Abstract
Background
Papillary renal cell carcinoma (pRCC) is the most common non-clear cell RCC, and associated with poor outcomes in the metastatic setting. In this study, we aimed to comprehensively evaluate the immune tumor microenvironment (TME), largely unknown, of patients with metastatic pRCC and identify potential therapeutic targets.
Methods
We performed quantitative gene expression analysis of TME using Microenvironment Cell Populations-counter (MCP-counter) methodology, on two independent cohorts of localized pRCC (n=271 and n=98). We then characterized the TME, using immunohistochemistry (n=38) and RNA-sequencing (RNA-seq) (n=30) on metastatic pRCC from the prospective AXIPAP trial cohort.
Results
Unsupervised clustering identified two “TME subtypes”, in each of the cohorts: the “immune-enriched” and the “immune-low”. Within AXIPAP trial cohort, the “immune-enriched” cluster was significantly associated with a worse prognosis according to the median overall survival to 8 months (95% CI, 6 to 29) versus 37 months (95% CI, 20 to NA, p=0.001). The two immune signatures, Teff and JAVELIN Renal 101 Immuno signature, predictive of response to immune checkpoint inhibitors (CPI) in clear cell RCC, were significantly higher in the “immune-enriched” group (adjusted p<0.05). Finally, five differentially overexpressed genes were identified, corresponding mainly to B lymphocyte populations.
Conclusion
For the first time, using RNA-seq and immunohistochemistry, we have highlighted a specific immune TME subtype of metastatic pRCC, significantly more infiltrated with T and B immune population. This “immune-enriched” group appears to have a worse prognosis and could have a potential predictive value for response to immunotherapy, justifying the confirmation of these results in a cohort of metastatic pRCC treated with CPI and in combination with targeted therapies.
Trial registration number
Keywords: Immunotherapy, Tumor Microenvironment, Renal Cell Carcinoma, Tumor Biomarkers, Gene Expression Profiling
WHAT IS ALREADY KNOWN ON THIS TOPIC
Papillary renal cell carcinoma is the most common non-clear cell RCC, and associated with poor outcomes in the metastatic setting. We aimed to comprehensively evaluate, by RNA sequencing and immunohistochemistry (IHC), the immune tumor microenvironment (TME), largely unknown, to identify potential therapeutic targets.
WHAT THIS STUDY ADDS
The identification of TME subtypes, and notably the “immune-enriched” group, could be done by IHC, and in particular by the CD3 marker, a reliable and inexpensive technique. This “immune-enriched” feature appears to be correlated with a poor prognosis but could indicate a potential predictive value for response to immunotherapy, alone or in combination, a treatment not currently recommended.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study could lead to the development of predictive biomarker-driven clinical trials in these rare tumors.
Introduction
Papillary renal cell carcinoma (pRCC) is the most common non-clear cell RCC (nccRCC) and represents up to 15% of renal cell carcinoma (RCC).1 2 The denomination nccRCC comprises a heterogeneous group of tumors with distinct histological and molecular characterization.3 Patients with metastatic pRCC (mpRCC) have significantly lower response rates, lower median progression-free survival (PFS) and overall survival (OS) than those with clear cell renal cell carcinoma (ccRCC).4 5 Based on the pathological assessment according to the WHO 2016 classification, pRCC have been routinely classified in two subtypes: type 1, commonly associated with multiple or bilateral small tumors with a favorable prognosis and few metastatic development, type 2, commonly more aggressive and associated with a dismal prognosis, and unclassified pRCC.6 Several studies aimed at investigating molecular events specific to pRCC subtypes.2 7 8
The therapeutic management of patients with metastatic nccRCC has historically been similar to metastatic ccRCC given the lack of dedicated trials.1 9 10 A few phase 2 studies, evaluating targeted therapies, have been carried out specifically in mpRCC. Those investigated sunitinib (SUPAP11), everolimus (RAPTOR12), cabozantinib (PAPMET13) and axitinib (AXIPAP14) as first-line agents. Cabozantinib treatment resulted in significantly longer PFS (median 9.0 months, 95% CI, 6% to 12%) than in the sunitinib group (5.6 months, 3% to 7%; HR for progression or death 0.60, 0.37 to 0.97, one-sided p=0.019). Response rate for cabozantinib was 23% versus 4% for sunitinib (two-sided p=0.010).13 As well, axitinib demonstrated encouraging efficacy in patients with mpRCC, especially in type 2 pRCC, with manageable toxicity. The progression-free rate at 24 weeks, primary end-point, was 45.2% (95% CI, 32.6% to not reached), the objective response rate (ORR) 28.6% (95% CI, 15.7% to 44.6%) including 7.7% in type 1 and 35.7% in type 2. Median OS was 18.9 months (95% CI, 12.8 to not reached).14 The clinical efficacy of immunotherapy, monotherapy or in combination, has since been established in metastatic RCC with a clear cell histologic component.15 16 However, these pivotal studies of immune checkpoint inhibitors (CPI) excluded nccRCC. Small retrospective cohorts report discordant results regarding nccRCC response to CPI.17–22 The activity of CPI, as a single agent appears variable in patients with metastatic pRCC, with ORR ranging from 8% to 25%. Indeed, in monotherapy, the KEYNOTE-427 cohort B study remains the largest prospective data set to date, showing promising antitumor activity with first-line pembrolizumab monotherapy in metastatic pRCC. At a median follow-up of 11.1 months (range 0.9–21.3), median PFS was 4.1 months and the median OS has not yet been reached with 72% of patients alive at 1 year in the entire nccRCC cohort. In 118 patients with mpRCC, ORR was 25.4% (95% CI, 17.9% to 34.3%). In combination, the preliminary analysis of KEYNOTE-B61, a single-arm, phase 2 study (NCT04704219) evaluating pembrolizumab and lenvatinib as first-line treatment for nccRCC, showed increased antitumor activity. The 6-month PFS rate was 72.3% (95% CI, 60.7% to 81.0%) and the 6-month OS rate was 87.8% (95% CI, 78.5% to 93.2%). In 51 patients with mpRCC, ORR was 52.9% (95% CI, 38.5% to 67.1%).23 Further activity could be gained from combinations, but optimal partners still need to be investigated.24
CPI therapy appears to be more effective in patients with pre-existing antitumor immune activation. According to Charoentong et al,25 immunogenicity can be represented by cytotoxic lymphocyte activity,26 being the ultimate effector mechanism of the antitumor immune response. Lymphocyte infiltration has been described in pRCC but seems to be less present than in ccRCC.25 Papillary RCC may have a different immunogenicity, and thus a different response to CPI than ccRCC. An heterogeneous expression of the immune checkpoint programmed death-ligand 1 (PD-L1) was described in pRCC.27 28 Characteristics of the immune infiltrate have also been described with limited information in pRCC,25 29 highlighting two predominant profiles: inflammatory cluster with elevated Th17 and Th1 genes, or depleted lymphocyte cluster with a more prominent macrophage signature, associated with a different prognosis.30 An immune signature based on the expression of Th2 genes, was also described in a rare pRCC subtype with the worst prognosis and harboring a Cytosine preceding Guanine (CpG) island methylator phenotype, questioning the potential role of CPI in this context.31 While further data is awaited from prospective trials, these studies constitute growing evidence that the immune tumor microenvironment (TME) may have a key role in pRCC and support the need to investigate the role of CPI in patients with metastatic pRCC.
Immune infiltration and expression of immune checkpoints may be critical factors to select patients, but the microenvironment of pRCC is still to be described.26 32–35
In ccRCC, several transcriptomic signatures were associated with predictive value of response to CPI,36 37 as Teff signature,38–42 or myeloid signature,38–40 42 or JAVELIN Renal 101 Immuno signature.40 42 Prospective studies are ongoing to validate these predictive transcriptomic signatures but their use in routine practice will represent a major challenge.43
Therefore, in this study, we comprehensively evaluated the TME of patients with pRCC, and identified key genes and B and T-cell subsets that are closely related to the TME of patients with pRCC and could be used as immunotherapeutic targets or predictive biomarkers.
Materials and methods
Study cohorts
We performed quantitative explorations of the immune infiltration gene expression on two independent cohorts of localized pRCC, as a discovery set.
We downloaded the gene expression RNA-sequencing (RNA-seq) data and clinical phenotype of kidney papillary cell carcinoma (KIRP) from The Cancer Genome Atlas (TCGA) portal (https://gdc.cancer.gov/). The transcriptome profiling of RNA expression was obtained by RNA‐seq according to the GRCh38 reference genome annotation, Ensembl V.80 of May 2015 (BiomaRt package). HTSeq-count data were converted to transcripts per million (TPM) (TCGAbiolinks package). Log2‐based transformation was used for the normalization of RNA expression profiles (TPM + 1). Clinical data were extracted from Ricketts et al.31 After the data were preprocessed, and the samples without clinical data excluded, 271 pRCC were enrolled. Updated survival data were extracted from Liu et al,44 with OS available for 266 patients. The same TCGA barcode structure is used for both clinical data and molecular data, enabling integrated analysis of patient-based clinical data and sample-based molecular data.
Gene expression microarray data were also obtained from an independent cohort of frozen tissue samples from 98 localized pRCC, with 47 type 1 pRCC, 45 type 2 pRCC and 6 unclassified pRCC.45 We performed our analyses using the already normalized data.
Then, we performed a post hoc analysis of the AXIPAP trial.14 This multicenter, single-arm, phase II trial enrolled patients with locally advanced or metastatic specifically confirmed pRCC, in first-line treatment. The study was registered on ClinicalTrials.gov. Fifty-six patients were screened, and 44 included (13 type 1, 30 type 2 and 1 non-specified, according to the WHO 2016 classification). For our ancillary study, clinical data cut-off was February 28, 2021, for the final analysis. The pRCC samples were all confirmed by expert central pathology review. Only formalin-fixed paraffin-embedded (FFPE) samples with sufficient materials were included.
Procedures in AXIPAP trial cohort
Immunohistochemistry
Paraffin blocks of pRCC have been centralized at the Department of Pathology (NRL, Rennes Hospital, France). They were sent with original pathology reports by different Departments of Pathology in France. An Hémalun Eosine Safran (HES) slide has been performed from each block and all the HES-stained slides were reviewed by a uropathologist (NRL).
The following pathological data were collected: histological type (according to the WHO 2016 classification), International Society of Urological Pathology (ISUP) nucleolar grade; presence of necrosis and sarcomatoid and/or rhabdoid component.
From each paraffin block, unstained sections were obtained for immunohistochemistry (IHC), and slides were labeled using the BenchMark ULTRA/Roche.
For angiogenesis analysis, the following antibodies were used: the anti-Vascular Endothelial Growth Factor (VEGF) antibody (clone SP28-M3281, Spring/Biosciences), and the anti-CD31 antibody (Clone JC70A; titer 1:500; DakoCytomation). We described two angiogenic phenotypes one and two according to the following publication in Human Pathology,46 and we defined the micro vessel density as the vessel sections per mm2.
For the immune microenvironment, we used the following antibodies: CD3 (MAb rabbit 2GV6, Roche), CD8 (clone C8/144B, M7103, DAKO), CD68 (clone PG-M1, Dako), and PD-L1 (E1L3N, Cell Signaling). We evaluated the tumor infiltrating lymphocytes (CD3, CD8) and macrophages (CD68) at the invasive margin defined as a 1 mm wide zone centered on the border of the malignant cells with the host tissue, and in the central tumor defined as the central tumor tissue surrounded by this zone. We scored the CD3/CD8/CD68 positive cells according to the proposal of the International ImmunoOncology Biomarkers Working Group,47 using a manual semi quantitative 4-point scale: score 0 (no CD3/CD8/CD68+ cells or very rare positive cells), score 1 (rare diffuse or focal positive cells), score 2 (diffuse numerous positive cells), and score 3 (diffuse and numerous positive cells with some aggregates). For PD-L1, we evaluated the % of positive tumor cells.
On HES, we also evaluated the tumor infiltrating mononuclear cells (lymphocytes, and plasma cells) both at the invasive margin and in the central tumor as previously described.
RNA-sequencing
RNA extraction was performed with COVARIS ME220 Focused-ultrasonicator, to allow a high quantity and high quality of RNA extracted. RNA libraries were prepared with the SureSelectXT RNA Direct Library Preparation kit and the SureSelectXT Human All Exon V6+UTR probes from Agilent. All libraries were sequenced on an Illumina NextSeq550 in paired-end mode (2×75 bp) with a target depth of 20 million fragments per sample.
Sequenced reads were trimmed with fastp V.0.20.1 and mapped to GRCh38 using HISAT2 V.2.1.0 both with default parameters. Reads overlapping genomic features were counted with featureCounts V.2.0.0 from the Subread package and Ensembl V.99. Only uniquely mapped and not duplicated reads were counted. Multiple overlaps of unique genomic feature were not counted. The counts data were converted to TPM. Log2‐based transformation was used for the normalization of RNA expression profiles (TPM + 1).
The R package “DESeq2” was applied to screen differentially expressed messenger RNAs (mRNAs) between different groups. Next, the p value was calculated by the false discovery rate (FDR)-corrected method. The mRNAs with | log2 fold-change | >2 and p adjusted <0.01 were filtrated as differentially expressed genes.
Identification of clusters
Based on transcriptomic markers, using Microenvironment Cell Populations-counter (MCP-counter)-methodology,48 49 that assesses the proportion of 10 immune and stromal cell populations in the TME, we applied unsupervised clustering by using pheatmap package and Ward‐d2 distance method.
We applied the same unsupervised clustering method to IHC immune markers.
Gene expression analyses
From the output of MCP-counter, we performed exploratory quantitative analyses to characterize the TME. We analyzed immune cell populations and immune markers including LAG3, TIGIT, CTLA4, PD-1 and PD-L1. Multiomics analyses were performed using available data from Chen et al 50 and Ricketts et al.31 We analyzed the Th2 prognostic signature (PMCH, AHI, PTGIS, CXCR6, EVI5, IL-26, MB, NEIL3, GSTA4, PHEX, SMAD2, CENPF, ANK1, ADCY1, LAIR2, SNRPD1, MICAL2, DHFR, WDHD1, BIRC5, SLC39A14, HELLS, LIMA1, CDC25C, CDC7, GATA3)31 51 in the TCGA cohort. Moreover, we analyzed three transcriptomic signatures described in ccRCC,38–40 between each cluster, in each cohort: angiogenesis (VEGFA, KDR, ESM1, PECAM1, ANGPTL4, CD34) signature, predictive of tyrosine kinase inhibitors (TKI) response, and Teff (CD8A, EOMES, PRF1, IFNG, CD274) and JAVELIN Renal 101 Immuno (CD3G, CD3E, CD8B, THEMIS, TRAT1, GRAP2, CD247, CD2, CD96, PRF1, CD6, IL7R, ITK, GPR18, EOMES, SIT1, NLRC3, CD244, KLRD1, SH2D1A, CCL5, XCL2, CST7, GFI1, KCNA3, PSTPIP1) signatures, predictive of response to CPI.
Statistical analysis
Data processing and statistical analyses were performed using R programming language (V.4.0.3). The heatmaps were generated by applying R package “pheatmap”. The data, RNA-seq and IHC, were normalized by the scale function, before clustering. The Kruskal-Wallis test was employed to compare differences between clusters. In all data analyses, a two-tailed p<0.05 was considered statistically significant, with p adjusted for Benjamini-Hochberg correction (*p<0.05 **p<0.01, ***p<0.001). Boxplots were drawn using the ggplot2 package. Kaplan-Meier survival analysis between different clusters was performed using the R survival and survminer packages. The group comparisons were performed with the logrank test with p<0.05, considered statistically significant.
Results
Gene expression analysis of localized pRCC reveal two distinct immune landscapes
The clinical characteristics of 271 patients from TCGA KIRP cohort were presented in table 1.
Table 1.
Characteristics of the localized papillary renal cell carcinoma population, from The Cancer Genome Atlas kidney papillary cell carcinoma cohort
| Characteristic | N=271* |
| Age | 61 (53–69) |
| Unknown | 23 |
| Gender | |
| Female | 67 (25) |
| Male | 204 (75) |
| Type | |
| Type 1 papillary RCC | 159 (59) |
| Type 2 papillary RCC | 78 (29) |
| Unclassified papillary RCC | 34 (13) |
| Stage | |
| Stage I | 163 (66) |
| Stage II | 21 (8.5) |
| Stage III | 48 (19) |
| Stage IV | 15 (6.1) |
| Unknown | 24 |
| Vital_status | |
| Alive | 231 (85) |
| Dead | 40 (15) |
| Survival_time (days) | 742 (403–1,465) |
| Unknown | 1 |
*Median (IQR); n (%).
RCC, renal cell carcinoma.
Discovery of TME subtypes in pRCC
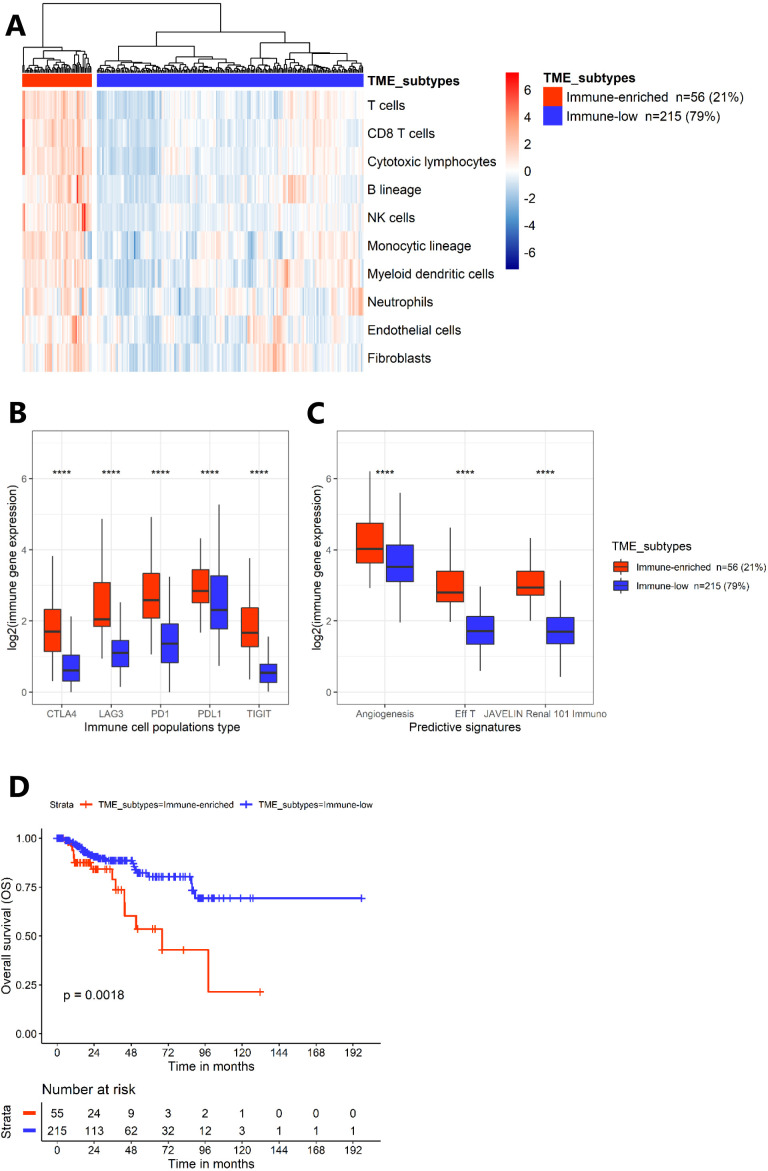
Using MCP-counter methodology,48 49 we estimated abundances of immune and stromal cell population from cell-specific transcriptomic markers. We applied unsupervised clustering to the abundance scores and identified two different clusters: 21% (n=56) of patients featured an “immune-enriched” tumor and 79% (n=215) an “immune-low” tumor, more heterogeneous (figure 1A). Notably, the “immune-enriched” cluster was significantly characterized by higher abundances of cytotoxic T cells, B cells and natural killer cells (online supplemental figure S1A). There was no significant difference between our two clusters in the number of mutations (data not shown), but there were significantly more copy number alterations of chromosome 7, corresponding to mesenchymal epithelial transition factor (MET) gene, in the “immune-low” cluster (p<0.01) (online supplemental figure S1B). Gene signature mRNA-based concerning the cell cycle, hypoxia, NRF2/ARE, TFE3 fusion, were significantly greater in expression in the “immune-enriched” cluster (p<0.001) (online supplemental figure S1C).
Figure 1.
Transcriptomic analyses from The Cancer Genome Atlas kidney papillary cell carcinoma cohort (n=271). (A) Heatmap representing unsupervised analysis from MCP-counter on normalized transcriptomic expression data (k=2), identifying the TME subtypes. (B) Boxplots representing the transcriptomic expression of five checkpoint markers according to clusters from the MCP-counter analysis. (C) Boxplots representing the three predictive gene signatures of response to treatment described in the clear-cell renal cell carcinoma (angiogenesis, effector T-cell (Eff T), JAVELIN Renal 101 Immuno), according to the clusters from MCP-counter. P values were obtained using the two-sided Mann-Whitney test (***p<0.001). (D) Kaplan-Meier survival curves (overall survival (OS)) according to clusters from MCP-counter analysis. CTLA-4, cytotoxic T-lymphocyte–associated antigen 4; LAG-3, lymphocyte activation gene-3; MCP, Microenvironment Cell Populations; NK, natural killer; PD-1, programmed death 1; PD-L1, programmed death-ligand 1; TIGIT, T cell immunoglobulin and ITIM domain; TME, tumor microenvironment.
jitc-2023-006885supp001.pdf (148.8KB, pdf)
jitc-2023-006885supp002.pdf (199.4KB, pdf)
We performed the same analysis in an independent cohort of 98 patients with localized pRCC. We confirmed the presence of “immune-enriched” and “immune-low” TMEs (8% vs 92%, respectively) (online supplemental figure S2A).
jitc-2023-006885supp003.pdf (159.6KB, pdf)
pRCC TME subtypes are associated with immune checkpoints and prognosis
Interestingly, in two independent cohorts, the immune checkpoints markers, LAG3, TIGIT, CTLA-4, PD-1, PD-L1 were significantly enriched in the “immune-enriched” cluster (adjusted p<0.001) (figure 1B and online supplemental figure S2B). Additionally, the three predictive signatures for response to TKI (angiogenesis) and CPI (Teff and JAVELIN Renal 101 Immuno) in ccRCC were significantly greater in the “immune-enriched” cluster (adjusted p<0.001) (figure 1C and online supplemental figure S2C).
The “immune-enriched” component was associated with a worse prognosis (median OS was 68 months (95% CI, 43.5 to NA) vs not reached, and 12-month OS=87% vs 97%, in “immune-enriched” vs “immune-low” clusters, respectively, p=0.002) (figure 1D). Similarly, the Th2 signature, associated with a poor prognosis in the literature,31 was significantly higher in the “immune-enriched” cluster (adjusted p<0.001) (online supplemental figure S1D).
Based on these exploratory results in the localized pRCC cohort, we investigated the immune infiltrate in a cohort of metastatic pRCC with treatment data, as a validation cohort.
Validation in patients with metastatic pRCC treated by axitinib
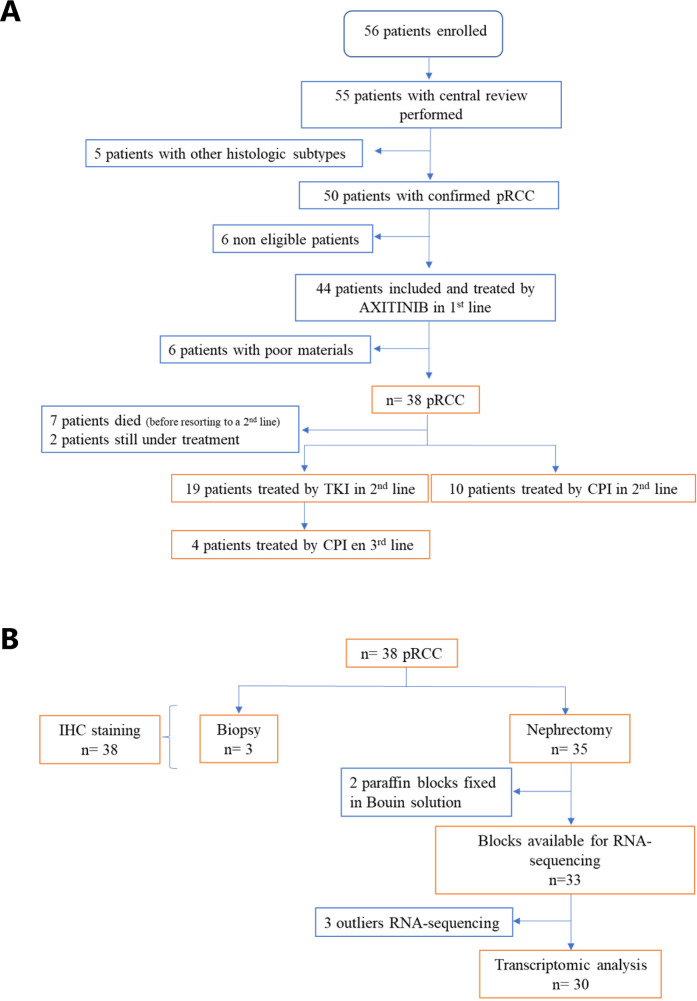
We performed a post hoc analysis of the AXIPAP trial14 from 38 patients with available tissue samples and clinically annotated treatment response on first line systemic therapy, second and subsequent line, represented in flow chart (figure 2A). Fourteen patients were treated with immunotherapy in second or third line including 12 with nivolumab (in the French national AcSé program). The clinical characteristics of these 38 patients were presented in table 2. Among the 38 cases, 1 (3%) case was from metastatic sites and 37 (97%) cases were primitive pRCC.
Figure 2.
(A) Flow chart of patients included in AXIPAP and their follow-up (clinical data cut-off was February 28, 2021) and (B) with materials available for IHC and RNA-seq analysis. CPI, checkpoint inhibitors; IHC, immunohistochemistry; pRCC, papillary renal cell carcinoma; RNA-seq, RNA-sequencing; TKI, tyrosine kinase inhibitors.
Table 2.
Characteristics of the metastatic papillary renal cell carcinoma population, from AXIPAP trial cohort
| Characteristic | N=38* |
| Age | 62 (53–69) |
| Gender | |
| Female | 6 (16) |
| Male | 32 (84) |
| Type | |
| Type 1 papillary RCC | 12 (32) |
| Type 2 papillary RCC | 26 (68) |
| Prior nephrectomy | 35 (92) |
| ECOG | |
| 0 | 19 (50) |
| 1 | 19 (50) |
| IMDC score | |
| Favorable risk group | 8 (22) |
| Intermediate risk group | 16 (44) |
| High-risk group | 12 (33) |
| Unknown | 2 |
| Vital_status | |
| Alive | 12 (32) |
| Dead | 26 (68) |
| Survival_time (months) | 18 (8–35) |
*Median (IQR); n (%).
ECOG, Eastern Cooperative Oncology Group; IMDC, International Metastatic RCC Database Consortium; RCC, renal cell carcinoma.
We performed IHC on 38 tumor samples, among which 30 samples had RNA of sufficient quality for downstream analyses (figure 2B).
Protein analyses of pRCC TME subtypes in metastatic patients treated by axitinib
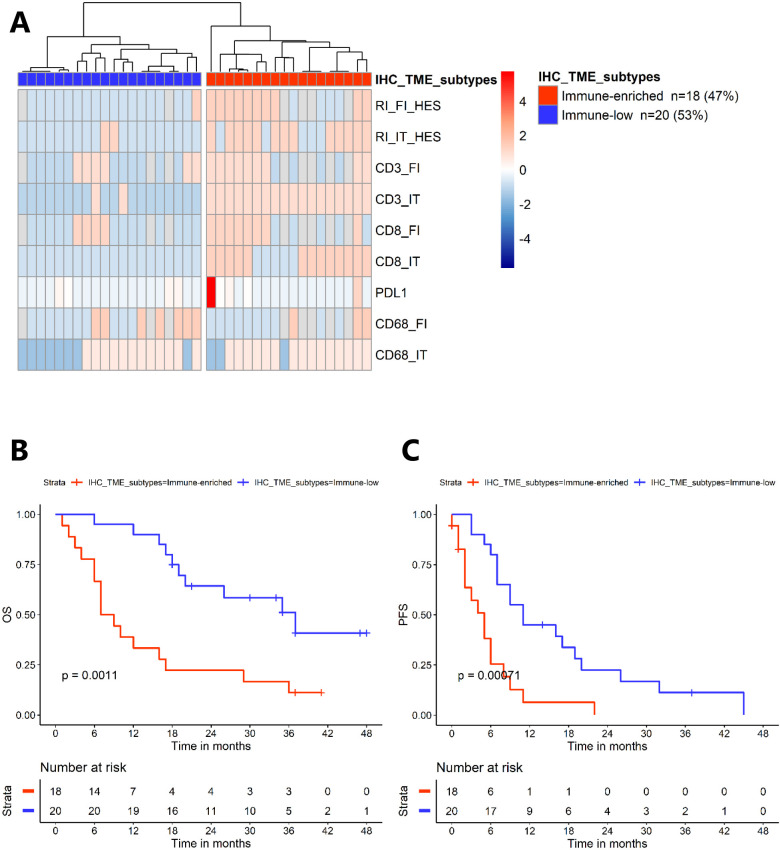
Using IHC, we scored T lymphocytes (CD3, CD8), macrophages (CD68) (from 0 to 3) and tumor-infiltrating lymphocytes (TILs) scoring, at the invasive front and intratumoral (IT), and the immune checkpoint ligand PD-L-1 (% of cells). Based on unsupervised clustering, we identified two groups: 47% (n=18) of patients appeared to have an “immune-enriched” tumor and 53% (n=20) had an “immune-low” tumor (figure 3A). The proportion of histological type 2 pRCC appeared to be higher in the “immune-enriched” subgroup (72% vs 65%, χ2 test p=0.63). Vascular density was similar in both groups (online supplemental figure S3).
Figure 3.
Immunohistochemistry (IHC) analyses from AXIPAP trial cohort (n=38). (A) Heatmap representing unsupervised analysis based on the immune markers performed in IHC (k=2), identifying the “IHC TME subtypes” with the “immune-enriched” group and the “immune-low” group. (B) Kaplan-Meier survival curves (overall survival (OS)) according to subgroups from the unsupervised analysis based on IHC immune markers. (C) Kaplan-Meier survival curves (progression-free survival (PFS)) according to subgroups from the unsupervised analysis based on IHC immune markers. FI, invasive front; HES, Hémalun Eosine Safran; IT, intratumoral; PD-L1, programmed death-ligand 1; TME, tumor microenvironment.
jitc-2023-006885supp004.pdf (123.5KB, pdf)
Interestingly, most samples with CD3 IT>1 marker were in the “immune- enriched” group (36/38, 95%).
pRCC TME subtypes “immune enriched” is associated with worse OS and lower response rates to axitinib
As identified with RNA-seq analyses, the IHC “immune-enriched” component featured a worse prognosis. Median OS was 8 months (95% CI, 6 to 29) versus 37 months (95% CI, 20 to NA), and 12-month OS was 33% versus 90%, in “immune-enriched” versus “immune-low” groups, respectively) (p=0.001) (figure 3B).
Similarly, the PFS was significantly worse in this same group with p=0.0007. Median PFS was 5 months (95% CI, 2 to 9) versus 11 months (95% CI, 7 to 26), and 12-month PFS was 6% versus 45%, in “immune-enriched” versus “immune-low” groups, respectively) (figure 3C). ORR to axitinib appeared to be lower in the IHC “immune-enriched” group (11% vs 30%, Fisher’s exact test p=0.2) (online supplemental figure S4A). Despite the limited data on responses to second and third line immunotherapy (n=14) in the AXIPAP cohort, we observed that the only partial response corresponded to a patient identified in the “immune-enriched” group. In the same group, we observed one stable disease and one progressive disease. In the “immune-low” group, we identified two stable diseases and seven progressive diseases. The ORR to immunotherapy was better in the “immune-enriched” group (33% vs 0%, Fisher’s exact test p=0.16) (online supplemental figure S4B). The two gene expression immune signatures, predictive of response to CPI in ccRCC, were significantly higher in the “immune-enriched” group (adjusted p<0.05) (online supplemental figure S4C). These results suggest that pRCC patients from the “immune-enriched” TME subtype could potentially benefit from immunotherapy.
jitc-2023-006885supp005.pdf (61KB, pdf)
Transcriptomic characterization of the pRCC TME subtypes defined by IHC
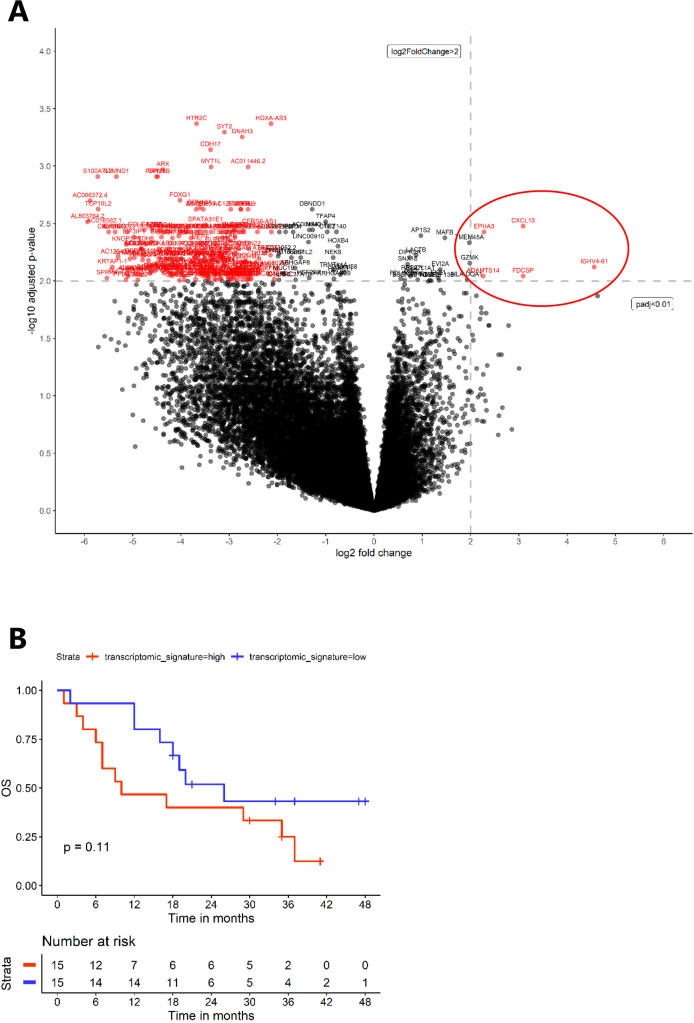
To identify the transcriptomic programs driving the pRCC TME subtypes, we performed differential expression analyses. Under the condition of log2 (fold-change) >2 and p adjusted <0.01, five differentially overexpressed genes were identified between the two groups identified by IHC in the AXIPAP cohort (figure 4A): IGHV4-61, CXCL13, FDCSP, ADAMTS14, EPHA3. Three of these genes directly indicate that B cells and tertiary lymphoid structures (TLS) are likely drivers of the pRCC “immune enriched” subtype. IGHV4-61, immunoglobulin heavy variable 4–61, is coding for the variable region of an immunoglobulin which indicates involvement of B cells. Further, CXCL13 (C-X-C motif chemokine ligand 13) is a chemo-attractant for B cells and T follicular helper cells. Finally, FDCSP (follicular dendritic cell secreted protein) is indicative of the presence of follicular dendritic cells, key component of mature TLS.
Figure 4.
Transcriptomic characterization of the papillary renal cell carcinoma tumor microenvironment subtypes defined by IHC. (A) Volcano plot representing the messenger RNAs differential-expression analysis (=32,378 genes) from DESeq2, between the two groups identified from IHC in the AXIPAP trial cohort (n=30). Under the condition of log2 (fold-change) >2 and p adjusted <0.01, five differentially overexpressed genes were identified between the two groups (red circle). (B) Kaplan-Meier survival curves (overall survival (OS)) according to subgroups with a “high transcriptomic signature” and “low transcriptomic signature”, defined as a median of the five significant differentially overexpressed genes. IHC, immunohistochemistry.
The prognosis of patients with a “high transcriptomic signature”, defined as a higher expression of these five genes, seemed to be associated with a dismal prognosis, even if the difference in survival was not significant with p=0.11. Median OS was 10 months (95% CI, 7 to NA) versus 26 months (95% CI, 18 to NA), and 12-month OS was 47% versus 80%, in “high signature” versus “low signature”, respectively (figure 4B).
According to the Gene Ontology terms analyses, and in particular biological process, similarly to the TCGA cohort (online supplemental figure S5A), the “immune-enriched” group was significantly associated with the immune-response and B-cell activation pathways (online supplemental figure S5B). Furthermore, from the top 50, 42 pathways were common between the “immune-enriched” and “immune-low” clusters in the TCGA cohort and the “immune-enriched” and “immune-low” groups in the AXIPAP cohort.
jitc-2023-006885supp006.pdf (95.6KB, pdf)
Discussion
We report the first comprehensive characterization of immune infiltration in pRCC in both localized and metastatic patients.
Using an unsupervised analysis on localized pRCC data, we identified distinct subsets of pRCC, including one population enriched in immune infiltrate. We confirmed this result in an independent localized pRCC cohort.45
Given that the immune infiltrate may differ between localized and metastatic disease,31 52 we subsequently characterized the TME of patients with metastatic pRCC. We conducted a comprehensive evaluation of immune infiltration, by IHC and RNA-seq, in a prospective metastatic pRCC trial cohort, annotated with systemic therapy treatment.14
In the metastatic cohort, we also identified an “immune-enriched” subgroup, by IHC, with the CD3 marker being a routinely applicable IHC staining. Our results provide further granularity to the previously presented analyses from the KEYNOTE-427-B cohort53 performed in 136 nccRCC, showing that the T-cell–inflamed gene expression profile signature was significantly associated with ORR, which may be predictive of a response to CPI.54 However, this signature remains based on RNA-seq, therefore limiting its clinical implementation in a daily practice.
In addition to identifying an “immune-enriched” subgroup pRCC, we highlighted its prognostic value, which was significantly worse than that of the identified “immune-low” population, both in RNA-seq and IHC. These results are similar to those of the ccRCC well described in literature.55 56
Our results as part of the AXIPAP clinical trial cohort, indicated that both ORR and PFS under axitinib appeared to be lower in the “immune-enriched” group. These findings suggest that other treatment options, such as immunotherapy, may be considered in this subgroup. We previously reported on CPI therapies limited activity in an unselected pRCC population,24 highlighting the need to identify predictive biomarkers to better select patients more likely to respond to CPI. In our analysis, the “immune-enriched” cluster was characterized by significantly higher gene expression of CPI markers. Interestingly, the study by Şenbabaoğlu et al showed that enriched in T cells ccRCC tumors and responding to immunotherapy had also high expression levels of immune response-related genes, including immune checkpoint genes such as PD-1, PD-L1 and CTLA-4.57 Moreover, the two predictive signatures for response to CPI (Teff and JAVELIN Renal 101 Immuno), described in ccRCC, were significantly greater in the “immune-enriched” cluster. Despite the limited data on responses to second and third line immunotherapy in our AXIPAP cohort, the ORR to immunotherapy appeared numerically higher in the “immune-enriched” group. Taken altogether, these results suggest that the “immune-enriched” feature may have potential predictive value for a favorable response to immunotherapy.
Furthermore, in order to characterize the difference between the two groups identified in IHC, we performed mRNAs differential expression analysis with strict significance criteria. We highlighted five differentially overexpressed genes in the “immune-enriched” group. The gene IGHV4-61 is part of B lymphocyte populations. The gene CXCL13, corresponds to chemokine (C-X-C motif) ligand 13 or BLC (B-lymphocyte chemoattractant) or BCA-1 (B cell-attracting chemokine 1). His role is selective chemotaxis for B cells, a product of follicular helper CD4+T cells and a contributor to TLS.58 The gene FDCSP, follicular dendritic cell secreted protein, is able to specifically bind to activated B cells and is intimately connected to chemokine pathways, particularly with the CXCL13.59 Follicular dendritic cells are found in the center of the germinal center of mature tertiary lymphoid structures60 where they are likely to present antigen to B cells. The gene ADAMTS14, a disintegrin and metalloproteinase with thrombospondin motifs 14, is mainly involved in extracellular matrix assembly and degradation. It is highly associated with several immune cells in ccRCC, such as activated dendritic cell, central memory CD8 T cell, central memory CD4 T cell, and activated CD4 T cell.61 Finally, the gene EPHA3, Eph receptor tyrosine kinases, formerly known as HEK, contribute to tumor development, modulating cell–cell adhesion and survival during invasion, neo-angiogenesis and metastasis.62 Therefore, the gene EPHA3 represents a potential source of tumor-specific antigens recognized on tumor cells that express human leukocyte antigens (HLA) class II molecules. It appears to function as a tumor-suppressor in ccRCC as in the tumor stromal microenvironment with mesenchymal stromal cells (MSCs).63 All these genes are related to the B population and TLS, recently described in the literature. Notably, tumors with mature TLS, a high density of B cells and plasma cells, as well as the presence of antibodies totumor-associated antigens are typically associated with favorable clinical outcomes and responses to immunotherapy compared with those lacking these characteristics.60
Our exploratory analysis of a transcriptomic signature, defined as a higher expression of these five genes, seemed to be associated with a dismal prognosis in OS. This result is consistent with the literature as these five genes are described in ccRCC, each of which is associated with a poor prognosis and decreased survival of patients.64–67 IT CXCL13+CD8+T cells abundance was associated with immune-evasive contexture. The abundance of CXCL13+CD8+T cells was shown to be an independent prognosticator and a potential immunotherapeutic target marker for ccRCC treatment.68 Luo et al reports that four TME-related genes (CD79A, CXCL13, IL-6 and CCL19) were identified as biomarkers for pRCC prognosis in localized pRCC from the TCGA cohort.69
Beyond their prognostic value, data from the recent literature showed that the B and TLS populations have a potential predictive value for response to immunotherapy in various cancers,66 70 demonstrating significantly higher expression of B-cell-related genes in responders versus non-responders.71 CXCL13 expression, as a surrogate for tumor TLS, is a relevant candidate predictive biomarker of response to CPI for patients with advanced-stage bladder cancer.72 Similarly, in the NIVOREN cohort, Carril-Ajuria et al, demonstrated for the first time, that a pre-existing high number of circulating baseline unswitched memory B cells is associated with higher probability of response to nivolumab and longer PFS and OS in patients with metastatic ccRCC.73
Our study has several limitations. As pRCC are rare tumors, our analyses were performed on a limited number of available samples. We can note the differences in clustering proportions between cohorts, which can be explained by metastatic tumors, known to be more inflammatory and aggressive than localized pRCC,52 the heterogeneity of techniques between RNA-seq, microarray, and IHC, and also a potential lack of power due to a limited number of available samples. Moreover, sequencing techniques, especially on paraffin blocks, are recent, and may generate biases in the results. Similarly, it would be interesting to be able to confirm the possibility of identifying the “immune-enriched” group from the CD3 marker in another independent cohort. Finally, the potential predictive value of response to immunotherapy of our identified “immune-enriched” group can only be formulated as a hypothesis since we have limited data from further CPI treatment. It will be necessary to validate our results and hypotheses in a prospective cohort of metastatic pRCC treated with CPI alone and in combination with a TKI.
Conclusion
In summary, for the first time, based on a comprehensive analysis, using RNA-seq and IHC, we identified a specific immune TME subtype of metastatic pRCC, significantly more infiltrated with cytotoxic T and B immune populations. The identification of this group could be done by IHC, and in particular by the CD3 marker, a reliable and inexpensive technique. This “immune-enriched” feature, with its defining markers highlighted, appears to be correlated with a poor prognosis but could indicate a potential predictive value for response to immunotherapy. This, however, requires a confirmation in metastatic pRCC treated with CPI alone and in combination with a TKI.
Acknowledgments
The authors thank Sabrina Fronteau for the blocks RNA-sequencing procedures of the AXIPAP trial cohort.
Footnotes
Contributors: LA and MdV-B contributed to the study conception and design. NR-L, SN, EB, JD, CP, ES-M, LA and MdV-B performed the data collection. NR-L, MM, LA and MdV-B performed the data analysis. MdV-B prepared the first draft of the manuscript. Guarantor of this work is LA. All authors revised the manuscript. All authors contributed to patient care and approved the final manuscript.
Funding: Research Grant: MERCUR-1.2 – IPSEN PHARMA and ARTuR Grant (French Kidney Cancer association) Grant recipient.
Competing interests: MdV-B: Research Grant: Ipsen. Board: AstraZeneca, AAA, BMS, Astellas. Travel expense: Pfizer. NR-L: Board: Ipsen, BMS, AstraZeneca. RF: Honoraria: Merck, Pfizer, Ipsen, MSD, Bayer. Board: Janssen, Merck, Pfizer. Expert: Ipsen, Janssen, Astellas. GG: Speaker bureau: Janssen, Amgen, BMS, Ipsen, AAA, AstraZeneca, Bayer, Pfizer, Merck, Astellas. Recipient my institution. Board: Janssen, Amgen, BMS, Curium, Bayer, Pfizer, Merck. Recipient my institution. Expert: BMS, Bayer, Pfizer, Merck. Recipient my institution. Travel expense: Janssen, BMS, AstraZeneca, Bayer, Pfizer Merck. Recipient: me. LG: Consulting or advisory role: BMS, MSD, Novartis, Ipsen, Janssen. CC: Consulting or advisory role: Pfizer, Novartis, BMS. FR: Consulting: Pfizer, Ipsen, BMS, MSD. AR: Consulting or advisory role: Pfizer, BMS, AstraZeneca, Roche, MSD, Ipsen. Travel expenses: Pfizer, BMS, AstraZeneca, Roche, MSD, Ipsen ; Institutional Grant: Pfizer. MG-G: Honoraria and travel expenses: Amgen, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck KGaA, MSD, Novartis, Pfizer, Roche and Sanofi. BE: Advisory role: BMS, Pfizer, Novartis, Oncorena, Immunicum. Research grants: BMS, Novartis, Aveo. SN: Honoraria: Pfizer, Ipsen, MSD, BMS, Eisaï. Travel expenses: Pfizer, Ipsen, MSD. Research grant (institution): Pfizer, Ipsen. LA: Advisory role (Institution): BMS, MSD, Pfizer, Novartis, Amgen, Astellas, Ipsen, Roche, Merck, AstraZeneca, Exelixis, Peloton therapeutics, Corvus pharmaceuticals, Janssen, Eisai, 4D Pharma. Travel expenses: Merck/Pfizer. MM, JD, CP, ES-M, GF, EB, FL: no conflict.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was conducted according to the declaration of Helsinki and the International Conference of Good Clinical Practices after local approval of the Ethic Committee of Lyon Sud-Est IV (approval ID : 15/022). All patients provided written informed consent before enrollment.
References
- 1. Albiges L, Flippot R, Rioux-Leclercq N, et al. Non-clear cell renal cell carcinomas: from shadow to light. J Clin Oncol 2018;36:JCO2018792531. 10.1200/JCO.2018.79.2531 [DOI] [PubMed] [Google Scholar]
- 2. Linehan WM, Spellman PT, Ricketts CJ, et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med 2016;374:135–45. 10.1056/NEJMoa1505917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Humphrey PA, Moch H, Cubilla AL, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol 2016;70:106–19. 10.1016/j.eururo.2016.02.028 [DOI] [PubMed] [Google Scholar]
- 4. Vera-Badillo FE, Templeton AJ, Duran I, et al. Systemic therapy for non-clear cell renal cell carcinomas: a systematic review and meta-analysis. Eur Urol 2015;67:740–9. 10.1016/j.eururo.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 5. de Velasco G, McKay RR, Lin X, et al. Comprehensive analysis of survival outcomes in non-clear cell renal cell carcinoma patients treated in clinical trials. Clin Genitourin Cancer 2017;15:652–60. 10.1016/j.clgc.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 6. Leroy X, Zini L, Leteurtre E, et al. Morphologic subtyping of papillary renal cell carcinoma: correlation with prognosis and differential expression of MUC1 between the two subtypes. Mod Pathol 2002;15:1126–30. 10.1097/01.MP.0000036346.88874.25 [DOI] [PubMed] [Google Scholar]
- 7. Klatte T, Pantuck AJ, Said JW, et al. Cytogenetic and molecular tumor profiling for type 1 and type 2 papillary renal cell carcinoma. Clinical Cancer Research 2009;15:1162–9. 10.1158/1078-0432.CCR-08-1229 [DOI] [PubMed] [Google Scholar]
- 8. Cheville JC, Lohse CM, Zincke H, et al. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 2003;27:612–24. 10.1097/00000478-200305000-00005 [DOI] [PubMed] [Google Scholar]
- 9. Giles RH, Choueiri TK, Heng DY, et al. Recommendations for the management of rare kidney cancers. Eur Urol 2017;72:974–83. 10.1016/j.eururo.2017.06.040 [DOI] [PubMed] [Google Scholar]
- 10. Graham J, Wells JC, Donskov F, et al. Cytoreductive nephrectomy in metastatic papillary renal cell carcinoma: results from the International metastatic renal cell carcinoma database consortium. Eur Urol Oncol 2019;2:643–8. 10.1016/j.euo.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ravaud A, Oudard S, De Fromont M, et al. First-line treatment with Sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: a phase II study (SUPAP) by the French genitourinary group (GETUG)†. Ann Oncol 2015;26:1123–8. 10.1093/annonc/mdv149 [DOI] [PubMed] [Google Scholar]
- 12. Escudier B, Molinie V, Bracarda S, et al. Open-label phase 2 trial of first-line everolimus monotherapy in patients with papillary metastatic renal cell carcinoma: RAPTOR final analysis. Eur J Cancer 2016;69:226–35. 10.1016/j.ejca.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 13. Pal SK, Tangen C, Thompson IM, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet 2021;397:695–703. 10.1016/S0140-6736(21)00152-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Negrier S, Rioux-Leclercq N, Ferlay C, et al. Axitinib in first-line for patients with metastatic papillary renal cell carcinoma: results of the multicentre, open-label, single-arm, phase II AXIPAP trial. Eur J Cancer 2020;129:107–16. 10.1016/j.ejca.2020.02.001 [DOI] [PubMed] [Google Scholar]
- 15. Choueiri TK, Escudier B, Powles T, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1814–23. 10.1056/NEJMoa1510016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koshkin VS, Barata PC, Zhang T, et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J Immunother Cancer 2018;6:9. 10.1186/s40425-018-0319-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McKay RR, Bossé D, Xie W, et al. The clinical activity of PD-1/PD-L1 inhibitors in metastatic non-clear cell renal cell carcinoma. Cancer Immunol Res 2018;6:758–65. 10.1158/2326-6066.CIR-17-0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yip SM, Wells C, Moreira R, et al. Checkpoint inhibitors in patients with metastatic renal cell carcinoma: results from the international metastatic renal cell carcinoma database consortium. Cancer 2018;124:3677–83. 10.1002/cncr.31595 [DOI] [PubMed] [Google Scholar]
- 20. Stukalin I, Wells JC, Graham J, et al. Real-world outcomes of nivolumab and cabozantinib in metastatic renal cell carcinoma: results from the international metastatic renal cell carcinoma database consortium. Current Oncology 2019;26:175–9. 10.3747/co.26.4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boileve A. Immune checkpoint inhibitors following targeted therapies in MITF family translocation renal cell carcinomas. Abstract ESMO 2017; 2017. [Google Scholar]
- 22. Chahoud J, Msaouel P, Campbell MT, et al. Nivolumab for the treatment of patients with metastatic non-clear cell renal cell carcinoma (NccRCC): a single-institutional experience and literature meta-analysis. Oncologist 2020;25:252–8. 10.1634/theoncologist.2019-0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Albiges L, Gurney HP, Atduev V, et al. 1448O phase II KEYNOTE-B61 study of pembrolizumab (Pembro) + Lenvatinib (Lenva) as first-line treatment for non-clear cell renal cell carcinoma (nccRCC). Ann Oncol 2022;33:S1204–5. 10.1016/j.annonc.2022.07.1551 [DOI] [Google Scholar]
- 24. de Vries-Brilland M, McDermott DF, Suárez C, et al. Checkpoint inhibitors in metastatic papillary renal cell carcinoma. Cancer Treat Rev 2021;99:102228. 10.1016/j.ctrv.2021.102228 [DOI] [PubMed] [Google Scholar]
- 25. Charoentong P, Finotello F, Angelova M, et al. Pan-cancer Immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep 2017;18:248–62. 10.1016/j.celrep.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 26. Rooney MS, Shukla SA, Wu CJ, et al. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015;160:48–61. 10.1016/j.cell.2014.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choueiri TK, Fay AP, Gray KP, et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol 2014;25:2178–84. 10.1093/annonc/mdu445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Motoshima T, Komohara Y, Ma C, et al. PD-L1 expression in papillary renal cell carcinoma. BMC Urol 2017;17:8. 10.1186/s12894-016-0195-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Danaher P, Warren S, Lu R, et al. Pan-cancer adaptive immune resistance as defined by the tumor inflammation signature (TIS): results from the cancer genome Atlas (TCGA). J Immunother Cancer 2018;6:63. 10.1186/s40425-018-0367-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thorsson V, Gibbs DL, Brown SD, et al. The immune landscape of cancer. Immunity 2018;48:812–30. 10.1016/j.immuni.2018.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ricketts CJ, De Cubas AA, Fan H, et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep 2018;23:3698. 10.1016/j.celrep.2018.06.032 [DOI] [PubMed] [Google Scholar]
- 32. Choueiri TK, Fishman MN, Escudier B, et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res 2016;22:5461–71. 10.1158/1078-0432.CCR-15-2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Braun DA, Hou Y, Bakouny Z, et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat Med 2020;26:909–18. 10.1038/s41591-020-0839-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee M, Samstein RM, Valero C, et al. Tumor mutational burden as a predictive biomarker for checkpoint inhibitor immunotherapy. Hum Vaccin Immunother 2020;16:112–5. 10.1080/21645515.2019.1631136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meylan M, Beuselinck B, Dalban C, et al. 700O kidney ccRCC immune classification (KIC) enhances the predictive value of T Effector (teff) and angiogenesis (Angio) signatures in response to Nivolumab (N). Annals of Oncology 2020;31:S553. 10.1016/j.annonc.2020.08.772 [DOI] [Google Scholar]
- 36. Simonaggio A, Epaillard N, Pobel C, et al. Tumor microenvironment features as predictive biomarkers of response to immune checkpoint inhibitors (ICI) in metastatic clear cell renal cell carcinoma (mccRCC). Cancers (Basel) 2021;13:231. 10.3390/cancers13020231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tucker MD, Rini BI. Predicting response to immunotherapy in metastatic renal cell carcinoma. Cancers (Basel) 2020;12:2662. 10.3390/cancers12092662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 2018;24:749–57. 10.1038/s41591-018-0053-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Motzer RJ, Banchereau R, Hamidi H, et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell 2020;38:803–17. 10.1016/j.ccell.2020.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Motzer RJ, Robbins PB, Powles T, et al. Avelumab plus Axitinib versus Sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN renal 101 trial. Nat Med 2020;26:1733–41. 10.1038/s41591-020-1044-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rini BI, Huseni M, Atkins MB, et al. Molecular correlates differentiate response to atezolizumab (Atezo) + bevacizumab (BEV) vs sunitinib (sun): results from a phase III study (Immotion151) in untreated metastatic renal cell carcinoma (mRCC). Ann Oncol 2018;29:viii724–5. 10.1093/annonc/mdy424.037 [DOI] [Google Scholar]
- 42. Motzer RJ, Choueiri TK, McDermott DF, et al. Biomarker analyses from the phase III checkmate 214 trial of nivolumab plus Ipilimumab (N+I) or Sunitinib (S) in advanced renal cell carcinoma (aRCC). J Clin Oncol 2020;38:5009. 10.1200/JCO.2020.38.15_suppl.5009 [DOI] [Google Scholar]
- 43. Vano Y-A, Elaidi R, Bennamoun M, et al. Nivolumab, nivolumab-Ipilimumab, and VEGFR-tyrosine kinase inhibitors as first-line treatment for metastatic clear-cell renal cell carcinoma (BIONIKK): a biomarker-driven, open-label, non-comparative, randomised, phase 2 trial. Lancet Oncol 2022;23:612–24. 10.1016/S1470-2045(22)00128-0 [DOI] [PubMed] [Google Scholar]
- 44. Liu J, Lichtenberg T, Hoadley KA, et al. An integrated TCGA Pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell 2018;173:400–16. 10.1016/j.cell.2018.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Albiges L, Guegan J, Le Formal A, et al. MET is a potential target across all papillary renal cell carcinomas: result from a large molecular study of pRCC with CGH array and matching gene expression array. Clin Cancer Res 2014;20:3411–21. 10.1158/1078-0432.CCR-13-2173 [DOI] [PubMed] [Google Scholar]
- 46. Edeline J, Mottier S, Vigneau C, et al. Description of 2 angiogenic phenotypes in clear cell renal cell carcinoma. Hum Pathol 2012;43:1982–90. 10.1016/j.humpath.2012.01.023 [DOI] [PubMed] [Google Scholar]
- 47. Hendry S. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group. Adv Anat Pathol 2017;24:311–35. 10.1097/PAP.0000000000000162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Becht E, Giraldo NA, Lacroix L, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol 2016;17:218. 10.1186/s13059-016-1070-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Petitprez F, Vano YA, Becht E, et al. Transcriptomic analysis of the tumor microenvironment to guide prognosis and immunotherapies. Cancer Immunol Immunother 2018;67:981–8. 10.1007/s00262-017-2058-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen F, Zhang Y, Şenbabaoğlu Y, et al. Multilevel genomics-based taxonomy of renal cell carcinoma. Cell Rep 2016;14:2476–89. 10.1016/j.celrep.2016.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013;39:782–95. 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 52. Baine MK, Turcu G, Zito CR, et al. Characterization of tumor infiltrating lymphocytes in paired primary and metastatic renal cell carcinoma specimens. Oncotarget 2015;6:24990–5002. 10.18632/oncotarget.4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McDermott DF, Lee J-L, Ziobro M, et al. First-line pembrolizumab (Pembro) monotherapy for advanced non-clear cell renal cell carcinoma (nccRCC): results from KEYNOTE-427 cohort B. J Clin Oncol 2019;37:546. 10.1200/JCO.2019.37.7_suppl.546 [DOI] [Google Scholar]
- 54. McDermott DF, Lee J-L, Donskov F, et al. Association of gene expression with clinical outcomes in patients with renal cell carcinoma treated with pembrolizumab in KEYNOTE-427. J Clin Oncol 2020;38:5024. 10.1200/JCO.2020.38.15_suppl.5024 [DOI] [Google Scholar]
- 55. Drake CG, Stein MN. The Immunobiology of kidney cancer. JCO 2018;36:3547–52. 10.1200/JCO.2018.79.2648 [DOI] [PubMed] [Google Scholar]
- 56. Giraldo NA, Becht E, Pagès F, et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clinical Cancer Research 2015;21:3031–40. 10.1158/1078-0432.CCR-14-2926 [DOI] [PubMed] [Google Scholar]
- 57. Şenbabaoğlu Y, Gejman RS, Winer AG, et al. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol 2016;17:231. 10.1186/s13059-016-1092-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hussain M, Adah D, Tariq M, et al. CXCL13/CXCR5 signaling axis in cancer. Life Sci 2019;227:175–86. 10.1016/j.lfs.2019.04.053 [DOI] [PubMed] [Google Scholar]
- 59. Marshall AJ, Du Q, Draves KE, et al. FDC-SP, a novel secreted protein expressed by follicular dendritic cells. J Immunol 2002;169:2381–9. 10.4049/jimmunol.169.5.2381 [DOI] [PubMed] [Google Scholar]
- 60. Fridman WH, Meylan M, Petitprez F, et al. B cells and tertiary Lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat Rev Clin Oncol 2022;19:441–57. 10.1038/s41571-022-00619-z [DOI] [PubMed] [Google Scholar]
- 61. Zhou T, Chen W, Wu Z, et al. A newly defined basement membrane-related gene signature for the prognosis of clear-cell renal cell carcinoma. Front Genet 2022;13:994208. 10.3389/fgene.2022.994208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Janes PW, Slape CI, Farnsworth RH, et al. Epha3 biology and cancer. Growth Factors 2014;32:176–89. 10.3109/08977194.2014.982276 [DOI] [PubMed] [Google Scholar]
- 63. Chiari R, Hames G, Stroobant V, et al. Identification of a tumor-specific shared antigen derived from an Eph receptor and presented to CD4 T cells on HLA class II molecules. Cancer Res 2000;60:4855–63. [PubMed] [Google Scholar]
- 64. Xu W, Ma C, Liu W, et al. Prognostic value, DNA variation and immunologic features of a tertiary lymphoid structure-related chemokine signature in clear cell renal cell carcinoma. Cancer Immunol Immunother 2022;71:1923–35. 10.1007/s00262-021-03123-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen Y, Ji H, Liu S, et al. Survival prognosis, tumor immune landscape, and immune responses of adamts14 in clear cell renal cell carcinoma and its potential mechanisms. Front Immunol 2022;13:790608. 10.3389/fimmu.2022.790608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen J, Chen S, Dai X, et al. Exploration of the underlying biological differences and targets in ovarian cancer patients with diverse immunotherapy response. Front Immunol 2022;13:1007326. 10.3389/fimmu.2022.1007326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. London M, Gallo E. Critical role of Epha3 in cancer and current state of Epha3 drug Therapeutics. Mol Biol Rep 2020;47:5523–33. 10.1007/s11033-020-05571-8 [DOI] [PubMed] [Google Scholar]
- 68. Dai S, Zeng H, Liu Z, et al. Intratumoral CXCL13 + CD8 + T CeLL infiltration determines poor clinical outcomes and immunoevasive contexture in patients with clear cell renal cell carcinoma. J Immunother Cancer 2021;9:e001823. 10.1136/jitc-2020-001823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Luo L, Zhou H, Su H. Identification of 4-genes model in papillary renal cell tumor microenvironment based on comprehensive analysis. BMC Cancer 2021;21:553. 10.1186/s12885-021-08319-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Petitprez F, de Reyniès A, Keung EZ, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020;577:556–60. 10.1038/s41586-019-1906-8 [DOI] [PubMed] [Google Scholar]
- 71. Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary Lymphoid structures promote immunotherapy response. Nature 2020;577:549–55. 10.1038/s41586-019-1922-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Groeneveld CS, Fontugne J, Cabel L, et al. Tertiary Lymphoid structures marker Cxcl13 is associated with better survival for patients with advanced-stage bladder cancer treated with immunotherapy. Eur J Cancer 2021;148:181–9. 10.1016/j.ejca.2021.01.036 [DOI] [PubMed] [Google Scholar]
- 73. Carril-Ajuria L, Desnoyer A, Meylan M, et al. Baseline circulating Unswitched memory B cells and B-cell related soluble factors are associated with overall survival in patients with clear cell renal cell carcinoma treated with Nivolumab within the NIVOREN GETUG-AFU 26 study. J Immunother Cancer 2022;10:e004885. 10.1136/jitc-2022-004885 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-006885supp001.pdf (148.8KB, pdf)
jitc-2023-006885supp002.pdf (199.4KB, pdf)
jitc-2023-006885supp003.pdf (159.6KB, pdf)
jitc-2023-006885supp004.pdf (123.5KB, pdf)
jitc-2023-006885supp005.pdf (61KB, pdf)
jitc-2023-006885supp006.pdf (95.6KB, pdf)
Data Availability Statement
No data are available.