Abstract
Objective
Few prospective cohort studies with relatively large numbers of patients with non-idiopathic pulmonary fibrosis (non-IPF) of idiopathic interstitial pneumonia (IIP) have been described. We aimed to assess disease progression and cause of death for patients with non-IPF IIPs or IPF under real-life conditions.
Methods
Data were analysed for a prospective multi-institutional cohort of 528 IIP patients enrolled in Japan between September 2013 and April 2016. Diagnosis of IPF versus non-IPF IIPs was based on central multidisciplinary discussion, and follow-up surveillance was performed for up to 5 years after patient registration. Survival and acute exacerbation (AE) were assessed.
Results
IPF was the most common diagnosis (58.0%), followed by unclassifiable IIPs (35.8%) and others (6.2%). The 5-year survival rate for non-IPF IIP and IPF groups was 72.8% and 53.7%, respectively, with chronic respiratory failure being the primary cause of death in both groups. AE was the second most common cause of death for both non-IPF IIP (24.1%) and IPF (23.5%) patients. The cumulative incidence of AE did not differ significantly between the two groups (p=0.36), with a 1-year incidence rate of 7.4% and 9.0% in non-IPF IIP and IPF patients, respectively. We found that 30.2% and 39.4% of non-IPF IIP and IPF patients, respectively, who experienced AE died within 3 months after an AE event, whereas 55.8% and 66.7% of such patients, respectively, died within 5 years after registration.
Conclusion
Closer monitoring of disease progression and palliative care interventions after AE are important for non-IPF IIP patients as well as for IPF patients.
Keywords: Clinical Epidemiology, Interstitial Fibrosis
What is already known on this topic
Several registries of patients with idiopathic pulmonary fibrosis (IPF) have been established, leading to increased understanding of disease progression. In contrast, limited information is available regarding idiopathic interstitial pneumonia (IIP) not due to IPF, especially with regard to acute exacerbation (AE).
What this study adds
This registry provides valuable longitudinal real-world data that describes the disease course of patients with non-IPF IIPs in addition to those with IPF. Non-IPF IIP patients had a better prognosis than did IPF patients. However, the incidence of AE in non-IPF IIP cases was similar to that in IPF cases, and AE is an important cause of death in non-IPF IIP patients as well as in IPF patients.
How this study might affect research, practice or policy
Our findings increase knowledge regarding the disease course and long-term outcomes for patients with non-IPF IIPs compared with IPF and highlight the significance of AE as a marker of disease progression and, for many individuals, limited life expectancy.
Introduction
Idiopathic interstitial pneumonias (IIPs) are a group of diffuse parenchymal lung diseases of unknown aetiology with various degrees of inflammation and fibrosis and are classified into eight histological subtypes. Diagnosis is based on medical history, physical examination, high-resolution CT imaging, pulmonary function tests and lung biopsy.1 Prognosis differs according to disease subtype and ranges from excellent to nearly always fatal. Although the prototype of progressive interstitial lung disease (ILD) is idiopathic pulmonary fibrosis (IPF), individuals with other fibrotic ILDs including non-IPF IIPs can similarly experience a decline in lung function with progressive symptoms and poor prognosis.2
Several IPF registries have been established around the world to enhance clinical research and to facilitate real-world studies.3–7 However, few prospective cohort studies focused on patients with non-IPF IIPs have been reported, especially with regard to acute exacerbation (AE). AE has been determined as a common cause of death in individuals with IPF8 9 and has been defined in this setting by an International Working Group, whereas a standard definition has not been formally established for AE of non-IPF IIPs. Although AE of IPF has been widely studied, information regarding the incidence and risk factors for AE of non-IPF IIPs has been limited.10
Considering the lack of knowledge regarding the disease course and long-term outcome for non-IPF IIPs as compared with IPF, we aimed to explore the characteristics, causes of death, AE and risk factors for mortality in individuals with both non-IPF IIPs and IPF followed up in the same multi-institutional registry.
Patients and methods
Study design
This prospective, multicentre observational study was performed at 29 general hospitals in the Fukuoka Tobacco-Related Lung Disease registry study group, Fukuoka, Japan.
Study sample
The main inclusion criteria were age >20 years and a diagnosis of IIPs or chronic obstructive lung disease (COPD).11 The diagnosis of IIPs was based on the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association (ATS/ERS/JRS/ALAT) IPF 2011 guidelines and the ATS/ERS IIPs 2013 guidelines.1 12 Diagnosis of COPD was based on the international criteria.13 The patients (regardless of whether newly or previously diagnosed) were sequentially enrolled after a diagnosis of IIP or COPD was made by respiratory specialists at the participating facilities. Total planned enrolment number was set at 1000 without statistical sample size calculation.
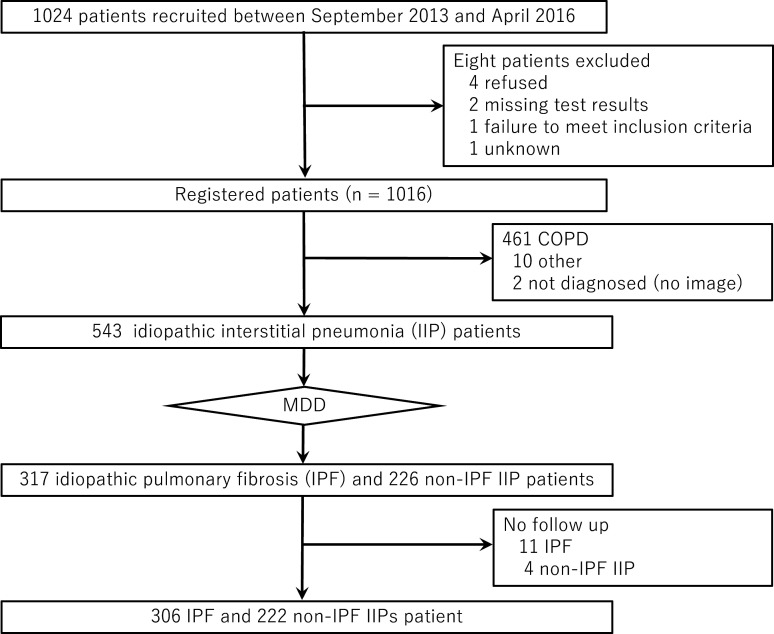
Overall, 1024 patients were recruited between 1 September 2013 and 30 April 2016, with 8 of these individuals being subsequently excluded owing to refusal (n=4), missing test results (n=2), failure to meet the inclusion criteria (n=1) or unknown reasons (n=1). The remaining 1016 patients were sequentially enrolled after a diagnosis of IIPs or COPD. All diagnoses of the enrolled patients were reevaluated by a central diagnosis committee comprising pulmonologists, subspecialist radiologists and histopathologists. A total of 543 patients was diagnosed with IIPs and 461 with COPD (figure 1). IPF and non-IPF IIPs were classified according to the ATS/ERS/JRS/ALAT guidelines after multidisciplinary discussion (MDD).1 12
Figure 1.
Flow diagram for the study. COPD, chronic obstructive lung disease; MDD, multidisciplinary discussion.
Follow-up surveillance was conducted annually for 5 years to evaluate disease progression, AE and death. Data on treatment, physical examinations, clinical assessments, pulmonary function tests and chest CT were collected annually. All examinations and investigations were performed as part of the routine care of each patient at the physician’s discretion and no additional visits or investigations were mandated for this study. A survival survey was conducted with a 5-year longitudinal follow-up.
AE of IIPs was defined when all of the following were present during the course of IIPs within 1 month after the exclusion of alternative diagnoses, including pulmonary infection, pneumothorax, malignancy, pulmonary embolus or heart failure: (1) worsening of dyspnoea, (2) new bilateral ground-glass opacities and/or consolidation superimposed on a background reticular or honeycomb pattern and (3) decreased partial pressure of oxygen in arterial blood (PaO2 >10 mm Hg).14 Patients with AE were identified by respiratory specialists stationed at each participating facility, using defined diagnostic criteria.
Patient involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Statistical analysis
The cumulative proportion of survival and cumulative incidence of exacerbation were estimated by the Kaplan-Meier method. HRs were estimated with a Cox regression model for evaluation of the association of background characteristics with survival or exacerbation. For selection of the model to predict survival or exacerbation, the backward stepwise method was adopted, with the significance level for removal set at 0.05. A two-tailed p value of <0.05 was considered statistically significant. All statistical analysis was performed with Stata software (V.17.0; Stata, College Station, TX, USA). See the online supplemental file 1 for full method.
bmjresp-2023-001864supp001.pdf (261KB, pdf)
Results
Study subjects
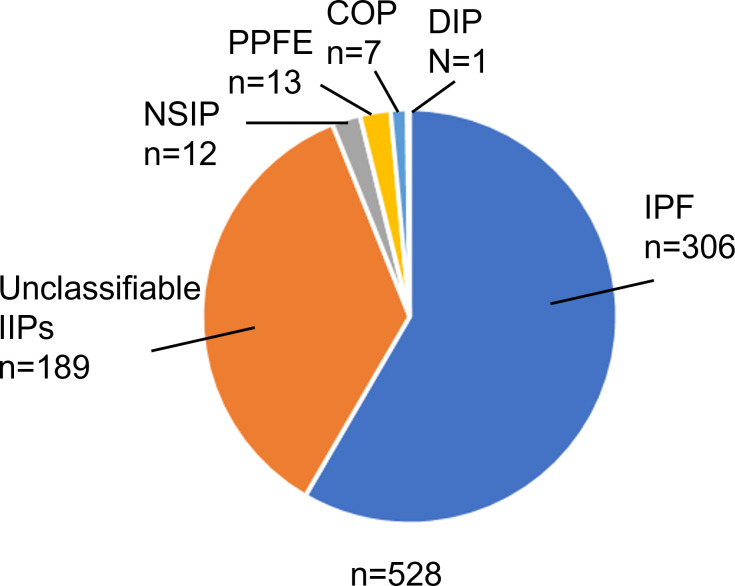
Between September 2013 and April 2016, a total of 543 patients was diagnosed with IIPs. After MDD, 317 patients were diagnosed with IPF and 226 patients with non-IPF IIPs. Eleven IPF patients and four non-IPF IIP patients were then excluded because of lack of follow-up, leaving a total of 306 patients with IPF and 222 patients with non-IPF IIPs for evaluation in the present study (figure 1). Among these IIP patients, IPF was the most common diagnosis (58.0%), followed by unclassifiable IIPs (35.8%), pleuroparenchymal fibroelastosis (2.4%), non-specific interstitial pneumonia (NSIP, 2.3%), cryptogenic organising pneumonia (1.3%) and desquamative interstitial pneumonia (0.2%) (figure 2).
Figure 2.
Diagnosis for the study patients after multidisciplinary discussion. COP, cryptogenic organising pneumonia; DIP, desquamative interstitial pneumonia; IIPs, idiopathic interstitial pneumonias; IPF, idiopathic pulmonary fibrosis; NSIP, non-specific interstitial pneumonia; PPFE, pleuroparenchymal fibroelastosis.
Baseline characteristics
The clinical characteristics and laboratory test results of patients with IPF or with non-IPF IIPs are shown in table 1. The mean±SD age was 72.7±7.2 years for IPF patients and 71.2±9.2 years for non-IPF IIP patients, and males predominated in both groups, although the proportion of females differed significantly between the two groups (21.6% vs 32.4%, respectively, p<0.01). The proportion of patients with a history of smoking was significantly greater for the IPF group than for the non-IPF IIP group (75.2% vs 63.5%, p<0.01). With regard to the distribution of modified Medical Research Council (mMRC) scores, 58.3% of IPF patients and 67.5% of non-IPF IIP patients showed a mild degree of dyspnoea as reflected by a score of 0 or 1. Fine crackles and finger clubbing were more common in IPF patients than in non-IPF IIP patients (p<0.001 for each). The mean±SD forced vital capacity (FVC) as a percentage of the predicted value was 83.6±20.8 in the IPF group and 82.9±19.9 in the non-IPF IIP group, whereas the mean±SD diffusion capacity of carbon monoxide as a percentage of the predicted value was 62.3±26.2 and 69.0±22.1, respectively. Laboratory test results revealed increased serum levels of Krebs von den Lungen-6 (KL-6) as well as of pulmonary surfactant protein (SP)-A and SP-D in both groups.
Table 1.
Baseline characteristics of patients with idiopathic pulmonary fibrosis (IPF) or non-IPF idiopathic interstitial pneumonias (IIPs) at registration
| Characteristic | IPF (n=306) | Non-IPF IIPs (n=222) | P value |
| Sex, n (%) | <0.01 | ||
| Male | 240 (78.4) | 150 (67.6) | |
| Female | 66 (21.6) | 72 (32.4) | |
| Age (years), mean±SD | 72.7±7.2 | 71.2±9.2 | 0.09 |
| <65, n (%) | 43 (14.1) | 49 (22.1) | |
| 65–74, n (%) | 136 (44.4) | 85 (38.3) | |
| >74, n (%) | 127 (41.5) | 88 (39.6) | |
| Smoking status, n (%) | <0.01 | ||
| Never | 76 (24.8) | 81 (36.5) | |
| Current or former | 230 (75.2) | 141 (63.5) | |
| FIP, n (%) | 1.00 | ||
| Yes | 16 (5.2) | 12 (5.4) | |
| No | 290 (94.8) | 210 (94.6) | |
| BMI (kg/m2), mean±SD | 23.2±3.3 | 23.1±3.5 | 0.71 |
| mMRC score, n (%) | 0.08 | ||
| 0 | 39 (17.9) | 23 (14.6) | |
| 1 | 88 (40.4) | 83 (52.9) | |
| 2 | 55 (25.2) | 27 (17.2) | |
| 3 | 24 (11.0) | 20 (12.7) | |
| 4 | 12 (5.5) | 4 (2.6) | |
| Fine crackles, n (%) | <0.001 | ||
| Yes | 265 (86.6) | 129 (58.1) | |
| No | 41 (13.4) | 93 (41.9) | |
| Clubbed fingers, n (%) | <0.001 | ||
| Yes | 66 (21.6) | 23 (10.4) | |
| No | 240 (78.4) | 199 (89.6) | |
| SpO2 (%) at rest, mean±SD | 96.3±1.8 | 96.6±1.8 | 0.013 |
| FVC (% predicted), mean±SD | 83.6±20.8 | 82.9±19.9 | 0.76 |
| DLco (% predicted), mean±SD | 62.3±26.2 | 69.0±22.1 | 0.06 |
| KL-6 (U/mL), median (IQR) | 759.0 (500.0–1162.0) | 695.5 (416.0–1129.0) | 0.09 |
| SP-A (ng/mL), median (IQR) | 60.5 (46.4–84.9) | 52.8 (39.5–84.8) | 0.18 |
| SP-D (ng/mL), median (IQR) | 186.0 (118.8–323.0) | 179.5 (115.5–278.0) | 0.70 |
| WBC (×109/L), median (IQR) | 6.400 (5.310–7.915) | 6.685 (5.500–8.250) | 0.11 |
| LDH (U/L), median (IQR) | 211.0 (187.5–240.0) | 208.5 (188.0–236.0) | 1.00 |
| Albumin (g/dL), median (IQR) | 4.000 (3.800–4.260) | 4.100 (3.900–4.300) | 0.016 |
| CRP (mg/dL), median (IQR) | 0.149 (0.070–0.335) | 0.130 (0.060–0.340) | 0.44 |
Numbers of patients for continuous parameters (IPF and non-IPF IIPs, respectively) were as follows: BMI (306 and 222), SpO2 (300 and 216), FVC (304 and 221), DLco (131 and 78), KL-6 (303 and 222), SP-A (86 and 65), SP-D (121 and 76), WBC (304 and 222), LDH (304 and 222), albumin (301 and 222) and CRP (304 and 222). The p values were determined with Fisher’s exact test or the χ2 test for categorical variables, and with Wilcoxon rank test in the case of continuous variables.
BMI, body mass index; CRP, C reactive protein; DLco, diffusion capacity of carbon monoxide; FIP, familial interstitial pneumonia; FVC, forced vital capacity; KL-6, Krebs von den Lungen-6; LDH, lactate dehydrogenase; mMRC, modified Medical Research Council; SP-A, surfactant protein-A; SP-D, surfactant protein-D; SpO2, percutaneous oxygen saturation; WBC, white cell count.
Survival time and outcomes of IPF and non-IPF IIP patients
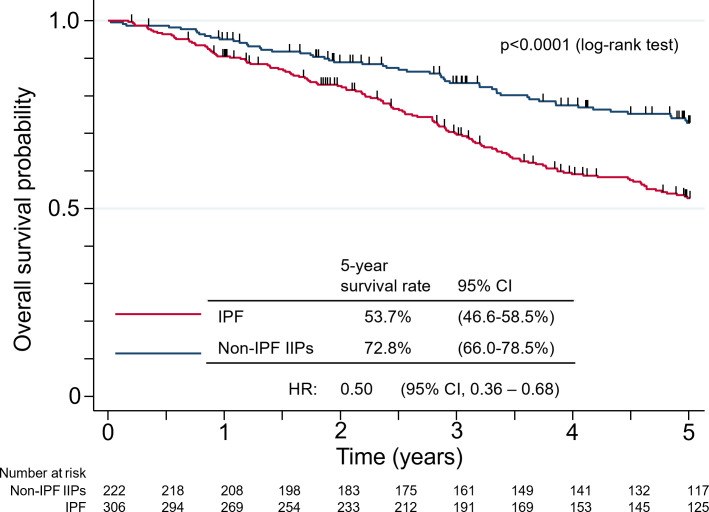
Of the 306 IPF patients, 125 patients had completed the 5-year follow-up and 132 had died over 1071.4 total person-years, whereas 117 patients of the 222 non-IPF IIP patients had completed 5 years of follow-up and 54 patients had died over 870.1 person-years (online supplemental figure S1). The 5-year survival rate was 53.7% (95% CI 46.6% to 58.5%) in the IPF group and 72.8% (95% CI 66.0% to 78.5%) in the non-IPF IIP group. Non-IPF IIP patients had a significantly longer overall survival compared with IPF patients (HR of 0.50 with a 95% CI 0.36 to 0.68, p<0.0001) (figure 3).
Figure 3.
Kaplan-Meier plots of survival probability from the time of registration for patients with idiopathic pulmonary fibrosis (IPF) or non-IPF idiopathic interstitial pneumonias (IIPs). The number of deaths was 132 for IPF and 54 for non-IPF IIPs, and the number of person-years was 1071.4 and 870.1, respectively.
bmjresp-2023-001864supp002.pdf (393.4KB, pdf)
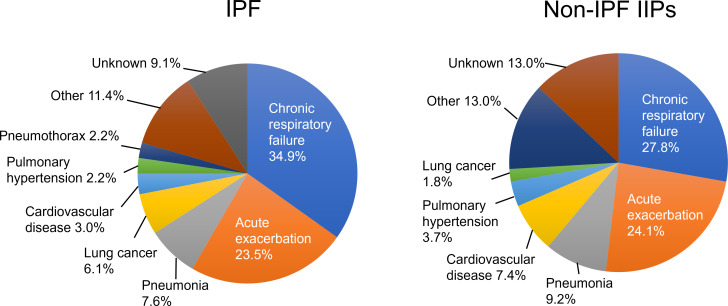
The most common reason for death in both groups was chronic respiratory failure due to IIP (IPF, 34.9%; non-IPF, 27.8%) (figure 4). Death due to AE was defined as death within 3 months after AE. The proportion of mortalities attributed to AE in cases of IPF and non-IPF IIP was 23.5% and 24.1%, respectively. Pneumonia was the cause of death in 8.3% of IPF patients and 9.2% of non-IPF IIP patients. Deaths from lung cancer were more frequent in the IPF group than in the non-IPF IIP group (6.1% vs 1.8%), whereas those from cardiovascular disease were more common in the non-IPF IIP group than in the IPF group (7.4% vs 3.0%). Pulmonary hypertension accounted for 2.2% of deaths in IPF patients and 3.7% of those in non-IPF IIP patients.
Figure 4.
Causes of death in patients with idiopathic pulmonary fibrosis (IPF) or non-IPF idiopathic interstitial pneumonias (IIPs).
Predictors of mortality in IPF and non-IPF IIP patients
The predictors of mortality in IPF and non-IPF IIP patients were examined by simple Cox regression analysis of baseline factors (online supplemental table S1). In multivariate Cox regression analysis (stepwise variable selection), a lower FVC (% predicted), higher mMRC score, higher serum lactate dehydrogenase level, lower body mass index and lower serum albumin concentration were independently associated with mortality of IPF patients (table 2). An older age, lower FVC (% predicted), higher serum KL-6 and C reactive protein (CRP) levels, and higher mMRC score were significant predictors of poor prognosis in non-IPF IIP patients (table 2).
Table 2.
Cox regression analysis for mortality of patients with idiopathic pulmonary fibrosis (IPF) or non-IPF idiopathic interstitial pneumonias (IIPs) according to baseline characteristics after stepwise selection
| Characteristic | HR | 95% CI | P value |
| IPF | |||
| FVC | 1.33 | 1.20 to 1.48 | <0.0001 |
| LDH | 1.84 | 1.21 to 2.80 | 0.004 |
| BMI | 0.93 | 0.89 to 0.99 | 0.015 |
| mMRC score | 1.46 | 1.23 to 1.74 | <0.0001 |
| Albumin | 0.61 | 0.40 to 0.94 | 0.025 |
| Non-IPF IIPs | |||
| Age | 1.10 | 1.06 to 1.14 | <0.0001 |
| FVC | 1.24 | 1.06 to 1.45 | 0.008 |
| log10(KL-6) | 3.30 | 1.36 to 7.98 | 0.008 |
| mMRC score | 1.56 | 1.19 to 2.06 | 0.002 |
| log10(CRP) | 1.71 | 1.05 to 2.79 | 0.031 |
BMI, body mass index; CRP, C reactive protein; FVC, forced vital capacity; KL-6, Krebs von den Lungen-6; LDH, lactate dehydrogenase; mMRC, modified Medical Research Council.
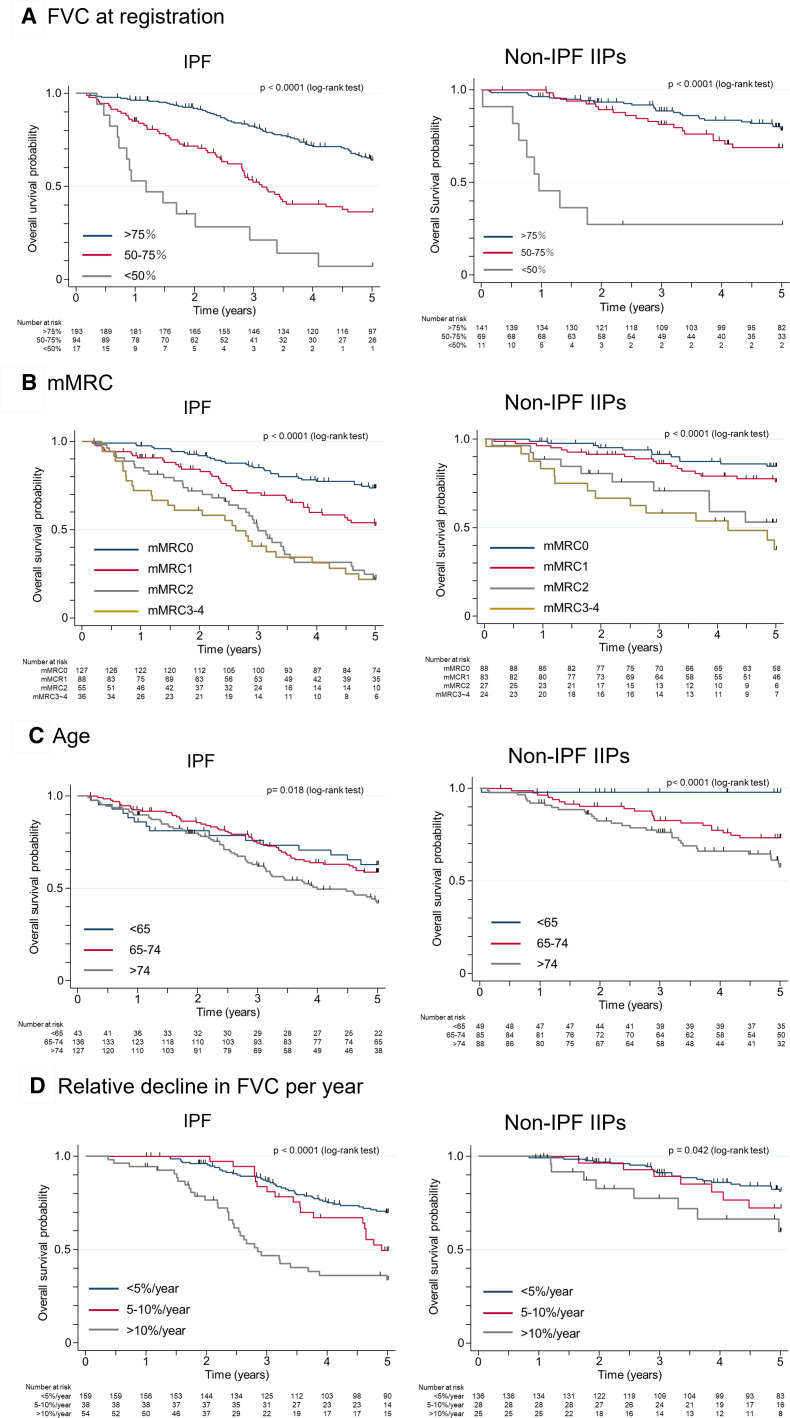
Kaplan-Meier survival curves for subgroup analysed retrospectively according to the representative independent predictors of mortality revealed by multivariate Cox regression analysis are shown in figure 5. The patients were divided into three groups on the basis of FVC (% predicted) at baseline, with the cumulative survival of IPF or non-IPF IIP patients with an FVC of <50% being significantly worse than that of corresponding patients with an FVC of 50%–75% or of >75% (p<0.0001) (figure 5A). The mMRC score was significantly associated with survival in both IPF and non-IPF IIP groups (figure 5B). An age of >74 years (figure 5C) were also significantly associated with mortality in both groups. The annual relative decline rate was determined by comparing the FVC (% predicted) values at baseline and 1 year later. Three hundred and four IPF patients (99%) and 221 non-IPF patients (99.5%) had their FVC measured at enrolment, whereas 251 IPF patients (82%) and 189 non-IPF patients (85%) had their FVC measured at the 1-year follow-up. Patients with an annual relative decline in FVC (% predicted) of >10% had a shorter survival compared with those with annual declines of 5%–10% or of <5% in both IPF (p<0.0001) and non-IPF IIP (p=0.042) groups (figure 5D).
Figure 5.
Kaplan-Meier plots of survival probability according to risk factors for mortality in patients with idiopathic pulmonary fibrosis (IPF) or non-IPF idiopathic interstitial pneumonias (IIPs). (A) Forced vital capacity (FVC, % predicted) at registration. (B) Modified Medical Research Council (mMRC) score at registration. (C) Age at registration. (D) Relative decline in FVC (% predicted) per year.
Incidence and predictors of AE in IPF and non-IPF IIP patients
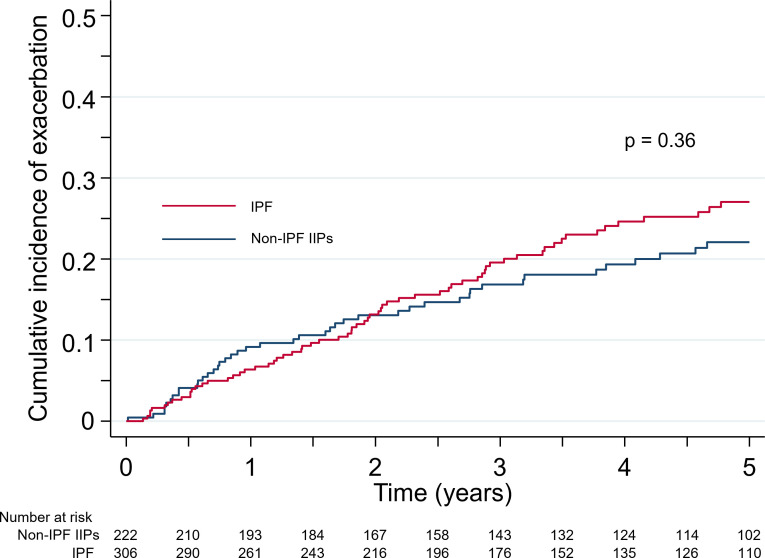
AE occurred in 66 (21.6%) of the 306 IPF patients and 43 (19.4%) of the 222 non-IPF IIP patients. The clinical characteristics and laboratory test results of patients with or without AE in both groups are shown in online supplemental table S2. Twenty-two (33.3%) and 12 (27.9%) of the patients with AE in the IPF and non-IPF IIP groups, respectively, experienced multiple episodes of AE (range, 2–5 episodes). The 1-year incidence rate of AE in IPF and non-IPF IIP patients was 9.0% (95% CI 7.3% to 10.9%) and 7.4% (95% CI 5.7% to 9.4%), respectively. There was no significant difference in the cumulative incidence rates of first-time AE between the two groups (p=0.36) (figure 6). Of the total of 66 patients who experienced AE in the IPF group, 26 (39.4%) individuals died within a period of 3 months after an AE event and 44 (66.7%) individuals died from any cause within 5 years. Similarly, among the 43 non-IPF IIP patients who experienced AE, the mortality rate within 3 months after an AE event was 30.2% and the 5-year mortality rate was 55.8%.
Figure 6.
Kaplan-Meier plots for the cumulative incidence of acute exacerbation in patients with idiopathic pulmonary fibrosis (IPF) or non-IPF idiopathic interstitial pneumonias (IIPs).
The predictors of the incidence of AE in IPF and non-IPF IIP patients as determined by simple Cox regression analysis of baseline factors are shown in online supplemental table S3. Multivariate Cox regression analysis revealed that, after stepwise selection, a low FVC (% predicted) and a high mMRC score were independently associated with the risk of AE in the IPF group, whereas a high mMRC score and a high serum CRP concentration were significant risk factors for AE in the non-IPF IIP group (table 3).
Table 3.
Cox regression analysis for the incidence of acute exacerbation in idiopathic pulmonary fibrosis (IPF) and non-IPF idiopathic interstitial pneumonia (IIP) patients according to baseline characteristics after stepwise selection
| Characteristic | HR | 95% CI | P value |
| IPF | |||
| FVC | 1.23 | 1.07 to 1.42 | 0.005 |
| mMRC score | 1.56 | 1.22 to 2.00 | <0.0001 |
| Non-IPF IIPs | |||
| mMRC score | 2.04 | 1.54 to 2.71 | <0.0001 |
| log10(CRP) | 1.88 | 1.10 to 3.20 | 0.021 |
CRP, C reactive protein; FVC, forced vital capacity; mMRC, modified Medical Research Council.
Discussion
We have performed a prospective cohort study to examine the disease course of non-IPF IIP patients together with IPF patients. Whereas several registries for IPF have been established worldwide,3–7 15 information regarding non-IPF IIPs, especially AE is limited. Our longitudinal follow-up study allowed accurate estimation of mortality, revealing that 72.8% of non-IPF IIP patients survived up to 5 years after registration, compared with 53.7% of IPF patients. Although non-IPF IIP patients had a better prognosis than did IPF patients, there was no significant difference in the incidence of AE between the two patient groups. AE was the second most common cause of death in non-IPF IIP patients as well as in IPF patients.
Non-IPF IIPs include several disease subtypes.1 If a specific diagnosis cannot be defined, even after thorough MDD, the disease is determined to be unclassifiable IIP. In the present study, 85.1% of non-IPF IIP patients were diagnosed with unclassifiable IIPs and 5.4% with idiopathic NSIP after MDD. Surgical lung biopsy was performed in only 41 (7.8%) of all 528 patients, accounting for the low proportion of cases diagnosed with idiopathic NSIP. The relatively old age of the patients in our registry and the unavailability of cryobiopsy in Japan during the observation period contributed to the low rate of histological assessment.
The incidence of AE in patients with non-IPF ILD has been found to be lower than that in IPF patients.16 17 However, there have been no prospective studies that focused on the incidence and prognosis of AE in patients with non-IPF IIPs and IPF followed in the same multi-institutional registry. The present registry revealed no difference in the cumulative incidence of AE between non-IPF IIP and IPF groups. We found that about one-third of non-IPF IIP and IPF patients who experienced AE died within 3 months after an AE event, whereas more than half of these patients died within 5 years after registration. Non-IPF IIP patients with AE had a shorter overall survival than IPF patients without AE (online supplemental figure S2). These data indicate that AE is an important prognostic event, and that closer monitoring and palliative care interventions after AE are crucial for patients with non-IPF IIPs as well as for those with IPF.
bmjresp-2023-001864supp003.pdf (342.9KB, pdf)
Multivariate Cox proportional analysis (table 3) as well as Kaplan-Meier analysis (online supplemental figure S3) showed that a high mMRC score at baseline was independently associated with the incidence of AE in both groups, indicating that baseline disease severity contributed to AE incidence in patients with non-IPF IIPs as well as in those with IPF. Consistent with our findings, increased dyspnoea as measured by the mMRC score or St. George’s Respiratory Questionnaire has been implicated as a risk factor for IPF exacerbation.18 19 We chose the mMRC score with its five grades of dyspnoea because of its simplicity. Our data revealed that dyspnoea in daily life, as assessed on the basis of the mMRC score at baseline, is an independent risk factor for AE in individuals with non-IPF IIPs as well as in those with IPF. Furthermore, our multivariate Cox proportional analysis of mortality revealed that a high mMRC score at registration was significantly associated with an unfavourable prognosis in non-IPF IIP patients as well as in IPF patients. Although an association of the mMRC score with survival in IPF patients has previously been described,20 21 our study revealed that the extent of dyspnoea was also an independent predictor of mortality in non-IPF IIP patients. In contrast to pulmonary function tests, the mMRC score is simple and convenient and can be determined quickly at any time. Our data suggest that a simple evaluation of dyspnoea grade can be an important tool for prediction of disease course including the incidence of AEs in patients with IIPs.
bmjresp-2023-001864supp004.pdf (339.5KB, pdf)
A progressive decline in lung function is accompanied by worsening of symptoms and is associated with an unfavourable prognosis in fibrotic ILDs.2 22 23 In the present study, we divided IIP patients into three groups on the basis of the relative decline in FVC (% predicted) per year (>10%, 5–10% or <5%). The proportion of patients who showed progressive ILD (annual FVC decline of >10%) was 21.5% for the IPF group and 13.2% for the non-IPF IIP group. Patients with an annual decline of >10% showed a significantly worse survival in both IPF and non-IPF IIP groups, indicating that an annual decline in FVC of >10% is a predictive factor for mortality in non-IPF IIP patients as well as in IPF patients. The 5-year survival rate of patients showing an annual FVC decline of >10% was 59.8% and 33.9% for non-IPF IIP and IPF groups, respectively. These results thus indicate that, despite a similar reduction in pulmonary function, disease development differs between the two groups, suggesting that divergence of pathogenesis between non-IPF IIPs and IPF likely contributes to the difference in 5-year survival rates.
In studies from 1998 to 2005, the 5-year survival rate of IPF patients ranged between 20% and 40%.24 The 5-year survival rate of IPF patients in our registry was 53.7%, consistent with recent data from other registries (45% for EMPIRE, 52% for Swedish IPF and 45% for Finnish IPF).4 7 25 In our registry, the mean FVC (% predicted) for IPF patients was >80%, and ~60% of IPF patients were asymptomatic or had mild symptoms as assessed by the mMRC score, suggesting that earlier diagnosis and increased recognition of IPF likely contributed to the favourable prognosis of individuals with this disease. A recent systematic review and meta-analysis showed that antifibrotic treatment also reduced the risk of death in IPF patients.26 In the present study, 100 (32.7%) IPF patients were treated with antifibrotic drugs (pirfenidone, n=64; nintedanib, n=25; pirfenidone and nintedanib, n=11), which is a lower percentage than in European countries.27 The proportion of IPF patients aged >74 years was 41.5%, with older patients in Japan tending not to receive antifibrotic drugs because of concerns about age-related side effects. In contrast to previous studies,26 the IPF patients treated with antifibrotic drugs in our study had a shorter overall survival than those not treated with these drugs (online supplemental figure S4). For 54.0% of the IPF patients treated with antifibrotic drugs in our registry, the treatment was initiated during the follow-up period because of disease progression. It is therefore possible that IPF patients with rapid progression were over-represented in the group treated with antifibrotic drugs.
bmjresp-2023-001864supp005.pdf (286.3KB, pdf)
This study has some limitations. First, the non-IPF IIP group is a heterogeneous population including various types of IIPs. Some patients with unclassifiable IIP may have been sebseqently diagnosed with IPF. We did not gather information regarding changes in the diagnoses. Second, we did not have information on the date of IIPs diagnosis. Both prevalent and incident cases were enrolled, which may have affected survival time analysis. Third, we were not able to enrol all IIP patients in the registry because individuals were included only if they provided informed consent. It is possible that patients with the most aggressive forms of disease are missing from the registry. Fourth, certain patients who experienced AE might have been unable to visit the hospitals included in the data collection due to acute respiratory failure and consequently received treatment at alternative healthcare facilities. Finally, despite the execution of annual protocolised assessments as a component of routine medical care, categorical data were missing.
In conclusion, we here report a prospective multicentre IIP registry with outcome follow-up extending up to 5 years. This registry provides valuable longitudinal real-world data that describe the disease course of patients with non-IPF IIPs in addition to those with IPF. Our study showed that the incidence of AE in non-IPF IIP cases was similar to that in IPF cases, and that AE is an important cause of death in non-IPF IIP patients as well as in IPF patients. Our results highlight the importance of prospective real-world data for improvement of IIP management.
Acknowledgments
The authors thank all the patients and their proxies who participated in this study. The authors are also grateful to Takatoshi Aoki (Department of Radiology, University of Occupational and Environmental Health, Japan, Kitakyushu, Japan), Kiminori Fujimoto (Department of Radiology, Kurume University School of Medicine, Kurume, Japan.), Mikiko Hashisako (Department of Anatomic Pathology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan) and Junya Fukuoka (Department of Pathology Informatics, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan) for helping with the diagnosis as members of central multidisciplinary discussion. The authors thank Clinical Research Support Center Kyushu for their official work regarding this study.
Footnotes
Contributors: KTsubouchi and IO contributed to the literature search, figures, study design, data collection, data analysis, data interpretation, and writing and approved the final version of the review. NH and YN contributed to the literature search, study design, data collection, and data interpretation and approved the final version of the review. STokunaga contributed to the literature search, figures, data analysis, and writing and approved the final version of the review. KI, STakata, HI, YK, MO, SK, KY, MKawasakii, MF, MY, TM, TH, HW, RT, MKomori, YM, KT and EH contributed to the literature search, and data collection, and data interpretation and approved the final version of the review. HY contributed to the literature search, data analysis, and writing and approved the final version of the review. IO accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This study was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology: the broad-area, network-based project to drive clinical research at Kyushu University Hospital, a grant from by Boehringer Ingelheim, and a grant to the Diffuse Lung Diseases Research Group from the Ministry of Health, Labor and Welfare, Japan.
Competing interests: Yes.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and this prospective, multicentre observational study was approved by the Institutional Review Board of Kyushu University (#25-135, 23 August 2013; #555-00, 27 August 2013) as well as by the institutional review boards of all participating hospitals. Participants gave informed consent to participate in the study before taking part.
References
- 1.Travis WD, Costabel U, Hansell DM, et al. An official American thoracic society/European respiratory society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022;205:e18–47. 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guenther A, Krauss E, Tello S, et al. The European IPF Registry (eurIPFreg): baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir Res 2018;19:141. 10.1186/s12931-018-0845-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao J, Kalafatis D, Carlson L, et al. Baseline characteristics and survival of patients of idiopathic pulmonary fibrosis: a longitudinal analysis of the swedish IPF registry. Respir Res 2021;22:40. 10.1186/s12931-021-01634-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behr J, Prasse A, Wirtz H, et al. Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: long-term results of the INSIGHTS-IPF Registry. Eur Respir J 2020;56:1902279. 10.1183/13993003.02279-2019 [DOI] [PubMed] [Google Scholar]
- 6.Jo HE, Glaspole I, Grainge C, et al. Baseline characteristics of idiopathic pulmonary fibrosis: analysis from the Australian idiopathic pulmonary fibrosis Registry. Eur Respir J 2017;49:1601592. 10.1183/13993003.01592-2016 [DOI] [PubMed] [Google Scholar]
- 7.Kaunisto J, Salomaa E-R, Hodgson U, et al. Demographics and survival of patients with idiopathic pulmonary fibrosis in the finnishIPF Registry. ERJ Open Res 2019;5:00170-2018. 10.1183/23120541.00170-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 2014;190:773–9. 10.1164/rccm.201403-0566OC [DOI] [PubMed] [Google Scholar]
- 9.Jeon K, Chung MP, Lee KS, et al. Prognostic factors and causes of death in Korean patients with idiopathic pulmonary fibrosis. Respir Med 2006;100:451–7. 10.1016/j.rmed.2005.06.013 [DOI] [PubMed] [Google Scholar]
- 10.Kolb M, Bondue B, Pesci A, et al. Acute exacerbations of progressive-Fibrosing interstitial lung diseases. Eur Respir Rev 2018;27:180071. 10.1183/16000617.0071-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogata-Suetsugu S, Hamada N, Tsuda T, et al. Characteristics of tobacco-related lung diseases in fukuoka Prefecture, Japan: a prospective, multi-institutional, observational study. Respir Investig 2020;58:74–80. 10.1016/j.resinv.2019.10.002 [DOI] [PubMed] [Google Scholar]
- 12.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Am J Respir Crit Care Med 2017;195:557–82. 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 14.Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:636–43. 10.1164/rccm.200703-463PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher JH, Kolb M, Algamdi M, et al. Baseline characteristics and comorbidities in the canadian registry for pulmonary fibrosis. BMC Pulm Med 2019;19:223. 10.1186/s12890-019-0986-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kershaw CD, Batra K, Torrealba JR, et al. Characteristics and evaluation of acute exacerbations in chronic interstitial lung diseases. Respir Med 2021;183. 10.1016/j.rmed.2021.106400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faverio P, Stainer A, Conti S, et al. Differences between acute exacerbations of idiopathic pulmonary fibrosis and other interstitial lung diseases. Diagnostics (Basel) 2021;11:1623. 10.3390/diagnostics11091623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondoh Y, Taniguchi H, Katsuta T, et al. Risk factors for acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2010;27:103–10. [PubMed] [Google Scholar]
- 19.Collard HR, Yow E, Richeldi L, et al. Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir Res 2013;14:73. 10.1186/1465-9921-14-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishiyama O, Taniguchi H, Kondoh Y, et al. A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur Respir J 2010;36:1067–72. 10.1183/09031936.00152609 [DOI] [PubMed] [Google Scholar]
- 21.Zappala CJ, Latsi PI, Nicholson AG, et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J 2010;35:830–6. 10.1183/09031936.00155108 [DOI] [PubMed] [Google Scholar]
- 22.Olson A, Hartmann N, Patnaik P, et al. Estimation of the prevalence of progressive fibrosing interstitial lung diseases: systematic literature review and data from a physician survey. Adv Ther 2021;38:854–67. 10.1007/s12325-020-01578-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown KK, Schlenker-Herceg R, Wells AU. Reply to comment on the natural history of progressive fibrosing interstitial lung diseases Eur Respir J 2020;56. 10.1183/13993003.03967-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim DS, Collard HR, King TE. Classification and natural history of the idiopathic interstitial Pneumonias. Proc Am Thorac Soc 2006;3:285–92. 10.1513/pats.200601-005TK [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran T, Šterclová M, Mogulkoc N, et al. The European Multipartner IPF Registry (EMPIRE): validating long-term prognostic factors in idiopathic pulmonary fibrosis. Respir Res 2020;21:11. 10.1186/s12931-019-1271-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petnak T, Lertjitbanjong P, Thongprayoon C, et al. Impact of antifibrotic therapy on mortality and acute exacerbation in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Chest 2021;160:1751–63. 10.1016/j.chest.2021.06.049 [DOI] [PubMed] [Google Scholar]
- 27.Maher TM, Molina-Molina M, Russell A-M, et al. Unmet needs in the treatment of idiopathic pulmonary fibrosis-insights from patient chart review in five European countries. BMC Pulm Med 2017;17:124. 10.1186/s12890-017-0468-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2023-001864supp001.pdf (261KB, pdf)
bmjresp-2023-001864supp002.pdf (393.4KB, pdf)
bmjresp-2023-001864supp003.pdf (342.9KB, pdf)
bmjresp-2023-001864supp004.pdf (339.5KB, pdf)
bmjresp-2023-001864supp005.pdf (286.3KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available.