Abstract
Chlamydiae are obligate intracellular bacteria that can inhibit apoptosis of their host cell. As shown recently, this inhibition is in part explained by the proteolytic degradation of the proapoptotic Bcl-2 family members (BH3-only proteins) Bim, Puma, and Bad upon chlamydial infection. In this study, we further explore this antiapoptotic mechanism. In cells infected with a Chlamydia trachomatis L2 strain, Bim, Puma, and Bad were degraded with similar kinetics, and the degradation of all three was blocked by inhibition of the proteasome. Furthermore, the BH3-only proteins Bmf, Noxa, and tBid were also targeted by chlamydial infection. The constitutively expressed Bmf disappeared during infection. When Noxa was experimentally induced, the levels were also reduced by infection with C. trachomatis. In death-receptor-induced apoptosis, cleaved and activated tBid was degraded, and this destruction was also prevented by inhibition of the proteasome. These results show that chlamydial infection leads to a broad degradation of BH3-only proteins. This loss of proapoptotic factors can explain the almost general protection of infected cells against apoptotic stimuli.
Chlamydiae are obligate intracellular bacteria and depend on host cell factors to replicate. They reside in a cytosolic vacuole in eukaryotic cells and have the ability to interfere with cellular functions in various ways. Three chlamydial species are pathogenic to humans: Chlamydia trachomatis, C. pneumoniae, and C. psittaci. Of these, C. trachomatis is of great importance as the cause of urogenital infections, leading to sexually transmitted diseases all over the world, and trachoma, an eye infection that is common in tropical countries (9).
Many pieces of evidence indicate that apoptosis is used as a defense against microbial pathogens (11, 20). Over the last few years, it has been recognized that cell death plays a role in the interplay between chlamydia and host cells; a balance of apoptosis/cell death-inducing and inhibiting factors determines the outcome of the infection (for a comprehensive recent review, see reference 3).
All chlamydial species investigated have the capacity to inhibit apoptosis in the infected cell (5, 6, 10), suggesting that apoptosis also plays a role in the host cell's response to chlamydial infection.
Cell death by apoptosis occurs when an intracellular signal transduction pathway is activated. In the apoptotic signaling pathway, the release of cytochrome c from the mitochondria is a central step that is required to activate caspases and to cause the morphological changes of apoptosis such as nuclear condensation. Only one situation is known in which apoptosis occurs in the absence of cytochrome c release: the activation of caspase-8 through death receptors (Fas, TNFR, and TRAILR) in some cells (type I cells). In type II cells, on the other hand, death receptor signaling also requires the release of cytochrome c (19).
Mitochondrial release of cytochrome c is regulated by the Bcl-2 family of proteins. Bcl-2-like proteins consist of three groups: the proapoptotic subfamily of Bax, Bak, and Bok (effectors of cytochrome c release); antiapoptotic proteins such as Bcl-2 and Bcl-xL (inhibitors of cytochrome c release); and the proapoptotic BH3-only proteins. The latter group is structurally diverse and has in common only a small domain termed the Bcl-2 homology domain 3 (BH3 domain). This group of proteins is required to initiate cytochrome c release (2). At present, eight confirmed members of BH3-only proteins are known (several more have been suggested). One or several of the BH3-only proteins are activated by a given stimulus. The activation of BH3-only proteins can involve transcriptional induction (this is the case for the proteins Puma, Noxa, and Bim), release from sequestration sites (Bim and Bmf), dephosphorylation (Bad), and proteolytic cleavage (Bid). The molecular details are uncertain, and there are probably differences in the precise action of various BH3-only proteins. However, all of them lead to the activation of Bax/Bak, and this activation requires the conserved BH3 domain (2).
The protection of chlamydia-infected cells against external stimuli is profound. Molecular analysis of this protection has shown that the release of cytochrome c is prevented (5, 7). The inhibition of apoptosis is limited to stimuli that act via this release and does not extend to death receptor-induced apoptosis in type I cells (6). Recent work further shows that the inhibition acts upstream of Bax/Bak activation (8, 22).
Significantly, chlamydial infection causes the proteolytic degradation of the BH3-only proteins Bim, Puma, and Bad through a proteasomal activity (8). In this process, a short region in Bim encompassing the BH3 domain was sufficient for degradation. Since the BH3 domain is the only part that is structurally conserved in all BH3-only proteins, we derived the model that all BH3-only proteins are destroyed by the same mechanism during infection. Here we test this model and provide a broader study of this process. Time course experiments suggest that it is indeed the same mechanism that leads to the degradation of Bim, Puma, and Bad. Analysis of the BH3-only proteins Bid, Noxa, and Bmf shows that degradation also extends to these family members and that the accessibility of the BH3 domain is required for destruction.
MATERIALS AND METHODS
Cell lines, bacterial organisms, and infection.
The human laryngeal carcinoma cell line Hep2 and the human T lymphocyte cell line Jurkat were obtained from the American Type Culture Collection. The cell line T-REx HeLa, which stably expresses the tetracycline-repressor, was purchased from Invitrogen. The human cervical adenocarcinoma cell line HeLa-fas (HeLa cells stably transfected to express human fas/APO-1/CD95 was kindly provided by H. Wajant, University of Stuttgart, Stuttgart, Germany [8]), mouse embryo fibroblasts (MEF) were kindly provided by Irmgard Förster, Technische Universität München, Munich, Germany. Cells were cultured at 37°C in 5% CO2 in Dulbecco modified Eagle medium for HeLa-fas, MEF, T-REx HeLa (plus 5 μg of blasticidin/ml), and Hep2 cells and in RPMI 1640 for Jurkat cells, each supplemented with 10% heat-inactivated fetal calf serum and 2 mM l-glutamine. HeLa T-REx Noxa clones were cultured in the additional presence of 125 μg of zeocin/ml.
The mycoplasma-free C. trachomatis strain L2 was obtained from the American Type Culture Collection. Chlamydiae were grown in Hep2 cells and purified as described previously (6, 8). Human cells were infected with C. trachomatis at a multiplicity of infection (MOI) of 3.
Induction of apoptosis.
HeLa-fas cells (3 × 105/well in six-well plates seeded the day before) or Jurkat cells (106 cells/well in 12-well plates) were infected with chlamydiae or not and then subjected to anti-CD95 monoclonal antibody (MAb) stimulation (CH11; Upstate Biotechnology) at 100 ng/ml.
For inducible expression, Noxa was cloned by PCR into the vector pCDNA4/TO. When stably transfected into cells that stably express the tetracycline repressor (HeLa T-REx cells), the expression can be induced by removal of the suppressor upon addition of tetracycline. This HeLa cell line was first infected or left uninfected and, at the indicated time points postinfection, the expression of Noxa was induced by the addition of tetracycline. Cells were harvested for Western blot analysis as indicated in the figure legends.
Western blot analysis.
Cells were harvested at the indicated time points. In some experiments cells were treated with the proteasome inhibitor MG132 (Calbiochem). For detergent extracts, 5 × 105 cells were lysed by incubation in 50 μl of Triton buffer (1% Triton X-100, 0.05 M PIPES-NaOH, 0.05 M HEPES [pH 7.0], 2 mM MgCl2, 1 mM EDTA, 10 mM dithiothreitol, and protease inhibitors [Roche]) for 30 min on ice. After centrifugation at 2,000 × g at 4°C for 10 min, loading buffer was added and the lysates were run on a 12% polyacrylamide gel. Proteins were transferred to nitrocellulose membranes, and membranes were probed with antibodies specific for Bax and Bak (both from Upstate Biotechnology), Bim (polyclonal antibody from rabbits; Sigma), Puma N terminus (antibody from rabbits; Sigma), Bid and Bad (both from Cell Signaling Technology), Bmf (kindly provided by Andreas Strasser, WEHI, Melbourne, Australia; now available from Alexis Biochemicals as clone 17A9), Noxa (Alexis), Bcl-2 (Pharmingen), Bcl-xL (Cell Signaling Technology), chlamydial HSP60 (Affinity BioReagents), or tubulin (Sigma). Proteins were visualized by using peroxidase-conjugated secondary antibodies and a chemiluminescence detection system (Perkin-Elmer/Life Sciences, Boston, Mass.).
RESULTS
Time course of degradation of the BH3-only proteins Bim, Puma, and Noxa in chlamydia-infected cells.
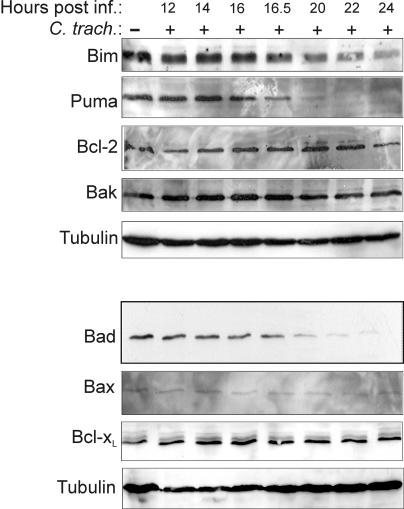
In a previous study we demonstrated that chlamydial infection causes the degradation of the BH3-only proteins Bim, Puma, and Bad. Bim disappearance became detectable approximately 16 h after infection with C. trachomatis and was nearly complete at about 24 h (the developmental cycle of C. trachomatis takes about 48 h). Figure 1 shows a time course study of the expression of Bim, Puma, and Bad. The disappearance of all three BH3-only proteins began at a very similar point in time (just after 16 h of infection), strongly suggesting that they are the target of the same activity. The apparent sensitivity to degradation, as measured by the time until complete disappearance, was similar for Puma and Bad and somewhat lower for Bim (Fig. 1). We also monitored the protein level of other members of the Bcl-2 family: the expression levels of the proapoptotic proteins Bax and Bak were unchanged, as were the levels of the antiapoptotic group members Bcl-2 and Bcl-xL, emphasizing the specificity of chlamydial targeting.
FIG. 1.
Time course of disappearance of Bim, Puma, and Bad during chlamydial infection. At the indicated time points postinfection with C. trachomatis Triton X-100 extracts of Hep2 cell samples were prepared and analyzed by Western blotting against the Bcl-2-family proteins Bim, Puma, Bad, Bcl-2, Bcl-xL, Bax, and Bak. Detection of tubulin served as a loading control. The top and bottom panels were obtained by reprobing two membranes that contained samples from the same experiments. Degradation of Bim, Puma, and Bad was observed in at least three experiments.
Degradation of Bim, Puma, and Bad requires proteasomal activity.
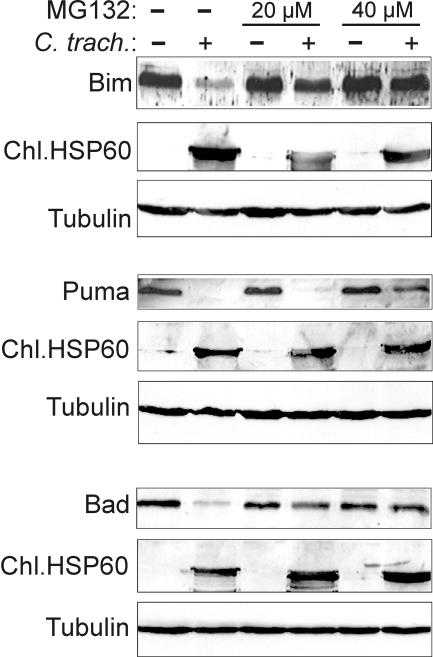
Proteolytic destruction by the cellular proteasome is an important means to regulate protein levels in cells. Typically, proteins are linked to the small peptide ubiquitin, which allows recognition and degradation by the proteasome. The Bim degradation during chlamydial infection was prevented by proteasome inhibitors (8), implicating the proteasome in Bim degradation. To analyze whether Puma and Bad are also degraded by this activity, cells were infected with C. trachomatis, and the levels of Bim, Puma, and Bad were assessed in the absence or presence of the proteasome inhibitor MG132. As shown in Fig. 2, MG132 blocked the disappearance of all three BH3-only proteins. The sensitivity of the inhibition to MG132 was somewhat different: whereas the degradation of Bim and Bad was already significantly diminished at 20 μM, this concentration had almost no effect on Puma degradation, and even at 40 μM Puma degradation was still detectable. These results confirm that a proteasomal activity induces the degradation of all three proteins.
FIG. 2.
Degradation of Bim, Puma, and Bad requires proteasomal activity. Hep2 cells were infected with C. trachomatis for 16 h or left uninfected. Then, the indicated amount of the proteasome inhibitor MG132 was added for 8 h. Cells were extracted, and extracts were analyzed by Western blotting with antibodies against Bim, Puma, and Bad. Chlamydial Hsp60 served as a control for chlamydial infection; tubulin served as a protein loading control.
Disappearance of Bmf in MEF cells upon infection with C. trachomatis.
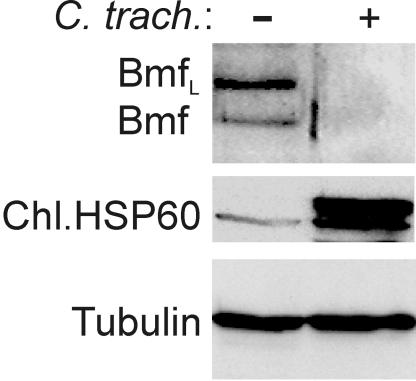
In addition to the BH3-only proteins investigated above, the proteins Bmf, Noxa, Bid, Hrk/DP5, and Bik/Nbk/Blk are confirmed members of this family (2). We were unable to detect unequivocal expression of Hrk or Bik with the available antibodies in a number of cell lines. For the detection of Bmf, only one MAb works reliably in our hands, and this antibody recognizes only mouse but not human Bmf. Bmf is expressed in nonapoptotic cells, where it is sequestered to the actin cytoskeleton. Stimuli that induce apoptosis via Bmf activation cause its release from this site of sequestration. To address the question of Bmf degradation, MEF were infected with C. trachomatis. As shown in Fig. 3, infection leads to reduction of Bmf below detection levels. Thus, like the other BH3-only proteins, Bmf is a target of chlamydial infection.
FIG. 3.
Disappearance of the BH3-only protein Bmf in chlamydia-infected cells. MEF were infected with C. trachomatis for 24 h. Cells were harvested, and Western blot analysis was performed with an antibody to Bmf (two isoforms of Bmf are detected). The identity of Bmf was confirmed in blots with lysate from Bmf-deficient mice (not shown). Chlamydial Hsp60 was tested as a control of infection; tubulin was tested as a loading control. The experiment was performed twice with very similar results.
Reduction of Noxa levels in chlamydia-infected cells.
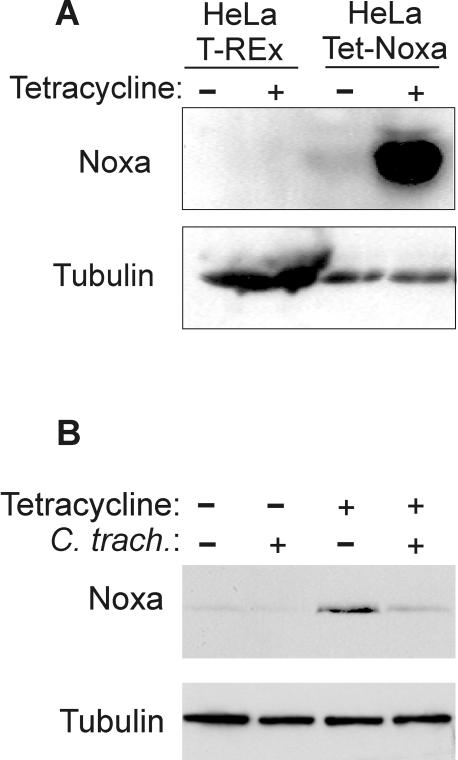
The BH3-only protein Noxa is regulated by transcriptional induction. Noxa protein is expressed to only very low levels in healthy cells but has been shown to be upregulated in some cell lines upon stress insults. We failed in our attempts to induce Noxa protein in HeLa cells by the reported Noxa stimuli of gamma irradiation and hypoxia (not shown). We therefore turned to a tetracycline-inducible system of Noxa induction. HeLa cells expressing the Tet repressor (23) were transfected with a vector in which human Noxa was placed under the control of this repressor. By adding tetracycline, the repressor is removed and Noxa can be induced. Clones were selected that stably carried this construct (HeLa Tet-Noxa cells, Fig. 4A). HeLa Tet-Noxa cells were infected for 24 h or left uninfected and Noxa was induced by the addition of tetracycline for 8 h (Noxa expression is not sufficient to induce apoptosis under this protocol). Cells were harvested and subjected to Western blotting. As shown in Fig. 4B, the Noxa levels were strongly diminished when cells had been infected with C. trachomatis. Earlier studies have shown that the promoter used to express Noxa is active also in chlamydia-infected cells (8) (expression of Bim and Puma using the same vector). The data therefore indicate that Noxa protein is also targeted by chlamydial infection.
FIG. 4.
Chlamydial infection prevents the appearance of Noxa protein. (A) Induction of Noxa by tetracycline in HeLa Tet-Noxa cells. Cells stably carrying a construct where Noxa was placed under a tetracycline-inducible promoter were treated with tetracycline for 24 h or left untreated (Noxa expression becomes detectable after about 2 h of treatment and is maximal at approximately 24 h [not shown]). Noxa was detected by Western blotting; tubulin was used as a loading control. (B) Induction of Noxa in infected cells. HeLa Tet-Noxa cells were infected with C. trachomatis or left uninfected. After 24 h, tetracycline was added for 8 h to some wells. Cells were harvested, and Western blot analysis was performed with a Noxa-specific antibody. As established earlier, treatment with antibiotics during this time frame does not affect the antiapoptotic effect of chlamydia (7).
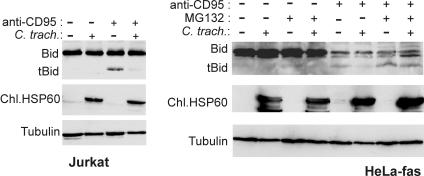
Proteolytic degradation of tBid during infection.
The BH3-only protein Bid is a mediator of death-receptor induced apoptosis. Although its activity is limited to so-called type II cells (19), it is required in these cells for cytochrome c release upon death receptor stimulation. We have previously investigated the chlamydial protection against death receptor-mediated apoptosis. When HeLa-fas or Jurkat (lymphoid) cells were stimulated with agonistic anti-fas/APO-1/CD95 antibodies, the release of cytochrome c was blocked in cells infected with chlamydia (since HeLa cells do not express high enough levels of Fas, we used a cell line stably transfected to express human Fas). However, the early events in the apoptotic pathway were not affected. Fas-induced processing of caspase-8 and caspase-8-mediated cleavage of Bid were found to be normal despite infection (tBid is not detected by most antibodies and could therefore not be analyzed in this earlier study) (6).
Bid is normally inactive and is activated only upon cleavage by caspase-8 to generate the active truncated form (i.e., tBid), which then activates Bax and thus causes cytochrome c release (14, 15). Since Bid is a BH3-only protein it might be expected that it is degraded upon infection by C. trachomatis, but this was not the case (6). However, structural studies revealed a possible explanation for this finding. In intact Bid, the BH3-domain was found to be hidden in the three-dimensional structure of the protein. Caspase-8-cleavage induced structural changes that caused the exposure of the BH3 domain on the surface of tBid (16).
Since chlamydial infection targets the BH3 domain in Bim (8), this led us to speculate that, unlike intact Bid, tBid would be degraded as it is generated during fas stimulation. As shown in Fig. 5, this is indeed the case. In untreated Jurkat cells (Fig. 5, left), as well as in HeLa-fas cells (Fig. 5, right), only inactive full-length Bid was detectable irrespective of infection with C. trachomatis. After stimulation with anti-fas antibodies for 6 h, active tBid could be detected in both cell types. However, in infected cells there was clearly less tBid present. This was not due to less Bid activation since Bid cleavage has been shown to be unaltered by infection in time course experiments in the same cells (6). Furthermore, when HeLa-fas cells were treated with anti-fas antibody in the presence of the proteasome inhibitor MG132, the disappearance of tBid was prevented (Fig. 5, right). Thus, chlamydial infection leads to the degradation of tBid but not Bid by a proteasomal activity.
FIG. 5.
Chlamydial infection leads to the degradation of activated (truncated) Bid. Jurkat or HeLa-fas cells were infected with C. trachomatis or left uninfected. After 24 h of infection cells were treated with MAb to the fas death receptor. In some experiments, the proteasome inhibitor MG132 (50 μM) was added at the same time, as indicated. After 6 h cells were harvested, and extracts were subjected to Western blotting and probed with an antibody that recognizes both Bid and tBid (although sensitivity for tBid is much lower than for Bid). Both Jurkat cells (left) and HeLa-fas cells (right) are shown. Chlamydial Hsp60 served as a control for chlamydial infection; detection of tubulin served as a loading control.
DISCUSSION
The protection of an infected cell by chlamydia against experimental apoptotic stimuli is well established. We have recently mapped this protection to the proteolytic destruction of the BH3-only protein Bim and have shown that the BH3-only proteins Puma and Bad also disappear during infection (8). Since a short region in the Bim protein encompassing its BH3 domain was the target of chlamydial infection, we speculated that the same mechanism could also apply to other BH3-only proteins. In the present study we show that this is indeed the case. The disappearance of Puma and Bad occurred with the same kinetics as found for Bim (8) and was inhibited in all three cases by blockade of the proteasome. In addition, chlamydial infection caused the disappearance of the constitutively expressed BH3-only protein Bmf, of the transcriptionally regulated protein Noxa upon induction, and of the cleavage-generated active BH3-only protein tBid. Members of the other classes of the Bcl-2 protein family were not affected in their expression. Infection with C. trachomatis thus leads to a broad but specific degradation of the BH3-only class of initiators of apoptosis.
Although some molecular details are still unclear, there is now ample evidence that BH3-only proteins are required for the release of cytochrome c during apoptosis (2). Experiments with mice deficient for individual BH3-only proteins support the model that a given apoptotic stimulus requires one (in some cases more than one) BH3-only protein. For instance, apoptosis induced by microtubule perturbation is mediated via Bim, and apoptosis upon cytokine withdrawal in lymphocytes is mediated via Bim and Puma, upon gamma irradiation at least to a major part via Puma, glucose withdrawal possibly via Bad, death receptor signaling (in type II cells) via Bid, and DNA-damage in part via Noxa (1, 4, 12, 21, 24). Although this has not been fully explored, the available evidence suggests that at least the vast majority of apoptotic stimuli depends on BH3-only proteins to induce apoptosis. By degradation of BH3-only proteins, chlamydial infection would therefore impose a very broad protection. In fact, the only death stimulus that has been reported not to be blocked by chlamydial infection is a death receptor signal in type I cells (6). This is not surprising, however, since this stimulus operates in the absence of cytochrome c release and therefore without requirement for BH3-only proteins (19). As reported recently, chlamydial infection fails to protect against experimental expression of BH3-only proteins from a strong promoter (8). This indicates that no other protective mechanism operates in infected cells and that therefore the degradation of BH3-only proteins is the relevant event.
Degradation of BH3-only proteins started approximately 16 h postinfection and was complete approximately 24 h postinfection. This correlates well with the time course of the build-up of protection against apoptosis. Fan et al. (5) reported that protection in cells infected with C. trachomatis L2 at an MOI of 5 started at ca. 14 h postinfection and infection with an MOI of 0.5 caused protection starting at approximately 24 h postinfection. We used an MOI of 3 and observed BH3-only degradation at 16 h, demonstrating a good correlation between BH3-only protein degradation and observed protection. The data suggest that a protection against apoptosis is not required earlier during infection, perhaps because the inclusion is a trigger for apoptosis only at later time points.
Although protection against apoptosis has been reported as a prominent feature of chlamydial infection, it has been suggested that apoptosis is also induced through the activation of Bax at later stages of chlamydial infection (18) and the clearance of chlamydia during vaginal infection of mice was enhanced in mice lacking Bax (17). Bax was furthermore under experimental conditions directly (in the absence of BH3-only proteins) activated by oxidative stress (13). It is therefore possible that chlamydial infection might, despite the absence of BH3-only proteins, cause the activation of Bax toward the end of the replicative cycle.
It is still unclear how chlamydia mediate degradation of BH3-only proteins. The finding that the disappearance could be blocked by inhibitors of the cellular proteasome suggests that a chlamydial factor modifies the proteins and thereby targets them for proteasomal destruction. It will have to be the focus of future work to delineate the molecular events leading to this destruction.
Acknowledgments
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (to G.H.) and through graduate school grant GRK 333.
We thank Juliane Vier and Christina Hilpert for expert technical assistance.
Editor: D. L. Burns
REFERENCES
- 1.Bouillet, P., D. Metcalf, D. C. Huang, D. M. Tarlinton, T. W. Kay, F. Kontgen, J. M. Adams, and A. Strasser. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286:1735-1738. [DOI] [PubMed] [Google Scholar]
- 2.Bouillet, P., and A. Strasser. 2002. BH3-only proteins: evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J. Cell Sci. 115:1567-1574. [DOI] [PubMed] [Google Scholar]
- 3.Byrne, G. I., and D. M. Ojcius. 2004. Chlamydia and apoptosis: life and death decisions of an intracellular pathogen. Nat. Rev. Microbiol. 2:802-808. [DOI] [PubMed] [Google Scholar]
- 4.Danial, N. N., C. F. Gramm, L. Scorrano, C. Y. Zhang, S. Krauss, A. M. Ranger, S. R. Datta, M. E. Greenberg, L. J. Licklider, B. B. Lowell, S. P. Gygi, and S. J. Korsmeyer. 2003. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 424:952-956. [DOI] [PubMed] [Google Scholar]
- 5.Fan, T., H. Lu, H. Hu, L. Shi, G. A. McClarty, D. M. Nance, A. H. Greenberg, and G. Zhong. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J. Exp. Med. 187:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer, S. F., T. Harlander, J. Vier, and G. Hacker. 2004. Protection against CD95-induced apoptosis by chlamydial infection at a mitochondrial step. Infect. Immun. 72:1107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer, S. F., C. Schwarz, J. Vier, and G. Hacker. 2001. Characterization of antiapoptotic activities of Chlamydia pneumoniae in human cells. Infect. Immun. 69:7121-7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer, S. F., J. Vier, S. Kirschnek, A. Klos, S. Hess, S. Ying, and G. Hacker. 2004. Chlamydia inhibit host cell-apoptosis by degradation of pro-apoptotic BH3-only proteins. J. Exp. Med. 200:905-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grayston, J. T., and S. Wang. 1975. New knowledge of chlamydiae and the diseases they cause. J. Infect. Dis. 132:87-105. [DOI] [PubMed] [Google Scholar]
- 10.Greene, W., Y. Xiao, Y. Huang, G. McClarty, and G. Zhong. 2004. Chlamydia-infected cells continue to undergo mitosis and resist induction of apoptosis. Infect. Immun. 72:451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacker, G., and S. F. Fischer. 2002. Bacterial antiapoptotic activities. FEMS Microbiol. Lett. 211:1-6. [DOI] [PubMed] [Google Scholar]
- 12.Hildeman, D. A., Y. Zhu, T. C. Mitchell, P. Bouillet, A. Strasser, J. Kappler, and P. Marrack. 2002. Activated T-cell death in vivo mediated by proapoptotic bcl-2 family member Bim. Immunity 16:759-767. [DOI] [PubMed] [Google Scholar]
- 13.Jungas, T., I. Motta, F. Duffieux, P. Fanen, V. Stoven, and D. M. Ojcius. 2002. Glutathione levels and BAX activation during apoptosis due to oxidative stress in cells expressing wild-type and mutant cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 277:27912-27918. [DOI] [PubMed] [Google Scholar]
- 14.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 15.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 16.McDonnell, J. M., D. Fushman, C. L. Milliman, S. J. Korsmeyer, and D. Cowburn. 1999. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell 96:625-634. [DOI] [PubMed] [Google Scholar]
- 17.Perfettini, J. L., T. Darville, G. Gachelin, P. Souque, M. Huerre, A. Dautry-Varsat, and D. M. Ojcius. 2000. Effect of Chlamydia trachomatis infection and subsequent tumor necrosis factor alpha secretion on apoptosis in the murine genital tract. Infect. Immun. 68:2237-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perfettini, J. L., J. C. Reed, N. Israel, J. C. Martinou, A. Dautry-Varsat, and D. M. Ojcius. 2002. Role of Bcl-2 family members in caspase-independent apoptosis during Chlamydia infection. Infect. Immun. 70:55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K. J. Tomaselli, K. M. Debatin, P. H. Krammer, and M. E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaux, D. L., G. Hacker, and A. Strasser. 1994. An evolutionary perspective on apoptosis. Cell 76:777-779. [DOI] [PubMed] [Google Scholar]
- 21.Villunger, A., E. M. Michalak, L. Coultas, F. Mullauer, G. Bock, M. J. Ausserlechner, J. M. Adams, and A. Strasser. 2003. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science 302:1036-1038. [DOI] [PubMed] [Google Scholar]
- 22.Xiao, Y., Y. Zhong, W. Greene, F. Dong, and G. Zhong. 2004. Chlamydia trachomatis infection inhibits both Bax and Bak activation induced by staurosporine. Infect. Immun. 72:5470-5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao, F., T. Svensjo, T. Winkler, M. Lu, C. Eriksson, and E. Eriksson. 1998. Tetracycline repressor, tetR, rather than the tetR-mammalian cell transcription factor fusion derivatives, regulates inducible gene expression in mammalian cells. Hum. Gene Ther. 9:1939-1950. [DOI] [PubMed] [Google Scholar]
- 24.Yin, X. M., K. Wang, A. Gross, Y. Zhao, S. Zinkel, B. Klocke, K. A. Roth, and S. J. Korsmeyer. 1999. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400:886-891. [DOI] [PubMed] [Google Scholar]