Abstract
Objectives
Patients with psoriatic arthritis (PsA) are at a significantly increased risk of hyperuricaemia and development of gout, and those with hyperuricaemia have been found to respond poorly to PsA treatment and have more peripheral and destructive joint damage. We present a comprehensive post hoc analysis using pooled data from the FUTURE 2–5 studies and the MAXIMISE study to further evaluate the impact of hyperuricaemia on clinical presentation/disease severity and response to secukinumab in patients with PsA.
Methods
Patients were stratified into two groups based on baseline serum uric acid (SUA) level (threshold of 360 µmol/L). A sensitivity analysis was also performed based on SUA thresholds of 300 µmol/L and 420 µmol/L. Demographics, clinical, radiological characteristics and comorbidities data were collected.
Results
At baseline, patients with hyperuricaemia were mostly male, reported a higher prevalence of hypertension, with more clinical dactylitis, more psoriasis and more severe skin disease compared with patients with normouricaemia. A similar proportion of patients in the normouricaemic and hyperuricaemic cohorts achieved American College of Rheumatology responses, resolution of enthesitis and dactylitis, inhibition of structural damage progression and improvement in health-related quality of life across all secukinumab doses at week 52.
Conclusion
Patients with PsA and hyperuricaemia have different clinical characteristics from patients with PsA and normouricaemia. Identification of these patients at an early stage may facilitate a personalised treatment approach and improved management of comorbidities. Furthermore, secukinumab provided a rapid and sustained response across all manifestations of PsA up to week 52, irrespective of baseline uricaemia status.
Keywords: arthritis, psoriatic; gout; biological therapy; immune system diseases; arthritis
WHAT IS ALREADY KNOWN ON THIS TOPIC
‘Psout’ has been proposed as a new nosological entity, consisting of an overlapping syndrome between gout and psoriatic arthritis (PsA).
WHAT THIS STUDY ADDS
Comorbidities, clinical symptoms and radiological signs of PsA in patients with hyperuricaemia appear to differ from those without concomitant hyperuricaemia.
Secukinumab up to week 52 is effective on all aspects of PsA irrespective of baseline uric acid level.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Patients with PsA and hyperuricaemia have a different clinical characteristics from patients with PsA and normouricaemia. Recognition of this may facilitate a personalised treatment approach and improved management of patient comorbidities.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease affecting both peripheral and axial skeleton.1 The disease is heterogeneous and characterised by varied clinical features including peripheral arthritis, dactylitis, enthesitis, axial disease, nail dystrophy and skin disease, with a significant impact on patients’ quality of life.2–4 It may be associated with various cardiac and metabolic comorbidities, including obesity, hypertension, insulin resistance or diabetes mellitus, as well as malignancies and mental illnesses.5 The pathogenic role of chronic hyperuricaemia in the development and maintenance of psoriasis and PsA is based on epidemiological, pathophysiological and clinical arguments, and does not appear to be coincidental. These arguments suggest a much closer interplay between PsA and gout than the simple coexistence of two common conditions in the same patient.6 7 Gout is characterised at a biochemical level by an excess of urate in the extracellular fluid, due to high levels of uric acid (UA) in the blood (hyperuricaemia), where the concentration of urate surpasses its solubility limit.8 The clinical manifestations of gout can include joint-related issues like recurrent flares of inflammatory arthritis (gout flare) and chronic arthropathy. It may also involve the accumulation of urate crystals in the form of tophaceous deposits and/or affect the kidneys, leading to conditions such as UA nephrolithiasis and chronic nephropathy.8 Hyperuricaemia is a necessary condition for the development of urate crystal deposition disease (gout), but it is not sufficient on its own. It is important to differentiate between hyperuricaemia and gout, which is the clinical syndrome associated with the deposition of urate crystals. Interestingly, most people with high UA levels never experience any clinical symptoms or events resulting from the deposition of urate crystals.
Patients with psoriasis and PsA are at significantly increased risk of hyperuricaemia and development of gout than the general population.2 5–7 9 The prevalence of hyperuricaemia in the general population is 2%—13% compared with 19% in patients with psoriasis and 32% in patients with PsA.9 10 Hyperuricaemia may also be the consequence of the metabolic comorbidities associated with PsA.6 10–12 While some studies have indicated that hyperuricaemia is associated mainly with components of the metabolic syndrome, others have shown a correlation with the extent of skin disease or longer PsA disease duration.9 13
UA is the final product of purine metabolism and plays an aetiological role in gout.8 It has been demonstrated that UA has a role in the pathophysiology of psoriasis14 and PsA.2 The internalisation of UA crystals by leukocytes leads to production of free radicals, release of cathepsin B, activation of the NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome15 and subsequent stimulation of innate immunity, leading to the secretion of the pro-inflammatory cytokine interleukin (IL)-1.16 As monosodium urate (MSU) crystals have been found in psoriatic skin plaques,17 they may represent central effectors of cell activation through stimulation of an innate immune response.
The injection of urate crystals in vivo leads to the production of T helper (Th)17 cells and regulatory T cells.18 Th17-derived proinflammatory cytokines, including IL-17A, have a critical role in the pathogenesis of psoriasis and PsA.19 In addition, hyperuricaemia might also derive from an increased UA production in the liver following either increased IL-17 production, which can lead to non-alcoholic fatty liver disease, or lower hepatic ATP production leading to hepatic steatosis.20
Gout results from the deposition of MSU around joints following long-standing, persistent, hyperuricaemia.8 The presence of urate crystals has also been reported in the synovial fluid of patients with PsA,21 22 thus highlighting the coexistence of both conditions in the same individual.
While it has been described that hyperuricaemia may worsen psoriasis or PsA, prospective clinical trials and observational studies evaluating the coexistence of these two conditions are limited. Clinical symptoms are confounding and radiological signs of PsA and gout are similar, making it difficult to differentiate the two in patients with advanced radiological lesions or less clearly defined phenotypes.23 Notably, PsA with hyperuricaemia has been shown to be more peripheral, destructive and challenging to treat,24 demonstrating a need for greater understanding of the relationship between elevated serum uric acid (SUA) levels in patients with PsA and management of both diseases.
Herein, we present a comprehensive post hoc analysis using pooled data from the 25–28FUTURE 2–5 studies and the MAXIMISE29 study to further evaluate the association between hyperuricaemia/gout and clinical presentation/disease severity in patients with PsA. We also evaluated the effect of the IL-17A inhibitor secukinumab on clinical response, radiographic progression and quality of life in patients with PsA with hyperuricaemia or normouricaemia.
Methods
Study design and patients
This post hoc analysis included patients with active PsA who participated in five phase III clinical trials: FUTURE 2 (N=397), FUTURE 3 (N=414), FUTURE 4 (N=341), FUTURE 5 (N=996) and MAXIMISE (n=498). The design, eligibility criteria, methodology and statistical analysis of these individual studies have been published previously.25–29 As the FUTURE 1 study did not include week 52 data from the secukinumab 300 mg group, and used intravenous loading rather than subcutaneous, it was not used as part of this analysis.30 While the FUTURE 2–5 studies included patients with active PsA, the MAXIMISE study specifically assessed axial manifestations in patients with PsA. All analyses were based on the pooled full analysis set (FAS) population of 2504 patients.
In the main analysis, patients were stratified into two groups based on baseline SUA level. Patients with hyperuricaemia were defined as having baseline SUA ≥360 µmol/L, whereas patients with normouricaemia were selected based on baseline SUA <360 µmol/L and without history of gout and/or UA-lowering therapies (ULTs). Different sensitivity analyses were performed by level of UA threshold in serum of 300 µmol/L and 420 µmol/L and in patients with or without history of gout and/or ULT. Patients with gout were defined as patients with either a history of gout and/or concomitant ULT or adverse events of gout. Information on the presence or absence of gout and on the use of ULTs was obtained from the data reported by investigators on patient medical history and previous or concomitant medications in the case report forms at patient screening, enrolment and follow-up visits.
All included studies were conducted in accordance with the Declaration of Helsinki (as revised in Brazil 2013) and were approved by the institutional review boards or independent ethics committees for each study site. Written informed consent was obtained from all participants before study inclusion.
Assessments and outcomes
Assessments at baseline included recording of demographics such as age, sex and body mass index (BMI). Medical history of PsA was further assessed by examining time since first diagnosis of PsA, previous antirheumatic treatments, clinical characteristics (total tender/swollen joint score, presence of enthesitis/dactylitis, mean Psoriasis Area and Severity Index (PASI) score for both mild and moderate to severe groups, presence of nail psoriasis, presence of axial disease, C reactive protein levels), van der Heijde-Modified Total Sharp Score (vdH-mTSS) and presence of comorbidities (hypertension and diabetes mellitus).
Efficacy, structural outcomes and patient-reported outcomes
The efficacy of secukinumab in patients with hyperuricaemia or gout was assessed in the pooled dataset of the FUTURE 2–5 studies and the MAXIMISE study across key manifestations of PsA, such as peripheral arthritis, enthesitis, dactylitis, spinal pain and discomfort, nail disease and psoriasis severity. Secukinumab efficacy was assessed across patient improvement in peripheral arthritis using the percentage of participants meeting American College of Rheumatology (ACR) response criteria. This represents a percentage improvement of ≥20% (ACR20), ≥50% (ACR50) and ≥70% (ACR70), at all scheduled postbaseline visits up to week 52. Treatment efficacy was further assessed by examining the percentage of patients with resolution of enthesitis using the Leeds Enthesitis Index (LEI) score or in the case of the MAXIMISE study, the Spondyloarthritis Research Consortium of Canada (SPARCC) score. The percentage of patients with resolution of dactylitis, at all scheduled postbaseline visits up to week 52, was examined using the Leeds Dactylitis Index (LDI) score. The LEI score is based on the presence or absence of pain or tenderness at enthesitis sites, while the LDI score is based on finger circumference across all dactylitic digits. The efficacy of secukinumab in patients with hyperuricaemia or gout was further assessed by the mean change from baseline using Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) response, using data from the MAXIMISE study only. The mean change in severity of nail disease was determined using the modified Nail Psoriasis Severity Index (mNAPSI) score. Finally, the percentage of patients observed to have experienced a 90% psoriasis resolution, or more, using PASI score, following treatment with secukinumab, was also examined.
Inhibition of structural damage progression was explored by mean change from baseline to week 52 in vdH-mTSS and the proportion of patients with no structural damage progression defined as a ≤0.5 change from baseline in vdH-mTSS.28 Physical function and quality of life were explored using the Health Assessment Questionnaire-Disability Index (HAQ-DI), the 36-Item Short Form Health Survey (SF-36) and the EuroQol-5 Dimension Health State Profile (EQ-5D) from baseline up to week 52.
Statistical analysis
All analyses were performed based on the pooled FAS from the FUTURE 2–5 studies and the MAXIMISE study and stratified by baseline SUA levels. Summary statistics for continuous variables included N, mean, SD, minimum, median and maximum. All efficacy analyses were performed at a descriptive level and are presented as observed. All descriptive efficacy summaries are presented by treatment group assigned at randomisation: secukinumab 300 mg (all FAS patients initially randomised into secukinumab 300 mg treatment groups across all five studies); secukinumab 150 mg (all FAS patients initially randomised into secukinumab 150 mg treatment groups, with loading doses, across all five studies); secukinumab 150 mg no load (all FAS patients initially randomised into secukinumab 150 mg treatment groups, without loading doses, in the FUTURE 4 and FUTURE 5 studies); placebo-secukinumab 300 mg (all FAS patients initially randomised into placebo group and later switched into secukinumab 300 mg treatment groups at week 16/24 according to the protocols); placebo-secukinumab 150 mg (all FAS patients initially randomised into placebo group and later switched into secukinumab 150 mg treatment groups at week 16/24 according to the protocols). In the absence of protocol prespecified statistical tests to compare the subgroups of patients with PsA with or without either hyperuricaemia or gout, we chose only to consider what appeared to us to be clinically relevant differences, such as the frequency of comorbidities, the frequency of dactylitis or more severe psoriasis.
Results
Demographics, and clinical and radiographic characteristics at baseline
Overall, 2504 patients with PsA were included in the analysis, of whom 822 (32.8%) and 356 (14.3%) had SUA levels of ≥360 and ≥420 µmol/L, respectively; 62 patients (2.5%) had a history of gout/ULT. Most patients with hyperuricaemia (SUA ≥360 µmol/L) were male (76%) with a higher BMI (30.90±5.86 vs 28.33±5.91; table 1). Patients with hyperuricaemia were associated with more frequent hypertension, compared with patients with normouricaemia (43.8% vs 31.3%; table 1). A higher proportion of patients with dactylitis were also observed in the hyperuricaemic group (34.5% vs 25.9% for the SUA threshold of ≥360 µmol/L; table 1).
Table 1.
Demographics and baseline clinical characteristics of patients with hyperuricaemia or normouricaemia by threshold
| Parameters (mean±SD) unless specified | SUA threshold 300 µmol/L | SUA threshold 360 µmol/L | SUA threshold 420 µmol/L | |||
| Hyperuricaemic (N=1495) | Normouricaemic (N=1025) | Hyperuricaemic (N=822) |
Normouricaemic (N=1682) | Hyperuricaemic (N=356) | Normouricaemic (N=2130) |
|
| Age (years) | 48.7±12.34 | 48.0±12.12 | 48.5±12.41 | 48.3±12.19 | 49.4±12.04 | 48.1±12.30 |
| Gender (male), n (%) | 978 (65.4) | 234 (22.8) | 625 (76.0) | 576 (34.2) | 278 (78.1) | 908 (42.6) |
| BMI (kg/m2) | 30.30±5.97 | 27.63±5.78 | 30.90±5.86 | 28.33±5.91 | 31.77±6.13 | 28.71±5.89 |
| History of hypertension; n (%) | 603 (40.3) | 295 (28.8) | 360 (43.8) | 526 (31.3) | 184 (51.7) | 694 (32.6) |
| History of diabetes mellitus; n (%) | 144 (9.6) | 88 (8.6) | 85 (10.3) | 144 (8.6) | 40 (11.2) | 182 (8.5) |
| MTX use at randomisation; n (%) | 601 (40.2) | 410 (40.0) | 321 (39.1) | 685 (40.7) | 132 (37.1) | 868 (40.8) |
| Naїve to TNF alpha inhibitors; n (%) | 865 (57.9) | 559 (54.5) | 477 (58.0) | 938 (55.8) | 198 (55.6) | 1205 (56.6) |
| TJC | 20.4±15.12 | 22.1±17.29 | 20.6±15.52 | 21.3±16.25 | 20.3±14.63 | 21.2±16.25 |
| SJC | 10.7±8.92 | 11.0±9.65 | 10.9±9.31 | 10.8±9.13 | 10.9±9.21 | 10.8±9.18 |
| Enthesitis; n (%)* | 761 (50.9) | 512 (50.0) | 412 (50.1) | 852 (50.7) | 187 (52.5) | 1063 (49.9) |
| Dactylitis; n (%)* | 478 (32.0) | 246 (24.0) | 284 (34.5) | 436 (25.9) | 135 (37.9) | 578 (27.1) |
| Evidence of current psoriasis†; n (%) | 659 (44.1) | 355 (34.6) | 397 (48.3) | 611 (36.3) | 192 (53.9) | 806 (37.8) |
| Mild | 247 (37.5) | 186 (52.4) | 135 (34.0) | 296 (48.4) | 56 (29.2) | 371 (46.0) |
| Moderate to severe | 412 (62.5) | 169 (47.6) | 262 (66.0) | 315 (51.5) | 136 (70.8) | 435 (53.9) |
| Mean PASI score‡ | 12.43±10.34 | 9.77±9.21 | 13.61±11.03 | 10.16±9.13 | 14.60±12.10 | 10.78±9.34 |
| mNAPSI score†; n (%) | ||||||
| ≥1 | 752 (60.4) | 434 (54.9) | 431 (62.7) | 745 (55.8) | 202 (65.0) | 962 (56.7) |
| ≥2 | 720 (57.8) | 422 (53.4) | 414 (60.2) | 718 (53.7) | 191 (61.4) | 929 (54.7) |
| >2 | 678 (54.5) | 392 (49.6) | 397 (57.7) | 664 (49.7) | 181 (58.2) | 869 (51.2) |
| Time since first diagnosis of PsA (years) | 6.8±7.79 | 6.7±7.45 | 6.90±7.86 | 6.70±7.58 | 7.6±8.10 | 6.6±7.60 |
| Serum uric acid (µmol/L) | 378.0±64.46 | 242.3±39.03 | 420.7±57.11 | 274.9±51.98 | 470.7±52.25 | 297.5±64.13 |
| CRP (mg/L) | 11.3±20.25 | 10.6±24.06 | 11.6±18.66 | 10.7±23.36 | 11.9±19.13 | 10.8±22.38 |
| vdH-mTSS§ | NA | NA | 12.2±29.68 | 13.6±33.94 | 17.4±41.46 | 12.3±30.40 |
*Enthesitis/dactylitis was assessed for the subset of patients with enthesitis/dactylitis at baseline.
†Subset from the FUTURE 2–5 studies.
‡Based on a subset of patients with psoriasis at baseline in the FUTURE 2–5 studies.
§Only FUTURE 5 study data.
BMI, body mass index; CRP, C reactive protein; mNAPSI, modified Nail Psoriasis Severity Index; MTX, methotrexate; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; SJC, swollen joint count; SUA, serum uric acid; TJC, tender joint count; TNF, tumour necrosis factor; ULT, uric acid–lowering therapy; vdH-mTSS, van der Heijde-Modified Total Sharp Score.
In a sensitivity analysis of the SUA threshold of ≥420 µmol/L, a higher incidence of diabetes mellitus was reported in patients with hyperuricaemia compared with patients with normouricaemia (11.2% vs 8.5%). In patients with hyperuricaemia and normouricaemia, the incidence of enthesitis was 52.5% versus 49.9% and the incidence of dactylitis was 37.9% versus 27.1%, respectively (table 1). Within the sensitivity analysis, using a SUA threshold of ≥420 µmol/L, a subset of patients reported having psoriasis currently (53.9% with hyperuricaemia and 37.8% with normouricaemia), of which a higher proportion of patients with hyperuricaemia had moderate to severe psoriasis, compared with patients with normouricaemia (70.8% vs 53.9%; table 1).
In the FUTURE 5 study, patients with a SUA level of ≥420 µmol/L appeared to have higher vdH-mTSS scores with large variability compared with patients with normouricaemia (17.4±41.46 vs 12.3±30.40; table 1). Overall, similar findings were observed in a sensitivity analysis of patients with history of gout and ULT therapy (SUA ≥420 µmol/L), with a higher incidence of diabetes mellitus (13.2% vs 8.5%), dactylitis (38.5% vs 27.1%) and psoriasis (52.1% vs 37.8%) (online supplemental table S1).
rmdopen-2023-003428supp001.pdf (35.4KB, pdf)
Clinical efficacy evaluations
Efficacy in musculoskeletal, axial disease and physical function
A similar proportion of patients achieved ACR20, ACR50 and ACR70 responses and resolution of enthesitis (as measured by LEI, or SPARCC in the case of the MAXIMISE study) and dactylitis (as measured by LDI) across all secukinumab doses at week 52, in both the hyperuricaemic and the normouricaemic groups (figure 1). Similarly, there was no major difference in mean change from baseline at week 52 (as measured by BASDAI response in the MAXIMISE study) with secukinumab 150 mg (hyperuricaemia group: −3.57; normouricaemia group: −4.18) and secukinumab 300 mg (hyperuricaemia group: −4.84; normouricaemia group: −4.26).
Figure 1.
Percentage improvement in ACR criteria and resolution of enthesitis and dactylitis at week 52. Baseline hyperuricaemia is defined as baseline serum uric acid ≥360 µmol/L. *Resolution to enthesitis/dactylitis was evaluated in patients with enthesitis/dactylitis at baseline. †Only MAXIMISE study data. ACR, American College of Rheumatology; LDI, Leeds Dactylitis Index; LEI, Leeds Enthesitis Index; n, number of patients; SEC, secukinumab; SPARCC, Spondyloarthritis Research Consortium of Canada.
Improvement in physical function as measured by HAQ-DI change from baseline to week 52 was consistent in patients in both hyperuricaemic and normouricaemic groups (figure 2).
Figure 2.
Mean change in patient-reported outcomes on QoL and physical function from baseline to week 52. Baseline hyperuricaemia is defined as baseline serum uric acid ≥360 µmol/L. *Not collected for the MAXIMISE study. EQ-5D, EuroQol-5 Dimension Health State Profile; HAQ-DI, Health Assessment Questionnaire-Disability Index; n, number of patients; QoL, quality of life; SEC, secukinumab; SF-36, 36-Item Short Form Health Survey.
Efficacy in skin and nail disease
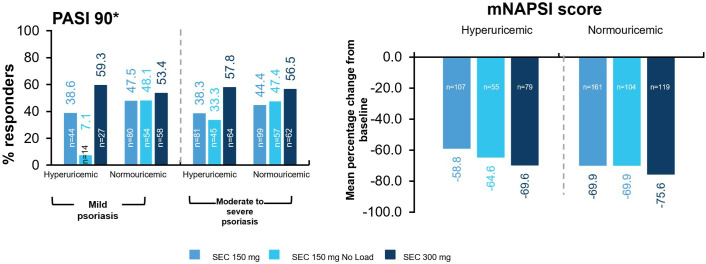
At week 52, weaker PASI 90 response rates were observed in patients with hyperuricaemia receiving secukinumab 150 mg and secukinumab 150 mg no load doses. However, PASI 90 response was comparable with secukinumab 300 mg irrespective of hyperuricaemia status, in patients with PsA with moderate to severe psoriasis (figure 3). Nail psoriasis (as measured by mNAPSI) was comparable at week 52 across all doses in both hyperuricaemic and normouricaemic groups (figure 3).
Figure 3.
PASI 90 response and mean percentage change in mNAPSI from baseline to week 52. Baseline hyperuricaemia is defined as baseline serum uric acid ≥360 µmol/L. Mean percentage change in mNAPSI from baseline was based on patients with mNAPSI score >2 at baseline in the FUTURE 2–5 studies only. mNAPSI, modified Nail Psoriasis Severity Index; n, number of patients; PASI, Psoriasis Area and Severity Index; SEC, secukinumab.
Efficacy in structural damage
Inhibition of structural damage progression (as evaluated by vdH-mTSS from baseline to week 52) and the proportion of non-radiographic progressors were comparable in the secukinumab 150 mg, secukinumab 150 mg no load and secukinumab 300 mg groups, in patients with hyperuricaemia and normouricaemia (figure 4A).
Figure 4.

Inhibition of radiographic progression from baseline to week 52. Baseline hyperuricaemia is defined as baseline serum uric acid level ≥360 µmol/L. *Only FUTURE 5 study data. BL, baseline; n, number of patients; SEC, secukinumab; vdH-mTSS, van der Heijde-Modified Total Sharp Score.
Health-related quality of life
Mean changes from baseline to week 52 in the SF-36 survey and the EQ-5D instrument were also comparable between the secukinumab 150 mg, secukinumab 150 mg no load and secukinumab 300 mg groups, irrespective of baseline hyperuricaemic status (figure 2).
Discussion
Here, we presented a comprehensive post hoc analysis using pooled data from the 25–28FUTURE 2–5 studies and the MAXIMISE29 study to further evaluate the association between hyperuricaemia/gout and clinical presentation/disease severity in patients with PsA. Patient demographics, clinical response, radiographic progression and quality of life were assessed.
As different thresholds of hyperuricaemia have been reported in the literature, we performed the main analysis on hyperuricaemia defined with a SUA threshold of ≥360 µmol/L and a sensitivity analysis in patients with hyperuricaemia with two different SUA cut-offs of 300 µmol/L and 420 µmol/L. The prevalence of hyperuricaemia in our main analysis represented 33% of patients with PsA, showing similar prevalence range to that reported previously by a French retrospective study, as well as in a study examining an Asian cohort.13 24 In contrast, the prevalence of patients with a previous history of gout or receiving ULT was slightly lower (3.5%) than the prevalence reported by the French retrospective study (6%).24
In a previously reported large-scale study of patients from the USA, consisting of two large male and female cohorts, who were monitored for the occurrence of gout, the condition was found to be present in 4.9% of men and 1.2% of women included in the study.31 A HR of 1.71 for gout was reported when compared with individuals without psoriasis.31 In those with PsA, the incidence of gout was even higher, and a HR of 4.95 was reported.31
In our analysis, the majority of patients with concomitant hyperuricaemia, irrespective of the SUA threshold selected, were males and had a higher BMI than normouricaemic patients, consistent with the epidemiology of patients with hyperuricaemia and gout.2 Patients with PsA with hyperuricaemia from the pooled FUTURE 2–525–28 studies and the MAXIMISE29 study dataset were more frequently associated with hypertension and diabetes mellitus, when compared with patients with PsA with normouricaemia. This is reflective of current literature, as different studies have shown that PsA with hyperuricaemia, and PsA with gout, share comorbidities.6 7 24 Hyperuricaemia is a necessary, but not a sufficient, precondition for the development of urate crystal deposition disease and should be thus distinguished from gout, the clinical syndrome. Most hyperuricaemic individuals never experience a clinical event resulting from urate crystal deposition. In a study of more than 2000 healthy males, followed for 15 years, the annual incidence of gout was only 0.5% for serum urate levels 7.0–8.9 mg/dL (about 420–534 µmol/L) and 0.1% for serum urate levels less than 7.0 mg/dL (about 420 µmol/L).32
In patients who are obese, hyperuricaemia results from both impaired excretion and overproduction of UA.33 The mechanism of overproduction of UA during insulin resistance lies in the increased fatty acid synthesis in the liver that causes de novo purine synthesis and accelerated UA production.34 Furthermore, insulin stimulates the renal UA transporters leading to hyperuricaemia.35 While a high prevalence of cardiovascular comorbidities including obesity, hypertension and diabetes mellitus have been described in patients with PsA,9 36–40 hyperuricaemia has also been described to be an independent risk factor for cardiovascular disease in PsA. Different pathophysiological mechanisms interplay between hyperuricaemia, PsA and other associated metabolic comorbidities. Recognition of this has recently led some authors to propose the term ‘psout’.6 7
Our descriptive analysis showed no relevant difference in the prevalence of polyarticular and enthesitis disease, although a higher frequency of clinical dactylitis and of moderate to severe skin psoriasis was found in patients with hyperuricaemia than in patients with normouricaemia. The increased prevalence of dactylitis in patients with hyperuricaemia found in our analysis was not previously reported in either the French retrospective cohort or the Asian study.13 24 Dactylitis is typical but not unique to PsA, and was found to be present in 5%–10% of patients in a gout study.41 Different pathogenic mechanisms of dactylitis are probably at work to explain the higher proportion of patients with dactylitis among patients with hyperuricaemia, but the deposition of MSU crystals around phalanges and tendons could offer one possible explanation, as tophus is often found in the extremities.
In contrast to previous studies,24 our analysis found no difference in peripheral joint involvement between patients with hyperuricaemia and patients with normouricaemia. It is important to note, however, that patients were required to have polyarthritis, defined as ≥3 tender joints and ≥3 swollen joints, to be included the FUTURE 2–5 studies. In addition, the mean swollen joints count at baseline in our analysis was 11, which may also account for the lack of difference found in our study.
The frequency and severity of skin psoriasis in this PsA dataset support different literature reports in psoriasis, where the number of psoriatic lesions has been shown to be significantly increased in patients with higher SUA levels compared with those with normal SUA levels. An association between both extent and change in PASI score and serum urate level has also been shown.42 Furthermore, a recent meta-analysis found that in European, American and Southeast Asian countries, patients with moderate to severe psoriasis or those with metabolic syndrome and obesity were more likely to have higher UA levels.14 In contrast with the literature reporting a higher rate of erosive and destructive disease,24 43 we could not identify any relevant differences in the baseline radiographic characteristics between patients with normouricaemia and patients with hyperuricaemia, except for patients with hyperuricaemic level ≥420 µmol/L who had a more severe baseline vdH-mTSS.
The updated recommendations of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis highlight the importance of comorbidities and their impact to be considered when deciding on appropriate treatment for patients with PsA.44 The presence of comorbidities like hyperuricaemia pose a challenge for the management of PsA, especially when biological therapies are considered, as these patients are more severe and more likely to be refractory to treatments. To date, however, there has been no dedicated study which specifically evaluated the efficacy of a biological therapy in patients with both hyperuricaemia and PsA. Short-term efficacy on skin psoriasis was shown with secukinumab in patients with moderate to severe plaque psoriasis and concomitant hyperuricaemia in a post hoc analysis of pooled data from the FIXTURE, ERASURE and SCULPTURE trials.32 Here, we examined whether there was any relevant difference in terms of efficacy of secukinumab in patients with hyperuricaemia compared with patients with normouricaemia with active PsA in our dataset from the FUTURE 2–525–28 studies and the MAXIMISE29 study. An early and sustained efficacy of secukinumab was shown up to week 52 across all key manifestations of PsA. Secukinumab was also associated with an improvement in health-related quality of life in both patients with hyperuricaemia, irrespective of the threshold of hyperuricaemia, and patients with normouricaemia.
We acknowledge that the findings from this current dataset have several limitations owing to: the post hoc nature of these analyses; the inclusion criteria of the FUTURE 2–5 studies which were primarily designed as PsA trials and excluded patients with uncontrolled comorbidities; no assessment of axial manifestations was performed, except in the MAXIMISE study; the lack of placebo group in the studies beyond week 16 (FUTURE 2–5) and week 12 (MAXIMISE). In addition, randomisation of patients to secukinumab 150 mg or 300 mg was not based on psoriasis severity. We were also unable to evaluate the impact of secukinumab combined with ULT on SUA levels or the control and prevention of cardiovascular and metabolic comorbidities. The absence of statistical tests and the descriptive nature of this study do not allow conclusions to be drawn, but our study does highlight the potential deleterious role of urate crystals which makes for more complex management of patients with PsA in some cases. The key strengths of our study, however, are the large sample size, the collection of data from a pooled clinical trial programme in PsA with concomitant hyperuricaemia, and the evaluation of patients’ clinical characteristics. This study is also the first to suggest the 52-week efficacy of secukinumab clinical response on PsA irrespective of SUA level.
Conclusion
In our descriptive analysis of pooled data from the 25–28FUTURE 2–5 studies and the MAXIMISE29 study, the majority of patients with PsA and concomitant hyperuricaemia were males, had a higher BMI and reported higher prevalence of both hypertension and diabetes mellitus. A numerically higher frequency of dactylitis and more severe psoriasis, as compared with patients with normouricaemia, was also reported. Recognition of these patients at an early stage could be beneficial for personalised treatment and improved management of their comorbidities. These analyses suggest that secukinumab provides a rapid and sustained response across all manifestations of PsA up to week 52 irrespective of baseline hyperuricaemia.
Acknowledgments
The authors thank the patients who participated in the studies and the study investigators. Manuscript writing support was provided by M.D Zuber Birajdar, Dhanya Mukundan and Rajeeb Ghosh, Novartis Healthcare Pvt Ltd, Hyderabad, India and Kate Killick, Novartis Ireland Ltd. The authors thank Andrew Franklin (Novartis Pharma AG, Basel, Switzerland) who provided editorial support and guidance during manuscript development.
Footnotes
Contributors: The authors were fully responsible for all content and editorial decisions and received no financial support or other form of compensation related to the development of this manuscript. RF is acting as guarantor. All authors read and approved the final manuscript.
Funding: This study was funded by Novartis Pharma AG, Basel, Switzerland.
Disclaimer: The opinions expressed in the manuscript are those of the authors; Novartis Pharmaceuticals had no influence on the manuscript contents.
Competing interests: RF: Consulting fees and advisory boards (Novartis); J-EG: Consulting fees and advisory boards (Novartis); CG and WB are employees and shareholders of Novartis.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The datasets generated during and/or analysed during the current study are not publicly available. Novartis is committed to sharing with qualified external researchers’ access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved on the basis of scientific merit. All data provided are anonymised to respect the privacy of patients who have participated in these trials in line with applicable laws and regulations. Data may be requested from the corresponding author of the manuscript.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All included studies were conducted in accordance with the Declaration of Helsinki (as revised in Brazil 2013) and were approved by the institutional review boards or independent ethics committees for each study site. Participants gave informed consent to participate in the study before taking part.
References
- 1.Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet 2018;391:2273–84. 10.1016/S0140-6736(18)30830-4 [DOI] [PubMed] [Google Scholar]
- 2.Tripolino C, Ciaffi J, Ruscitti P, et al. Hyperuricemia in psoriatic arthritis: epidemiology, pathophysiology, and clinical implications. Front Med (Lausanne) 2021;8:737573. 10.3389/fmed.2021.737573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belasco J, Wei N. Psoriatic arthritis: what is happening at the joint? Rheumatol Ther 2019;6:305–15. 10.1007/s40744-019-0159-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol 2018;14:405–17. 10.1080/1744666X.2018.1468252 [DOI] [PubMed] [Google Scholar]
- 5.Perez-Chada LM, Merola JF. Comorbidities associated with psoriatic arthritis: review and update. Clin Immunol 2020;214:108397. 10.1016/j.clim.2020.108397 [DOI] [PubMed] [Google Scholar]
- 6.Felten R, Duret P-M, Gottenberg J-E, et al. At the crossroads of gout and psoriatic arthritis: "Psout" Clin Rheumatol 2020;39:1405–13. 10.1007/s10067-020-04981-0 [DOI] [PubMed] [Google Scholar]
- 7.Messer L, Felten R, Duret P-M, et al. Quelle Taxonomie des maladies Inflammatoires en Rhumatologie? - le concept de Psoutte. Med Sci (Paris) 2021;37:927–32. 10.1051/medsci/2021118 [DOI] [PubMed] [Google Scholar]
- 8.Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers 2019;5:69. 10.1038/s41572-019-0115-y [DOI] [PubMed] [Google Scholar]
- 9.AlJohani R, Polachek A, Ye JY, et al. Characteristic and outcome of psoriatic arthritis patients with hyperuricemia. J Rheumatol 2018;45:213–7. 10.3899/jrheum.170384 [DOI] [PubMed] [Google Scholar]
- 10.Gisondi P, Targher G, Cagalli A, et al. Hyperuricemia in patients with chronic plaque psoriasis. J Am Acad Dermatol 2014;70:127–30. 10.1016/j.jaad.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 11.Bruce IN, Schentag CT, Gladman DD. Hyperuricemia in Psoriatic arthritis: prevalence and associated features. J Clin Rheumatol 2000;6:6–9. [PubMed] [Google Scholar]
- 12.Kwon HH, Kwon IH, Choi JW, et al. Cross-sectional study on the correlation of serum uric acid with disease severity in Korean patients with psoriasis. Clin Exp Dermatol 2011;36:473–8. 10.1111/j.1365-2230.2010.03988.x [DOI] [PubMed] [Google Scholar]
- 13.Lai TL, Yim CW, Wong PY, et al. Hyperuricemia in Asian psoriatic arthritis patients. Int J Rheum Dis 2018;21:843–9. 10.1111/1756-185X.13265 [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Liu L, Sun X, et al. Updated evidence of the association between elevated serum uric acid level and psoriasis. Front Med (Lausanne) 2021;8:645550. 10.3389/fmed.2021.645550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid Crytals activate the NALP3 inflammasome. Nature 2006;440:237–41. 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- 16.Tsuruta N, Imafuku S, Narisawa Y. Hyperuricemia is an independent risk factor for psoriatic arthritis in psoriatic patients. J Dermatol 2017;44:1349–52. 10.1111/1346-8138.13968 [DOI] [PubMed] [Google Scholar]
- 17.Goldman M. Uric acid in the etiology of psoriasis. Am J Dermatopathol 1981;3:397–404. 10.1097/00000372-198100340-00014 [DOI] [PubMed] [Google Scholar]
- 18.Dai X-J, Tao J-H, Fang X, et al. Changes of Treg/Th17 ratio in spleen of acute gouty arthritis rat induced by MSU crystals. Inflammation 2018;41:1955–64. 10.1007/s10753-018-0839-y [DOI] [PubMed] [Google Scholar]
- 19.Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol 2018;55:379–90. 10.1007/s12016-018-8702-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan X, Xu C, Lin Y, et al. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 Inflammasome-dependent mechanism. J Hepatol 2016;64:925–32. 10.1016/j.jhep.2015.11.022 [DOI] [PubMed] [Google Scholar]
- 21.Geneva-Popova M, Popova-Belova S, Popova V, et al. Assessment of crystals in the synovial fluid of psoriatic arthritis patients in relation to disease activity. Diagnostics (Basel) 2022;12:1260. 10.3390/diagnostics12051260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliviero F, Scanu A, Galozzi P, et al. Prevalence of calcium pyrophosphate and monosodium urate crystals in synovial fluid of patients with previously diagnosed joint diseases. Joint Bone Spine 2013;80:287–90. 10.1016/j.jbspin.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 23.Galozzi P, Oliviero F, Scanu A, et al. “Acute joint swelling in psoriatic arthritis: flare or "Psout"-a 10-year-Monocentric study on Synovial fluid”. Exp Biol Med (Maywood) 2022;247:1650–6. 10.1177/15353702221110666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widawski L, Fabacher T, Spielmann L, et al. Psoriatic arthritis with hyperuricemia: more peripheral, destructive, and challenging to treat. Clin Rheumatol 2022;41:1421–9. 10.1007/s10067-022-06061-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-Interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1137–46. 10.1016/S0140-6736(15)61134-5 [DOI] [PubMed] [Google Scholar]
- 26.Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized. Arthritis Res Ther 2018;20:47. 10.1186/s13075-018-1551-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kivitz AJ, Nash P, Tahir H, et al. Efficacy and safety of subcutaneous secukinumab 150mg with or without loading regimen in psoriatic arthritis: results from the FUTURE 4 study. Rheumatol Ther 2019;6:393–407. 10.1007/s40744-019-0163-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mease P, van der Heijde D, Landewé R, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomized, double-blind, phase III FUTURE 5 study. Ann Rheum Dis 2018;77:890–7. 10.1136/annrheumdis-2017-212687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomized, phase 3 MAXIMISE trial. Ann Rheum Dis 2021;80:582–90. 10.1136/annrheumdis-2020-218808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mease PJ, Kavanaugh A, Reimold A, et al. Secukinumab provides sustained improvements in the signs and symptoms of Psoriatic arthritis: final 5-year results from the phase 3 FUTURE 1 study. ACR Open Rheumatol 2020;2:18–25. 10.1002/acr2.11097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merola JF, Wu S, Han J, et al. Psoriasis, psoriatic arthritis and risk of gout in US men and women. Ann Rheum Dis 2015;74:1495–500. 10.1136/annrheumdis-2014-205212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the normative aging study. Am J Med 1987;82:421–6. 10.1016/0002-9343(87)90441-4 [DOI] [PubMed] [Google Scholar]
- 33.Gong M, Wen S, Nguyen T, et al. Converging relationship of obesity and hyperuricemia with special reference to metabolic disorders and plausible therapeutic implications. Diabetes Metab Syndr Obes 2020;13:943–62. 10.2147/DMSO.S232377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuura F, Yamashita S, Nakamura T, et al. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism 1998;47:929–33. 10.1016/s0026-0495(98)90346-8 [DOI] [PubMed] [Google Scholar]
- 35.Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol 2013;25:210–6. 10.1097/BOR.0b013e32835d951e [DOI] [PubMed] [Google Scholar]
- 36.Ogdie A, Schwartzman S, Husni ME. Recognizing and managing comorbidities in psoriatic arthritis. Curr Opin Rheumatol 2015;27:118–26. 10.1097/BOR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 37.Eder L, Dey A, Joshi AA, et al. Cardiovascular diseases in psoriasis and psoriatic arthritis. J Rheumatol Suppl 2019;95:20–7. 10.3899/jrheum.190114 [DOI] [PubMed] [Google Scholar]
- 38.Jamnitski A, Symmons D, Peters MJL, et al. Cardiovascular comorbidities in patients with psoriatic arthritis. Ann Rheum Dis 2013;72:211–6. 10.1136/annrheumdis-2011-201194 [DOI] [PubMed] [Google Scholar]
- 39.Edson-Heredia E, Zhu B, Lefevre C, et al. Prevalence and incidence rates of cardiovascular, autoimmune, and other diseases in patients with psoriatic or psoriatic arthritis: a retrospective study using clinical practice research datalink. J Eur Acad Dermatol Venereol 2015;29:955–63. 10.1111/jdv.12742 [DOI] [PubMed] [Google Scholar]
- 40.Scriffignano S, Perrotta FM, De Socio A, et al. Role of Comorbidities in Spondyloarthritis including Psoriatic arthritis. Clin Rheumatol 2019;38:3–10. 10.1007/s10067-018-4332-7 [DOI] [PubMed] [Google Scholar]
- 41.Andracco R, Zampogna G, Parodi M, et al. Dactylitis in gout. Ann Rheum Dis 2010;69:316. 10.1136/ard.2009.107755 [DOI] [PubMed] [Google Scholar]
- 42.El Farargy SM, Ghanayem NM, Yehia HY. Association between serum uric acid concentration and clinical features of psoriasis. Menoufia Med J 2021;34:1255–8. 10.4103/mmj.mmj_163_19 [DOI] [Google Scholar]
- 43.Catanoso MG, Macchioni P, Marchesoni A, et al. Fri0350 factors associated with peripheral erosive radiographic disease in a consecutive series of 794 PSA patients. Ann Rheum Dis 2020;79:770–1. 10.1136/annrheumdis-2020-eular.6088 [DOI] [Google Scholar]
- 44.Coates LC, Corp N, van der Windt DA, et al. GRAPPA treatment recommendations: 2021 update. J Rheumatol 2022;49:52–4. 10.3899/jrheum.211331 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003428supp001.pdf (35.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The datasets generated during and/or analysed during the current study are not publicly available. Novartis is committed to sharing with qualified external researchers’ access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved on the basis of scientific merit. All data provided are anonymised to respect the privacy of patients who have participated in these trials in line with applicable laws and regulations. Data may be requested from the corresponding author of the manuscript.