Abstract
Introduction
Several studies have reported that exposure to antibiotics can lead to asthma during early childhood. However, the association between antibiotic use and risk of asthma in the adult population remains unclear. This study aimed to investigate the association between antibiotic use and asthma in adults.
Methods
We used data from the National Health Insurance Service (NHIS)-Health Screening Cohort, which included participants aged ≥40 years who had health screening examination data in 2005–2006. A total of 248 961 participants with a mean age of 55.43 years were enrolled in this retrospective cohort study. To evaluate antibiotic exposure from the NHIS database for 5 years (2002–2006), cumulative usage and multiclass prescriptions were identified, respectively. During the follow-up period (2007–2019), 42 452 patients were diagnosed with asthma. A multivariate Cox proportional hazard regression model was used to assess the association between antibiotic use and newly diagnosed asthma.
Results
Participants with antibiotic use for ≥91 days showed a higher risk of asthma (adjusted HR (aHR) 1.84, 95% CI 1.72 to 1.96) compared with participants who did not use antibiotics (n=38 450), with a duration-dependent association (ptrend<0.001). Furthermore, ≥4 antibiotic class user group had an increased risk of asthma (aHR 1.44, 95% CI 1.39 to 1.49) compared with one class of antibiotic use (n=64 698). Also, one class of antibiotic use had a higher risk of asthma (aHR 1.21, 95% CI 1.17 to 1.26) compared with non-users, and it also showed a duration-dependent relationship in all classes, including 1, 2, 3 and ≥4 class group (ptrend<0.001). The duration-response relationship between antibiotic use and increased risk of asthma remained in our sensitivity analyses with the washout and shifting of the index date.
Conclusions
The duration-response pattern observed in antibiotic use and asthma may suggest the implication of proper antibiotic use and management in adults.
Keywords: asthma epidemiology, asthma pharmacology, asthma
WHAT IS ALREADY KNOWN ON THIS TOPIC
Numerous studies have shown that antibiotic use in newborns and early childhood can cause allergic reactions such as asthma; however, studies on the effects of antibiotics on asthma in adults are limited.
WHAT THIS STUDY ADDS
Similar to the results of a study in early childhood, our study showed a duration-dependent relationship between the use of antibiotics and asthma in adults aged over 40 years.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study suggests that the cumulative use of antibiotics in adults is associated with asthma; therefore, it can be used to guide and manage the proper use of antibiotics.
Introduction
Asthma is an inflammatory disorder characterised by airway hyper-responsiveness and variable airflow restriction with respiratory symptoms.1 2 The prevalence of asthma has been steadily increasing worldwide across all age groups over the past four decades.3 4 In adults aged >64 years, asthma is an increasingly serious health problem with improved longevity in the total population.5 The mortality rates for asthma were the highest in the ≥65 years age group. In addition, 40% of older adult patients with asthma experience their first onset after the age of 40 years.4 Nevertheless, the pathophysiology of asthma in adults is relatively understudied.4 6 7 Thus, identifying and managing the risk factors for asthma to reduce the adverse effects and related mortality among middle-aged adults are important.
Previous studies have suggested that high antibiotic exposure increases the risk of asthma. Accumulating pathophysiological and clinical evidence suggests that antibiotic use increases the prevalence of asthma in early childhood.8–11 Antibiotics trigger dysbiosis of bacterial communities, and early microbiome composition skews the developing immune system towards dysfunctional allergic responses.12–14 The association between antibiotic use and development of asthma in early childhood may be explained by the hygiene hypothesis, which states that exposure to endotoxins in early childhood can lead to a decrease in T-helper 2 (Th2)-biased responses with the continual production of IgE.10 A large twin study investigated the relationship between antibiotics and asthma based on prescription claims data considering environmental and genetic factors.15 However, these associations have not been found in previous studies, particularly in adult populations. Although some studies did not consider the use of antibiotics to reduce respiratory symptoms, indicating asthmatic prognosis,16 17 other studies based on self-reported antibiotic usage databases could have a high risk of recall bias.18 19 Further studies are required to address these limitations.
Given the growing need for epidemiological evidence on the relationship between antibiotic exposure and the risk of asthma in adults, we aimed to evaluate the risk of incident asthma. We conducted an analysis of asthma risk using data from the National Health Insurance Service-Health Screening Cohort (NHIS-HEALS) in Korean middle-aged to older-aged adults based on (1) the cumulative antibiotic prescription days and (2) the number of classes of antibiotics.
Methods
Study population
We collected data from the NHIS-HEALS (NHIS-2022-2-088) from 2002 to 2019. The NHIS offers compulsory health insurance and covers approximately 97% of the population.10 In South Korea, biennial health screening programmes are offered to individuals aged ≥40 years and their dependents by the NHIS. From these health-screening programmes, the NHIS-HEALS includes data such as demographic variables, laboratory results, health behaviours, drug prescriptions, healthcare visits and mortality information for 2002–2019.
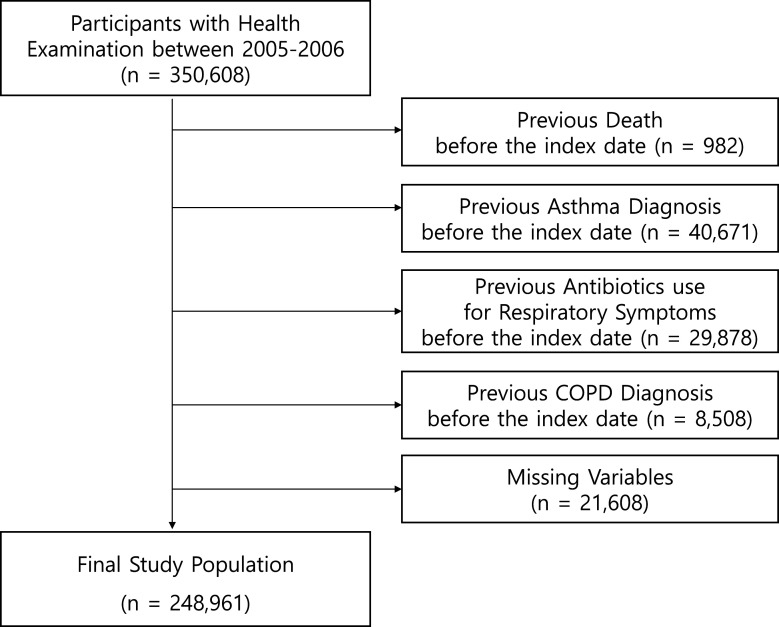
We included 350 608 participants with health screening data from 2005 to 2006. The participants who passed away before the index date of 1 January 2007 (n=982), who had an asthma diagnosis before the index date (n=40 671), who received antibiotics for respiratory symptoms before the index date (n=29 878), who had a previous chronic obstructive pulmonary disease (COPD) diagnosis before the index date (n=8508) and participants with missing variables (n=21 608) were excluded (figure 1).
Figure 1.
Selection of cohort study participants.COPD, chronic obstructive pulmonary disease.
Asthma diagnosed before the index date of 1 January 2007 was excluded according to the International Classification of Diseases, 10th edition (ICD-10) with J45 or J46 codes in the main diagnosis. To avoid reverse causation and potential biases of confounding by indication, we excluded participants who received antibiotics for respiratory symptoms, such as cough, wheezing, chest and acute upper or lower respiratory infections, including pneumonia.9 Antibiotics for respiratory symptoms were identified with the J00-J22, R05, R062 and R0989 codes (ICD-10) with simultaneous prescriptions of antibiotics based on Anatomical Therapeutic Chemical (ATC) described in online supplemental table 1. COPD and asthma have similar overlapping symptoms, such as airway obstruction and inflammatory processes, making them difficult to diagnose in primary care settings.20 Therefore, patients with COPD with the J44 code (ICD-10) were excluded. Ultimately, 248 961 participants were included in this retrospective cohort study as the baseline sample.
bmjresp-2023-001643supp001.pdf (194.9KB, pdf)
Key variables
Outcome variable
The main outcome of this study was newly diagnosed cases of asthma between 2007 and 2019. Asthma diagnoses were defined based on the medical treatment data using the ICD-10. Individuals newly diagnosed with asthma (ICD-10: J45) or status asthmaticus (ICD-10: J46) were considered with newly diagnosed asthma. We selected participants who were prescribed asthma medications, including tablet-based β2-agonists, inhaled β-agonists, leukotriene receptor antagonists, inhaled corticosteroids (ICSs) and ICSs with long-acting β2-agonists.21–23 The participants were censored by an asthma event, death or 31 December 2019, whichever occurred first.
Exposure variable
The cumulative antibiotic prescription days and number of antibiotic classes from 2002 to 2006 were exposure variables that were defined individually in the NHIS database. Antibiotics were defined based on the ATC established by WHO. The antibiotics investigated in this study included tetracyclines, macrolides, fluoroquinolones, penicillins, sulfonamides, lincosamides, vancomycin, carbapenems, cephalosporins, monobactams and linezolid, which are presented in . Antibiotic prescriptions for cumulative days were grouped into 0, 1–14, 15–30, 31–90 and ≥91 days, and the antibiotic classes were divided into 0, 1, 2, 3 and ≥4.
Covariates
The covariates included age, sex, residence, household income, smoking status, alcohol intake, physical activity, body mass index (BMI; kg/m2), total cholesterol (mg/dL), systolic blood pressure (mm Hg), fasting serum glucose (mg/dL), number of healthcare visits, Carlson Comorbidity Index (CCI), previous atopic dermatitis (AD), previous allergic rhinitis (AR) and infectious diseases. To adjust for the indication of antibiotic use for infectious diseases, we included intra-abdominal infections; urinary tract infections; intestinal infectious diseases; skin, soft tissue, bone and joint infections and others24 25 using ICD-10 codes along with the prescription of one or more antibiotics (Online supplemental table 2).
Household income was divided into quartiles based on insurance premium data and depicted from lowest to highest levels. BMI, blood pressure and total cholesterol levels were adjusted as surrogate markers for obesity and metabolic syndrome, which could be major risk factors for asthma.26 The CCI, which considers a participant’s health status based on various comorbid conditions, was adjusted using health claims records. The healthcare visit variable was the sum of the days the examinee visited a nursing institution for all types of treatment (first visit, revisit and admission) from 2002 to 2006.10 27 28
We drew a directed acyclic graph that included all covariates to determine the appropriate covariates and avoid duplicate variables (Online supplemental figure 1).
Statistical analysis
The clinical characteristics of the participants were compared with the cumulative number of days for which antibiotics were prescribed. The adjusted HRs (aHRs) and 95% CIs of asthma incidence and effects of antibiotic use were estimated using Cox proportional hazards regression. The proportional hazard assumption was tested statistically and visually using the Schoenfeld residual method. To identify the effects of antibiotic use on asthma incidence, we applied multivariate Cox proportional hazard regression analysis using two models.24 25 29 Model 1 was adjusted for baseline characteristics, such as age, sex, residence, household income, smoking status, alcohol intake, physical activity, BMI, total cholesterol, systolic blood pressure, fasting serum glucose, CCI, number of healthcare visits, previous AD and previous AR. Last, we adjusted model 2 for the variables in model 1 plus infectious diseases such as intra-abdominal infections; urinary tract infections; intestinal infectious diseases; skin, soft tissue, bone and joint infections and others. In model 1, which did not incorporate infection adjustment that prompted antibiotic use, the analysis encompasses cases without antibiotic prescriptions. In model 2, where infection adjustment was applied, the analysis did not include the antibiotic non-user but rather focused on the group who were prescribed antibiotics. Non-users of antibiotics were used as references to evaluate the risk of asthma and use of antibiotics. Additionally, each of the 1–14 antibiotic users and only one class of antibiotic users were used as references in all adjusted models, including infectious disease as an additional covariate (Online supplemental table 2).
For sensitivity analysis, we applied washout periods of 3, 4 and 5 years to minimise the likelihood of reverse causation and reduce protopathic bias. That is, we did not consider asthma events during the first 3–5 years of the follow-up period. We applied a washout period to prevent misinterpretation that the prestatus of the patient with asthma was prescribed antibiotics, which can be regarded as antibiotic use and can lead to asthma. In addition, we increased the exposure period to 6 and 7 years through shifting the index date and simultaneously shortening the follow-up period to adjust for antibiotic exposure after the index date. We attempted to verify whether the use of antibiotics after the index date showed the same tendency as an increased risk of asthma through increasing antibiotic exposure. Furthermore, stratified subgroup analyses were conducted for major covariates such as age, sex, residence, BMI, CCI, previous AD and history of AR. In addition, we conducted subgroup analyses for <2 and ≥2 years of asthma incidence based on cumulative days of antibiotics prescribed. Finally, we analysed a single specific class of antibiotic use and asthma risk compared with non-users of antibiotics. All statistical analyses were performed using SAS V.9.4 (SAS Institute, Cary, North Carolina, USA) and R V.3.3.3 (www.r-project.org) software. Statistical significance was determined using a two-sided cut-off value of p<0.05.
Patient and public involvement statement
This study was a retrospective cohort study using data from the NHIS database, and because of the retrospective nature of the study, individual patients or the public were not allowed to access the study. The patients were not involved in the recruitment process or study. Data will be disseminated to the study participants through research publications and press release portals.
Results
Table 1 shows the baseline characteristics of 248 961 cohort participants. During the observation period, 42 452 participants developed asthma. The median and IQR of follow-up times were summarised for each cumulative day of antibiotic administration. The antibiotic non-user group included 38 450 participants, and the antibiotic user groups categorised by cumulative days of antibiotic prescription consisted of 112 068 (1–14 days), 56 494 (15–30 days), 37 252 (31–90 days) and 4697 (≥91 days) participants. Compared with the antibiotic non-user group, higher antibiotic use had an increased tendency of number of healthcare visits (none, 39.05±51.99; ≥91 days, 176.03±120.70), CCI with ≥2 (none, 25.91%; ≥91 days, 65.94%) and previous AR (none, 34.88%; ≥91 days, 78.45%).
Table 1.
Baseline characteristics of the study population
| Characteristics | Total population | Cumulative days of antibiotics prescribed for 5 years before the index date | ||||
| None | 1–14 days | 15–30 days | 31–90 days | ≥91 days | ||
| Number of participants, n | 248 961 | 38 450 | 112 068 | 56 494 | 37 252 | 4697 |
| Asthma events, n | 42 452 | 3975 | 16 963 | 11 167 | 9058 | 1289 |
| Median (IQR) of follow-up times | 13.00 (12.93, 13.00) |
13.00 (13.00, 13.00) |
13.00 (13.00, 13.00) |
13.00 (11.58, 13.00) |
13.00 (9.25, 13.00) |
13.00 (7.43, 13.00) |
| Age, years, mean±SD | 55.43±8.91 | 54.51±8.75 | 55.07±8.79 | 55.82±8.97 | 56.62±9.14 | 57.21±9.32 |
| Sex, n (%) | ||||||
| Men | 141 223 (56.72) | 24 674 (64.17) | 65 154 (58.14) | 29 737 (52.64) | 18 834 (50.56) | 2824 (60.12) |
| Women | 107 738 (43.28) | 13 776 (35.83) | 46 914 (41.86) | 26 757 (47.36) | 18 418 (49.44) | 1873 (39.88) |
| Residence, n (%) | ||||||
| Capital | 41 843 (16.81) | 6911 (17.97) | 18 449 (16.46) | 9407 (16.65) | 6272 (16.84) | 804 (17.12) |
| Metropolitan | 112 290 (45.10) | 17 293 (44.98) | 50 672 (45.22) | 25 475 (45.09) | 16 751 (44.97) | 2099 (44.69) |
| Rural | 94 828 (38.09) | 14 246 (37.05) | 42 947 (38.32) | 21 612 (38.26) | 14 229 (38.20) | 1794 (38.19) |
| Household income, n (%) | ||||||
| First quartile (highest) | 91 666 (36.82) | 14 506 (37.73) | 41 064 (36.64) | 20 636 (36.53) | 13 696 (36.77) | 1764 (37.56) |
| Second quartile | 73 016 (29.33) | 10 791 (28.07) | 32 969 (29.42) | 16 875 (29.87) | 10 997 (29.52) | 1384 (29.47) |
| Third quartile | 51 547 (20.70) | 8063 (20.97) | 23 255 (20.75) | 11 693 (20.70) | 7585 (20.36) | 951 (20.25) |
| Fourth quartile (lowest) | 32 732 (13.15) | 5090 (13.24) | 14 780 (13.19) | 7290 (12.90) | 4974 (13.35) | 598 (12.73) |
| Smoking status, n (%) | ||||||
| Never smoker | 176 845 (71.03) | 25 538 (66.42) | 78 353 (69.92) | 41 592 (73.62) | 27 993 (75.14) | 3369 (71.73) |
| Past smoker | 22 918 (9.21) | 3666 (9.53) | 10 407 (9.29) | 4956 (8.77) | 3381 (9.08) | 508 (10.82) |
| Current smoker | 49 198 (19.76) | 9246 (24.05) | 23 308 (20.80) | 9946 (17.61) | 5878 (15.78) | 820 (17.46) |
| Alcohol intake, times/week, n (%) | ||||||
| 0 | 181 804 (73.03) | 26 596 (69.17) | 80 521 (71.85) | 42 254 (74.79) | 28 769 (77.23) | 3664 (78.01) |
| 1–2 | 42 198 (16.95) | 7528 (19.58) | 19 690 (17.57) | 8894 (15.74) | 5403 (14.50) | 683 (14.54) |
| 3–4 | 16 000 (6.43) | 2804 (7.29) | 7588 (6.77) | 3412 (6.04) | 1974 (5.30) | 222 (4.73) |
| ≥5 | 8959 (3.60) | 1522 (3.96) | 4269 (3.81) | 1934 (3.42) | 1106 (2.97) | 128 (2.73) |
| Physical activity, times/week, n (%) | ||||||
| 0 | 123 288 (49.52) | 18 906 (49.17) | 55 658 (49.66) | 28 003 (49.57) | 18 390 (49.37) | 2331 (49.63) |
| 1–2 | 68 684 (27.59) | 11 336 (29.48) | 31 168 (27.81) | 15 222 (26.94) | 9725 (26.11) | 1233 (26.25) |
| 3–4 | 31 045 (12.47) | 4657 (12.11) | 13 812 (12.32) | 7132 (12.62) | 4840 (12.99) | 604 (12.86) |
| ≥5 | 25 944 (10.42) | 3551 (9.24) | 11 430 (10.20) | 6137 (10.86) | 4297 (11.53) | 529 (11.26) |
| Body mass index, kg/m2, mean±SD | 23.95±2.86 | 23.75±2.90 | 23.90±2.85 | 24.03±2.86 | 24.12±2.85 | 24.17±2.90 |
| Total cholesterol, mg/dL, mean±SD | 198.75±36.68 | 198.16±36.60 | 198.63±36.59 | 198.93±36.65 | 199.40±36.94 | 199.03±37.63 |
| Systolic blood pressure, mm Hg, mean±SD | 126.04±16.68 | 126.93±17.14 | 126.06±16.68 | 125.68±16.58 | 125.64±16.38 | 125.90±16.31 |
| Fasting serum glucose, mg/dL, mean±SD | 98.32±27.21 | 97.82±26.27 | 98.15±26.88 | 98.48±27.16 | 98.96±28.49 | 99.82±32.20 |
| Number of healthcare visits*, mean±SD | 73.13±75.99 | 39.05±51.99 | 58.47±60.32 | 85.02±74.26 | 121.36±94.51 | 176.03±120.70 |
| Charlson Comorbidity Index, n (%) | ||||||
| 0 | 74 640 (29.98) | 18 829 (48.97) | 37 771 (33.70) | 11 981 (21.21) | 5472 (14.69) | 587 (12.50) |
| 1 | 67 324 (27.04) | 9658 (25.12) | 31 579 (28.18) | 15 965 (28.26) | 9109 (24.45) | 1013 (21.57) |
| ≥2 | 106 997 (42.98) | 9963 (25.91) | 42 718 (38.12) | 28 548 (50.53) | 22 671 (60.86) | 3097 (65.94) |
| Previous atopic dermatitis, n (%) | ||||||
| No | 240 200 (96.48) | 37 639 (97,89) | 108 618 (96.92) | 54 230 (95.99) | 35 322 (94.82) | 4391 (93.49) |
| Yes | 8761 (3.52) | 811 (2.11) | 3450 (3.08) | 2264 (4.01) | 1930 (5.18) | 306 (6.51) |
| Previous allergic rhinitis, n (%) | ||||||
| No | 108 289 (43.50) | 25 039 (65.12) | 53 410 (47.66) | 19 342 (34.24) | 9486 (25.46) | 1012 (21.55) |
| Yes | 140 672 (56.50) | 13 411 (34.88) | 58 658 (52.34) | 37 152 (65.76) | 27 766 (74.54) | 3685 (78.45) |
| Intra-abdominal infections, n (%) | ||||||
| No | 248 837 (99.95) | 38 450 (100.00) | 112 035 (99.97) | 56 458 (99.94) | 37 206 (99.88) | 4688 (99.81) |
| Yes | 124 (0.05) | 33 (0.03) | 36 (0.06) | 46 (0.12) | 9 (0.19) | |
| Urinary tract infections, n (%) | ||||||
| No | 247 005 (99.21) | 38 450 (100.00) | 111 658 (99.63) | 55 905 (98.96) | 36 455 (97.86) | 4537 (96.59) |
| Yes | 1956 (0.79) | 410 (0.37) | 589 (1.04) | 797 (2.14) | 160 (3.41) | |
| Intestinal infectious diseases, n (%) | ||||||
| No | 248 118 (99.66) | 38 450 (100.00) | 111 693 (99.67) | 56 273 (99.61) | 37 029 (99.40) | 4673 (99.49) |
| Yes | 843 (0.34) | 375 (0.33) | 221 (0.39) | 223 (0.60) | 24 (0.51) | |
| Skin, soft tissue, bone and joint infections, n (%) | ||||||
| No | 247 457 (99.40) | 38 450 (100.00) | 111 604 (99.59) | 56 018 (99.16) | 36 772 (98.71) | 4613 (98.21) |
| Yes | 1504 (0.60) | 464 (0.41) | 476 (0.84) | 480 (1.29) | 84 (1.79) | |
| Other infectious diseases, n (%) | ||||||
| No | 247 751 (99.51) | 38 450 (100.00) | 111 852 (99.81) | 56 200 (99.48) | 36 743 (98.63) | 4506 (95.93) |
| Yes | 1210 (0.49) | 216 (0.19) | 294 (0.52) | 509 (1.37) | 191 (4.07) | |
*The number of healthcare visits is the summation of days the examinee visited a nursing institution for all types of treatment (first visit, revisit and admission) from 2002 to 2006; n indicates number of people.
Table 2 shows the relationship between the cumulative number of days of antibiotic prescription and incidence of asthma. The adjusted multivariable Cox proportional hazards model (model 1) showed an increased risk of asthma in the ≥91 days of the antibiotic-exposed group, compared with the antibiotic non-user (aHR 1.84, 95% CI 1.72 to 1.96). The adjusted multivariable Cox proportional hazard model adjusting for infectious diseases (model 2) showed an increased risk of asthma in the ≥91 days of antibiotic exposure (aHR 1.46, 95% CI 1.38 to 1.55). In model 2, the cumulative days of 1–14 groups were used as a reference for the antibiotic user groups with the lowest usage. A clear duration-dependent pattern between antibiotic use and asthma incidence was observed in both models (p<0.001).
Table 2.
Risk for asthma according to cumulative days of antibiotics prescribed
| Cumulative days of antibiotics prescribed for 5 years before the index date | P for trend | |||||
| None | 1–14 days | 15–30 days | 31–90 days | ≥91 days | ||
| Number of participants, n | 38 450 | 112 068 | 56 494 | 37 252 | 4697 | |
| Events, n | 3975 | 16 963 | 11 167 | 9058 | 1289 | |
| Person-years | 454 255 | 1 288 569 | 629 744 | 399 519 | 48 171 | |
| Incidence/1000 person-years | 8.75 | 13.16 | 17.73 | 22.67 | 26.76 | |
| aHR (95% CI) | ||||||
| Model 1* | 1.00 (ref) | 1.28 (1.23 to 1.32) | 1.49 (1.44 to 1.55) | 1.69 (1.63 to 1.76) | 1.84 (1.72 to 1.96) | <0.001 |
| Model 2† | 1.00 (ref) | 1.17 (1.15 to 1.20) | 1.34 (1.30 to 1.37) | 1.46 (1.38 to 1.55) | <0.001 | |
The aHRs were calculated by Cox proportional hazards regression after adjustments for multivariate variables. Model 1 was adjusted for age, sex, residence, household income, smoking status, alcohol intake, physical activity, body mass index, total cholesterol, systolic blood pressure, fasting serum glucose, Charlson Comorbidity Index, the number of healthcare visits, previous atopic dermatitis and previous allergic rhinitis. Model 2 was adjusted for the variables in model 1 plus infectious diseases (intra-abdominal infections, urinary tract infections, intestinal infectious diseases, skin, soft tissue, bone and joint infections and others); n, indicates number of people.
*Antibiotics non-user group was set as a reference group.
†Antibiotics 1–14 days user group was set as a reference group.
aHR, adjusted HR; ref, reference.
Table 3 presents the sensitivity analysis of asthma risk based on the cumulative number of days antibiotics were prescribed. We conducted a washout period analysis using 3-year, 4-year and 5-year periods. Considering the 3-year, 4-year and 5-year washout periods, the aHRs in ≥91 days were 1.71 (95% CI 1.58 to 1.84), 1.67 (95% CI 1.54 to 1.82) and 1.62 (95% CI 1.48 to 1.78) in model 1. In model 2, with additional adjustments for infectious diseases, an increasing trend remained. We also conducted analyses through shifting the index date to consider exposure to antibiotics after the index date. Adjusted for 6-year and 7-year antibiotic exposure, the aHRs in ≥91 days were 2.12 (95% CI 1.99 to 2.27) and 2.33 (95% CI 2.16 to 2.51) when compared with non-users. It remained in model 2 with additional adjustments for infectious diseases. Compared with the 5-year baseline exposure, the 6-year and 7-year antibiotic exposure remained at an increased risk of asthma incidence based on the cumulative days of antibiotics prescribed.
Table 3.
Sensitivity analysis of risk for asthma according to cumulative days of antibiotics prescribed
| aHR (95% CI) | Cumulative days of antibiotics prescribed for 5 years before the index date | P for trend | ||||||
| Total | Events | None | 1–14 days | 15–30 days | 31–90 days | ≥91 days | ||
| Washout period (years) | ||||||||
| Model 1* | ||||||||
| 3 | 237 630 | 31 121 | 1.00 (ref) |
1.26 (1.21 to 1.31) |
1.46 (1.40 to 1.52) |
1.61 (1.54 to 1.69) |
1.71 (1.58 to 1.84) |
<0.001 |
| 4 | 234 170 | 27 661 | 1.00 (ref) |
1.25 (1.20 to 1.31) |
1.45 (1.39 to 1.52) |
1.60 (1.53 to 1.68) |
1.67 (1.54 to 1.82) |
<0.001 |
| 5 | 230 763 | 24 254 | 1.00 (ref) |
1.24 (1.19 to 1.30) |
1.44 (1.37 to 1.51) |
1.60 (1.52 to 1.68) |
1.62 (1.48 to 1.78) |
<0.001 |
| Model 2† | ||||||||
| 3 | 200 084 | 28 050 | 1.00 (ref) |
1.17 (1.13 to 1.20) |
1.30 (1.26 to 1.34) |
1.38 (1.28 to 1.48) |
<0.001 | |
| 4 | 196 930 | 24 896 | 1.00 (ref) |
1.16 (1.13 to 1.20) |
1.29 (1.25 to 1.34) |
1.35 (1.25 to 1.46) |
<0.001 | |
| 5 | 193 821 | 21 787 | 1.00 (ref) |
1.16 (1.13 to 1.20) |
1.30 (1.25 to 1.34) |
1.32 (1.22 to 1.44) |
<0.001 | |
| Shifting the index date | ||||||||
| Model 1* | ||||||||
| 6-year exposure | 233 495 | 36 462 | 1.00 (ref) |
1.36 (1.29 to 1.43) |
1.65 (1.57 to 1.73) |
1.91 (1.82 to 2.01) |
2.12 (1.99 to 2.27) |
<0.001 |
| 7-year exposure | 224 315 | 31 620 | 1.00 (ref) |
1.38 (1.29 to 1.48) |
1.64 (1.54 to 1.76) |
2.00 (1.87 to 2.14) |
2.33 (2.16 to 2.51) |
<0.001 |
| Model 2† | ||||||||
| 6-year exposure | 211 131 | 34 602 | 1.00 (ref) |
1.22 (1.19 to 1.25) |
1.42 (1.38 to 1.46) |
1.58 (1.51 to 1.67) |
<0.001 | |
| 7-year exposure | 210 207 | 30 671 | 1.00 (ref) |
1.20 (1.16 to 1.24) |
1.46 (1.42 to 1.51) |
1.71 (1.63 to 1.79) |
<0.001 | |
The aHRs were calculated by Cox proportional hazards regression after adjustments for multivariate variables. Model 1 was adjusted for age, sex, residence, household income, smoking status, alcohol intake, physical activity, body mass index, total cholesterol, systolic blood pressure, fasting serum glucose, Charlson Comorbidity Index, the number of healthcare visits, previous atopic dermatitis and previous allergic rhinitis. Model 2 was adjusted for the variables in model 1 plus infectious diseases (intra-abdominal infections, urinary tract infections, intestinal infectious diseases, skin, soft tissue, bone and joint infections and others).
*Antibiotics non-user group was set as a reference group.
†Antibiotics 1–14 days user group was set as a reference group.
aHR, adjusted HR; ref, reference.
Table 4 describes the association between the number of antibiotic classes and the incidence of asthma. In model 1, more than four antibiotic class users showed an increased risk of asthma (aHR 1.72, 95% CI 1.65 to 1.80) compared with the antibiotic non-user group. In model 2, participants who used ≥4 antibiotic classes showed a higher risk of asthma (aHR 1.44, 95% CI 1.39 to 1.49) compared with one class of antibiotic use.
Table 4.
Risk for asthma according to the number of antibiotic classes prescribed
| Number of antibiotic classes prescribed during 5 years before the index date | P for trend | |||||
| None | 1 | 2 | 3 | ≥4 | ||
| Number of participants, n | 38 450 | 64 698 | 69 746 | 50 420 | 25 647 | |
| Events, n | 3975 | 9023 | 12 196 | 10 800 | 6458 | |
| Person-years | 454 255 | 747 146 | 788 765 | 555 094 | 274 997 | |
| aHR (95% CI) | ||||||
| Model 1* | 1.00 (ref) |
1.21 (1.17 to 1.26) |
1.39 (1.34 to 1.44) |
1.56 (1.51 to 1.62) |
1.72 (1.65 to 1.80) |
<0.001 |
| Model 2† | 1.00 (ref) |
1.15 (1.12 to 1.18) |
1.30 (1.27 to 1.34) |
1.44 (1.39 to 1.49) |
<0.001 | |
The aHRs were calculated by Cox proportional hazards regression after adjustments for multivariate variables. Model 1 was adjusted for age, sex, residence, household income, smoking status, alcohol intake, physical activity, body mass index, total cholesterol, systolic blood pressure, fasting serum glucose, Charlson Comorbidity Index, the number of healthcare visits, previous atopic dermatitis and previous allergic rhinitis. Model 2 was adjusted for the variables in model 1 plus infectious diseases (intra-abdominal infections, urinary tract infections, intestinal infectious diseases, skin, soft tissue, bone and joint infections and others); n indicates number of people.
Antibiotics were divided into 11 classes consisting of penicillin, cephalosporin, macrolide, fluoroquinolone, sulfonamides, tetracyclines and lincosamides or others.
*Antibiotics non-user group was set as a reference group.
†Antibiotics 1–14 days user group was set as a reference group.
aHR, adjusted HR; ref, reference.
Online supplemental table 3 depicts the subgroup analyses stratified by major covariates, such as age, sex, residence, BMI, CCI, previous AD and previous AR. In addition, regarding sex, a previous study reported that different directions exist for the modifiable risk of asthma; therefore, a stratified analysis for sex was conducted7 30 (Online supplemental table 4). The risk of asthma showed an increasing trend in the overall stratified covariates as the cumulative days of antibiotic use increased (p<0.001). In the longer antibiotic use group (≥91 days), those who have CCI group (≥2) showed a higher risk of asthma (aHR 1.91, 95% CI 1.75 to 2.08) than those who do not have CCI (aHR 1.50, 95% CI 1.23 to 1.83). In all the antibiotic user groups, those who did not have previous AD or AR had a higher risk of asthma than those who had previous AD or AR.
Online supplemental table 5 presents the subgroup analyses for the <2 and ≥2 years of newly diagnosed asthma incidence based on the cumulative number of days of antibiotics prescribed. For ≥2 years, the ≥91 days of antibiotic exposure seemed to have a higher risk of asthma (aHR 1.37, 95% CI 1.28 to 1.47) compared with 1–14 days. The aHR was higher in the asthma incidence for ≥2 years than for <2 years for all cumulative days of antibiotic prescription.
Online supplemental table 6 describes the risk of a single specific class of antibiotics compared with the antibiotic non-user group. The results showed that only users of macrolides (aHR 1.14, 95% CI 1.07 to 1.23), fluoroquinolones (aHR 1.16, 95% CI 1.10 to 1.23), penicillins (aHR 1.20, 95% CI 1.15 to 1.26) and cephalosporins (aHR 1.18, 95% CI 1.12 to 1.24) had a higher risk of asthma compared with non-users of antibiotics.
Discussion
In our retrospective cohort study comprising adults aged ≥40 years, increasing cumulative prescription days and the number of classes of antibiotic use increased the risk of asthma incidence. We also adjusted for potential confounders, including specific infectious diseases that could indicate antibiotic use. Our findings were consistent with those of additional analyses with different washout periods until asthma development or an extended duration of antibiotic exposure. To the best of our knowledge, this is the first study to report a positive relationship between antibiotic exposure and new-onset asthma in adults.
Our results correspond to those of previous cohort studies showing that antibiotic use in early life can induce the development of asthma, although most of them focused on asthma incidence in early childhood.31–33 A prospective cohort study based on 493 785 children born in 2006–2010 by Örtqvist et al34 showed an increasing relationship between exposure to antibiotics during infancy or childhood and subsequent asthma risk. However, our study stands out, as we considered a different age group (≥40 years) and investigated antibiotic exposures for 5 years and more (6 years, 7 years).
The relevant mechanism proposed for the relationship between antibiotics and asthma in adults has not been completely elucidated; however, the following mechanisms have been suggested. Antibiotics may affect global alterations in microbial communities35 36 and can cause allergic reactions. The microbial diversity hypothesis suggests that a decline in the variability of microbiota consequently increases allergy.37 Changes in the gastrointestinal microbiota composition, particularly the reduction of Lachnospira and Clostridium, are associated with allergic diseases such as asthma.38 39 The gut microbiota, such as Bacteroides fragilis, influences the balance between Th1 and Th2 and confers protection against allergic airway disorders.40–42 The gut-lung axis strongly suggests a major role in respiratory diseases resulting from interactions between the different microbiome compositions of both the gut and lungs.43 In addition, specific airway microbiome composition such as increased Proteobacteria, Haemophilus parainfluenzae and Neisseria may lead to bronchial hyper-responsiveness and airway allergic responses in adults with asthma.44–46 The hygiene hypothesis states that exposure to endotoxins during early life can induce Th1 lymphocytes, reduce Th2-biased responses and produce persistent IgE.10 47 Antibiotics administered in early childhood can induce allergic reactions through increasing IgE levels and Th2-mediated responses via reducing the Th1 response, which reacts competitively. Other studies have shown that both adult and neonatal mice treated with antibiotic have elevated IgE levels in the blood, and increased Th2 responses can result in severe airway inflammation that can be aggravated by basophil-bound IgE in a papain model of allergic airway inflammation.8 48 However, these biological plausibilities have not been verified in humans. Further human-targeted studies are required to elucidate these pathophysiological mechanisms.
Our study had several strengths. We conducted a large population-based study using the NHIS database. It offers numerous precise prescriptions or medical records that can minimise the recall bias of antibiotic use. Sensitivity analyses, such as the washout period and shifting index date with a follow-up duration of >10 years, can strengthen the credibility of our results. In addition, we performed a stratified analysis of major covariates such as age, sex, residence, BMI, CCI, previous AD and previous AR. Furthermore, to increase the accuracy of new-onset asthma detection during the follow-up period, we defined asthma diagnosis based on ICD-10 codes and asthma drug prescriptions. In addition, we excluded previous COPD diagnoses and antibiotic use for respiratory symptoms from our sample selection process to exclude participants with possible underlying asthma precursor symptoms. Moreover, we examined both the cumulative use and number of classes of antibiotics, which fortified our hypothesis. We also described the association between a single specific class of antibiotics and asthma risk. Our findings are consistent with those of a previous study that reported an increased risk of asthma development associated with the use of penicillins and macrolides.49
In the stratified analysis, the residential area and BMI were unlikely to affect the relationship between antibiotics and asthma. However, age, sex, CCI, previous AD and previous AR may influence interactions between antibiotics and asthma. We included previous AD and AR as covariates because these factors are prerequisites for asthma development.50 51 AD is generally considered a precursor to other atopic disorders,52 and our study supports the hypothesis that AD is influenced by the association between antibiotics and asthma.
However, this study has some limitations. First, some risk factors for asthma, such as air pollution, occupational risk, genetics and family history, were not considered as the risk factors were not available in our claims data. Additionally, overcrowded living environments with air pollution or poor hygiene conditions can lead to antibiotic use and asthma.53 To overcome air pollution and living environments as confounders, we used residence variables (capital, metropolitan and rural) as surrogate factors and conducted stratified analyses. Work environment is a potential risk factor for asthma; however, occupational asthma is often underdiagnosed.54 55 Therefore, follow-up studies that include potential covariates of asthma are required. Second, although we excluded participants with antibiotic use for respiratory symptoms and a previous diagnosis of COPD, bias due to reverse causation in patients with undiagnosed asthma could exist. To overcome this shortcoming, we conducted a sensitivity analysis with an adjusted 3-year to 5-year washout period. Third, confusing viral infections and asthma symptoms with antibiotic use may lead to missed cases where antibiotics are wrongly prescribed for asthma, despite clinical guidelines stating otherwise. Viral infections could easily be confused with asthma symptoms, and they could occur simultaneously regarding the upper respiratory tract because they both invoke coughing, sneezing and even wheezing. Reversible airway limitation is the key pathophysiology of asthma, but this aspect could also easily be found in viral infections. Therefore, the misapplication of antibiotics for asthma may be a potential bias and limitation in our study. Fourth, accuracy uncertainty exists in the definition of asthma in the NHIS database. For example, differentiating the diagnosis of asthma and COPD is difficult in primary care56 57 because COPD is a respiratory disease that is difficult to distinguish from asthma. In addition, inconsistencies between the diagnoses of infectious diseases and prescribed antibiotics may exist in the claims database. Finally, measures using self-reported questionnaires, such as health behaviours (drinking, smoking and physical activity), could be partially inaccurate. Therefore, this could lead to under-reporting of risky health behaviours.
This retrospective cohort study showed an association between the cumulative use of antibiotics, number of antibiotics used and asthma incidence in adults aged ≥40 years. Our results suggest that antibiotic use could possibly increase the risk of asthma in a duration-response relationship; therefore, using antibiotics appropriately and responsibly with caution in clinical settings to prevent newly diagnosed asthma, particularly in adults, is necessary.
Footnotes
Contributors: SMP is the guarantor of this work and, as such, has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. JChoi, SJP, YJP, JH and SMP conceptualised and designed the study. Acquisition of data was done by SJP and SMP. Analysis and interpretation of data was done by JChoi, SJ, YJP, JH and SMP. The drafting of the manuscript was done by JChoi. Critical revision of the manuscript was done by JChoi, SJP, YJP, JH, SJ, YC, JChang, SMK, JH and SMP. Statistical analysis was done by JChoi, SJP, YJP, JH. Administrative, technical or material support was given by SJ, YC, JChang, SMK, JH and SMP.
Funding: This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1F1A1063346). SJP received a scholarship from the BK21 FOUR education programme from the NRF.
Disclaimer: The funding sources had no role in design and conduct of the study; collection, management, analysis and interpretation of the data and preparation, review or approval of the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The datasets presented in this article are available from the Korean National Health Insurance Service (https://nhiss.nhis.or.kr). Requests for access to the datasets are needed, and qualified researchers can apply the data from the NHIS for research purposes.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Owing to the retrospective nature of the study, using the anonymised NHIS database based on rigorous confidentiality guidelines, the requirement for informed consent was waived by the Institutional Review Board of Seoul National University Hospital. All methods were performed according to the relevant guidelines and regulations, and the study strictly adhered to the principles of the Declaration of Helsinki.
References
- 1.Hargreave FE, Nair P. The definition and diagnosis of asthma. Clin Experimental Allergy 2009;39:1652–8. 10.1111/j.1365-2222.2009.03321.x Available: https://onlinelibrary.wiley.com/toc/13652222/39/11 [DOI] [PubMed] [Google Scholar]
- 2.Mims JW. Definitions and pathophysiology. Int Forum Allergy Rhinol 2015;5. 10.1002/alr.21609 Available: https://onlinelibrary.wiley.com/toc/20426984/5/S1 [DOI] [PubMed] [Google Scholar]
- 3.Rodrigo GJ, Rodrigo C, Hall JB. Acute asthma in adults: a review. Chest 2004;125:1081–102. 10.1378/chest.125.3.1081 [DOI] [PubMed] [Google Scholar]
- 4.Hanania NA, King MJ, Braman SS, et al. Asthma in the elderly: Current understanding and future research needs—a report of a national Institute on aging (NIA) workshop. J Allergy Clin Immunol 2011;128(3 Suppl):S4–24. 10.1016/j.jaci.2011.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson PG, McDonald VM, Marks GB. Asthma in older adults. The Lancet 2010;376:803–13. 10.1016/S0140-6736(10)61087-2 [DOI] [PubMed] [Google Scholar]
- 6.Song W-J, Kang M-G, Chang Y-S, et al. Epidemiology of adult asthma in Asia: toward a better understanding. Asia Pac Allergy 2014;4:75–85. 10.5415/apallergy.2014.4.2.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S, Jung S-Y, Kwon J-W. Sex differences in the association between asthma incidence and Modifiable risk factors in Korean middle-aged and older adults: NHIS-HEALS 10-year cohort. BMC Pulm Med 2019;19:1–10. 10.1186/s12890-019-1023-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wypych TP, Marsland BJ. Antibiotics as Instigators of microbial dysbiosis: implications for asthma and allergy. Trends Immunol 2018;39:697–711. 10.1016/j.it.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 9.Patrick DM, Sbihi H, Dai DLY, et al. Decreasing antibiotic use, the gut Microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir Med 2020;8:1094–105. 10.1016/S2213-2600(20)30052-7 [DOI] [PubMed] [Google Scholar]
- 10.Han K, Kim SW. Effects of antibiotics on the development of asthma and other allergic diseases in children and adolescents. Allergy Asthma Immunol Res 2018;10:457–65. 10.4168/aair.2018.10.5.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Droste JHJ, Wieringa MH, Weyler JJ, et al. Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease Clin Experimental Allergy 2000;30:1548–53. 10.1046/j.1365-2222.2000.00939.x [DOI] [PubMed] [Google Scholar]
- 12.Strzępa A, Lobo FM, Majewska-Szczepanik M, et al. Antibiotics and autoimmune and allergy diseases: causative factor or treatment? Int Immunopharmacol 2018;65:328–41. 10.1016/j.intimp.2018.10.021 [DOI] [PubMed] [Google Scholar]
- 13.Francino MP. Antibiotics and the human gut Microbiome: Dysbioses and accumulation of resistances. Front Microbiol 2015;6. 10.3389/fmicb.2015.01543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsakok T, McKeever TM, Yeo L, et al. Does early life exposure to antibiotics increase the risk of Eczema? A systematic review. Br J Dermatol 2013;169:983–91. 10.1111/bjd.12476 [DOI] [PubMed] [Google Scholar]
- 15.Slob EMA, Brew BK, Vijverberg SJH, et al. Early-life antibiotic use and risk of asthma and Eczema: results of a discordant twin study. Eur Respir J 2020;55. 10.1183/13993003.02021-2019 [DOI] [PubMed] [Google Scholar]
- 16.Pitter G, Ludvigsson JF, Romor P, et al. Antibiotic exposure in the first year of life and later treated asthma, a population based birth cohort study of 143,000 children. Eur J Epidemiol 2016;31:85–94. 10.1007/s10654-015-0038-1 [DOI] [PubMed] [Google Scholar]
- 17.Ahmadizar F, Vijverberg SJH, Arets HGM, et al. Early life antibiotic use and the risk of asthma and asthma exacerbations in children. Pediatr Allergy Immunol 2017;28:430–7. 10.1111/pai.12725 [DOI] [PubMed] [Google Scholar]
- 18.Risnes KR, Belanger K, Murk W, et al. Antibiotic exposure by 6 months and asthma and allergy at 6 years: findings in a cohort of 1,401 US children. Am J Epidemiol 2011;173:310–8. 10.1093/aje/kwq400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foliaki S, Pearce N, Björkstén B, et al. Antibiotic use in infancy and symptoms of asthma, Rhinoconjunctivitis, and Eczema in children 6 and 7 years old: International study of asthma and allergies in childhood phase III. J Allergy Clin Immunol 2009;124:982–9. 10.1016/j.jaci.2009.08.017 [DOI] [PubMed] [Google Scholar]
- 20.Postma DS, Rabe KF. The asthma–COPD overlap syndrome. N Engl J Med 2015;373:1241–9. 10.1056/NEJMra1411863 [DOI] [PubMed] [Google Scholar]
- 21.Kim SY, Lim H, Lim J-S, et al. Analysis of the relationship between adult asthma and stroke: a longitudinal follow-up study using the Korean national sample cohort. Biomed Res Int 2019. 10.1155/2019/8919230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J-H, Wee J-H, Choi HG, et al. Association between Statin medication and asthma/asthma exacerbation in a national health screening cohort. J Allergy Clin Immunol Pract 2021;9:2783–91. 10.1016/j.jaip.2021.04.014 [DOI] [PubMed] [Google Scholar]
- 23.Kim SY, Min C, Oh DJ, et al. Bidirectional association between asthma and Migraines in adults: two longitudinal follow-up studies. Sci Rep 2019;9:1–9. 10.1038/s41598-019-54972-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SJ, Park YJ, Chang J, et al. Association between antibiotics use and diabetes incidence in a nationally representative retrospective cohort among Koreans. Sci Rep 2021;11:1–10. 10.1038/s41598-021-01125-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim M, Park SJ, Choi S, et al. Association between antibiotics and dementia risk: A retrospective cohort study. Front Pharmacol 2022;13. 10.3389/fphar.2022.888333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol 2018;141:1169–79. 10.1016/j.jaci.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen K, Zahran H, Iqbal S, et al. Factors associated with asthma control among adults in five New England States, 2006–2007. J Asthma 2011;48:581–8. 10.3109/02770903.2011.576744 [DOI] [PubMed] [Google Scholar]
- 28.Mäkikyrö EMS, Jaakkola MS, Jaakkola JJK. Subtypes of asthma based on asthma control and severity: a latent class analysis. Respir Res 2017;18:24.:24. 10.1186/s12931-017-0508-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim M, Park SJ, Choi S, et al. Association of antibiotic use with risk of lung cancer: A nationwide cohort study. J Infect Public Health 2023;16:1123–30. 10.1016/j.jiph.2023.05.006 [DOI] [PubMed] [Google Scholar]
- 30.Kynyk JA, Mastronarde JG, McCallister JW. Asthma, the sex difference. Curr Opin Pulm Med 2011;17:6–11. 10.1097/MCP.0b013e3283410038 [DOI] [PubMed] [Google Scholar]
- 31.Celedón JC, Fuhlbrigge A, Rifas‐Shiman S, et al. Antibiotic use in the first year of life and asthma in early childhood. Clin Experimental Allergy 2004;34:1011–6. 10.1111/j.1365-2222.2004.01994.x Available: https://onlinelibrary.wiley.com/toc/13652222/34/7 [DOI] [PubMed] [Google Scholar]
- 32.Kozyrskyj AL, Ernst P, Becker AB. Increased risk of childhood asthma from antibiotic use in early life. Chest 2007;131:1753–9. 10.1378/chest.06-3008 [DOI] [PubMed] [Google Scholar]
- 33.Hoskin-Parr L, Teyhan A, Blocker A, et al. Antibiotic exposure in the first two years of life and development of asthma and other allergic diseases by 7.5 yr: a Dose‐Dependent relationship. Pediatr Allergy Immunol 2013;24:762–71. 10.1111/pai.12153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Örtqvist AK, Lundholm C, Kieler H, et al. Antibiotics in fetal and early life and subsequent childhood asthma: nationwide population based study with Sibling analysis. BMJ 2014;349:g6979. 10.1136/bmj.g6979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding C, He J. Effect of antibiotics in the environment on microbial populations. Appl Microbiol Biotechnol 2010;87:925–41. 10.1007/s00253-010-2649-5 [DOI] [PubMed] [Google Scholar]
- 36.Modi SR, Collins JJ, Relman DA. Antibiotics and the gut Microbiota. J Clin Invest 2014;124:4212–8. 10.1172/JCI72333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Legatzki A, Rösler B, von Mutius E. Microbiome diversity and asthma and allergy risk. Curr Allergy Asthma Rep 2014;14:466. 10.1007/s11882-014-0466-0 [DOI] [PubMed] [Google Scholar]
- 38.Gholizadeh P, Mahallei M, Pormohammad A, et al. Microbial balance in the intestinal Microbiota and its association with diabetes, obesity and allergic disease. Microb Pathog 2019;127:48–55. 10.1016/j.micpath.2018.11.031 [DOI] [PubMed] [Google Scholar]
- 39.Frati F, Salvatori C, Incorvaia C, et al. The Role of the Microbiome in Asthma: The Gut 2018;20:123. 10.3390/ijms20010123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ni J, Friedman H, Boyd BC, et al. Early antibiotic exposure and development of asthma and allergic rhinitis in childhood. BMC Pediatr 2019;19. 10.1186/s12887-019-1594-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bengmark S. Gut Microbiota, immune development and function. Pharmacol Res 2013;69:87–113. 10.1016/j.phrs.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 42.Russell SL, Gold MJ, Hartmann M, et al. Early life Antibiotic‐Driven changes in Microbiota enhance susceptibility to allergic asthma. EMBO Rep 2012;13:440–7. 10.1038/embor.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enaud R, Prevel R, Ciarlo E, et al. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom Crosstalks. Front Cell Infect Microbiol 2020;10:9. 10.3389/fcimb.2020.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut Microbiome. Cell Host & Microbe 2015;17:592–602. 10.1016/j.chom.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hufnagl K, Pali-Schöll I, Roth-Walter F, et al. Dysbiosis of the gut and lung Microbiome has a role in asthma. Semin Immunopathol 2020;42:75–93. 10.1007/s00281-019-00775-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goleva E, Jackson LP, Harris JK, et al. The effects of airway Microbiome on corticosteroid responsiveness in asthma. Am J Respir Crit Care Med 2013;188:1193–201. 10.1164/rccm.201304-0775OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renz H, Blümer N, Virna S, et al. The immunological basis of the hygiene hypothesis. Allergy and Asthma in Modern Society: A Scientific Approach 2006;91:30–48. [DOI] [PubMed] [Google Scholar]
- 48.Russell SL, Gold MJ, Willing BP, et al. Perinatal antibiotic treatment affects murine Microbiota, immune responses and allergic asthma. Gut Microbes 2013;4:158–64. 10.4161/gmic.23567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin Y-C, Chen Y-C, Kuo C-H, et al. Antibiotic exposure and asthma development in children with allergic rhinitis. J Microbiol Immunol Infect 2020;53:803–11. 10.1016/j.jmii.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 50.Spergel JM. From Atopic dermatitis to asthma: the Atopic March. Annals of Allergy, Asthma & Immunology 2010;105:99–106. 10.1016/j.anai.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 51.Zheng T, Yu J, Oh MH, et al. The Atopic March: progression from Atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res 2011;3:67. 10.4168/aair.2011.3.2.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lowe AJ, Leung DYM, Tang MLK, et al. The skin as a target for prevention of the Atopic March. Ann Allergy Asthma Immunol 2018;120:145–51. 10.1016/j.anai.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 53.Ayuk AC, Eze JN, Edelu BO, et al. The prevalence of allergic diseases among children with asthma: what is the impact on asthma control in South East Nigeria Niger J Clin Pract 2018;21:632–8. 10.4103/njcp.njcp_343_17 [DOI] [PubMed] [Google Scholar]
- 54.Toskala E, Kennedy DW. Asthma risk factors. Int Forum Allergy Rhinol 2015;5 Suppl 1(Suppl 1):S11–6. 10.1002/alr.21557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan-Yeung M, Malo J-L. Occupational asthma. N Engl J Med 1995;333:107–12. 10.1056/NEJM199507133330207 [DOI] [PubMed] [Google Scholar]
- 56.Tho NV, Park HY, Nakano Y. Asthma–COPD overlap syndrome (ACOS): a diagnostic challenge. Respirology 2016;21:410–8. 10.1111/resp.12653 [DOI] [PubMed] [Google Scholar]
- 57.Chang J, Mosenifar Z. Differentiating COPD from asthma in clinical practice. J Intensive Care Med 2007;22:300–9. 10.1177/0885066607304445 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2023-001643supp001.pdf (194.9KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The datasets presented in this article are available from the Korean National Health Insurance Service (https://nhiss.nhis.or.kr). Requests for access to the datasets are needed, and qualified researchers can apply the data from the NHIS for research purposes.