Abstract
In this study we investigated the in vitro responses of peripheral blood mononuclear preparations and purified monocytes to Clostridium difficile toxin A. In contrast to the responses of T and B cells, exposure to toxin A led to a rapid loss of monocytes in a time- and dose-dependent fashion (the majority of cells were lost within 24 h of exposure to >100 ng of toxin per ml). Transmission electron microscopy, flow cytometry, and fluorescence microscopy after propidium iodide and Hoechst staining showed that cell death in purified preparations of monocytes following exposure to 100 and 1,000 ng of toxin A per ml occurred by apoptosis. Further studies showed that 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazole-carbocyanine iodide aggregates were retained within toxin A-exposed monocyte mitochondria, but cytochrome c was released, suggesting that the apoptotic cascade was triggered in the absence of mitochondrial permeability transition. There was also an increase in caspase-3 activity in toxin A-stimulated monocytes. Following exposure to very high concentrations of toxin A (30 μg/ml), monocyte cell death was predominantly of the necrotic type, with rapid extracellular release of lactate dehydrogenase. These studies demonstrated that C. difficile toxin A has a cell-specific effect, in which monocytes exhibit greater susceptibility than lymphocytes and their death is induced in a concentration-dependent manner.
Clostridium difficile, an anaerobic gram-positive bacterium, is the etiological agent of antibiotic-associated pseudomembranous colitis (3, 30) and the most common cause of nosocomial diarrhea. It causes an acute inflammatory colitis via two large secreted toxins, toxins A and B. Toxin A is a 308-kDa molecule and consists of an N-terminal glucosyltransferase domain, an ATP-binding domain, and a C-terminal receptor binding domain (19, 37). Purified toxin A causes fluid secretion and intestinal inflammation in animals (42, 49). In vitro exposure of intestinal epithelial monolayers to toxin A induces cytokine expression (5, 17, 39), rapid loss of barrier function (23, 45), and subsequent cell death by apoptosis (6, 39). In primary human intestinal epithelial cells, toxin A induces cell death within 24 h (39). In vivo, the release of proinflammatory cytokines and exposure to luminal microbial products, following loss of the epithelial barrier, are believed to lead to recruitment of circulating polymorphonuclear cells, monocytes, and lymphocytes. We showed previously that isolated normal human intestinal lamina propria T cells exposed to C. difficile toxin A underwent cell death by apoptosis, which was prominent after 72 h (38).
Apoptosis, or programmed cell death, plays an important part in the development and homeostasis of multicellular organisms. Morphologically, apoptosis is characterized by nuclear condensation (and often fragmentation) into spherical bodies containing dense chromatin (27). DNA fragmentation leads to a subdiploid DNA content, which can be demonstrated by flow cytometry (10, 44). In contrast, cellular necrosis (also referred to as oncosis) is characterized by dilation of mitochondria and loss of plasma membrane integrity, leading to cell swelling and eventual rupture (40, 52). At the molecular level, apoptosis is mediated by a family of cysteine proteases known as caspases (1, 20). Caspases can be activated by cell surface receptor binding (caspase-8) or intracellularly after release of cytochrome c from mitochondria (caspase-9) (13, 15), leading to eventual activation of the downstream executioner molecules caspase-3, caspase-6, and caspase-7 (33, 48). The executioner caspases mediate a series of events, such as cleavage of cytoskeleton proteins (31, 46) and activation of nucleases, resulting in DNA fragmentation (35).
The aims of the present study were to investigate the responses of peripheral blood monocytes to various concentrations of purified C. difficile toxin A and to determine the mode of cell death exhibited by susceptible cells.
MATERIALS AND METHODS
Purification of toxin A.
Toxin A was purified from a toxigenic strain of C. difficile, VPI 10463, as previously described (25). Following culture at 37°C for 48 h in brain heart infusion broth, the crude culture filtrate was applied to a bovine thyroglobulin affinity chromatography column, and this was followed by two sequential anion-exchange chromatography steps on Q-Sepharose-FF (Amersham Biosciences, Uppsala, Sweden) and Mono Q columns (Amersham Biosciences). Purified fractions were assessed for cytotoxicity by using the Vero cell assay (25) and for hemagglutinating activity (with rabbit erythrocytes) as described by Krivan et al. (29). Aliquots of purified toxin were stored at −80°C prior to use.
Isolation of PBMNCs and monocyte purification.
Peripheral blood mononuclear cells (PBMNCs) were obtained from blood drawn from healthy donors by density gradient centrifugation over Histopaque (Sigma-Aldrich Co. Ltd., Poole, United Kingdom). The peripheral blood cells were washed twice in phosphate-buffered saline (PBS) (Gibco Invitrogen, Paisley, United Kingdom) prior to use. For studies requiring purified monocytes, PBMNCs were depleted of T cells, B cells, and dendritic cells by labeling with specific hapten-conjugated antibodies and anti-hapten coupled magnetic MicroBeads in a monocyte depletion kit (Miltenyi Biotec GmBH, Bergisch Gladbach, Germany) used according to the manufacturer's instructions. The magnetically labeled nonmonocytic cells were depleted by retention on an LS MACS column in a magnetic field generated by a Vario MACS separator (Miltenyi Biotech GmBH), which allowed the unlabeled monocytes to be collected. The purity of monocyte preparations was >95%.
PBMNCs or purified monocytes were divided into aliquots containing 1 × 106 cells/ml in RPMI 1640 medium (Gibco Invitrogen) supplemented with 10% fetal calf serum (Gibco Invitrogen), 0.1 mg of streptomycin sulfate (Sigma-Aldrich) per ml, 0.1 mg of penicillin (Brittania Pharmaceuticals Ltd., Poole, United Kingdom) per ml, 49 μg of gentamicin (Roussel Laboratories, Uxbridge, United Kingdom) per ml, and 100 mM l-glutamine (Sigma-Aldrich) and cultured at 37°C in the presence of 5% CO2 for various times in the presence of different concentrations of purified C. difficile toxin A.
THP-1 cell culture.
THP-1 cells, a human monocytic cell line (passages 15 to 25) obtained from the American Type Culture Collection, were cultured at 37°C in the presence of 5% CO2 in RPMI 1640 medium (Gibco Invitrogen) supplemented with 10% fetal calf serum (Gibco Invitrogen), 0.1 mg of streptomycin sulfate (Sigma-Aldrich) per ml, 0.1 mg of penicillin (Brittania Pharmaceuticals Ltd.) per ml, 49 μg of gentamicin (Roussel Laboratories) per ml, 100 mM l-glutamine (Sigma-Aldrich), and 2 μM mercaptoethanol (Sigma-Aldrich).
Analysis of T-cell, B-cell, and monocyte populations.
The proportions of T-cells, B-cells, and monocytes in PBMNC populations were analyzed by flow cytometry. PBMNCs, incubated in the absence and presence of a range of toxin A concentrations, were pelleted by centrifugation (200 × g for 5 min) and fixed with 4% formaldehyde in PBS for 15 min at room temperature. Following two washes with PBS, the cells were incubated for 45 min at 4°C with the following fluorochrome-conjugated antibodies: anti-CD14-allophycocyanin (APC) and anti-CD14-phycoerythrin (PE) (for monocytes), anti-CD19-peridinin chlorophyll protein-cyanin 5.5 (PC5) (for B lymphocytes), and anti-CD3-fluorescein isothiocyanate (FITC) (for T lymphocytes). The isotype control antibodies used were polyclonal mouse anti-human immunoglobulin G1-PC5, -PE, -APC, and -FITC (all antibodies were obtained from Immunotech, Marseille, France). Unbound antibodies were removed from the cells by two further washes in PBS, and the cells were resuspended in 0.5% formaldehyde prior to analysis. Samples were analyzed with a Beckman Coulter Altra flow cytometer (Beckman Coulter, High Wycombe, United Kingdom) equipped with a 488-nm argon ion laser. The green fluorescence (FITC) was collected with a 525-band pass (BP) filter, the yellow fluorescence (PC5) was collected with a 575-BP filter, and the red fluorescence was collected with a 675-BP filter by using 488-nm excitation for PC5, 633-nm excitation for APC, and offset lasers.
Hoechst 33342 DNA staining.
The nuclear morphology of control and toxin A-treated monocytes and THP-1 cells was assessed by fluorescence microscopy after staining with Hoechst 33342 dye (36). Toxin-exposed and untreated cells were centrifuged (at 200 × g for 5 min) and fixed for 15 min in 4% formaldehyde at room temperature. THP-1 cells incubated with 30 μM etoposide (Sigma-Aldrich) for 5 h were used as a positive apoptotic control. Other controls included exposure to heat-inactivated (95°C for 1 h) toxin A and lipopolysaccharide (LPS) (from Escherichia coli O127:B8; Sigma-Aldrich Co. Ltd.).
The fixed cell suspensions (approximately 0.2 × 106 cells) were then spun onto a glass slide by using a Shandon/Lipshaw cytospin centrifuge (Thermo Shandon, Pittsburgh, Pa.) at 1,000 rpm for 8 min and stained with 10 μg of Hoechst 33342 dye (Molecular Probes, Eugene, Oreg.) per ml in PBS by incubation in the dark at room temperature for 10 min. The slides were rinsed with distilled water and air dried before coverslips were mounted by using PBS containing glycerol (50%, vol/vol). Slides were monitored for fluorescence with a fluorescence microscope (Carl Zeiss, Ltd., Welwyn Garden City, United Kingdom) by using a blue (488-nm) filter and a magnification of ×100. Apoptotic cells were identified by brightly staining condensed chromatin and fragmented nuclei (10). A total of 200 cells per sample were analyzed, and the numbers of apoptotic cells were expressed as percentages of the total cells.
LDH assay.
Purified monocytes were incubated in phenol red-free RPMI 1640 medium (Sigma-Aldrich) in the absence or presence of various concentrations of toxin A for 2, 5, and 24 h. Following centrifugation, the levels of lactate dehydrogenase (LDH) enzyme activity in supernatant samples were measured by the CytoTox 96 cytotoxicity assay (Promega Corporation, Madison, Wis.). The absorbance at 490 nm corresponded to the experimentally released LDH. Intracellular LDH activity was also measured after cell lysis (with the lysis solution contained in the kit). The extracellular release of LDH was expressed as a percentage of total cellular LDH activity (supernatant activity plus intracellular activity).
Flow cytometry. (i) Propidium iodide staining.
Propidium iodide staining of purified monocytes was performed as previously described (44). Cells from each stimulation reaction were pelleted by centrifugation (200 × g for 5 min) prior to fixation and permeabilization with ice-cold 70% ethanol. The cells were then washed twice in PBS containing 0.1% sodium azide and 0.5% bovine serum albumin (Sigma-Aldrich) before incubation with 0.25 μg of RNase at 37°C for 30 min. Propidium iodide (100 μg/ml; Sigma-Aldrich) was then added, and the propidium iodide fluorescence emission was analyzed with a Beckman Coulter Altra flow cytometer (Beckman Coulter) at 617 nm after excitation with a blue laser at 488 nm. Events accounting for less than 5% of the total diploid DNA content were gated out to prevent acquisition of individual apoptotic bodies. Events that fell within the marked hypodiploid region (apoptotic events) were enumerated by the statistics function of the WinMDI V2.8 software, and the results were expressed as a percentage of the total events. The percentages of apoptotic events identified by this method after incubation with or without toxin A were calculated for each time examined.
(ii) Cytofluorometric detection of alterations in mitochondrial membrane potential.
The integrity of the monocyte mitochondrial membrane after stimulation with toxin A was assessed with the lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazole-carbocyanine iodide (JC-1). JC-1 is able to selectively enter mitochondria, where it exists as a J-aggregate, emitting at 590 nm (red/yellow) after excitation at 488 nm (8). Changes in the mitochondrial membrane potential and subsequent depolarization result in dissociation of the aggregate into the monomeric form of the dye, causing a shift in emission to 530 nm (green), which can be detected by flow cytometry (8).
Purified monocytes were divided into aliquots containing 1 × 106 cells/ml in supplemented RPMI medium and incubated with or without 1,000 ng of toxin A for 5 min to 5 h at 37°C in the presence of 5% CO2. Cells were then washed twice with PBS prior to staining with 1 μM JC-1 in dimethyl sulfoxide (DMSO) (Invitrogen Ltd., Paisley, United Kingdom) for 30 min in the dark at 37°C. A negative control consisted of an aliquot of cells incubated with an equal volume of DMSO (Sigma-Aldrich). A positive control consisted of monocytes incubated in the presence of 100 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP) (Sigma-Aldrich), an uncoupler of oxidative phosphorylation in mitochondria (18, 26), for 30 min in the dark at 37°C. A minimum of 500,000 cells were analyzed with a Beckman Coulter Altra flow cytometer (Beckman Coulter). The WinMDI V2.8 software was used for data analysis.
(iii) Detection of activated caspase-3.
Monocytes purified from PBMNCs of healthy donors (by the magnetic cell sorting magnetic depletion method) were divided into aliquots containing 1 × 106 cells/ml in supplemented RPMI medium and incubated with and without 1,000 ng of toxin A per ml for 5 h at 37°C in the presence of 5% CO2. After incubation the cells were fixed with 4% formaldehyde in PBS for 5 min at room temperature, and this was followed by permeabilization with saponin buffer (0.04% saponin, 50 mM glucose, 0.1% sodium azide) in PBS. Intracellular active caspase-3 subunits were detected by incubation with phycoerythrin (PE)-conjugated anti-active human caspase-3 antibody (BD Pharmingen, San Diego, Calif.) in the presence of rabbit serum (Sigma Ltd.) to block nonspecific binding. Unbound antibody was washed from the cells with saponin prior to analysis by single-color flow cytometry with a Beckmann Coulter Altra flow cytometer by using a 488-nm argon ion laser and a 575-BP filter. Monocytes incubated in the presence of 500 nM staurosporine (Sigma Ltd.) were used as a positive control.
Confocal microscopy.
An assessment of the cytochrome c release from within the mitochondrial inner membrane was carried out by staining cytochrome c with an FITC-conjugated antibody. Monocytes purified by the magnetic depletion method were divided into aliquots containing 1 × 106 cells/ml in supplemented RPMI medium and incubated with or without 1,000 ng of toxin A per ml for 5 and 24 h at 37°C in the presence of 5% CO2. Monocytes (1 × 106 cells/ml) incubated in the presence of the apoptosis inducer etoposide (30 μM; Sigma Ltd.) in DMSO for 5 h at 37°C in the presence of 5% CO2 were used as a positive control. Cells were washed twice in HEPES buffer (10 mM HEPES [Sigma Ltd.] in distilled H2O [pH 7.4]) by centrifugation at 1,200 rpm (200 × g) and then fixed in 4% formaldehyde in PBS at room temperature for 15 min. The cells were then permeabilized in saponin buffer containing 3% goat serum (Sigma Ltd.), 1% glycine (BDH, Poole, United Kingdom), and 0.05% saponin (Sigma Ltd.) before incubation with mouse anti-human cytochrome c monoclonal antibody (5 μg/ml; Promega, Southampton, United Kingdom) in saponin buffer overnight at 4°C. After the preparation was washed in saponin buffer, FITC-conjugated goat anti-mouse secondary antibody (10 μg/ml; Alexis Biochemicals, Lausen, Switzerland) in saponin buffer was applied for 1 h at room temperature. The cells were subsequently washed with 10 mM HEPES buffer before resuspension in a 1:1 PBS-glycerol solution and applied to a Superfrost microscope slide (Menzel Gläser, Braunschweig, Germany). The slides were viewed with a Zeiss LSM 510 confocal microscope (Carl Zeiss), and FITC-labeled cytochrome c was visualized after excitation with a 488-nm argon single-photon laser.
Toxin A binding studies.
The fluorescein isothiocyanate isomer (5 μg; Sigma Ltd.) was incubated with purified toxin A (50 μg) in 0.1 M borate buffer (pH 8.5) for 18 h at 4°C. The reaction was terminated by adding 0.2 M glycine. Following dialysis against 1.5 M Tris HCl and determination of the protein concentration, the cytotoxic activity of FITC-labeled toxin A was confirmed by demonstration of a cytopathic effect in Vero cells. Labeled toxin was stored at 4°C in the dark until it was used. For binding studies, PBMNCs (at concentration of 1 × 106 cells/ml), with or without prior fixation (2% formaldehyde in PBS for 10 min), were incubated with FITC-labeled toxin A (at a concentration of 10,000 ng/ml) for 30 min at 4°C. Labeled cells were analyzed by flow cytometry, and the monocyte and lymphocyte populations were gated on the basis of forward and side scatter characteristics. Further analysis was performed by using the WinMDI V2.8 software.
Toxin A in stool samples.
Stool samples were collected during flexible sigmoidoscopy from 18 patients with C. difficile-associated diarrhea (22). No bowel preparation was performed prior to sigmoidoscopy, but 10 to 20 ml of 0.9% NaCl was often applied to obtain better views of the mucosa. The concentrations of toxin A in stool samples aspirated during sigmoidoscopy were determined by using a C. difficile Tox-A Test enzyme immunoassay kit (TechLab Inc., Blacksburg, Va.). In brief, 100 μl of sample was added to each well of an assay plate containing immobilized affinity-purified polyclonal anti-toxin A antibody and 50 μl of conjugate solution (mouse monoclonal anti-toxin A antibody coupled to horseradish peroxidase, both supplied with the kit). Standards, prepared from dilutions of purified toxin A (0.1 pg to 50 μg in PBS), were similarly added to prepared wells. Subsequent steps were performed as instructed by the manufacturer. The concentration of toxin A in each stool sample was obtained by extrapolation from the standard curve.
Statistical analysis.
Data were analyzed by using analysis of variance and the Student paired t test.
RESULTS
C. difficile toxin A induces rapid loss of monocytes but not T and B cells.
Freshly isolated peripheral blood mononuclear cells were analyzed by flow cytometry following culture in control medium only or in the presence of 1,000 ng of toxin A per ml. CD14 events (monocyte specific) were lost within 24 h, but CD3 events (T cells) and CD19 events (B cells) did not change significantly over the same time (Table 1). Following exposure to 1,000 ng of toxin A per ml, approximately 50% of CD14-positive events (specific for monocytes) were lost after 2 h, and more than 90% were lost after 5 h. By contrast, the majority of CD3-positive events (T cells) and CD19-positive events (B cells) were still present after 72 h of exposure to the same concentration of the toxin (data not shown). An analysis in which anti-CD33 antibody was used as a monocyte marker produced results similar to those obtained with antibody to CD14 (data not shown).
TABLE 1.
Flow cytometric analysis of PBMNCs exposed to C. difficile toxin Aa
| Time (h) | % of cells
|
|||||
|---|---|---|---|---|---|---|
| CD3
|
CD19
|
CD14
|
||||
| Control | 1,000 ng of toxin A/ml | Control | 1,000 ng of toxin A/ml | Control | 1,000 ng of toxin A/ml | |
| 0 | 58.9 ± 0.8 | 58.9 ± 0.7 | 12.3 ± 1.5 | 12.1 ± 1.5 | 8.9 ± 1.8 | 8.4 ± 1.3 |
| 2 | 59.5 ± 1.9 | 60.4 ± 2.9 | 10.9 ± 4.1 | 11.1 ± 4.1 | 8.3 ± 1.2 | 4.6 ± 1.8 |
| 5 | 57.1 ± 3.9 | 62.3 ± 3.4 | 9.8 ± 2.7 | 11.4 ± 3.9 | 9.3 ± 1.7 | 0.4 ± 0.3 |
| 24 | 59.3 ± 4.0 | 58.4 ± 6.9 | 12.6 ± 2.8 | 10.6 ± 0.6 | 9.2 ± 2.4 | 0.2 ± 0.1 |
Data for events specific for T cells (CD3), B cells (CD19), and monocytes (CD14) in control and toxin A-exposed PBMNCs are shown. Following isolation, PBMNCs were exposed to 1,000 ng of purified C. difficile toxin A per ml for 2 to 24 h before analysis by flow cytometry. The data are means ± standard errors of the means for cells from three experiments.
The toxin A carrier buffer, Tris-buffered saline, did not have a distinguishable effect on PBMNCs compared to medium alone (data not shown); therefore, subsequent experiments were carried out by using culture medium alone as a negative control.
Exposure of PBMNCs to various concentrations of toxin A (1 to 1,000 ng/ml) led to the loss CD14 events in a dose- and time-dependent manner (Table 2). Over the study period (24 h), significant loss of CD14 events occurred at concentrations of toxin A that were >10 ng/ml.
TABLE 2.
Incubation with toxin A decreases the numbers of CD14-positive events in a time- and dose-dependent mannera
| Concn of toxin A (ng/ml) | No. of CD14-positive events (mean ± SEM)
|
||
|---|---|---|---|
| 2 h | 5 h | 24 h | |
| 0 | 8,308 ± 1,218 | 9,354 ± 1,795 | 9,192 ± 2,390 |
| 1 | 8,420 ± 1,293 | 7,860 ± 1,320 | 7,850 ± 2,393 |
| 10 | 8,400 ± 1,213 | 9,223 ± 2,298 | 4,430 ± 547 |
| 100 | 8,297 ± 1,138 | 7,460 ± 2,300 | 260 ± 80b |
| 1,000 | 4,638 ± 1,854b | 400 ± 340c | 230 ± 152d |
PBMNCs were labeled with anti-CD14-PE antibodies after incubation with 10-fold concentrations of toxin for 2, 5, and 24 h. Cells were then analyzed by single-color flow cytometry, and CD14-positive events were enumerated (n = 3).
P < 0.05.
P < 0.001.
P < 0.01.
Binding to FITC-labeled toxin A.
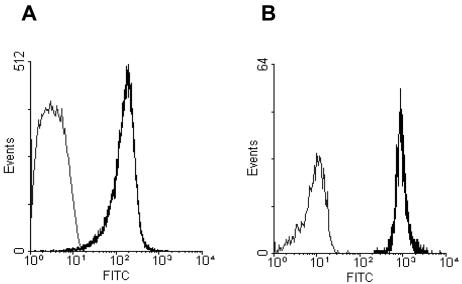
PBMNCs incubated with FITC-labeled toxin A for 30 min at 4°C were studied by flow cytometry. Monocyte and lymphocyte populations were analyzed after application of specific gates based on forward and side scatter characteristics. For cells labeled with FITC-labeled toxin A, the fluorescence intensity of monocytes was approximately 10-fold greater than that of lymphocytes (Fig. 1), implying that there was greater binding to the former cells by the labeled toxin. Differences with similar magnitudes between labeled monocytes and lymphocytes were also seen if the PBMNCs were fixed prior to incubation with the FITC-labeled toxin (data not shown).
FIG. 1.
FITC-labeled toxin A binding to monocytes and lymphocytes. PBMNCs were incubated with 10,000 ng of FITC-labeled toxin A per ml for 30 min at 4°C before analysis by flow cytometry. Lymphocyte (A) and monocyte (B) gates were applied based on forward and side scatter characteristics. The grey lines indicate the results for unlabeled cells, and the black lines indicate the results for cells incubated with FITC-labeled toxin A. The fluorescence intensity of labeled monocytes was approximately 10-fold greater than that of labeled lymphocytes.
Characterization of monocyte cell death in response to C. difficile toxin A.
In order to characterize (and quantify) the type of cell death occurring following exposure to toxin A, monocytes were purified by magnetic depletion of other mononuclear cell types in the PBMNC samples. The monocyte morphology was studied by fluorescence microscopy (following Hoechst 33342 staining) and by electron microscopy, and the cellular DNA content was analyzed by flow cytometry.
(i) Hoechst 33342 staining.
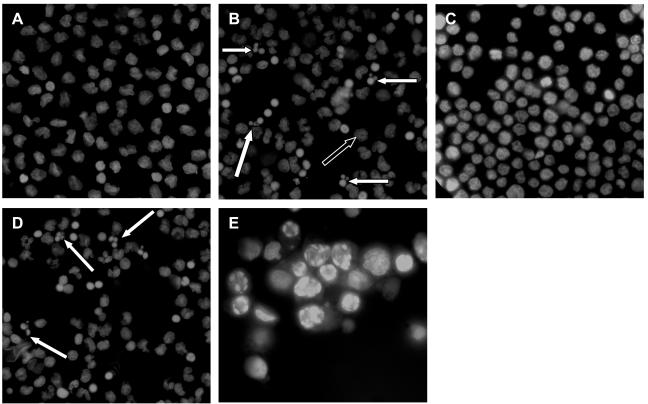
Purified monocyte preparations were incubated in the presence of a range of toxin A concentrations (1 to 1,000 ng/ml) for 2, 5, and 24 h, and this was followed by labeling of the cellular DNA with the fluorescent marker Hoechst 33342 dye. Following exposure to 1,000 ng of toxin A per ml, cells with the characteristic condensed chromatin structure of apoptotic nuclei were prominent (Fig. 2B). Apoptotic cell death in response to 1,000 ng of toxin A per ml was also seen in THP-1 cells (Fig. 2D). Following incubation with toxin A, a number of nonapoptotic cells (many with more diffuse nuclear staining than the controls) were also seen, and based on findings obtained by transmission electron microscopy (TEM) (see below), these cells were probably cells undergoing necrotic cell death. THP-1 cells incubated in the presence of 30 μM etoposide for 5 h provided a positive control for apoptotic cell death (Fig. 2E). Dose-response studies showed that significant monocyte apoptosis was seen only at concentrations of >10 ng/ml (Table 3).
FIG. 2.
Monocyte cell death in response to C. difficile toxin A. Purified monocytes and THP-1 cells were incubated for 24 h in the absence of toxin A (A and C) or in the presence of 1,000 ng of toxin A per ml (B and D). THP-1 cells were also incubated with 30 μM etoposide for 5 h (positive control) (E). Cytospin preparations of cells were fixed and stained with 10 μg of Hoechst 33342 dye per ml before visualization with a fluorescence microscope with UV light excitation. Cells exhibiting characteristic features of apoptotic cell death are indicated by solid arrows. A number of nonapoptotic cells with diffuse nuclear staining (open arrow) were also seen after exposure to toxin A, and these cells were probably cells undergoing necrosis (see Fig. 3C).
TABLE 3.
C. difficile toxin A induces monocyte apoptosisa
| Toxin concn (ng/ml) | % of apoptotic monocytes after staining with Hoechst dye (mean ± SEM)
|
|
|---|---|---|
| 5 h | 24 h | |
| 0 | 3.34 ± 2.4 | 3.3 ± 4.8 |
| 1 | 1.36 ± 0.6 | 0.47 ± 0.35 |
| 10 | 4.86 ± 4.1 | 1.03 ± 0.68 |
| 100 | 15.8 ± 17.4 | 35.6 ± 16.2 |
| 1,000 | 44.7 ± 18.1b | 42.7 ± 3.1b |
Purified monocytes were exposed to various concentrations of purified toxin for 5 or 24 h. Cytospin preparations of the cells were fixed, stained with 10 μg of Hoechst dye per ml, and analyzed by fluorescence microscopy. The percentages of cells with characteristic features of apoptosis were determined. The data are means ± standard errors of the means for three experiments.
P < 0.05 compared to control.
Studies of Hoechst dye-labeled control and toxin A-exposed (1,000 ng/ml) THP-1 cells also revealed that there was significant induction of apoptosis after 24 h. The percentages of apoptotic cells in control and toxin A-exposed preparations were as follows: at 2 h, 1.15% ± 0.87% and 0.84% ± 0.64%, respectively; at 5 h, 1.03% ± 0.69% and 6.77% ± 2.49%, respectively; and at 24 h, 7.97% ± 0.51% and 30.33% ± 4.78%, respectively (P < 0.05).
To confirm that the apoptotic effect seen was specific to toxin A and not due to contaminating LPS, monocytes were incubated for 5 h in the presence of either 1,000 ng of LPS per ml, 1,000 ng of heat-inactivated (95°C, 1 h) toxin A per ml, 1,000 ng of untreated toxin A per ml, or control medium. Cells were assessed for apoptosis by using the fluorescent marker Hoechst 33342 dye. Neither LPS nor heat-inactivated toxin A induced apoptosis above the basal levels (1.16% ± 0.49% and 1.04% ± 0.91%, respectively), compared to the 40.7% ± 18.6% apoptotic cells resulting from incubation with 1,000 ng of toxin A per ml.
(ii) Transmission electron microscopy.
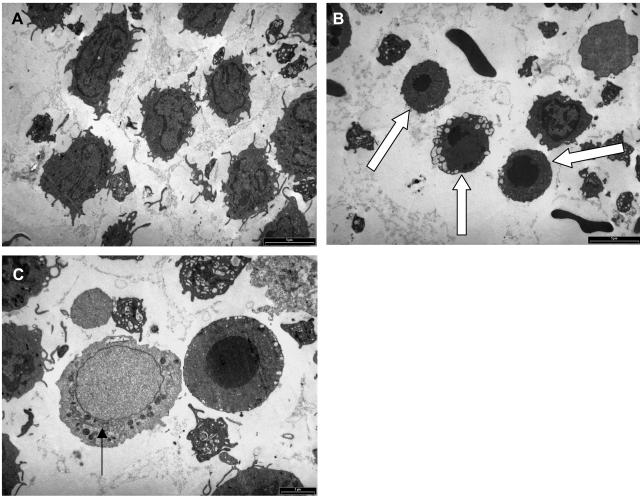
TEM studies of purified preparations of monocytes exposed to 1,000 ng of toxin A per ml for 5 and 24 h revealed characteristic ultrastructural features of apoptotic cell death (Fig. 3B and C), with nuclear condensation (nuclear fragmentation was also seen) into spherical structures containing dense chromatin and also membrane-bound apoptotic bodies. Some cells with features of necrotic cell death were also seen (Fig. 3C), but they were less numerous (after 5 h of exposure of monocytes to 1,000 ng of toxin A per ml, 40.9 and 25.5% of the cells had morphological features of apoptosis and necrosis, respectively). Following exposure to 30 μg of toxin A per ml for 2 h, TEM studies almost exclusively showed features of necrotic cell death, with an increase in the volume of the cells and a loss of nuclear chromatin (data not shown). After 5 h of exposure to 30 μg of toxin per ml, mostly cell debris was seen (data not shown). No ultrastructural evidence of apoptotic cell death was seen at either time in response to the highest concentration of toxin A.
FIG. 3.
Monocytes exhibit ultrastructural features of apoptotic (B and C) and necrotic (C) cell death following exposure to 1,000 ng of C. difficile toxin A per ml. Purified monocytes were incubated for 5 h with control medium (A) or medium containing 1,000 ng of toxin A per ml (B) before they were processed for electron microscopy. Cells exhibiting morphological features characteristic of apoptosis are indicated by arrows in panel B. In panel C, the arrow indicates a cell undergoing necrotic death, with swelling of the nucleus and a lack of dense chromatin. By contrast, the adjacent cell shows features of apoptotic death, with a condensed spherical nucleus containing dense chromatin.
(iii) DNA analysis by flow cytometry.
The DNA content of purified monocytes exposed to toxin A (or control medium) was analyzed by flow cytometry, after permeabilization and labeling with the DNA-specific fluorochrome propidium iodide. Apoptotic cells that have a subdiploid DNA content can be identified by their reduced fluorescence due to DNA degradation and leakage from the cell (9, 44) and are detected in a broad hypodiploid or sub-G1 region on a histogram. Following exposure of purified preparations of monocytes to 1,000 ng of toxin A per ml for 5 and 24 h, events within hypodiploid DNA regions (M1 regions in Fig. 4A, panels iii and iv) were expressed as percentages of the total propidium iodide-fluorescent events. After 2 h of incubation with 1,000 ng of toxin A per ml, the mean percentage of hypodiploid events (which represented apoptotic monocytes) was similar to control values. By 5 h the mean percentage of hypodiploid events in toxin A-exposed monocytes was 6.92% ± 3.76%, and the value increased to 20.8% ± 4.87% after 24 h of incubation. Control values remained less than 2% at all times studied.
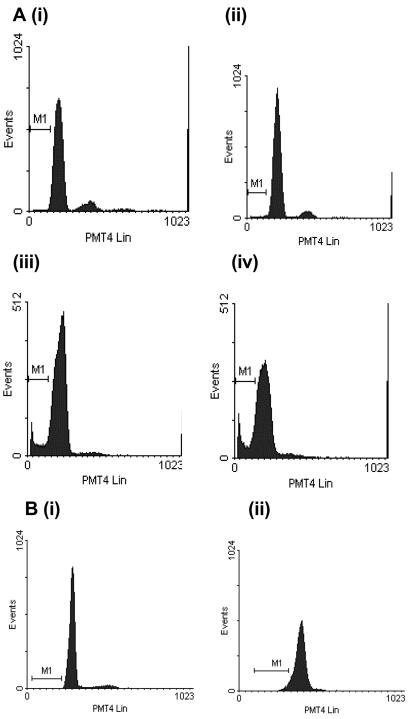
FIG. 4.
Representative DNA fluorescence profiles of propidium iodide-labeled purified preparations of control and toxin A-exposed monocytes. After incubation, the cells were fixed, permeabilized, and treated with RNase before labeling with propidium iodide and analysis of propidium iodide fluorescence. In panel A, the monocytes were incubated in the absence of C. difficile toxin A (panel i) or in the presence of 1,000 ng of C. difficile toxin A per ml for 2 h (panel ii), 5 h (panel iii), or 24 h (panel iv). In panel B, monocytes were exposed to control medium (panel i) or 30 μg of toxin A per ml (panel ii) for 5 h prior to propidium iodide labeling. Control monocytes (panels i in panels A and B) and monocytes exposed to 30 μg of toxin A per ml (panel ii in panel B) did not exhibit apoptotic characteristics, as shown by a lack of events in the hypodiploid region (M1). By contrast, many events characteristic of apoptotic cell death were seen in the hypodiploid (or sub-G1) region in monocytes incubated with 1,000 ng of toxin A per ml for 5 h (panel iii in panel A) and 24 h (panel iv in panel A).
In contrast to the results described above, flow cytometric analysis of propidium iodide-labeled nuclei of monocytes exposed to 30 μg of toxin A per ml for 5 h did not show significant events in the hypodiploid region (Fig. 4B, panel ii).
LDH assay.
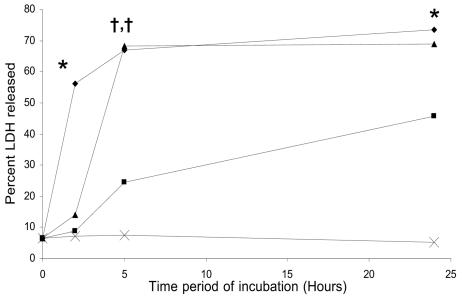
The C. difficile toxin A-induced effect on monocyte membrane integrity was assessed by measurement of the extracellular release of the stable cytosolic enzyme LDH. In contrast to the effects of lower concentrations, exposure to 30 μg of toxin A per ml led to rapid (by 2 h) and nearly maximal extracellular release of LDH (Fig. 5). Following exposure to 100 and 1,000 ng of the toxin per ml, the release of LDH was slower and occurred in a dose-dependent fashion.
FIG. 5.
LDH release by monocytes exposed to C. difficile toxin A. Purified preparations of monocytes were incubated in the absence of toxin A (×) or in presence of 100 ng of toxin A per ml (▪), 1,000 ng of toxin A per ml (▴), or 30 μg of toxin A per ml (♦) for 2, 5, or 24 h. LDH levels were measured in supernatant samples and cell lysates. Extracellular release of LDH (levels in supernatant samples) was expressed as a percentage of the total cellular LDH activity (supernatant activity plus intracellular activity). The data are the means from three experiments. Compared to the control, there was significantly greater release of LDH following exposure to 30 μg of toxin A per ml for 2 h (56.1% ± 7.6%; P = 0.02), for 5 h (68.8% ± 9.3%; P = 0.01), and for 24 h (73.5% ± 8.4%; P = 0.01) and following incubation with 1,000 ng of the toxin per ml for 5 h (68.2% ± 7.8%; P < 0.01) and for 24 h (68.8% ± 9.8%; P = 0.01).
Studies with mitochondria.
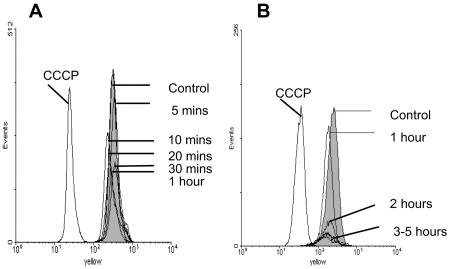
The effect of toxin exposure on the monocyte mitochondrial outer membrane function was studied by flow cytometry. Mitochondrial membrane depolarization was detected by a shift in fluorescence emission of the lipophilic cationic probe JC-1. Changes in the mitochondrial membrane potential, as induced by the uncoupling agent CCCP, caused a reduction in the aggregate form of JC-1 within mitochondria (emissions at 590 nm) by dissociation into the monomeric form (Fig. 6). By contrast, exposure of purified monocytes to toxin A (1,000 ng/ml) for 5 min to 5 h did not induce a significant change in emission of JC-1 (Fig. 6).
FIG. 6.
JC-1 fluorescence in monocytes exposed to 1,000 ng of toxin A per ml for 5 to 60 min (A) and for 1 to 5 h (B). CCCP (positive control), but not toxin A, induced a leftward shift in red/yellow fluorescence, consistent with mitochondrial membrane depolarization. The data are representative of the data from three experiments.
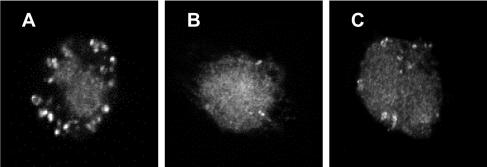
Release of cytochrome c from the mitochondrial inner membrane was visualized by using FITC-conjugated antibody and confocal fluorescence microscopy. Cytochrome c detection was confined to mitochondria in monocytes exposed to control medium (Fig. 7A), but in cells exposed to toxin A (1,000 ng/ml) for 5 h cytochrome c was seen throughout the cytoplasm (Fig. 7B), implying that it was released from mitochondria. This was also the case in monocytes incubated with 30 μM etoposide for 5 h (positive control) (Fig. 7C).
FIG. 7.
Cytochrome c expression by control and toxin A-exposed monocytes. Purified monocytes were exposed to control medium (A), 1,000 ng of toxin A per ml (B), or 30 μM etoposide (positive control) (C) for 5 h prior to fixation, permeabilization, and incubation with anti-cytochrome c antibody. Following application of FITC-conjugated goat anti-mouse antibody, the cells were examined by confocal fluorescence microscopy. Representative images are shown. Cytochrome c labeling is confined to mitochondria in panel A. By contrast, in monocytes exposed to toxin A and etoposide for 5 h (B and C), cytochrome c labeling occurs almost exclusively in the cytoplasm.
Detection of activated caspase-3.
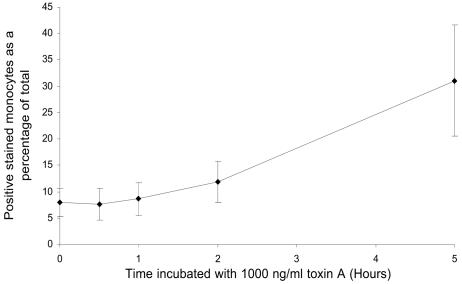
The presence of intracellular activated caspase-3 was investigated by flow cytometry by using a specific PE-conjugated antibody (Fig. 8). A marker region (M1) was constructed to encompass only positively stained events, and this was applied to subsequent histograms. The numbers of events falling within this marked region were determined, and the results were expressed as percentages of the total events. Following exposure to toxin A for 5 h, the percentage of monocytes expressing activated caspase-3 was significantly greater than the percentage for the control cells (31.1% ± 10.5% versus 7.9% ± 2.6%; P < 0.05).
FIG. 8.
Activation of caspase-3 in monocytes exposed to C. difficile toxin A. Purified monocytes were incubated in the absence or in the presence of 1,000 ng of toxin A per ml for 0.5, 1, 2, and 5 h at 37°C prior to fixation, permeabilization, incubation with PE-conjugated anti-active caspase-3 antibody, and analysis by flow cytometry. Monocytes exhibiting positive intracellular active caspase-3 fluorescence were enumerated, and the data were expressed as a percentage of the total number of cells analyzed. The data are means ± standard errors of the means for three experiments. Compared to unstimulated cells, a significantly greater proportion of monocytes exposed to 1,000 ng of toxin A per ml for 5 h exhibited intracellular expression of activated caspase-3 (7.9% ± 2.6% versus 31.1% ± 10.5%; P < 0.05).
Toxin A concentrations in stool samples of patients with C. difficile-associated diarrhea.
The amounts of toxin A present in stool samples obtained during flexible sigmoidoscopy from 18 patients with C. difficile-associated diarrhea were determined by using an enzyme immunoassay. The median concentration of the toxin was 4.3 ng/ml, and the range was 0.6 to 19,000 ng/ml.
DISCUSSION
Our study showed that monocytes are much more sensitive to the cytotoxic effects of C. difficile toxin A than circulating T or B cells are. Flow cytometry studies showed that within 5 h of exposure of PBMNCs to 1,000 ng of toxin A per ml (3.24 nM), >90% of monocyte-specific CD14 events were lost (a similar effect was seen in response to 24 h of incubation with 100 ng of toxin A per ml). By contrast, there was no significant change in CD3 (T-cell-specific) and CD19 (B-cell-specific) events in the same PBMNC preparations. These findings are consistent with our previous studies in which human intestinal lamina propria cells were used, in which exposure to C. difficile toxin A led to much more rapid loss of macrophages than of T cells (38). The reason for the greater sensitivity of monocytes/macrophages than of lymphocytes to the cytotoxic effect of toxin A remains to be determined, but our studies suggest that greater binding (likely due to increased surface receptor expression) and subsequent internalization are a possibility. Thus, following incubation (at 4°C) with FITC-labeled toxin A, the fluorescence intensity of monocytes was approximately 10-fold greater than that of lymphocytes. The difference is unlikely to be due to internalization of the labeled toxin by monocytes because studies performed with fixed cells gave similar results. We also believe that the nearly 10-fold difference in fluorescence between toxin-bound monocytes and toxin-bound lymphocytes is unlikely to be due to the dissimilarity in cell size because monocytes have only approximately twice the surface area of lymphocytes (based on average diameters of 9 μm for lymphocytes and 12.5 μm for monocytes).
Further studies showed that a large number of monocytes exposed to 100 and 1,000 ng of toxin A per ml underwent cell death by apoptosis, as confirmed by analysis of cells after DNA labeling (Hoechst 33342 dye and propidium iodide) and TEM ultrastructural studies. Since this finding has not been reported previously, different methods were used to confirm monocyte cell death by apoptosis in response to toxin A (100 and 1,000 ng/ml). Interestingly, TEM studies also showed that some cells underwent death by necrosis, which may partially explain the apparent disparity between data obtained (following exposure to 1,000 ng of toxin per ml for 5 h) by flow cytometry (showing >90% loss of CD14 events [Table 2]) and the proportion of monocytes showing morphological features of apoptotic cell death (mean, 44.7% [Table 3]). Other reasons for the apparent disparity may include the early loss of CD14 (prior to cell death (51) and the fact that apoptosis (and likely necrosis) in response to toxin A appears to be a continuous process with heterogeneity in susceptibility between monocytes. Thus, because cell death leads to rapid degradation and disruption of cellular constituents, the proportion of cells showing characteristic morphological features of apoptosis at a specific time (e.g., 5 or 24 h [Table 3]) is based on the total cell population at that time and not the number of cells present in the original preparation. The analysis of monocyte DNA by flow cytometry revealed 6.9 and 20.8% hyodiploid events (means) after 5 and 24 h of exposure, respectively, to 1,000 ng of toxin A per ml (Fig. 4A). The reason that these percentages of hypodiploid events are lower than the proportion of cells with morphological features of apoptosis (44.7 and 42.7%, respectively [Table 3]) could be the lack of detection by flow cytometry of very small fragments of DNA and the loss of such fragments from the cells. It is interesting that monocytes incubated for 5 h with 30 μg of toxin A per ml did not show significant events in the hypodiploid region (Fig. 4B). Cell death by necrosis following exposure to the highest concentration of the toxin was confirmed by TEM (data not shown). Moreover, during incubation of purified monocytes with 30 μg of toxin A per ml within 2 h there was nearly maximal release of LDH, further supporting the concept of cell death by necrosis following rapid loss of membrane integrity at high toxin concentrations. By contrast, exposure of monocytes to 1,000 ng of toxin A per ml did not induce significant release of LDH after 2 h, but maximal release occurred at 5 h. The latter release of LDH likely reflected a combination of necrotic cell death in a proportion of monocytes (as shown by TEM in Fig. 3C) and loss of membrane integrity during the late stages of apoptotic cell death.
Monocyte cell death by necrosis in response to a very high concentration of toxin A has been reported previously (51), but monocyte cell death by apoptosis at lower concentrations of the toxin is a new finding. The induction of apoptotic cell death at low concentrations and the induction of necrosis at high concentrations have also been reported in response to Staphylococcus aureus alpha-toxin (2) and Clostridium perfringens enterotoxin (7). For S. aureus alpha-toxin it has been postulated (2) that the interaction of large amounts of the toxin with the cell plasma membrane leads to the formation of large pores and the loss of intracellular ATP required in the apoptotic cell death pathway (12, 32). Since toxin A also induces depletion of ATP (16, 34), a similar mechanism could explain the predominance of necrotic cell death in response to very high concentrations of toxin A. The induction of both types of cell death (apoptosis and necrosis) in monocyte preparations exposed to 1,000 ng of toxin A per ml could be due to heterogeneity in toxin A binding to the cell surface. Thus, the smaller proportion of monocytes that bind large amounts of the toxin at the cell surface would be postulated to undergo necrosis due to rapid depletion of ATP caused by large pores in the cell membrane.
We also investigated changes in mitochondrial function in monocytes but could find no evidence of depolarization in response to concentrations of toxin A that induced apoptosis in many cells (1,000 ng/ml). These findings differ from those reported for CHO cells, in which there was a decrease in mitochondrial membrane potential 15 min after exposure to toxin A (16). The lack of such changes in our study could reflect cell-specific effects of the toxin.
In agreement with previous studies with epithelial cells (6, 34), we found that cytochrome c was released from mitochondria following exposure to the toxin. Although cytochrome c release is often associated with mitochondrial depolarization (41, 53), it may also occur in the absence of such depolarization (4, 28, 54). Cytochrome c release and activation of caspase-3 may occur after initiation of the apoptosis pathway by either the intrinsic (mitochondrion-mediated) or extrinsic (death receptor-mediated) pathway, suggesting that either of these mechanisms may be involved in toxin A-induced monocyte apoptosis. A possible candidate mechanism for toxin A-induced activation of caspase-3 is via the proapoptotic molecule Bid. Bid has been found to mediate mitochondrial damage that is followed by cytochrome c release in the T84 epithelial cell line (6). While further studies are required to characterize the mechanism of toxin A-induced apoptosis in monocytes, our studies suggest that although expression of tumor necrosis factor alpha is induced (38), this molecule is unlikely to be involved, as neutralization of it did not affect the apoptotic response (data not shown).
The sensitivity of monocytes to the cytotoxic effects of toxin A is similar to that of primary human intestinal epithelial cells (39, 43, 47). For both of these cell types, significant cytotoxicity is seen only after exposure to 10 ng or more of toxin A per ml. However, at concentrations of <10 ng/ml, there is a loss of transepithelial resistance (23, 34), and the cells are induced to express transforming growth factor β (23). These findings support the concept that there are distinct intracellular mechanisms by which C. difficile toxin A mediates its effects on host cells (17, 21, 24, 34, 50).
The concentrations of toxin A in stool samples of patients with C. difficile-associated diarrhea ranged from 0.6 ng/ml to 19 μg/ml (median, 4.3 ng/ml). Although local concentrations at the intestinal epithelial surface may not be accurately reflected by the concentrations present in a stool sample, one can postulate the in vivo significance of an in vitro response by host cells to different concentrations of the toxin. Exposure to low concentrations of toxin A induces expression of the anti-inflammatory cytokine transforming growth factor β and much slower loss of epithelial barrier function (23). By contrast, high concentrations of toxin readily overcome the epithelial barrier and induce the expression of proinflammatory cytokines, such as tumor necrosis factor alpha and interleukin-8 (14, 21, 38) in lamina propria monocytes/macrophages. The induction of proinflammatory cytokines is prominent following exposure to 1,000 ng or more of toxin A per ml (14, 38, 50). In our study, we showed that in addition to a large number of monocytes undergoing apoptosis, a smaller proportion of these cells exposed to 1,000 ng of toxin A per ml undergo necrotic cell death. We believe that it is likely that proinflammatory cytokines are expressed by monocytes destined to undergo cell death by necrosis. While cells undergoing apoptosis should be rapidly cleared via phagocytosis by neighboring cells (11), the cells dying by necrosis should further exacerbate the inflammatory response by releasing not only proinflammatory cytokines but also other intracellular contents. Thus, exposure of the colonic mucosa to very high concentrations of C. difficile toxins may lead to a severe inflammatory response (as seen in patients with pseudomembranous colitis) via induction of epithelium- and monocyte-derived proinflammatory cytokine release and rapid necrotic cell death in monocytes. The relative resistance of cells of the adaptive immune system (T and B cells) to toxin A-induced cell death compared to cells of the innate immune system (monocytes/macrophages and epithelial cells) suggests that antibody-mediated protection by the former system may be of major importance during exposure of the host to high concentrations of C. difficile toxins.
Acknowledgments
This work was supported by the Community Fund via the Digestive Disorders Foundation and the Medical Research Council.
We thank Trevor Gray, Division of Pathology, for assistance with the electron microscopy studies.
Editor: J. T. Barbieri
REFERENCES
- 1.Alnemri, E. S., D. J. Livingston, D. W. Nicholson, G. Salvesen, N. A. Thornberry, W. W. Wong, and J. Yuan. 1996. Human ICE/CED-3 protease nomenclature. Cell 87:171. [DOI] [PubMed] [Google Scholar]
- 2.Bantel, H., B. Sinha, W. Domschke, G. Peters, K. Schulze-Osthoff, and R. U. Janicke. 2001. Alpha-toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 155:637-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett, J. G., T. W. Chang, M. Gurwith, S. L. Gorbach, and A. B. Onderdonk. 1978. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N. Engl. J. Med. 298:531-534. [DOI] [PubMed] [Google Scholar]
- 4.Bossy-Wetzel, E., D. D. Newmeyer, and D. R. Green. 1998. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 17:37-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branka, J. E., G. Vallette, A. Jarry, C. Bou-Hanna, P. Lemarre, P. N. Van, and C. L. Laboisse. 1997. Early functional effects of Clostridium difficile toxin A on human colonocytes. Gastroenterology 112:1887-1894. [DOI] [PubMed] [Google Scholar]
- 6.Brito, G. A., J. Fujji, B. A. Carneiro-Filho, A. A. Lima, T. Obrig, and R. L. Guerrant. 2002. Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. J. Infect. Dis. 186:1438-1447. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti, G., X. Zhou, and B. A. McClane. 2003. Death pathways activated in CaCo-2 cells by Clostridium perfringens enterotoxin. Infect. Immun. 71:4260-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cossarizza, A., M. Baccarani-Contri, G. Kalashnikova, and C. Franceschi. 1993. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1). Biochem. Biophys. Res. Commun. 197:40-45. [DOI] [PubMed] [Google Scholar]
- 9.Darzynkiewicz, Z., S. Bruno, G. Del Bino, W. Gorczyca, M. A. Hotz, P. Lassota, and F. Traganos. 1992. Features of apoptotic cells measured by flow cytometry. Cytometry 13:795-808. [DOI] [PubMed] [Google Scholar]
- 10.Darzynkiewicz, Z., X. Li, and J. Gong. 1994. Assays of cell viability: discrimination of cells dying by apoptosis. Methods Cell Biol. 41:15-38. [DOI] [PubMed] [Google Scholar]
- 11.Fadok, V. A., D. L. Bratton, and P. M. Henson. 2001. Phagocyte receptors for apoptotic cells: recognition, uptake, and consequences. J. Clin. Investig. 108:957-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari, D., A. Stepczynska, M. Los, S. Wesselborg, and K. Schulze-Osthoff. 1998. Differential regulation and ATP requirement for caspase-8 and caspase-3 activation during CD95- and anticancer drug-induced apoptosis. J. Exp. Med. 188:979-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferri, K. F., and G. Kroemer. 2001. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 3:E255-E263. [DOI] [PubMed] [Google Scholar]
- 14.Flegel, W. A., F. Muller, W. Daubener, H. G. Fischer, U. Hadding, and H. Northoff. 1991. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Infect. Immun. 59:3659-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green, D., and G. Kroemer. 1998. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 8:267-271. [DOI] [PubMed] [Google Scholar]
- 16.He, D., S. J. Hagen, C. Pothoulakis, M. Chen, N. D. Medina, M. Warny, and J. T. LaMont. 2000. Clostridium difficile toxin A causes early damage to mitochondria in cultured cells. Gastroenterology 119:139-150. [DOI] [PubMed] [Google Scholar]
- 17.He, D., S. Sougioultzis, S. Hagen, J. Liu, S. Keates, A. C. Keates, C. Pothoulakis, and J. T. Lamont. 2002. Clostridium difficile toxin A triggers human colonocyte IL-8 release via mitochondrial oxygen radical generation. Gastroenterology 122:1048-1057. [DOI] [PubMed] [Google Scholar]
- 18.Heytler, P. G. 1963. Uncoupling of oxidative phosphorylation by carbonyl cyanide phenylhydrazones. I. Some characteristics of m-Cl-CCP action on mitochondria and chloroplasts. Biochemistry 2:357-361. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann, J. F., and K. Aktories. 2000. Molecular mechanisms of action of the large clostridial cytotoxins, p. 305-331. In K. Aktories and I. Just (ed.), Handbook of experimental pharmacology, vol. 145. Bacterial protein toxins. Springer-Verlag, Berlin, Germany.
- 20.Jacobson, M. D., and G. I. Evan. 1994. Apoptosis. Breaking the ICE. Curr. Biol. 4:337-340. [DOI] [PubMed] [Google Scholar]
- 21.Jefferson, K. K., M. F. Smith, Jr., and D. A. Bobak. 1999. Roles of intracellular calcium and NF-kappa B in the Clostridium difficile toxin A-induced up-regulation and secretion of IL-8 from human monocytes. J. Immunol. 163:5183-5191. [PubMed] [Google Scholar]
- 22.Johal, S. S., J. Hammond, K. Solomon, P. D. James, and Y. R. Mahida. 2004. Clostridium difficile associated diarrhoea in hospitalised patients: onset in the community and hospital and role of flexible sigmoidoscopy. Gut 53:673-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johal, S. S., K. Solomon, S. Dodson, S. P. Borriello, and Y. R. Mahida. 2004. Differential effects of varying concentrations of Clostridium difficile toxin A on epithelial barrier function and expression of cytokines. J Infect. Dis. 189:2110-2119. [DOI] [PubMed] [Google Scholar]
- 24.Just, I., M. Wilm, J. Selzer, G. Rex, C. von Eichel-Streiber, M. Mann, and K. Aktories. 1995. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J. Biol. Chem. 270:13932-13936. [DOI] [PubMed] [Google Scholar]
- 25.Kamiya, S., P. J. Reed, and S. P. Borriello. 1989. Purification and characterisation of Clostridium difficile toxin A by bovine thyroglobulin affinity chromatography and dissociation in denaturing conditions with or without reduction. J. Med. Microbiol. 30:69-77. [DOI] [PubMed] [Google Scholar]
- 26.Kasianowicz, J., R. Benz, and S. McLaughlin. 1984. The kinetic mechanism by which CCCP (carbonyl cyanide m-chlorophenylhydrazone) transports protons across membranes. J. Membr. Biol. 82:179-190. [DOI] [PubMed] [Google Scholar]
- 27.Kerr, J. F., A. H. Wyllie, and A. R. Currie. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26:239-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kluck, R. M., E. Bossy-Wetzel, D. R. Green, and D. D. Newmeyer. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132-1136. [DOI] [PubMed] [Google Scholar]
- 29.Krivan, H. C., G. F. Clark, D. F. Smith, and T. D. Wilkins. 1986. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Galα1-3Galβ1-4GlcNAc. Infect. Immun. 53:573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larson, H. E., A. B. Price, P. Honour, and S. P. Borriello. 1978. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet i:1063-1066. [DOI] [PubMed] [Google Scholar]
- 31.Lazebnik, Y. A., A. Takahashi, R. D. Moir, R. D. Goldman, G. G. Poirier, S. H. Kaufmann, and W. C. Earnshaw. 1995. Studies of the lamin proteinase reveal multiple parallel biochemical pathways during apoptotic execution. Proc. Natl. Acad. Sci. USA 92:9042-9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leist, M., B. Single, A. F. Castoldi, S. Kuhnle, and P. Nicotera. 1997. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J. Exp. Med. 185:1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 34.Liu, T. S., M. W. Musch, K. Sugi, M. M. Walsh-Reitz, M. J. Ropeleski, B. A. Hendrickson, C. Pothoulakis, J. T. Lamont, and E. B. Chang. 2003. Protective role of HSP72 against Clostridium difficile toxin A-induced intestinal epithelial cell dysfunction. Am. J. Physiol. Cell Physiol. 284:C1073-C1082. [DOI] [PubMed] [Google Scholar]
- 35.Liu, X., H. Zou, C. Slaughter, and X. Wang. 1997. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89:175-184. [DOI] [PubMed] [Google Scholar]
- 36.Loontiens, F. G., P. Regenfuss, A. Zechel, L. Dumortier, and R. M. Clegg. 1990. Binding characteristics of Hoechst 33258 with calf thymus DNA, poly[d(A-T)], and d(CCGGAATTCCGG): multiple stoichiometries and determination of tight binding with a wide spectrum of site affinities. Biochemistry 29:9029-9039. [DOI] [PubMed] [Google Scholar]
- 37.Lyerly, D. M., and T. D. Wilkins. 1995. Clostridium difficile, p. 867-891. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenberg, and R. L. Geurrant (ed.), Infections of the gastrointestinal tract. Raven Press, New York, N.Y.
- 38.Mahida, Y. R., A. Galvin, S. Makh, S. Hyde, L. Sanfilippo, S. P. Borriello, and H. F. Sewell. 1998. Effect of Clostridium difficile toxin A on human colonic lamina propria cells: early loss of macrophages followed by T-cell apoptosis. Infect. Immun. 66:5462-5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahida, Y. R., S. Makh, S. Hyde, T. Gray, and S. P. Borriello. 1996. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut 38:337-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majno, G., and I. Joris. 1995. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 146:3-15. [PMC free article] [PubMed] [Google Scholar]
- 41.Martinou, J. C., and D. R. Green. 2001. Breaking the mitochondrial barrier. Nat. Rev. Mol. Cell Biol. 2:63-67. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell, T. J., J. M. Ketley, S. C. Haslam, J. Stephen, D. W. Burdon, D. C. A. Candy, and R. Daniel. 1986. Effect of toxin A and B of Clostridium difficile on rabbit ileum and colon. Gut 27:78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore, R., C. Pothoulakis, J. T. LaMont, S. Carlson, and J. L. Madara. 1990. C. difficile toxin A increases intestinal permeability and induces Cl-secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 259:G165-G172. [DOI] [PubMed] [Google Scholar]
- 44.Nicoletti, I., G. Migliorati, M. C. Pagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139:271-279. [DOI] [PubMed] [Google Scholar]
- 45.Nusrat, A., C. von Eichel-Streiber, J. R. Turner, P. Verkade, J. L. Madara, and C. A. Parkos. 2001. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect. Immun. 69:1329-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orth, K., A. M. Chinnaiyan, M. Garg, C. J. Froelich, and V. M. Dixit. 1996. The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A. J. Biol. Chem. 271:16443-16446. [PubMed] [Google Scholar]
- 47.Riegler, M., R. Sedivy, C. Pothoulakis, G. Hamilton, J. Zacherl, G. Bischof, E. Cosentini, W. Feil, R. Schiessel, J. T. LaMont, et al. 1995. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J. Clin. Investig. 95:2004-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stennicke, H. R., J. M. Jurgensmeier, H. Shin, Q. Deveraux, B. B. Wolf, X. Yang, Q. Zhou, H. M. Ellerby, L. M. Ellerby, D. Bredesen, D. R. Green, J. C. Reed, C. J. Froelich, and G. S. Salvesen. 1998. Pro-caspase-3 is a major physiologic target of caspase-8. J. Biol. Chem. 273:27084-27090. [DOI] [PubMed] [Google Scholar]
- 49.Triadafilopoulos, G., C. Pothoulakis, M. J. O'Brien, and J. T. LaMont. 1987. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology 93:273-279. [DOI] [PubMed] [Google Scholar]
- 50.Warny, M., A. C. Keates, S. Keates, I. Castagliuolo, J. K. Zacks, S. Aboudola, A. Qamar, C. Pothoulakis, J. T. LaMont, and C. P. Kelly. 2000. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J. Clin. Investig. 105:1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warny, M., and C. P. Kelly. 1999. Monocytic cell necrosis is mediated by potassium depletion and caspase-like proteases. Am. J. Physiol. 276:C717-C724. [DOI] [PubMed] [Google Scholar]
- 52.Wyllie, A. H., and P. Golstein. 2001. More than one way to go. Proc. Natl. Acad. Sci. USA 98:11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zamzami, N., and G. Kroemer. 2001. The mitochondrion in apoptosis: how Pandora's box opens. Nat. Rev. Mol. Cell Biol. 2:67-71. [DOI] [PubMed] [Google Scholar]
- 54.Zhuang, J., and G. M. Cohen. 1998. Release of mitochondrial cytochrome c is upstream of caspase activation in chemical-induced apoptosis in human monocytic tumour cells. Toxicol. Lett. 102-103:121-129. [DOI] [PubMed] [Google Scholar]