Abstract
Helicobacter pylori is an important human pathogen that causes gastritis and is strongly associated with gastric ulcers, gastric adenocarcinomas, and mucosa-associated lymphoid tissue lymphomas. In response to H. pylori, interleukin-8 (IL-8) is secreted from host cells to attract components of the innate and adaptive immune systems to the site of infection. Toll-like receptor 2 (TLR2) and TLR5 have been shown to recognize H. pylori and to initiate signaling pathways that result in enhanced activation of NF-κB. Here, we evaluated the contribution of mitogen-activated protein kinase signaling pathways to TLR2-dependent and TLR5-dependent secretion of IL-8. Secretion of IL-8 from H. pylori-infected HEK293 cells was augmented by the expression of TLR2 or TLR5. While H. pylori infection resulted in the activation of ERK, JNK, and p38, the enhanced IL-8 secretion from TLR2- and TLR5-expressing cells coincided with increased p38 activation and phosphorylation of the transcription factor ATF2. When p38 activity was inhibited in TLR2- or TLR5-expressing cells, H. pylori-dependent IL-8 secretion returned to the level observed in infected parental HEK293 cells that did not express TLR2 or TLR5; inhibition of p38 had no effect on IL-8 secretion from infected parental HEK cells. In contrast, inhibition of JNK and/or ERK resulted in substantially less IL-8 secretion from infected cells, independent of TLR2 or TLR5 expression. Based on these data, we propose that H. pylori induces IL-8 secretion through a dual mechanism that includes a TLR2/5-independent component involving the activities of JNK and ERK and a TLR2/5-dependent component that requires p38 activity.
The innate immune system is the first line of defense against invading pathogens. The sentinels of the innate immune system are the Toll-like receptors (TLRs). The TLRs survey the cellular environment for molecular patterns commonly associated with pathogens. Once a TLR interacts with its ligand, the receptor complex initiates signaling cascades that lead to transcription and secretion of antimicrobials and immune-modulating cytokines and chemokines. The innate immune responses activate and instruct the adaptive immune system to respond in a pathogen-appropriate manner (2, 10, 23).
Infection by Helicobacter pylori can cause gastritis and is also highly associated with gastric ulcers, gastric adenocarcinomas, and mucosa-associated lymphoid tissue lymphomas (6, 14). Upon H. pylori infection, gastric epithelial cells respond to H. pylori by activating many signaling cascades. These lead to cytokine and chemokine secretion, which recruit innate and adaptive immune cells to the site of infection. Despite a vigorous host immune response, H. pylori infection is persistent and can be lifelong without medical intervention.
Interleukin-8 (IL-8) is an important chemokine in mediating the inflammatory response to H. pylori. Aihara et al. demonstrated that both the NF-κB and activating protein 1 (AP-1) DNA binding sites within the IL-8 promoter are required for optimal transcription in response to infection of gastric epithelial cells by H. pylori (3). During H. pylori infections, both NF-κB and members of the mitogen-activated protein kinase (MAPK) family become activated (11, 12, 15, 18). Activated MAPKs then phosphorylate AP-1 complexes, which results in increased AP-1-dependent transcription. As such, signaling pathways that activate NF-κB and/or AP-1 could result in increased IL-8 secretion.
In an in vivo H. pylori infection, gastric epithelial cells may be the first cells to induce innate immune signaling pathways. These cells can express TLR2 and TLR5, among other TLRs (11). Our previous work demonstrated that H. pylori lipopolysaccharide (LPS) and flagellin are TLR2 and TLR5 agonists, respectively, and that expression of TLR2 or TLR5 results in enhanced NF-κB activation upon in vitro H. pylori infection of gastric epithelial cells. We also noted variability in TLR expression within gastric epithelial cell lines. In addition, IL-8 mRNA levels were found to be elevated in TLR2-expressing epithelial cells upon H. pylori infection (20). Based on these initial findings, we hypothesized that, in addition to activating NF-κB, TLRs might be important for increased IL-8 secretion from H. pylori-infected cells through their ability to enhance AP-1 activation. Here, we evaluated the contribution of TLR2 and TLR5 expression to IL-8 secretion from H. pylori-infected cells, using HEK293 cells or the same cells expressing TLR2 or TLR5 as a model system, and tested the role of MAPK signaling pathways in the TLR-mediated responses to H. pylori infection.
MATERIALS AND METHODS
Reagents.
The synthetic lipopeptide Pam3CSK4 and Salmonella enterica serovar Typhimurium flagellin were purchased from InvivoGen (San Diego, Calif.). Anisomycin, epidermal growth factor (EGF), and the MEK inhibitor U0126 were obtained from Sigma-Aldrich (St. Louis, Mo.). The JNK inhibitor SP600125 and the p38 inhibitor SB202190 were purchased from Calbiochem (La Jolla, Calif.). Protein A-Sepharose beads were purchased from Amersham Biosciences (Piscataway, N.J.). The enhanced chemiluminescence (ECL) kit was purchased from Perkin-Elmer Life Sciences (Boston, Mass.). The following antibodies were purchased from Cell Signaling Technologies (Beverly, Mass.): anti-AKT, anti-ATF2, anti-Elk-1, anti-ERK1/2, anti-JNK, anti-c-Jun, anti-p38, anti-phospho-AKT, anti-phospho-ATF2, anti-phospho-Elk-1, anti-phospho-c-Jun, and anti-phospho-p38. Anti-phospho-ERK1/2 was purchased from Sigma-Aldrich, anti-phospho-JNK was purchased from Promega (Madison, Wis.), and anti-human TLR2 and anti-human TLR5 antibodies were obtained from InvivoGen. Secondary antibodies conjugated to horseradish peroxidase (HRP), anti-rabbit immunoglobulin G (IgG)-HRP, or anti-mouse IgG-HRP were purchased from Amersham Biosciences.
Cell and H. pylori culture.
Human embryonic kidney cells of the HEK293 line (HEK) were obtained from American Type Culture Collection (Manassas, Va.). HEK cells were routinely cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Gibco, Carlsbad, Calif.) and 1× penicillin-streptomycin (Gibco). HEK293 cells stably transfected with human TLR2, (HEK-hTLR2; TLR2) or with human TLR5 (HEK-hTLR5; TLR5) were purchased from InvivoGen. TLR2 and TLR5 cells were cultured in DMEM supplemented with 10% FBS, 1% penicillin-streptomycin, and 10 of blasticidin (InvivoGen)/ml at 37°C in 7.5% CO2.
H. pylori strain 26695 was routinely grown on sheep blood agar plates (BAP) (Remel, Lenexa, Kans.) in 10% CO2 at 37°C. For in vitro infections, H. pylori was cultured for 24 h on BAP, harvested with a sterile cotton swab, and resuspended in 1 ml of brucella broth per plate. The bacteria were pelleted at 3,000 × g for 5 min and then resuspended in 5 ml of brucella broth. This wash procedure was repeated, and the bacteria were resuspended in 2 ml of brucella broth. The number of viable bacteria was determined by serial dilution of the bacterial suspension, plating onto BAP, and incubation for 72 h at 37°C.
In vitro infections.
For biochemical assays, 6 × 106 epithelial cells were plated in 60-mm-diameter dishes in normal growth medium. The next day, the cells were washed with phosphate-buffered saline, pH 7.4, and subsequently serum starved in serum-free DMEM for 4 h without antibiotics. The medium was then exchanged for fresh DMEM containing 2% FBS. The eukaryotic cells were then stimulated with either 100 ng of EGF/ml for 10 min, 10 μg of anisomycin/ml for 1 h, or H. pylori at a multiplicity of infection (MOI) of approximately 100:1 for 1 h. Following treatment, the cells were washed with cold DMEM and then lysed on ice with 1 ml of cold lysis buffer (50 mM Tris, pH 7.3, 150 mM NaCl, 1% Triton X-100, 0.5 mM EDTA, 0.5 mM EGTA, 10% glycerol) supplemented with protease and phosphatase inhibitors (100 μM leupeptin, 1 mM phenylmethylsulfonyl fluoride, 0.15 U of aprotinin/ml, and 1 mM sodium orthovanadate).
For enzyme-linked immunosorbent assays (ELISAs), 3 × 105 cells/well were plated into individual wells of a 24-well dish in normal growth medium without antibiotics. The following day, cells were treated in triplicate with Pam3CSK4, S. enterica serovar Typhimurium flagellin, or H. pylori at an MOI of 100:1. Twenty-four-hour culture supernatants were collected, clarified by centrifugation, and evaluated for IL-8 secretion by ELISA, as described below.
MAPK inhibitor studies.
The kinase inhibitor studies were performed with the inhibitors SB202190 (p38 inhibitor), SP600125 (JNK inhibitor), and U0126 (MEK1/2 inhibitor) at a final concentration of 10 μM in dimethyl sulfoxide (DMSO) (Sigma-Aldrich). Cells were treated with the inhibitor(s) or DMSO for 1 h at 37°C prior to infection or treatment.
Immunoprecipitation and immunoblotting.
Immunoprecipitations were performed by incubating 1 mg of cell lysate with 5 μl of anti-human TLR2, 5 μl of anti-human TLR5, or 4 μl of anti-ATF2 overnight at 4°C with rotation. The immune complexes were recovered by incubation with 60 μl of protein A-Sepharose bead slurry for a further 4 h at 4°C. The immunoprecipitates were washed twice in cold lysis buffer and twice in cold Tris-buffered saline (150 mM NaCl, 50 mM Tris, pH 7.5). The immune complexes were then resuspended in 2× Laemmli sample buffer and boiled for 10 min.
For immunoblots, cell lysates were boiled in Laemmli sample buffer for 10 min. Cell lysates and immunoprecipitates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10% polyacrylamide) and transferred to nitrocellulose. The membranes were blocked following manufacturer's recommendations. Membranes were incubated overnight with primary antibodies at 4°C as indicated followed by secondary anti-rabbit IgG or anti-mouse IgG conjugated to HRP (1:1,000) for 1 h at room temperature. Immunoreactive bands were detected by ECL and radiography. Films in the linear range of detection were used to analyze the relative activation of the MAPKs or ATF2. Bands were quantified by densitometry and normalized for total protein with a Molecular Dynamics densitometer and ImageQuant 5.0 software (Amersham Biosciences).
IL-8 ELISA.
Clarified culture supernatants from 24-h infections or treatments were diluted 1:2 to 1:10, as needed. Supernatants were analyzed for IL-8 protein with a Quantikine human IL-8 ELISA kit (R&D Systems, Minneapolis, Minn.), as directed by the manufacturer.
Statistical analyses.
All ELISA experiments were performed in triplicate and were repeated a minimum of three times. Data from the repeated experiments were pooled, and the natural log of the values was taken. The data set was analyzed for statistical significance by analysis of variance (ANOVA). P values reported are derived from the combined data from all repetitions of each experiment.
RESULTS
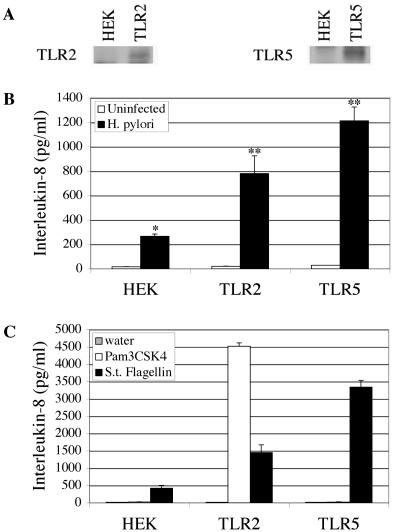
H. pylori induces increased IL-8 secretion from epithelial cells that express TLR2 or TLR5. Previously, we demonstrated that expression of TLR2 or TLR5 in gastric epithelial cells resulted in enhanced NF-κB activation upon infection by H. pylori (20). In light of these data and the fact that IL-8 mRNA levels were also shown to be elevated in TLR2-expressing cells, we hypothesized that TLR expression might result in enhanced IL-8 secretion from infected cells. To test this hypothesis, we took advantage of HEK293 cell lines that stably express TLR2 or TLR5 and HEK293 cells that do not express these receptors (Fig. 1A). To assess the contribution of TLR2 or TLR5 expression to IL-8 secretion, we measured the IL-8 protein levels of culture supernatants from 24-h H. pylori infections of HEK, TLR2, or TLR5 cells by ELISA. As expected, HEK cells were observed to secrete IL-8 in response to H. pylori infection (P ≤ 0.0001) (Fig. 1B). The level of IL-8 secreted from infected TLR2 or TLR5 cells was significantly higher than that secreted by parental HEK cells (P ≤ 0.0001).
FIG. 1.
H. pylori induces increased IL-8 secretion from epithelial cells that express TLR2 or TLR5. (A) Expression of TLR2 and TLR5 in HEK293 cells. TLR2 and TLR5 immune complexes were separated by SDS-PAGE and immunoblotted with either anti-TLR2 or anti-TLR5 antibodies (1:200), as indicated. (B) TLR expression enhances IL-8 secretion from H. pylori-infected cells. Culture supernatants from 24-h H. pylori infections were analyzed for IL-8 secretion by ELISA. The data shown are from one representative experiment performed in triplicate. This experiment was repeated three times, each experiment showing similar results. The data from these three independent experiments were pooled and analyzed by ANOVA to assess statistical significance. *, P ≤ 0.0001 for H. pylori-infected HEK cells versus uninfected HEK cells; **, P ≤ 0.0001 for H. pylori-infected TLR2 and TLR5 cells versus H. pylori-infected HEK cells. (C) Purified TLR ligands induce IL-8 secretion in cells expressing TLRs. Epithelial cells were treated with sterile water, 100 μg of Pam3CSK4/ml or 1 μg of S. enterica serovar Typhimurium (S.t.) flagellin/ml for 24 h at 37°C. Culture supernatants were assayed for IL-8 by ELISA.
To determine whether engagement of the TLRs would be sufficient to induce IL-8 secretion from these cells, HEK, TLR2, and TLR5 cells were treated with the TLR2-specific ligand Pam3CSK4 or the TLR5-specific ligand S. enterica serovar Typhimurium flagellin for 24 h and culture supernatants were assayed for IL-8 by ELISA. Pam3CSK4 induced IL-8 secretion from TLR2 cells, but not from HEK or TLR5 cells (Fig. 1C, white bars). S. enterica serovar Typhimurium flagellin induced a robust IL-8 response from TLR5 cells (black bar). Flagellin-stimulated HEK and TLR2 cells also secreted significant, albeit lower, levels of IL-8. This level of IL-8 could be due to residual TLR5 expression, as we have previously noted low endogenous TLR5 mRNA in HEK cells (20) and/or contamination of the flagellin preparation. Taken together, these data indicate that secretion of IL-8 in response to H. pylori infection is enhanced by the presence of TLR2 or TLR5 and that engagement of the receptors alone is sufficient to induce IL-8 secretion from these cells.
TLR2- and TLR5-expressing cells show increased p38 activation in response to infection by H. pylori.
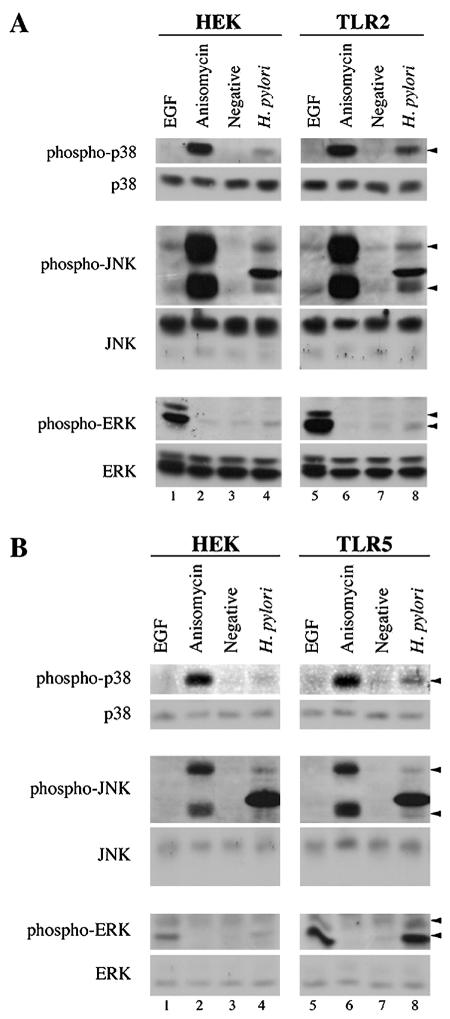
Since both the AP-1 and NF-κB sites are required for optimal expression of the IL-8 gene following H. pylori infection (3) and NF-κB activation was enhanced by TLR2 or TLR5 expression (20), we hypothesized that TLR2 or TLR5 expression also might increase MAPK signaling upstream of AP-1 and thus contribute to the enhanced IL-8 secretion. To investigate the effect of TLR2 or TLR5 expression on the H. pylori-dependent activation of MAPK family members, we measured activation of the MAPKs in cell lysates from infected HEK, TLR2, and TLR5 cells using phosphorylation-specific antibodies. In HEK cells, H. pylori infection induced the activation of p38, JNK, and ERK (Fig. 2A, upper panels, compare lanes 3 and 4). Infected TLR2 cells also showed an H. pylori-dependent activation of all three MAPKs (upper panels, compare lanes 7 and 8). However, while JNK and ERK were activated to equivalent levels in HEK and TLR2 cells, p38 activation in response to H. pylori infection was consistently fourfold to sixfold greater in TLR2 than in HEK cells, as determined by densitometry (upper panel, compare lanes 4 and 8). All three MAPK family members responded equivalently to conventional agonists (EGF for ERK and anisomycin for JNK and p38; compare lanes 1 and 2 with lanes 5 and 6), indicating that there are no intrinsic differences between the HEK and TLR2 cell lines in their ability to activate the MAPKs.
FIG. 2.
TLR2- and TLR5-expressing cells show increased p38 activation in response to infection by H. pylori. Cells were infected with H. pylori for 1 h. Fifty micrograms of cell lysate was separated by SDS-PAGE and analyzed by immunoblot. Activation of the MAPKs was detected with phosphorylation-specific antibodies: anti-phospho-p38 (1:250), anti-phospho-JNK (1:500), and anti-phospho-ERK1/2 (1:1,000). Total MAPKs were detected with anti-p38, anti-JNK, and anti-ERK1/2 (1:1,000 for all). Positive controls for activation of ERK (EGF) and JNK and p38 (anisomycin) were used as indicated. Arrows indicate phosphorylated forms of the MAPKs. (A) Response of HEK and TLR2 cells to infection by H. pylori. (B) Response of HEK and TLR5 cells to infection by H. pylori.
As with TLR2 cells, H. pylori infection of TLR5 cells resulted in activation of all three MAPK family members (Fig. 2B, upper panels, compare lanes 7 and 8). As was the case in TLR2 cells, the activation of p38 in TLR5 cells in response to H. pylori infection was enhanced beyond the level observed in infected HEK cells (upper panel, compare lanes 4 and 8). In this case, H. pylori-infected TLR5 cells exhibited a two- to fourfold increase in p38 activation relative to infected HEK cells, as measured by densitometry. JNK activation in response to H. pylori was similar in HEK and TLR5 cells (middle panels). ERK activation was increased in infected TLR5 cells; however, TLR5 cells also displayed a reproducible increase in ERK phosphorylation in response to EGF, suggesting that the TLR5 cells might be more responsive to ERK stimuli in general. Importantly, the difference in p38 activation could not be accounted for by a hyperresponsiveness of TLR5 cells to p38 stimuli, since p38 was activated to equivalent levels in HEK and TLR5 cells following anisomycin treatment (compare lanes 2 and 6).
p38 plays a key role in TLR2- and TLR5-dependent secretion of IL-8 from H. pylori-infected cells.
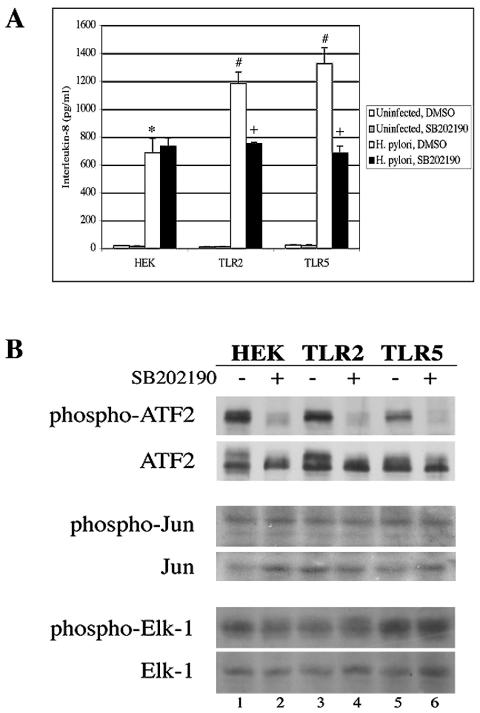
The importance of p38 activity on H. pylori-induced IL-8 secretion was tested with the selective p38 inhibitor SB202190. This inhibitor had no effect on H. pylori-dependent IL-8 secretion in HEK cells (Fig. 3A, black bar). However, treatment of TLR2 or TLR5 cells with SB202190 significantly reduced IL-8 secretion in response to H. pylori infection to the level observed in infected HEK cells (P ≤ 0.02). This suggests that the TLR2- and TLR5-dependent augmentation in IL-8 secretion following H. pylori infection is dependent on p38 activity. However, p38 does not appear to be essential for the induction of IL-8 by HEK cells following H. pylori infection, nor is it required to elicit the residual IL-8 response in infected TLR2 and TLR5 cells that remains following treatment with SB202190. The activity and specificity of this inhibitor were verified by examining the phosphorylation state of ATF2 to measure p38 activity, c-Jun to measure JNK activity, and Elk-1 to measure ERK activity in EGF- or anisomycin-treated cells following a 24-h incubation with SB202190. Whereas p38 activity was reduced in all three cell lines in the presence of the inhibitor (Fig. 3B, top panel), the kinase activities of JNK and ERK were not affected (middle and bottom panels).
FIG. 3.
p38 plays a key role in the TLR2- and TLR5-dependent secretion of IL-8 from H. pylori-infected cells. (A) Inhibition of p38 reduces IL-8 secretion from infected cells. HEK, TLR2, and TLR5 cells were pretreated with 10 μM SB202190 for 1 h and then infected with H. pylori for a further 24 h. Culture supernatants were analyzed for IL-8 secretion by ELISA. The graph shown illustrates the results from one experiment performed in triplicate. The data from three independent experiments were pooled and analyzed for statistical significance with the ANOVA test. *, P ≤ 0.0001 for H. pylori-infected HEK cells treated with DMSO versus uninfected HEK cells treated with DMSO; #, P ≤ 0.02 for H. pylori-infected TLR2 cells treated with DMSO and H. pylori-infected TLR5 cells treated with DMSO versus H. pylori-infected HEK cells treated with DMSO; +, P ≤ 0.02 for H. pylori-infected TLR2 cells treated with SB202190 versus H. pylori-infected TLR2 cells treated with DMSO and H. pylori-infected TLR5 cells treated with SB202190 versus H. pylori-infected TLR5 cells treated with DMSO. (B) SB202190 inhibits p38 kinase activity, but not the activities of JNK or ERK. A total of 106 cells of each cell line were treated with 10 μM SB202190 for 23 h. The cells were then stimulated with EGF to stimulate ERK signaling or with anisomycin to stimulate JNK and p38 signaling and incubated for 1 h. ATF2 immune complexes were resolved by SDS-PAGE and immunoblotted with anti-phospho-ATF2 (1:250) to detect p38 kinase activity. Fifty micrograms of total cell lysates was separated by SDS-PAGE, and JNK kinase activity was measured indirectly with anti-phospho-c-Jun (1:500) and ERK kinase activity was measured indirectly with anti-phospho-Elk-1 (1:500). Total ATF2, c-Jun, and Elk-1 were measured with anti-ATF2, anti-c-Jun, and anti-Elk-1 antibodies (all 1:1,000).
JNK and ERK play key roles in H. pylori-dependent IL-8 secretion from infected cells.
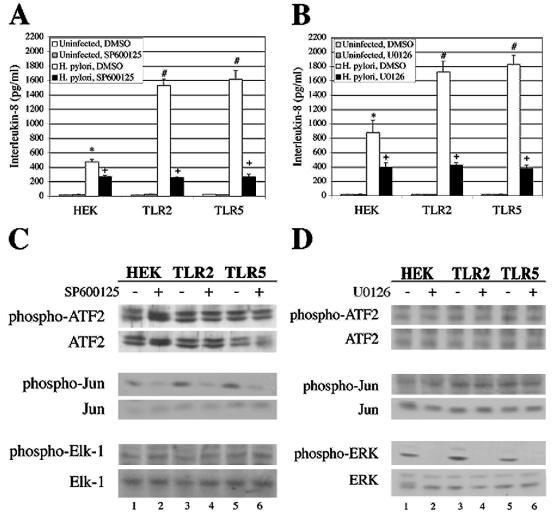
Since expression of TLR2 or TLR5 did not alter the extent to which H. pylori activated JNK or ERK, we hypothesized that JNK and ERK may not be required for the TLR2- and TLR5-dependent increase in IL-8 following infection. To test this hypothesis, cell lines were treated with SP600125 to inhibit JNK or U0126 to inhibit MEK1/2, an upstream activator of ERK, and IL-8 secretion from H. pylori-infected cells was measured. Each of the H. pylori-infected cell lines secreted significantly less IL-8 in the presence of the JNK or MEK inhibitors than DMSO-treated cells (Fig. 4A and B, black bars; P ≤ 0.0001 and P ≤ 0.005, respectively). Moreover, the augmentation of IL-8 production observed in TLR2- and TLR5-infected cells was ablated in the presence of the JNK and MEK inhibitors. The specificity of the inhibitors was confirmed (Fig. 4C and D) as described above. These data indicate that JNK and ERK play key roles in H. pylori-dependent secretion of IL-8 from epithelial cells and that the engagement of TLR2 or TLR5 by H. pylori does not overcome the requirement for JNK or ERK in the production of IL-8 in response to H. pylori infection.
FIG. 4.
JNK and ERK play key roles in H. pylori-dependent IL-8 secretion from infected cells. (A) Inhibition of JNK reduces IL-8 secretion from infected cells. HEK, TLR2, and TLR5 cells were pretreated with 10 μM SP600125 for 1 h and then infected with H. pylori for a further 24 h. Culture supernatants were analyzed for IL-8 secretion by ELISA. The graph represents the data from one representative experiment performed in triplicate. Data from three independent experiments were pooled and analyzed for statistical significance by the ANOVA test. *, P ≤ 0.0001 for H. pylori-infected HEK cells treated with DMSO versus uninfected HEK cells treated with DMSO; #, P ≤ 0.0001 for H. pylori-infected TLR2 cells treated with DMSO and H. pylori-infected TLR5 cells treated with DMSO versus H. pylori-infected HEK cells treated with DMSO; +, P ≤ 0.0001 for H. pylori-infected HEK cells treated with SP600125 versus H. pylori-infected HEK cells treated with DMSO; H. pylori-infected TLR2 cells treated with SP600125 versus H. pylori-infected TLR2 cells treated with DMSO; and H. pylori-infected TLR5 cells treated with SP600125 versus H. pylori-infected TLR5 cells treated with DMSO. (B) Inhibition of ERK signaling inhibits IL-8 secretion from infected cells. HEK, TLR2, and TLR5 cells were pretreated with 10 μM U0126 for 1 h and then infected with H. pylori for a further 24 h. Culture supernatants were analyzed for IL-8 secretion by ELISA. The graph represents the data from one representative experiment performed in triplicate. Data from three independent experiments were pooled and analyzed for statistical significance by the ANOVA test. *, P ≤ 0.0001 for H. pylori-infected HEK cells treated with DMSO versus uninfected HEK cells treated with DMSO; #, P ≤ 0.02 for H. pylori-infected TLR2 cells treated with DMSO and H. pylori-infected TLR5 cells treated with DMSO versus H. pylori-infected HEK cells treated with DMSO; +, P ≤ 0.005 for H. pylori-infected HEK cells treated with U0126 versus H. pylori-infected HEK cells treated with DMSO; H. pylori-infected TLR2 cells treated with U0126 versus H. pylori-infected TLR2 cells treated with DMSO; and H. pylori-infected TLR5 cells treated with U0126 versus H. pylori-infected TLR5 cells treated with DMSO. (C) SP600125 inhibits JNK kinase activity, but not the activities of p38 or ERK. SP600125-treated cell lysates were analyzed for p38, JNK, and ERK kinase activities as described in the legend of Fig. 3B. (D) U0126 inhibits MEK signaling but not the activities of p38 or JNK. U0126-treated cell lysates were analyzed for p38 and JNK kinase activities as previously described in the legend to Fig. 3B, while MEK kinase activity was indirectly measured with anti-phospho-ERK1/2.
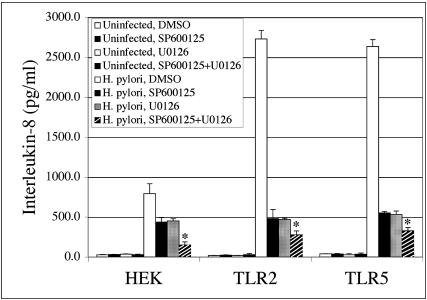
Although treatment with the JNK and ERK inhibitors resulted in a dramatic inhibition in H. pylori-induced IL-8 secretion, it did not reduce IL-8 secretion to the levels observed in uninfected cells. To determine whether the residual IL-8 secretion was due to the activity of MAPK family members not targeted by the inhibitors, HEK, TLR2, and TLR5 cells were simultaneously treated with SP600125 and U0126 and infected with H. pylori, and IL-8 levels were determined on 24-h supernatants by ELISA. Combined treatment with both the JNK and MEK inhibitors resulted in decreased IL-8 secretion below the level observed in the presence of either inhibitor alone (P ≤ 0.05; Fig. 5, compare hatched bars to black and gray bars). The greater inhibition observed in cells treated with both inhibitors suggests that JNK and ERK appear to each play a critical role in promoting IL-8 secretion from H. pylori-infected cells. However, treatment with both inhibitors did not fully reduce H. pylori-dependent IL-8 secretion to the levels observed for uninfected cells. The residual IL-8 secretion observed under these conditions may indicate that there is a JNK/ERK-independent pathway that remains active in the presence of both inhibitors or that the inhibitors did not fully block these signaling pathways under the conditions of the assay.
FIG. 5.
Simultaneous inhibition of JNK and MEK results in further reduction of H. pylori-dependent IL-8 secretion from infected HEK cells. HEK, TLR2, and TLR5 cells were pretreated with 10 μM SP600125, 10 μM U0126, or both for 1 h and were then infected with H. pylori for a further 24 h at 37°C. Culture supernatants were analyzed for IL-8 secretion by ELISA. The graph illustrates the data from one representative experiment performed in triplicate. Data from three independent experiments were pooled and analyzed for statistical significance by ANOVA. *, P ≤ 0.05 for H. pylori infection and treatment with SP600125 + U0126 versus H. pylori infection and treatment with SP600125 alone and for H. pylori infection and treatment with SP600125 + U0126 versus H. pylori infection and treatment with U0126 alone in each cell line.
ATF2 is phosphorylated in response to H. pylori infection of TLR2 or TLR5 cells, but not of HEK cells.
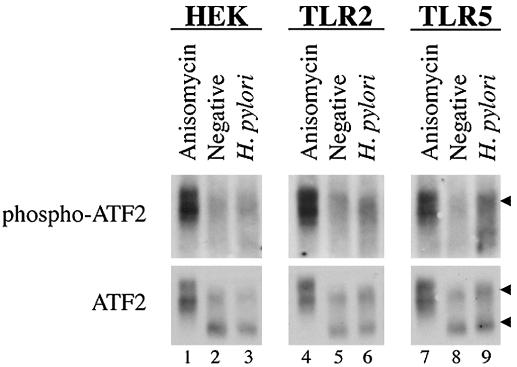
One of the downstream targets of p38 is the transcription factor ATF2 (8). ATF2 can homodimerize or heterodimerize with c-Jun. Phosphorylation of ATF2 and c-Jun enhances binding to AP-1 binding sites on DNA and transactivation (8). Since the AP-1 binding site in the IL-8 promoter is required for optimal transcription in response to infection (3), we hypothesized that the p38-dependent increase in IL-8 secretion exhibited by TLR2- and TLR5-expressing cells might result from increased phosphorylation of ATF2. To test this hypothesis, ATF2 was immunoprecipitated from H. pylori-infected HEK, TLR2, and TLR5 cell lysates, and its phosphorylation state was determined by immunoblotting with an antibody that recognizes the phosphorylated form of ATF2.
While phosphorylation of ATF2 in response to anisomycin was readily apparent in all three cell lines (Fig. 6, lanes 1, 4, and 7, top panels and slower-migrating species in bottom panels), there was no detectable phosphorylation of ATF2 in response to H. pylori infection of HEK cells (compare lanes 2 and 3). In contrast, a slight but reproducible H. pylori-dependent increase in ATF2 phosphorylation was evident in TLR2 and TLR5 cells (compare lanes 6 and 9 with lanes 5 and 8). These data suggest that the elevated p38 activation that is evident in H. pylori-infected TLR2- or TLR5-expressing cells might lead to ATF2 phosphorylation, which could then result in more activated AP-1 complexes and increased IL-8 secretion from these cells.
FIG. 6.
ATF2 is phosphorylated in response to H. pylori infection of TLR2 or TLR5 cells, but not HEK cells. HEK, TLR2, and TLR5 cells were infected with H. pylori for 1 h. ATF2 immune complexes were separated by SDS-PAGE and analyzed by immunoblot. Activation of ATF2 was detected with anti-phospho-ATF2 (1:250). The membrane was stripped and reprobed for total ATF2 (anti-ATF2 at 1:1,000). Anisomycin was used as a positive control for ATF2 activation, as indicated. An arrow in the top panels indicates phosphorylated ATF2. Arrows in the bottom panels indicate total ATF2.
DISCUSSION
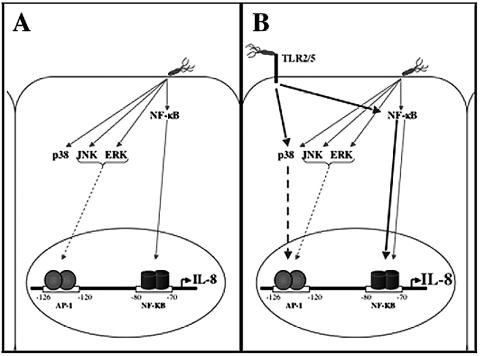
The data presented in this report showing that ERK, JNK, and p38 were consistently activated upon infection with H. pylori are in agreement with several groups who have also reported H. pylori-dependent activation of these MAPKs (1, 9, 12, 13, 15). However, while it is clear from these reports that H. pylori infection activates these MAPKs, no receptor has yet been implicated in the initiation of the MAPK cascades. Here we describe two cellular receptors for H. pylori, TLR2 and TLR5, which initiate signaling events leading to p38 activation and enhanced IL-8 secretion. This augmentation of IL-8 secretion was shown to be dependent on the activation of p38 and coincided with phosphorylation of the transcription factor ATF2. In contrast to p38, JNK and ERK were required for H. pylori-dependent IL-8 secretion, and TLR2 or TLR5 expression had no effect on the activation of these molecules following infection. Based on these data, we propose that H. pylori induces IL-8 secretion through a dual mechanism that includes a TLR2/5-independent component involving the activities of JNK and ERK (Fig. 7A and B, gray arrows) and a TLR2/5-dependent component that requires p38 activity (Fig. 7B, bold, black arrows).
FIG. 7.
Model depicting the dual molecular mechanisms for H. pylori-induced IL-8 secretion from TLR2- or TLR5-expressing epithelial cells. (A) H. pylori infection of epithelial cells results in activation of NF-κB, the MAPKs, and AP-1, which leads to IL-8 transcription and secretion (thin gray arrows). (B) In addition to the signal pathways described in panel A, H. pylori recognition by TLR2- or TLR5-expressing cells results in enhanced activation of NF-κB, p38, and AP-1 complexes and the subsequent increase in IL-8 transcription and secretion (bold, black arrows).
p38 activity has been implicated in H. pylori-induced inflammation in several studies. Bhattacharyya et al. demonstrated that inhibition of p38 activity in H. pylori-extract-treated macrophages resulted in a dramatic reduction of IL-8 secretion and Sabroe et al. reported that TLR2-dependent IL-8 secretion was dependent on p38 activity in neutrophils (4, 17). These results are particularly interesting since macrophages and neutrophils express many TLRs, including TLR2 and TLR5, and are recruited to the sites of H. pylori infection. Once exposed to H. pylori, macrophages and neutrophils secrete additional chemokines, which recruit more immune cells and thus contribute to the tissue damage observed in H. pylori-infected stomachs. Together with our data showing an important role for p38 in promoting IL-8 secretion from epithelial cells, these reports provide strong evidence that p38 is a critical component of the signaling pathways leading to enhanced inflammation and IL-8 secretion from H. pylori-infected cells.
In addition to the in vitro experiments discussed above, two studies support a role for p38 in H. pylori-induced inflammation in animal models of infection and inflammation. Slomiany et al. reported that intragastric application of H. pylori LPS induced a strong inflammatory response and mucosal ulceration in rats; however, inflammation and ulceration were reduced more than 60% when the rats were given H. pylori LPS and a p38 inhibitor. This was in contrast to treatment with an ERK inhibitor, which resulted in a more modest reduction in inflammation (19). This suggests that p38 may play a more significant role than ERK in H. pylori LPS-induced inflammation in animals. Since H. pylori LPS is a TLR2 agonist (20), we would propose from our data that the p38-mediated inflammation observed in the Slomiany study could be due to TLR2 signaling through p38. In the second study, Takahashi et al. reported that administration of a p38 inhibitor to Mongolian gerbils markedly reduced IL-8 and other chemokine secretion, neutrophil infiltration, and mucosal injury observed in the H. pylori-infected animals (22). Since H. pylori disease pathology is caused by inflammation, any factor enhancing inflammation contributes to H. pylori disease. As such, our data and the results of Slominay and Takahashi suggest that TLR-dependent activation of p38 results in more severe disease in H. pylori infection.
Our data suggest that one of the possible mechanisms by which increased p38 activation in TLR2- and TLR5-expressing cells might result in elevated IL-8 production is through increased phosphorylation of ATF2. ATF2 phosphorylation can also be mediated through JNK and phosphatidlyinositol 3-kinase (PI 3-kinase) pathways. Since both of these molecules can be activated in response to H. pylori infection (16, 21), they may also contribute to ATF2 phosphorylation and activity. However, JNK activation by H. pylori was not found to be enhanced in TLR2 or TLR5 cells (Fig. 2A and B), and PI3 kinase activity, as measured by phosphorylation of AKT, was similarly unaffected by TLR2 or TLR5 expression (data not shown). Thus, we propose that ATF2 becomes phosphorylated in H. pylori-infected TLR2 and TLR5 cells through the activity of p38 and not by the JNK or PI 3-kinase pathways.
We demonstrated that H. pylori infection stimulated ATF2 phosphorylation in TLR2 and TLR5, but not HEK cells. The lack of phosphorylated ATF2 in infected HEK cells was unexpected, since p38 was activated in those cells. It is possible that H. pylori activation of the TLR2 or TLR5 pathways may provide specificity for p38-dependent phosphorylation of ATF2. Alternatively, there may be a threshold of p38 activation that is required in order to promote phosphorylation of ATF2. Finally, ATF2 may be phosphorylated in HEK cells in response to H. pylori infection, but the amount may be below the level of detection. This possibility is currently being tested with more sensitive chromatin immunoprecipitation assays.
Previously, Naumann et al. and Meyer-ter-Vehn et al. reported that c-Jun and c-Fos, but not ATF2, were components of the AP-1 dimer that were induced in AGS cells by H. pylori infection (12, 15). On the other hand, Yamada et al. reported p38-dependent phosphorylation of ATF2 in MKN cells infected by H. pylori (25). These differing results can potentially be reconciled by our findings and those we reported previously (20). Since AGS cells do not contain detectable TLR2 mRNA (20), these cells may not undergo a hyperactivation of p38 in response to H. pylori and thus would not be expected to contain phosphorylated ATF2. In contrast, the MKN cells used by Yamada et al. and the TLR2 cells used here both express TLR2 mRNA (20) and as such promote TLR2-dependent p38 phosphorylation of ATF2 in H. pylori-infected cells.
We propose that phosphorylation of ATF2 by p38 in TLR2 and TLR5 cells may contribute to the enhanced IL-8 secretion observed upon H. pylori infection (Fig. 7B). Exactly how this occurs is not currently known. We suggest that increased levels of phosphorylated ATF2 could lead to larger pools of activated AP-1 complexes in TLR-expressing cells (ATF2/ATF2, ATF2/c-Jun, and c-Fos/c-Jun). This could result in increased AP-1 occupancy at the IL-8 promoter and increased IL-8 transcription in these cells (Fig. 7B, bold and dashed arrows). Alternatively, there could be a qualitative effect of ATF2-containing AP-1 complexes on IL-8 transcription. For example, ATF2/ATF2 or ATF2/c-Jun could replace c-Fos/c-Jun complexes at the IL-8 promoter. These ATF2-containing AP-1 complexes could have greater transactivation potential than the c-Fos/c-Jun AP-1 complexes, thus resulting in increased IL-8 transcription. These possibilities are currently being tested with chromatin immunoprecipitation assays.
Another possible mechanism by which p38 might enhance IL-8 secretion is by increasing NF-κB transcriptional activity. Carter et al. and Goebeler et al. reported that p38 is able to regulate the transcriptional activity of NF-κB at the monocyte chemoattractant protein 1 promoter. In these studies, p38 activity was found to enhance NF-κB transcriptional activity by modulating the assembly and activity of the transcriptional complex. Pharmacological inhibition of p38 did not affect the activation of NF-κB, but instead inhibited the recruitment of TATA-binding protein and blocked the enhancing effect of the transcriptional coactivator, p300 (5, 7).
Finally, it is possible that the enhanced p38 activation and phosphorylation of ATF2 that are observed in TLR2 and TLR5 cells following H. pylori infection could result in transcription at other promoters whose products could then stimulate IL-8 transcription and/or mRNA stability. For example, MAPKAP K2 (MK2), which can be phosphorylated by p38, has been shown to extend the half-life of IL-8 mRNAs by binding to AU-rich elements in IL-8 mRNA (24). Although the role of MK2 in H. pylori infection has not been assessed, it is possible that TLR2/5-dependent activation of p38 may lead to increased IL-8 secretion via MK2 stabilization of IL-8 mRNA. One argument against this possibility is that Yu et al. reported that IL-8 mRNA transcription was only slightly reduced and the half-life of IL-8 mRNA was unaffected by inhibition of p38 in flagellin-stimulated cells, even though IL-8 secretion was significantly decreased; the mechanism responsible for these observations was not proposed (26).
While it is clear that H. pylori can induce inflammation, most H. pylori infections of humans are chronic and can be lifelong without medical intervention. A pertinent question is why is the immune response ineffective against H. pylori? Why can other bacterial infections, such as those caused by Salmonella, be cleared by the immune response but those caused by H. pylori cannot? By studying the signaling pathways activated in H. pylori infection of human cells, it may be possible to identify bacterial virulence factors and/or host molecules or pathways unique to H. pylori infection that induce a host response that is inflammatory but functionally ineffective.
Acknowledgments
A.M.T. was supported in part by the Cell and Molecular Biology training grant (5T32-GM008136). This work was supported by National Institutes of Health grants AI51291 to J.B.G., in part by AI050733 to A.H.B., and by a Pilot and Feasibility Project from the Digestive Health Research Center at the University of Virginia to A.H.B. and J.B.G.
We would like to thank our colleagues in the lab for helpful discussions and Mark Smolkin for advice on the statistical analyses.
Editor: J. N. Weiser
REFERENCES
- 1.Abdel-Latif, M. M. M., H. J. Windle, K. A. Fitzgerald, Y. S. Ang, D. Ní Eidhin, M. Li-Weber, K. Sabra, and D. Kelleher. 2004. Helicobacter pylori activates the early growth response 1 protein in gastric epithelial cells. Infect. Immun. 72:3549-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal, S., A. Agrawal, B. Doughty, A. Gerwitz, J. Blenis, T. Van Dyke, and B. Pulendran. 2003. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 171:4984-4989. [DOI] [PubMed] [Google Scholar]
- 3.Aihara, M., D. Tsuchimoto, H. Takizawa, A. Azuma, H. Wakebe, Y. Ohmoto, K. Imagawa, M. Kikuchi, N. Mukaida, and K. Matsushima. 1997. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line, MKN45. Infect. Immun. 65:3218-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharyya, A., S. Pathak, S. Datta, S. Chattopadhyay, J. Basu, and M. Kundu. 2002. Mitogen-activated protein kinases and nuclear factor-kappaB regulate Helicobacter pylori-mediated interleukin-8 release from macrophages. Biochem. J. 368:121-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter, A. B., K. L. Knudtson, M. M. Monick, and G. W. Hunninghake. 1999. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP). J. Biol. Chem. 274:30858-30863. [DOI] [PubMed] [Google Scholar]
- 6.Covacci, A., J. L. Telford, G. Del Giudice, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 7.Goebeler, M., R. Gillitzer, K. Kilian, K. Utzel, E. B. Brocker, U. R. Rapp, and S. Ludwig. 2001. Multiple signaling pathways regulate NF-kappaB-dependent transcription of the monocyte chemoattractant protein-1 gene in primary endothelial cells. Blood 97:46-55. [DOI] [PubMed] [Google Scholar]
- 8.Karin, M., Z. Liu, and E. Zandi. 1997. AP-1 function and regulation. Curr. Opin. Cell Biol. 9:240-246. [DOI] [PubMed] [Google Scholar]
- 9.Keates, S., A. C. Keates, M. Warny, R. M. Peek, Jr., P. G. Murray, and C. P. Kelly. 1999. Differential activation of mitogen-activated protein kinases in AGS gastric epithelial cells by cag+ and cag− Helicobacter pylori. J. Immunol. 163:5552-5559. [PubMed] [Google Scholar]
- 10.Komai-Koma, M., L. Jones, G. S. Ogg, D. Xu, and F. Y. Liew. 2004. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc. Natl. Acad. Sci. USA 101:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda, S., H. Yoshida, K. Ogura, Y. Mitsuno, Y. Hirata, Y. Yamaji, M. Akanuma, Y. Shiratori, and M. Omata. 2000. H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterol. 119:97-108. [DOI] [PubMed] [Google Scholar]
- 12.Meyer-ter-Vehn, T., A. Covacci, M. Kist, and H. L. Pahl. 2000. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J. Biol. Chem. 275:16064-16072. [DOI] [PubMed] [Google Scholar]
- 13.Mitsuno, Y., H. Yoshida, S. Maeda, K. Ogura, Y. Hirata, T. Kawabe, Y. Shiratori, and M. Omata. 2001. Helicobacter pylori induced transactivation of SRE and AP-1 through the ERK signaling pathway in gastric cancer cells. Gut 49:18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moss, S. F., and S. Sood. 2003. Helicobacter pylori. Curr. Opin. Infect. Dis. 16:445-451. [DOI] [PubMed] [Google Scholar]
- 15.Naumann, M., S. Wessler, C. Bartsch, B. Wieland, A. Covacci, R. Haas, and T. F. Meyer. 1999. Activation of activator protein 1 and stress response kinases in epithelial cells colonized by Helicobacter pylori encoding the cag pathogenicity island. J. Biol. Chem. 274:31655-31662. [DOI] [PubMed] [Google Scholar]
- 16.Re, F., and J. L. Strominger. 2001. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276:37692-37699. [DOI] [PubMed] [Google Scholar]
- 17.Sabroe, I., L. R. Prince, E. C. Jones, M. J. Horsburgh, S. J. Foster, S. N. Vogel, S. K. Dower, and M. K. Whyte. 2003. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J. Immunol. 170:5268-5275. [DOI] [PubMed] [Google Scholar]
- 18.Sharma, S. A., M. K. Tummuru, M. J. Blaser, and L. D. Kerr. 1998. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J. Immunol. 160:2401-2407. [PubMed] [Google Scholar]
- 19.Slomiany, B. L., and A. Slomiany. 2001. Role of ERK and p38 mitogen-activated protein kinase cascades in gastric mucosal inflammatory responses to Helicobacter pylori lipopolysaccharide. IUBMB Life 51:315-320. [DOI] [PubMed] [Google Scholar]
- 20.Smith, M. F., Jr., A. Mitchell, G. Li, S. Ding, A. M. Fitzmaurice, K. Ryan, S. Crowe, and J. B. Goldberg. 2003. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J. Biol. Chem. 278:32552-32560. [DOI] [PubMed] [Google Scholar]
- 21.Strassheim, D., K. Asehnoune, J. S. Park, J. Y. Kim, Q. He, D. Richter, K. Kuhn, S. Mitra, and E. Abraham. 2004. Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of Toll-like receptor 2-stimulated neutrophils. J. Immunol. 172:5727-5733. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi, S., Y. Keto, T. Fujita, T. Uchiyama, and A. Yamamoto. 2001. FR167653, a p38 mitogen-activated protein kinase inhibitor, prevents Helicobacter pylori-induced gastritis in Mongolian gerbils. J. Pharmacol. Exp. Ther. 296:48-56. [PubMed] [Google Scholar]
- 23.Vasselon, T., and P. A. Detmers. 2002. Toll receptors: a central element in innate immune responses. Infect. Immun. 70:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winzen, R., M. Kracht, B. Ritter, A. Wilhelm, C. Y. Chen, A. B. Shyu, M. Muller, M. Gaestel, K. Resch, and H. Holtmann. 1999. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 18:4969-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada, H., T. Aihara, and S. Okabe. 2001. Mechanism for Helicobacter pylori stimulation of interleukin-8 production in a gastric epithelial cell line (MKN 28): roles of mitogen-activated protein kinase and interleukin-1beta. Biochem. Pharmacol. 61:1595-1604. [DOI] [PubMed] [Google Scholar]
- 26.Yu, Y., H. Zeng, S. Lyons, A. Carlson, D. Merlin, A. S. Neish, and A. T. Gewirtz. 2003. TLR5-mediated activation of p38 MAPK regulates epithelial IL-8 expression via posttranscriptional mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G282-G290. [DOI] [PubMed] [Google Scholar]