Abstract
We have previously correlated Chlamydia trachomatis antiapoptotic activity with the blockade of mitochondrial cytochrome c release and the inhibition of Bax and Bak activation. We now report that C. trachomatis infection leads to degradation of Bik, Puma, and Bim, three upstream proapoptotic BH3-only proteins of the Bcl-2 family that can transmit death signals to mitochondria by inhibiting the Bcl-2 antiapoptotic proteins and/or activating the Bcl-2 proapoptotic members, such as Bax and Bak. This observation has provided new information on the chlamydial antiapoptosis mechanisms.
Chlamydia trachomatis infects both the eyes, leading to blindness in patients in developing countries, and the urogenital tract, leading to sexually transmitted diseases worldwide. However, it is still not entirely clear how C. trachomatis infection causes disease. It is hypothesized that the chlamydia-induced pathologies in humans are due mainly to the inflammatory responses triggered by chlamydia-infected cells (13). The question is why the chlamydia-infected cells are not eliminated in immunocompetent individuals. Studies in the past 5 years have revealed that C. trachomatis has evolved various strategies for protecting the infected cells from host immune recognition (18-20) and effector mechanisms (3, 5-7). For example, it has been shown that C. trachomatis can inhibit host cell apoptosis programs (3, 5, 6), which may benefit long-term survival of chlamydia in the infected host. We have recently correlated the chlamydial antiapoptotic activity with the inhibition of Bax and Bak activation in chlamydia-infected cells (17). Bax and Bak proteins are proapoptotic Bcl-2 family members with multiple Bcl-2 homology (BH) domains (14), and the activation of Bax or Bak can cause mitochondrial cytochrome c release and apoptosis (8-10, 16). A prototype of Bcl-2 protein has four BH domains, including BH4, BH3, BH1, and BH2, from the N terminus to the C terminus. The several dozens of Bcl-2 family members that share at least one common BH domain can be categorized into three subfamilies based on their functional and structural features (11, 14). The antiapoptotic Bcl-2 subfamily contains members such as Bcl-2 and Bcl-xL. The proapoptotic Bcl-2 multidomain subfamily members, including Bax and Bak, share more than one BH domain. The proapoptotic Bcl-2 BH3-only subfamily members, including Bid, Bad, Bik, Puma, Bim, Bmf, Noxa, and Hrk, share only the common BH3 domain (2). How these Bcl-2 family members interact with each other to regulate mitochondrial cytochrome c release is not entirely clear. It is thought that the BH3-only domain proteins that are normally associated with intracellular structures can sense cell death signals from either extrinsic (e.g., Bid) or intrinsic (e.g., Bim, Bmf, Puma, and Bik) sources by undergoing transcriptional and/or posttranslational changes and translocating to mitochondria (2, 14). These BH3-only domain proteins can transmit the death signals to mitochondria by either inhibiting the Bcl-2 antiapoptotic members or activating the proapoptotic Bcl-2 multidomain members, such as Bax and Bak (2). To determine whether chlamydial infection interrupts host cell apoptosis pathways upstream of Bax and Bak, we evaluated the effects of chlamydial infection on protein levels of various proapoptotic BH3-only proteins.
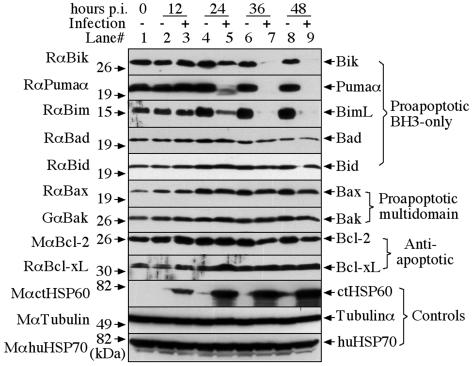
We used Western blotting to monitor the protein levels of nine different Bcl-2 family members, representing all three Bcl-2 subfamilies in HeLa cells, with or without C. trachomatis serovar L2 infection at a multiplicity of infection (MOI) of 5 (Fig. 1). At various times after infection, the cell samples were harvested via trypsinization and the cell pellets were solubilized in a sodium dodecyl sulfate sample buffer (with a brief sonication to break down genome DNA). The parallel HeLa cell cultures without infection were harvested and analyzed similarly along with the infected samples. The following antibodies were used for the Western blotting: rabbit anti-BimL (sc-11425), rabbit anti-Bid (sc-11423), rabbit anti-Bax (sc-493), goat anti-Bak (sc-1035), and mouse anti-HSP70 (sc-24), which were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif..); rabbit anti-Bik (4592) and rabbit anti-Bcl-xL (2762) from Cell Signaling (Beverly, Mass.); and rabbit anti-Pumaα (P4618) and mouse anti-tubulin α subunit (T5168) from Sigma (St. Louis, Mo.). Mouse anti-Bad (610392) and anti-Bcl-2 (610539) from BD Transduction (Mississauga, Ontario, Canada). A mouse monoclonal antibody (clone BC7.1; unpublished data) was used to detect chlamydial heat shock protein 60. These primary antibody bindings were probed with the corresponding secondary antibodies conjugated with horseradish peroxidase and were visualized by using standard enhanced chemiluminescence (Santa Cruz Biotechnology). It is clear that the BH3-only proteins Bik, Puma, and Bim, but not Bad and Bid, were degraded starting at the middle cycle of the L2 infection (at ∼24 h postinfection), which is largely consistent with the time course of chlamydial antiapoptotic activity (5). It is not known why the BH3-only proteins Bad and Bid were not degraded by chlamydia. The facts that Bad is regulated via phosphorylation (1) and that Bid is regulated via proteolytic cleavage (12) may suggest that chlamydia can use alternative approaches to modulate Bad and Bid function. Interestingly, none of the proteins from either the proapoptotic multidomain or the antiapoptotic subfamilies was degraded by chlamydia, which is consistent with previous observations (5, 17). These results together have demonstrated that chlamydia can selectively target a subset of the BH3-only proteins for degradation.
FIG. 1.
Degradation of Bik, Puma, and Bim during chlamydial infection. HeLa cells infected with C. trachomatis serovar L2 (MOI = 5) were harvested at various time points postinfection (hours p.i.) as indicated on top of the figure. The cell pellets were solubilized in a sodium dodecyl sulfate sample buffer (with a brief sonication to break down genomic DNA) for Western blot detection of nine different Bcl-2 family members representing all three Bcl-2 subfamilies and three control molecules as listed on the left and right of the figure. The parallel HeLa cell cultures without infection were harvested and analyzed similarly along with the infected samples. Although the amount of C. trachomatis heat shock protein 60 (ctHSP60) increased as infection progressed, the amounts of α-tubulin and human heat shock protein 70 (huHSP70) remained the same between different samples. Degradation of Bik, Puma, and Bim were detected in the chlamydia-infected but not in the uninfected HeLa cell cultures.
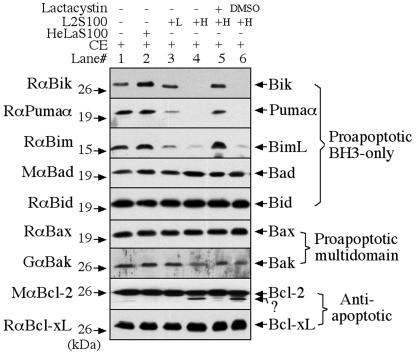
To confirm that the disappearance of Bik, Puma, and Bim in the infected cells is due to degradation at the protein level, we used a cell-free degradation assay as described previously (20) to measure the ability of the cytosolic fractions made from the chlamydia-infected cells to degrade the Bcl-2 proteins (Fig. 2). We used detergent-solubilized cytosolic extracts (CE) of uninfected HeLa cells as substrates (4). Cytosolic soluble protein fractions prepared as described previously (5) from either the chlamydia-infected cells (L2S100) or uninfected HeLa cells (HeLaS100) were used as the source of enzymes. Although the same amount (2 μg of total protein) of CE was used for each single reaction, L2S100 was used at two different concentrations, 0.1 and 5 μg. After the substrate interacts with the enzyme for 1 h at 37°C in a total volume of 20 μl, the residual Bcl-2 family proteins in the substrate were monitored by Western blotting with the corresponding specific antibodies. Among the nine Bcl-2 family proteins detected, only Bik, Puma, and Bim were degraded by L2S100, which is consistent with the results presented in Fig. 1. However, the control HeLaS100 did not show any degradation activity, demonstrating that degradation of Bik, Puma, and Bim is dependent on chlamydial infection. Furthermore, the degradation activity was inhibited by lactacystin (EMD Bioscience, San Diego, Calif.), an irreversible proteasome inhibitor, but not by the solvent dimethyl sulfoxide (Sigma) that was used to dissolve lactacystin. These observations together have demonstrated that the disappearance of Bik, Puma, and Bim proteins in chlamydia-infected cells is due to degradation by a lactacystin-sensitive proteolytic activity in the infected cell cytosol. It is not clear at this time how the minor bands migrating below Bcl-2 bands were generated (lanes 4 and 6). However, the bulk of the Bcl-2 proteins were still intact, supporting our conclusion that the antiapoptotic Bcl-2 molecule is not degraded by chlamydia.
FIG. 2.
Degradation of Bik, Puma, and Bim by a proteolytic activity in the cytosol of chlamydia-infected cells. Detergent-solubilized CE made from uninfected HeLa cells were used as substrates. Cytosolic soluble protein fractions prepared as described previously (5) from either the chlamydia-infected cells (L2S100) or uninfected HeLa cells (HeLaS100) were used as the sources of enzymes. After the substrate-enzyme interactions, the residual Bcl-2 family proteins were monitored by Western blotting. Although the same amount (2 μg of total protein) of CE was used for each single reaction, L2S100 was used at two different concentrations, 0.1 μg (L) and 5 μg (H). Degradation of Bik, Puma, and Bim was inhibited by lactacystin, an irreversible proteasome inhibitor, but not by the solvent dimethyl sulfoxide (DMSO), which was used to dissolve lactacystin.
Although the chlamydial antiapoptotic activity was first described more than 5 years ago by us (5), the mechanisms are still unknown. We have recently correlated the chlamydial antiapoptotic activity with the chlamydial inhibition of Bax and Bak (17), two proapoptotic Bcl-2 proteins with multiple BH domains. Activation of either Bax or Bak can result in mitochondrial cytochrome c release. Cells deficient in both Bax and Bak are profoundly resistant to apoptosis induced with a wide spectrum of proapoptotic stimuli (15), which is consistent with the observation that cells infected with chlamydia resist apoptosis induced by many different proapoptotic stimuli (5). The present finding that chlamydia selectively targets a subset of proapoptotic BH3-only proteins for degradation has allowed us to map the chlamydial antiapoptotic activity one step upstream of Bax and Bak. However, degradation of these three BH3 proteins alone cannot explain the overall chlamydial antiapoptotic activity. We are aware that in addition to these BH3-only proteins, many other factors can also regulate Bax and Bak function and affect mitochondrial cytochrome c release. It is likely that chlamydia may have evolved additional mechanisms for inhibiting host cell apoptosis. We are identifying the enzyme(s) responsible for the chlamydial degradation of Bik, Puma, and Bim as well as searching for chlamydial alternative strategies for blocking host cell apoptosis.
Acknowledgments
This work was supported in part by grants (to G.Z.) from the National Institutes of Health (R01AI47997, R01HL64883, and R21AI57450).
Editor: D. L. Burns
REFERENCES
- 1.Aho, T. L., J. Sandholm, K. J. Peltola, H. P. Mankonen, M. Lilly, and P. J. Koskinen. 2004. Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett. 571:43-49. [DOI] [PubMed] [Google Scholar]
- 2.Bouillet, P., and A. Strasser. 2002. BH3-only proteins—evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J. Cell Sci. 115:1567-1574. [DOI] [PubMed] [Google Scholar]
- 3.Dean, D., and V. C. Powers. 2001. Persistent Chlamydia trachomatis infections resist apoptotic stimuli. Infect. Immun. 69:2442-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong, F., H. Su, Y. Huang, Y. Zhong, and G. Zhong. 2004. Cleavage of host keratin 8 by a chlamydia-secreted protease. Infect. Immun. 72:3863-3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan, T., H. Lu, H. Hu, L. Shi, G. A. McClarty, D. M. Nance, A. H. Greenberg, and G. Zhong. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J. Exp. Med. 187:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer, S. F., T. Harlander, J. Vier, and G. Hacker. 2004. Protection against CD95-induced apoptosis by chlamydial infection at a mitochondrial step. Infect. Immun. 72:1107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greene, W., Y. Xiao, Y. Huang, G. McClarty, and G. Zhong. 2004. Chlamydia-infected cells continue to undergo mitosis and resist induction of apoptosis. Infect. Immun. 72:451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffiths, G. J., L. Dubrez, C. P. Morgan, N. A. Jones, J. Whitehouse, B. M. Corfe, C. Dive, and J. A. Hickman. 1999. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol. 144:903-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heimlich, G., A. D. McKinnon, K. Bernardo, D. Brdiczka, J. C. Reed, R. Kain, M. Krönke, and J. M. Jürgensmeier. 2004. Bax-induced cytochrome c release from mitochondria depends on α-helices-5 and -6. Biochem. J. 378:247-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jürgensmeier, J. M., Z. Xie, Q. Deveraux, L. Ellerby, D. Bredesen, and J. C. Reed. 1998. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 95:4997-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanave, C., M. Santamaria, and C. Saccone. 2004. Comparative genomics: the evolutionary history of the Bcl-2 family. Gene 333:71-79. [DOI] [PubMed] [Google Scholar]
- 12.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 13.Stephens, R. S. 2003. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 11:44-51. [DOI] [PubMed] [Google Scholar]
- 14.Tsujimoto, Y. 2003. Cell death regulation by the Bcl-2 protein family in the mitochondria. J. Cell. Physiol. 195:158-167. [DOI] [PubMed] [Google Scholar]
- 15.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolter, K. G., Y. T. Hsu, C. L. Smith, A. Nechushtan, X. G. Xi, and R. J. Youle. 1997. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139:1281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao, Y., Zhong, Y., Greene, W., Dong, F., Zhong, G. 2004. Chlamydia trachomatis infection inhibits both Bax and Bak activation induced by staurosporine. Infect. Immun. 72:5470-5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong, G., P. Fan, H. Ji, F. Dong, and Y. Huang. 2001. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J. Exp. Med. 193:935-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong, G., T. Fan, and L. Liu. 1999. Chlamydia inhibits interferon γ-inducible major histocompatibility complex class II expression by degradation of upstream stimulatory factor 1. J. Exp. Med. 189:1931-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong, G., L. Liu, T. Fan, P. Fan, and H. Ji. 2000. Degradation of transcription factor RFX5 during the inhibition of both constitutive and interferon γ-inducible major histocompatibility complex class I expression in chlamydia-infected cells. J. Exp. Med. 191:1525-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]