5 × 104 cells. The nitrocellulose membrane-immobilized proteins were detected with antibodies recognizing different CPAFs. Due to CPAF sequence variations between different chlamydial species, antibodies were first screened for their specific recognition of each type of CPAF (Fig. 2A). The monoclonal antibody (MAb) 100a recognized the C-terminal fragments of CPAF from C. trachomatis (Fig. 2A, panel a, lanes 4 and 5), while the MAb 54b recognized the N-terminal fragments of both CPAF from C. trachomatis (Fig. 2A, panel b, lanes 4 and 5) and CPAF from C. muridarum species (Fig. 2A, panel b, lane 3). We have previously shown that these CPAF C- and N-terminal fragments can form active CPAF molecules in solutions when they dimerize (5, 6). Therefore, we can use the MAb 54b to monitor active CPAF production in cells infected with either C. trachomatis or C. muridarum. As shown previously (9, 12), EB3.1, the MAb specific to CPAF from C. pneumoniae species, detected the C-terminal fragment of CPAF from C. pneumoniae (Fig. 2A, panel c, lane 6). A mouse polyclonal antibody raised with CPAF from C. psittaci recognized both the C- and N-terminal fragments of CPAF from C. psittaci (Fig. 2A, panel d, lane 1) and CPAF from C. caviae species (Fig. 2A, panel d, lane 2) but showed no cross-reactivity with any other CPAFs. We then used the MAbs 54b and EB3.1 and the anti-CPAF from C. psittaci antiserum to monitor the expression of CPAF in cultures infected with chlamydia for various periods of time (Fig. 2B). Active CPAF fragments (C-terminal fragments, N-terminal fragments, or both) were detected at both 24 and 48 h after infection with five different strains representing four chlamydial species (Fig. 2B, lanes 1 to 10). The exception is C. pneumoniae AR39. No obvious CPAF or CPAF active fragment was detected in cells infected with C. pneumoniae for 24 h (Fig. 2B, lane 11). However, by 48 h after infection, a C-terminal fragment of CPAF from C. pneumoniae was clearly detected (Fig. 2B, lane 12) and, importantly, gradually increased as the infection progressed (Fig. 2B, lanes 13 to 15). The delayed expression of CPAF by AR39 obviously reflects the slow growth rate of the C. pneumoniae species. The above observations have clearly demonstrated that the five chlamydial species not only expressed CPAF proteins but also produced active fragments during infection. To further confirm the functionality of the chlamydia-expressed CPAFs, we used a cell-free degradation assay (27) to compare the chlamydia-infected cell lysates (prepared and used in the above Western blot experiment) for their ability to degrade RFX5 in a nuclear extract (Fig. 3). The RFX5 was significantly degraded by all lysates made from chlamydia-infected cells regardless of the chlamydial species that infected the cells (Fig. 3, lanes 3 to 32) but not from normal HeLa cells (Fig. 3, lane 2). However, obvious variations in the levels of enzymatic activity between CPAFs from different species were noted, which may reflect the differences in the amounts of active CPAF produced in different cultures. For example, cells infected with AR39 for 24 h displayed no detectable RFX5 degradation activity (Fig. 3, lanes 23 and 24), but the degradation activity gradually increased in AR39-infected cells as infection progressed (Fig. 3, lanes 25 to 32). The activity patterns of AR39 CPAF perfectly match the patterns of CPAF quantity detected in Fig. 2B (lanes 11 to 15). These observations have confirmed that functional CPAF was produced in cells infected with all chlamydial species regardless of their differences in tissue tropism and replication cycle.
FIG. 2.
Detection of endogenous CPAF proteins expressed in chlamydia-infected cells. (A) HeLa cells infected with the various chlamydial organisms indicated at the top of the figure were lysed with an MLB lysis buffer 48 h after infection. The lysates were used as antigens to screen the various anti-CPAF antibodies on a Western blot. Please note that the monoclonal antibodies 100a, 54b, and EB3.1 recognized the 35-kDa C-terminal fragment of C. trachomatis CPAF (CPAFct/c), the 29-kDa N-terminal fragments of C. trachomatis and C. muridarum CPAF (CPAFct/n and CPAFcm/n), and the 35-kDa C-terminal fragment of C. pneumoniae CPAF (CPAFcp/c), respectively. The polyclonal antibody (pAb) raised against CPAF from C. psittaci 6BC (CPAFci) recognized both the C- and N-terminal fragments of CPAF from C. psittaci (CPAFci/c and CPAFci/n) and C. caviae (CPAFcc/c and CPAFcc/n). ns, nonspecific binding. (B) The antibodies listed above except 100a were used to monitor CPAF expression in cells infected with various chlamydia strains for various periods of time, as indicated at the top of the figure, on a Western blot. The infected cell lysates were prepared in the way described above and were loaded with 1 μl per lane after the total amounts of proteins in each sample were equalized.
FIG. 3.
RFX5 degradation by endogenous CPAFs. Cells infected with different chlamydial species and strains, as indicated in the figure, were lysed as described in the legend to Fig. 2 and used as the source of enzyme to digest RFX5 in the nuclear extract (NE) in a cell-free degradation assay. To compare the relative enzymatic activities between samples, different amounts of lysates were used for each sample. A 1-μl sample of 1:10 diluted lysates was considered to be 0.1 μl of the original lysates. “1H” indicates that 1 μl of lysates made from normal HeLa cells was used as a negative control. Please note that the RFX5 degradation activity (this figure) correlates with the presence of active CPAF fragments detected in the same lysates (Fig. 2B).
We have thus demonstrated that in addition to the two human chlamydial species C. trachomatis and C. pneumoniae, the species C. muridarum, C. psittaci, and C. caviae, which mainly infect animals, also encode and express functional CPAF. First, CPAF genes were identified in all of these chlamydial species, and the interspecies identity of CPAF at the amino acid level is at least 46%. Second, the CPAF genes cloned from these animal species degraded the human transcription factor RFX5 as efficiently as the CPAF of human chlamydial species did, suggesting that all CPAFs possess a similar proteolytic activity. Third, both the active CPAF fragments and CPAF activity were detected in cells infected with these animal-tropic chlamydial species, indicating that the endogenous CPAF expressed by these chlamydial species can undergo a similar activation process to acquire the proteolytic ability. The fact that CPAF is a functionally conserved molecule in all chlamydia species suggests that all chlamydia species may be selected by a common selection pressure, for example, to evade host adaptive immune recognition during their intracellular replication. The human RFX5 was used as the substrate for measuring the function of CPAF from all chlamydial species because the constitutively expressed transcription factor RFX5 is highly conserved between eukaryotic species. Besides participating in evading host adaptive immunity, CPAF may also have other important functions, since it has been recently shown that the Parachlamydia sp. UWE25 isolated from an amoeba encodes a CPAF homologue with a ∼30% amino acid sequence identity to CPAFs of chlamydial species. Although we do not yet know whether the UWE25 CPAF possesses the ability to degrade human RFX5, the identification of a CPAF homologue in UWE25 suggests that CPAF may be required for chlamydia's own biological process and/or for the intimate interactions with host cells. This is because the single-celled amoeba does not have adaptive immunity but may impose a stringent intracellular environment for chlamydial replication. We have recently shown that CPAF from C. trachomatis can cleave both head and tail domains off cytokeratin 8, a major subunit of intermediate filaments in epithelial cells, which may cause solubilization of a portion of the intermediate filaments so that chlamydial inclusions can expand (7). It will be interesting to know whether UWE25 CPAF also possesses the ability to solubilize the intracellular cytoskeleton of amoebas.
Acknowledgments
This work was supported in part by grants (to G.Z.) from the U.S. National Institutes of Health (R01 AI47997 and R01 HL64883).
Editor: F. C. Fang
REFERENCES
- 1.Blessing, E., L. A. Campbell, M. E. Rosenfeld, N. Chough, and C. C. Kuo. 2001. Chlamydia pneumoniae infection accelerates hyperlipidemia induced atherosclerotic lesion development in C57BL/6J mice. Atherosclerosis 158:13-17. [DOI] [PubMed] [Google Scholar]
- 2.Campbell, L. A., and C. C. Kuo. 2004. Chlamydia pneumoniae—an infectious risk factor for atherosclerosis? Nat. Rev. Microbiol. 2:23-32. [DOI] [PubMed] [Google Scholar]
- 3.Cotter, T. W., Q. Meng, Z. L. Shen, Y. X. Zhang, H. Su, and H. D. Caldwell. 1995. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4704-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean, D., and V. C. Powers. 2001. Persistent Chlamydia trachomatis infections resist apoptotic stimuli. Infect. Immun. 69:2442-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong, F., M. Pirbhai, Y. Zhong, and G. Zhong. 2004. Cleavage-dependent activation of a chlamydia-secreted protease. Mol. Microbiol. 52:1487-1494. [DOI] [PubMed] [Google Scholar]
- 6.Dong, F., J. Sharma, Y. Xiao, Y. Zhong, and G. Zhong. 2004. Intramolecular dimerization is required for the chlamydia-secreted protease CPAF to degrade host transcriptional factors. Infect. Immun. 72:3869-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong, F., H. Su, Y. Huang, Y. Zhong, and G. Zhong. 2004. Cleavage of host keratin 8 by a chlamydia-secreted protease. Infect. Immun. 72:3863-3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everett, K. D., R. M. Bush, and A. A. Andersen. 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 49:415-440. [DOI] [PubMed] [Google Scholar]
- 9.Fan, P., F. Dong, Y. Huang, and G. Zhong. 2002. Chlamydia pneumoniae secretion of a protease-like activity factor for degrading host cell transcription factors required for major histocompatibility complex antigen expression. Infect. Immun. 70:345-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan, T., H. Lu, H. Hu, L. Shi, G. A. McClarty, D. M. Nance, A. H. Greenberg, and G. Zhong. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J. Exp. Med. 187:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackstadt, T., E. R. Fischer, M. A. Scidmore, D. D. Rockey, and R. A. Heinzen. 1997. Origins and functions of the chlamydial inclusion. Trends Microbiol. 5:288-293. [DOI] [PubMed] [Google Scholar]
- 12.Heuer, D., V. Brinkmann, T. F. Meyer, and A. J. Szczepek. 2003. Expression and translocation of chlamydial protease during acute and persistent infection of the epithelial HEp-2 cells with Chlamydophila (Chlamydia) pneumoniae. Cell. Microbiol. 5:315-322. [DOI] [PubMed] [Google Scholar]
- 13.Howard, L. V., M. P. O'Leary, and R. L. Nichols. 1976. Animal model studies of genital chlamydial infections. Immunity to re-infection with guinea-pig inclusion conjunctivitis agent in the urethra and eye of male guinea-pigs. Br. J. Vener. Dis. 52:261-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu, H., G. N. Pierce, and G. Zhong. 1999. The atherogenic effects of chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumoniae. J. Clin. Investig. 103:747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, L., H. Hu, H. Ji, A. D. Murdin, G. N. Pierce, and G. Zhong. 2000. Chlamydia pneumoniae infection significantly exacerbates aortic atherosclerosis in an LDLR−/− mouse model within six months. Mol. Cell. Biochem. 215:123-128. [DOI] [PubMed] [Google Scholar]
- 16.Paavonen, J., C. W. Critchlow, T. DeRouen, C. E. Stevens, N. Kiviat, R. C. Brunham, W. E. Stamm, C. C. Kuo, K. E. Hyde, L. Corey, et al. 1986. Etiology of cervical inflammation. Am. J. Obstet. Gynecol. 154:556-564. [DOI] [PubMed] [Google Scholar]
- 17.Pal, S., E. M. Peterson, and L. M. de la Maza. 2004. New murine model for the study of Chlamydia trachomatis genitourinary tract infections in males. Infect. Immun. 72:4210-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 2001. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect. Immun. 69:6240-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajalingam, K., H. Al-Younes, A. Muller, T. F. Meyer, A. J. Szczepek, and T. Rudel. 2001. Epithelial cells infected with Chlamydophila pneumoniae (Chlamydia pneumoniae) are resistant to apoptosis. Infect. Immun. 69:7880-7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Read, T. D., R. C. Brunham, C. Shen, S. R. Gill, J. F. Heidelberg, O. White, E. K. Hickey, J. Peterson, T. Utterback, K. Berry, S. Bass, K. Linher, J. Weidman, H. Khouri, B. Craven, C. Bowman, R. Dodson, M. Gwinn, W. Nelson, R. DeBoy, J. Kolonay, G. McClarty, S. L. Salzberg, J. Eisen, and C. M. Fraser. 2000. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 28:1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Read, T. D., G. S. Myers, R. C. Brunham, W. C. Nelson, I. T. Paulsen, J. Heidelberg, E. Holtzapple, H. Khouri, N. B. Federova, H. A. Carty, L. A. Umayam, D. H. Haft, J. Peterson, M. J. Beanan, O. White, S. L. Salzberg, R. C. Hsia, G. McClarty, R. G. Rank, P. M. Bavoil, and C. M. Fraser. 2003. Genome sequence of Chlamydophila caviae (Chlamydia psittaci GPIC): examining the role of niche-specific genes in the evolution of the Chlamydiaceae. Nucleic Acids Res. 31:2134-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma, J., Y. Niu, J. Ge, G. N. Pierce, and G. Zhong. 2004. Heat-inactivated C. pneumoniae organisms are not atherogenic. Mol. Cell. Biochem. 260:147-152. [DOI] [PubMed] [Google Scholar]
- 23.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 24.Tanzer, R. J., D. Longbottom, and T. P. Hatch. 2001. Identification of polymorphic outer membrane proteins of Chlamydia psittaci 6BC. Infect. Immun. 69:2428-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanrompay, D., P. Butaye, C. Sayada, R. Ducatelle, and F. Haesebrouck. 1997. Characterization of avian Chlamydia psittaci strains using omp1 restriction mapping and serovar-specific monoclonal antibodies. Res. Microbiol. 148:327-333. [DOI] [PubMed] [Google Scholar]
- 26.Xiao, Y., Y. Zhong, W. Greene, F. Dong, and G. Zhong. 2004. Chlamydia trachomatis infection inhibits both Bax and Bak activation induced by staurosporine. Infect. Immun. 72:5470-5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong, G., P. Fan, H. Ji, F. Dong, and Y. Huang. 2001. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J. Exp. Med. 193:935-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong, G., T. Fan, and L. Liu. 1999. Chlamydia inhibits interferon gamma-inducible major histocompatibility complex class II expression by degradation of upstream stimulatory factor 1. J. Exp. Med. 189:1931-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong, G., L. Liu, T. Fan, P. Fan, and H. Ji. 2000. Degradation of transcription factor RFX5 during the inhibition of both constitutive and interferon gamma-inducible major histocompatibility complex class I expression in chlamydia-infected cells. J. Exp. Med. 191:1525-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]