Abstract
We demonstrate that Mycobacterium tuberculosis grown in vitro is sensitive to glutathione and its derivative S-nitrosoglutathione. Furthermore, our infection studies with J774.1 macrophages indicate that glutathione is essential for the control of the intracellular growth of M. tuberculosis. This study indicates the important role of glutathione in the control of macrophages by M. tuberculosis.
Tuberculosis is one of the most prevalent infectious diseases in the world (3). The situation is exacerbated by the emergence of mutlidrug-resistant strains and an ever-growing number of highly susceptible immunocompromised individuals arising from the AIDS pandemic (3). The production of reactive nitrogen intermediates by murine macrophages is considered to be a relatively effective host defense mechanism against Mycobacterium tuberculosis (1, 4, 7, 8, 9, 13, 16).
In this study, we show that a virulent laboratory strain of M. tuberculosis, H37Rv, grown in vitro is sensitive to glutathione (GSH) and nitrosoglutathione (GSNO) at physiological concentrations. While GSH at a 5 mM concentration is bacteriostatic to H37Rv (Fig. 1; Table 1), GSNO at a 5 mM concentration is bactericidal (Fig. 1; Table 1), as was observed by determining optical density (OD) and numbers of CFU. H37Rv was grown in 7H9 containing albumin dextrose complex (ADC) and Tween 80 to mid-log phase and then diluted to an OD of approximately 0.1. The bacterial suspensions were then treated with 5 mM GSH (Sigma) or 5 mM GSNO (Sigma) or left untreated. The OD was measured every day for 5 days (Fig. 1). Each day, an aliquot was taken from every sample, diluted 100-fold, and plated on 7H11 plates containing ADC for determination of numbers of CFU (Table 1). All experiments were performed three times in triplicate.
FIG. 1.
Growth of M. tuberculosis in the presence and absence of GSH (A) and GSNO (B) as determined by measuring OD. The H37Rv (Rv) cell suspension was treated with 5 mM GSH or 5 mM GSNO or left untreated. The OD was measured every day for 5 days (d.) (A). All experiments were repeated three times in triplicate.
TABLE 1.
Growth of H37Rv in 7 Hg medium
| H37Rv treatment | Mean no. of CFUa ± SD on:
|
|||||
|---|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| None | 2.8 × 104 ± 8.81 × 102 | 6.4 × 104 ± 4.1 × 103 | 3.3 × 105 ± 1.8 × 104 | 7.3 × 105 ± 1.2 × 104 | 7.2 × 106 ± 1.6 × 105 | 9.8 × 107 ± 2.0 × 105 |
| 5 mM GSH | 2.6 × 104 ± 1.2 × 103 | 2.1 × 104* ± 1.2 × 103 | 2.4 × 104* ± 5.5 × 102 | 2.4 × 104* ± 2.0 × 10 | 2.4 × 104* ± 6.2 × 102 | 2.7 × 104* ± 6.2 × 102 |
| 5 mM GSNO | 2.6 × 104* ± 1.8 × 104 | 8.6 × 103* ± 8.8 × 102 | 3.5 × 103* ± 3.1 × 102 | 3.2 × 103* ± 1.6 × 103 | 3.0 × 103* ± 6.2 × 102 | 2.6 × 103* ± 2.3 × 102 |
Asterisks indicate statistical significance.
The mechanism of action of the antimycobacterial activity of GSH is not certain. One possibility is that the presence of a high concentration of GSH may result in an imbalance in a bacterium containing an alternative thiol for regulating reduction or oxidation activity (i.e., mycothiol). We also consider the hypothesis of Spallholz in this connection (14). According to Spallholz, “GSH is structurally similar to the precursor of the antibiotics produced in fungi in the genera Penicillium and Cephalasporium.” Its potential conversion to the penicillin-like derivative glutacillin, a β-lactam form of GSH, raises the intriguing question of whether GSH was once a universal penem-like precursor of antibiotics in cells of many life forms (14).
GSH is a precursor for GSNO, and GSNO may represent one of the most important active forms of nitric oxide (NO) as an antimicrobial agent (6, 10, 11, 12).
Based on the results of our in vitro studies, we hypothesized that the control of the growth of H37Rv in murine macrophages is mediated in part by GSNO and GSH, generated by macrophages during oxidative or nitrosative stress. We tested this hypothesis by performing in vitro infection studies with a murine macrophage cell line, J774.1. J774.1 macrophages were maintained in vitro and infected with H37Rv, according to methods described by Venketaraman et al. (15). J774.1 cells were stimulated for GSNO synthesis as follows. Gamma interferon (IFN-γ) and lipopolysaccharide (LPS) stimulation result in NO production by macrophages (5) and subsequently in the formation of GSNO. Infected macrophages were therefore maintained in tissue culture medium with and without IFN-γ (100 U/ml) and LPS (1 μg/ml). Infected-macrophage cultures were terminated at 1 and 72 h to allow study of the intracellular viability of H37Rv.
To prove that the macrophage killing of H37Rv is mediated by GSH or GSNO, macrophages were treated with IFN-γ (100 U/ml), LPS (1 μg/ml), and buthionine sulfoximine (BSO; 500 μM), and the growth of H37Rv inside IFN-γ-, LPS-, and BSO-treated macrophages was compared with the growth of H37Rv inside unstimulated and IFN-γ- and LPS-treated macrophages. BSO specifically inhibits the activity of a γ-glutamyl-cysteinyl synthetase enzyme that catalyzes the first-step reaction in the synthesis of GSH. The intracellular viability of H37Rv was determined by plating the macrophage lysates on 7H11 enriched with ADC to count mycobacterial colonies.
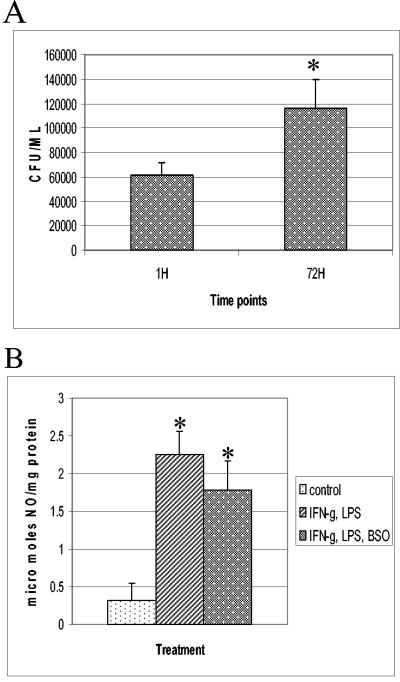
Figure 2 shows the results of these experiments, which are presented as averages of results of six different experiments performed in triplicate. We observed significant growth of M. tuberculosis inside unstimulated J774.1 macrophages between 1 and 72 h (Fig. 2A). Stimulation of J774.1 cells with IFN-γ-LPS resulted in a static effect on the growth of intracellular M. tuberculosis (Fig. 2B). Addition of BSO (500 μM) to IFN-γ- and LPS-treated J774.1 cells resulted in significant growth of H37Rv, similar to what occurred with untreated macrophages and elimination of the static effect brought about by IFN-γ-LPS treatment (Fig. 3A). These findings indicate that intracellular GSH and GSNO play a significant role in the killing of intracellular H37Rv in J774.1 cells.
FIG. 2.
Growth of H37Rv in untreated (A) and IFN-γ-LPS-treated (B) J774.1 cells. Experiments with H37Rv-infected macrophages, maintained in the absence (A) and presence (B) of IFN-γ plus LPS, were terminated at 1 and 72 h to determine the growth of H37Rv inside J774.1 cells. * denotes a statistically significant increase in the number of CFU between 1 and 72 h (P < 0.0006). Data are averages of results from six different experiments performed in triplicate.
FIG. 3.
(A) Growth of H37Rv in IFN-γ-LPS-BSO-treated J774.1 cells. Experiments with H37Rv-infected macrophages treated with IFN-γ plus LPS and BSO were terminated at 1 and 72 h to determine the levels of growth of H37Rv inside J774.1 cells. Statistical significance was calculated with the Statview program. * represents a statistically significant increase in the number of CFU between 1 and 72 h (P < 0.0083). Data are averages of results from from six different experiments performed in triplicate. (B) NO estimation in J774.1 cells. Nitrite levels in macrophage supernatants were determined spectrophotometrically by a Greiss reaction. Data are averages of results from five different experiments. * indicates a statistically significant increase in nitrite levels between control and IFN-γ-LPS- or IFN-γ-LPS-BSO-treated macrophages. For values for the control versus those after IFN-γ-LPS treatment, P was <0.0001. For values for the control versus those after IFN-γ-LPS-BSO treatment, P was <0.0030.
IFN-γ-LPS treatment is likely to induce several antimicrobial mechanisms within macrophages. In order to demonstrate that GSH and GSNO contribute to a great extent in the growth inhibition of H37Rv inside IFN-γ- and LPS-treated macrophages, we measured nitrite and GSH levels in macrophages treated with IFN-γ-LPS and IFN-γ-LPS-BSO.
Nitrite was detected spectrophotmetrically by a Greiss reaction (15). Stimulation of J774.1 macrophages with IFN-γ-LPS resulted in a significant fourfold increase in NO generation (Fig. 3B) compared to NO generation in unstimulated macrophages. Treatment of J774.1 cells with IFN-γ-LPS-BSO also resulted in a significant and almost fourfold increase in NO generation (Fig. 3B) compared to NO generation in unstimulated macrophages.
If NO is the primary species responsible for controlling mycobacterial growth in murine macrophages, then we should observe the inhibition of growth of M. tuberculosis in IFN-γ-, LPS-, and BSO-treated macrophages. However, we observed a significant growth of intracellular M. tuberculosis (Fig. 3A).
To prove that GSH and GSNO contribute to a great extent in the inhibition of the growth of H37Rv inside IFN-γ-LPS-treated macrophages, we measured GSH in macrophages under different treatments. GSH was assayed by two methods: spectrophotometry (15) and fluorescent detection of monochlorobimane (MCB) staining. J774.1 cells were cultured in 5-ml tissue culture flasks for 24 h at 37°C in the presence or absence of IFN-γ-LPS or IFN-γ-LPS-BSO. Macrophages were stained with MCB (60 μM) and incubated at 37°C for 30 min. MCB reacts with intracellular GSH to form glutathione-S bimane, a fluorescent adduct retained by the cells and detectable by fluorescence-activated cell sorting using a 351 nM excitation (2). Our results show similar trends by both techniques. As shown in Fig. 4, maximum levels of GSH were observed in untreated macrophages. Treatment of J774.1 cells with IFN-γ, LPS, and BSO caused a significant decrease in intracellular GSH levels (Fig. 4), possibly leading to an inhibition of GSNO formation, and hence we observed a significant increase in the intracellular growth of H37Rv.
FIG. 4.
Estimation of GSH levels in J774.1 cells by flow cytometry (A) and spectrophotometry (B). (A) GSH was quantitated in J774.1 cells by staining with monochlorobimane. * signifies a statistically significant decrease in the number of fluorescent cells in IFN-γ-LPS-BSO-treated macrophages compared to that for control or IFN-γ-LPS-treated macrophages. For values for the control versus those after IFN-γ-LPS-BSO treatment, P was <0.0001. For values after IFN-γ-LPS treatment versus those after IFN-γ-LPS-BSO treatment, P was <0.0026. Data are averages of results from three different experiments. (B) GSH was also assayed by spectrophotometry. Data are averages from three different experiments. * designates a statistically significant decrease in intracellular GSH levels in IFN-γ-LPS-BSO-treated macrophages compared to those in control macrophages or IFN-γ-LPS-treated macrophages. For values for the control versus those after IFN-γ-LPS—BSO treatment, P was <0.0024. For values after IFN-γ-LPS treatment versus IFN-γ-LPS-BSO treatment, P was <0.0028.
Acknowledgments
This work was supported by an American Heart Association grant (awarded to Vishwanath Venketaraman) and by Public Health Service grant R01AI34436 (awarded to N. D. Connell).
Editor: J. L. Flynn
REFERENCES
- 1.Adams, L. B., M. C. Dinauer, D. E. Morgenstern, and J. L. Krahenbuhl. 1997. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber. Lung Dis. 78:237-246. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, M. T., M. Roederer, I. Tjioe, and L. A. Herzenberg. 1996. p. 1-9. In L. A. Herzenberg, C. Blackwell, and D. Weir (ed.), The handbook of experimental immunology, 5th ed., vol. 1B. Blackwell Scientific, Boston, Mass.
- 3.Bloom, B. R., and C. J. Murray. 1992. Tuberculosis: commentary on a reemergent killer. Science 257:1055-1064. [DOI] [PubMed] [Google Scholar]
- 4.Chan, E. D., J. Chan, and N. W. Schluger. 2001. What is the role of nitric oxide in murine and human host defense against tuberculosis? Current knowledge. Am. J. Respir. Cell. Mol. Biol. 25:606-612. [DOI] [PubMed] [Google Scholar]
- 5.Chan, J., R. Zin, S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Groote, M. A., D. Grange, Y. Xu, G. Campbell, R. Prince, and F. C. Fang. 1995. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc. Natl. Acad. Sci. USA 92:6399-6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 9.Flynn, J. L., C. A. Scanga, K. E. Tanaka, and J. Chan. 1998. Effects of aminoguanidine on latent murine tuberculosis. J. Immunol. 160:1796-1803. [PubMed] [Google Scholar]
- 10.Incze, K., J. Farkas, V. Mihalyi, and E. Zukal. 1974. Antibacterial effect of cysteine-nitrosothiol and possible percursors thereof. Appl. Microbiol. 27:202-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jagannath, C., J. K. Actor, and R. L. Hunter, Jr. 1998. Induction of nitric oxide in human monocytes and monocyte cell lines by Mycobacterium tuberculosis. Nitric Oxide 2:74-86. [DOI] [PubMed] [Google Scholar]
- 12.MacMicking, J., Q. W. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323-350. [DOI] [PubMed] [Google Scholar]
- 13.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spallholz, J. E. 1987. Glutathione: is it an evolutionary vestige of the penicillins? Med. Hypoth. 23:253-257. [DOI] [PubMed] [Google Scholar]
- 15.Venketaraman, V., Y. K. Dayaram, A. G. Amin, R. Ngo, R. M. Green, M. T. Talaue, J. Mann, and N. D. Connell. 2003. Role of glutathione in macrophage control of mycobacteria. Infect. Immun. 71:1864-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu, K., C. Mitchell, Y. Xing, R. S. Magliozzo, B. R. Bloom, and J. Chan. 1999. Toxicity of nitrogen oxides and related oxidants on mycobacteria: M. tuberculosis is resistant to peroxynitrite anion. Tuber. Lung Dis. 79:191-198. [DOI] [PubMed] [Google Scholar]