Keywords: monkey, neurophysiology, posterior parietal cortex, population analysis, prefrontal cortex
Abstract
Information represented in working memory is reflected in the firing rate of neurons in the prefrontal cortex and brain areas connected to it. In recent years, there has been an increased realization that population measures capture more accurately neural correlates of cognitive functions. We examined how single neuron firing in the prefrontal and posterior parietal cortex of two male monkeys compared with population measures in spatial working memory tasks. Persistent activity was observed in the dorsolateral prefrontal and posterior parietal cortex and firing rate predicted working memory behavior, particularly in the prefrontal cortex. These findings had equivalents in population measures, including trajectories in state space that became less separated in error trials. We additionally observed rotations of stimulus representations in the neuronal state space for different task conditions, which were not obvious in firing rate measures. These results suggest that population measures provide a richer view of how neuronal activity is associated with behavior, largely confirming that persistent activity is the core phenomenon that maintains visual-spatial information in working memory.
NEW & NOTEWORTHY Recordings from large numbers of neurons led to a reevaluation of neural correlates of cognitive functions, which traditionally were defined based on responses of single neurons or averages of firing rates. Analysis of neuronal recordings from the dorsolateral prefrontal and posterior parietal cortex revealed that properties of neuronal firing captured in classical studies of persistent activity can account for population representations, though some population characteristics did not have clear correlates in single neuron activity.
INTRODUCTION
The ability to maintain and manipulate information in memory over a period of seconds is commonly referred to as working memory (1, 2). Early neurophysiological experiments in nonhuman primates identified neurons that not only respond to sensory stimuli but also remain active during a period after the stimuli were no longer present; this “persistent activity” represents a neural correlate of working memory (3, 4). Persistent activity has since been widely replicated in a variety of working memory tasks (5), including in human recordings (6). Computational models typically simulate persistent activity by networks of neurons with recurrent connections between units similarly tuned for spatial location (7, 8). Activation in the network behaves as a continuous attractor (9), with initial activation by the stimulus appearance generating a bump (peak) of activity in the network, which is maintained in the delay period, but may drift randomly. The position of the bump at the end of the delay period determines the recalled location (10). Drifts in neuronal activity from trial to trial account for corresponding deviations of behavior.
Although analysis of this sort has placed emphasis on the activity of individual neurons or averages of firing rate across multiple neurons that generate persistent activity, in recent years, it has been recognized that populations of neurons encode information that may not be readily visible in single neuron responses (11, 12). More generally, exploring the dynamics of population responses with dimensionality reduction methods has revealed latent variables that more accurately capture information about stimuli and tasks than individual responses (13, 14).
We were therefore motivated to compare aspects of single neuron and population activity that represent information and covary with behavior. We analyzed recordings obtained from the dorsolateral prefrontal and posterior parietal cortex of monkeys as they performed spatial working memory tasks, for which strong evidence exists that single neuron activity is predictive of behavior (10, 15). We examined neurons with persistent activity with conventional methods of firing rate analysis and used dimensionality reduction methods to obtain population measures, allowing us to compare the two types of variables in the same dataset.
METHODS
Data were obtained from two male rhesus monkeys (Macaca mulatta), weighing 7–9 kg, as previously documented (16). Monkeys were either single-housed or pair-housed in communal rooms with sensory interactions with other monkeys. All experimental procedures followed guidelines set by the US Public Health Service Policy on Humane Care and Use of Laboratory Animals and the National Research Council’s Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Wake Forest University Institutional Animal Care and Use Committee.
Experimental Setup
Monkeys sat with their heads fixed in a primate chair while viewing a monitor positioned 60 cm away from their eyes with dim ambient illumination and were required to fixate on a 0.2° white square appearing in the center of the screen.
To receive a liquid reward (typically fruit juice), the animals had to maintain fixation on the square while visual stimuli were presented at peripheral locations. Any break of fixation immediately terminated the trial, and no reward was given. Eye position was monitored throughout the trial using a noninvasive, infrared eye position scanning system (model RK-716; ISCAN, Burlington, MA). The system achieved a <0.3° resolution around the center of vision. Eye position was sampled at 240 Hz, digitized, and recorded. The visual stimulus display, monitoring of eye position, and synchronization of stimuli with neurophysiological data were performed with in-house software implemented in the MATLAB environment (MathWorks, Natick, MA), using the Psychophysics Toolbox (17).
Behavioral Task
The task involved two stimuli appearing in sequence, requiring the monkey to make an eye movement to the remembered location of either the first or the second depending on the color of fixation point (Fig. 1). The animals maintained fixation for 1 s, and then two white squares (1.5° in size) were displayed sequentially for 0.5 s, with a 1.5 s intervening delay period between them. The first stimulus (cue) was displayed pseudorandomly at one of two locations, always drawn from eight possible locations along a circular ring of 12° eccentricity. The second stimulus location was either the same as the first, or was displaced by an angle of 45°, 90°, or 180°; a final condition involved no second stimulus presentation. After a second delay period of 1.5 s, the monkeys were required to saccade to the location of the first stimulus if the fixation point was white in color (remember-first condition), and to the location of the second stimulus if the fixation point was blue (remember-second condition). In each daily session, the two possible locations of the first stimulus were the same for the remember-first (Fig. 1A) and remember-second tasks (Fig. 1B), as were the locations of the second stimulus relative to the first.
Figure 1.

Successive frames show the sequence of stimulus presentations in the remember first-remember-second task. A: in the remember-first task, the fixation point was white and the monkey had to remember the position of first stimulus (cue) and make a saccade toward it at the end of the trial while ignoring the second stimulus (distractor). The first stimulus could appear at either in the receptive field of the neuron (shown as the left location) or in the diametric position (not shown here), and depending on the position of the first stimulus, the distractor could appear at five different positions: at the same position as cue, at a location offset by 45°, 90°, and 180°, or no distractor at all (null condition). B: in the remember-second task, the fixation point was blue and the monkey had to remember and make a saccade toward the second stimulus ignoring the first stimulus (distractor in this case). The cue could appear at the preferred or diametric location of the neuron followed by a second stimulus as before. In this case, the null condition involved no stimulus presentation in the first interval.
To minimize the uncertainty about the stimulus to be remembered, the remember-first and remember-second conditions were presented in blocks of trials. The animal was required to perform 10 correct trials of the remember-first task, involving presentation of the two stimuli at each possible combination used in the block. The monkeys were rewarded with fruit juice after making a correct saccade. When running these sessions, the monkey was typically tested first with a single-stimulus oculomotor delayed response task (4) to map the receptive field of neurons recorded, so as to guide the selection of the location of stimuli. However, multiple neurons were recorded simultaneously, and it was not possible to optimize the stimulus location for all neurons in a session. We thus identified, post hoc, the location that elicited a better response compared with its diametric for each neuron. We refer to this as the “preferred location” of the neuron, in a relative sense.
Surgery and Neurophysiology
Two 20 mm diameter recording cylinders were implanted over the dorsolateral prefrontal cortex and the posterior parietal cortex of the same hemisphere in each monkey (Fig. 2). Extracellular activity of single units was recorded using arrays of 2–8 microelectrodes in each cylinder, which were either glass-coated, (250 μm diameter, impedance of 1 MΩ at 1 kHz, Alpha-Omega Engineering, Nazareth, Israel) or epoxylite-coated tungsten microelectrodes (125 or 250 μm diameter, impedance of 4 MΩ at 1 kHz, FHC Bowdoin, ME). Electrodes were advanced individually into the cortex with a microdrive system (EPS drive, Alpha-Omega Engineering, Nazareth, Israel). The electrical signal from each electrode was amplified, band-pass filtered between 500 Hz and 8 kHz, and recorded with a modular data acquisition system of 25-μs resolution (APM system, FHC, Bowdoin, ME). The anatomical location of electrode penetration was determined based on MR imaging of the brain. Neuronal data were collected from areas 8a and 46 of the dorsolateral prefrontal cortex (dlPFC) including both banks of the principal sulcus, and areas 7a and lateral intraparietal area (LIP) of the posterior parietal cortex (PPC) in the lateral bank of the intraparietal sulcus.
Figure 2.
A schematic diagram of the monkey brain. Recordings were performed from the highlighted areas. In posterior parietal cortex (PPC), recordings were done from area 7a and lateral intraparietal area (LIP). In dorsolateral prefrontal cortex (dlPFC), recordings sampled area 8 and 46. AS, arcuate sulcus; IPS, intraparietal sulcus; PS, principal sulcus; STS, superior temporal sulcus.
Neural Data Processing
A semiautomated cluster analysis relied on the KlustaKwik algorithm (18), which applied principal component analysis of the waveforms to sort recorded spike waveforms into separate units. To ensure a stable firing rate in the analyzed recordings, we identified recordings in which a significant effect of trial sequence was evident at the baseline firing rate (ANOVA, P < 0.05), e.g., due to a neuron disappearing or appearing during a run, as we were collecting data from multiple electrodes. Data from these sessions were truncated so that analysis was only performed on a range of trials with stable firing rate. Less than 10% of neurons were corrected in this way.
Firing Rate Analysis
Data analysis was implemented with the MATLAB computational environment (MathWorks, Natick, MA). Neuron analysis involved, initially, determining the mean firing rate of each neuron in each trial epoch: 1 s of fixation; 0.5 s of cue presentation; 1.5 s of first delay period; 0.5 s of sample presentation; and 1.5 s of second delay period. Next, we compared responses of each neuron in the 1 s baseline, fixation period with the cue presentation period, and the delay period following it. Any neuron that had significantly greater responses during the first delay period was identified as a task-responsive neuron (one-tailed paired t test; P < 0.05).
To quantify the trial-to-trial association between neuronal activity and behavior, we analyzed trials that involved presentation of the stimulus in the neuron’s preferred direction and its diametric and trials that resulted in correct choices and incorrect choices using receiver operating characteristic (ROC) analysis (19, 20). For the latter analysis, firing rates of trials involving the same sequences of stimuli were pooled separately for correct and error outcomes, and an ROC curve was computed from these two distributions of firing rates. The area under the ROC curve thus constructed is referred to in the literature as “choice probability” (19, 20) and represents a measure of correlation between the behavioral choice that results in a correct or error response and neuronal activity. As we defined it for our analysis, a value of 1 indicates that higher firing rates of the neuron predict perfectly the behavioral choices of the subject; a value of 0.5 indicates no correlation between the two; a value of 0 indicates that lower firing rates of a neuron predict the behavioral choices. Time-resolved choice probabilities were computed from the spikes in 500 ms time windows, stepped by 50 ms intervals. This analysis was done separately for the preferred and nonpreferred cue conditions.
Dimensionality Reduction
We applied principal component analysis (PCA) to analyze the neural population activity during the remember-first and remember-second tasks. PCA was performed on the mean firing rate of neurons, for data collected from both PFC and PPC. Neurons from each brain region were pooled and data were collected for L = 10 different conditions in the remember-first task. Each task condition was defined by the combination of the first and second stimulus locations: five conditions involved cue presentation at the neuron’s preferred location, and five at its diametric location; each condition was then followed by a different second stimulus location (as in Fig. 1A). The remember-second trials involved another 10 task conditions, structured in a similar fashion (as in Fig. 1B). For correct trajectories, we selected neurons with at least six correct trials per task condition. Then, for each neuron, the average firing rate of the remember-first trials (and remember-second trials) during a given time point formed a column entry (10 × 1 vector) for the population activity matrix A1 (A2 for remember-second trials). During each time point, we defined the corresponding activity matrix A1 (A2) for each prefrontal region as a 10 × N matrix, where 10 is the number of task conditions and N is the number of neurons. We then aligned the activity matrices (A1 and A2) into one single matrix B, which is a 20 × N matrix with the first 10 rows containing the activities in remember-first task and the rest containing for remember-second. Then, we normalized B by subtracting the mean across each column to guarantee the matrix was zero-centered. The PCA was applied on the centered data using singular value decomposition (SVD). We visualized data in the space defined by the first two principal components.
To create population activity trajectories, we used the binned firing rates of each neuron for each task condition, which comprised a matrix of N × 20 × number of bins. For individual trajectories, we selected the respective task condition (matrix of N × number of bins size) and multiplied with the inverse of PC1 and PC2 which were of N × 1 size each giving us two PC components of 1 × number of bins size. Then, we plotted them with respect to time.
For PCA analysis of error trials, we collapsed the 10 conditions of the remember-first task into two groups (and did the same for the remember-second task): those with the cue appearing at the neuron’s preferred location and those in the opposite. Neurons with at least one error trial in each of the four groups were used in this analysis. Then, we used binned firing rates as described earlier to produce correct and error trajectories. To measure the distance between correct and error trajectories, we projected the trajectories to the state space defined by the first two components and determined the average positions of the projected points across the delay period. In this way, we obtained a point for each of four groups as described earlier and measured Euclidean distances between the points. corresponding to the best cue and diametric cue conditions. To make the method robust, we repeated the analysis by choosing 75% of the selected neurons and measuring average distance each time as described earlier. We repeated this analysis for 100 times and plotted the distribution of average distances.
RESULTS
Data were analyzed from two monkeys trained to perform the remember-first and remember-second tasks (Fig. 1). The task requires the monkeys to observe two stimuli presented in sequence, with intervening delay periods between them, and to make an eye movement to the remembered location of either the first or the second stimulus depending on the color of the fixation point (white or blue, respectively). Performance in the task depended on the relative location of the two stimuli; the most challenging condition involved presentation of two stimuli at the adjacent locations (45° apart) in the remember-first task, when monkeys tended to frequently saccade the location of the second stimulus, rather than the first, as we have reported previously (16). With the exception of this stimulus condition, monkey HE achieved 85–95% correct performance for the rest of the conditions; monkey GR achieved 75–95% performance in these conditions.
Extracellular neurophysiological recordings were collected from areas 8a and 46 of the dorsolateral prefrontal cortex and areas 7a and LIP of the posterior parietal cortex (Fig. 2). We identified neurons with significantly elevated responses during the first delay period of the task, as these were defined previously (16). We focused on data recorded from the randomized variant of the task, where the second stimulus could appear at a variable location relative to the first as illustrated in Fig. 1. A total of 205 neurons from dlPFC and 241 from PPC were recorded with this stimulus set. Of those, 75 neurons exhibited persistent activity, i.e., significantly elevated firing rate in the delay period after the first stimulus presentation in the dlPFC compared with the baseline fixation firing rate (47 and 28 neurons, from monkeys GR and HE, respectively) and 85 such neurons did so in the PPC (21 and 64, from monkeys GR and HE, respectively).
Single Neuron Activity in Correct and Error Trials
We examined separately the activity of neurons that generated persistent activity and those that did not in correct and error trials of the remember-first task. When the first stimulus, which the monkey needed to remember, appeared in each neuron’s preferred location, firing rate of prefrontal neurons that generated persistent activity was generally higher in correct compared with error trials (Fig. 3A). Mean firing rate averaged across the entire first delay period was significantly higher in correct than in error trials (two-tailed paired t test, t176 = 2.93, P = 0.004). In other words, trials in which persistent activity was diminished were more likely to result in errors. This evidence is consistent with prior results that have linked persistent, delay period activity with behavior in working memory tasks (4, 15, 21), now demonstrated in the context of a new task.
Figure 3.

A: averaged peristimulus time histogram (PSTH) of neuronal spike discharges from the PFC neurons that showed significantly elevated cue delay activity (n = 75) in the remember-first task when the cue appeared in the preferred location of the neurons. Here, conditions from all possible locations of the second stimulus were pooled together, so responses to the second stimulus reflect the average of all stimulus locations tested. The solid line indicates mean activity of neurons in correct trials, whereas the dashed line represents the averaged error trials activity. Shaded areas indicate standard error (SE). The three colored bars indicate presentation of first stimulus, second stimulus, and response period, respectively. B: as in A, for the remember-first task when the cue appeared in the diametric position. C: as in A, for remember-second task when the cue appeared at the preferred location. D: as in C, for remember-second task when the cue appeared at the diametric location. PFC, prefrontal cortex.
This difference in firing rate was not a general effect but depended on task conditions and differed between neuronal populations. The difference between correct and error trials was much more diminished following the presentation of a stimulus in a neuron’s nonpreferred location (Fig. 3B) and it did not reach statistical significance (two-tailed paired t test, t124 = 0.462, P = 0.6). On the other hand, a trend in the opposite direction was evident in the remember-second task, for a stimulus appearing in the neuron’s preferred location (Fig. 3C), though the difference was not significant (two-tailed paired t test, t162 = 0.306, P = 0.76). When the monkey had to ignore this first stimulus, higher than average firing rate at the end of the first delay period was more likely to lead to an error. Finally, error trials involving appearance of a stimulus in a nonpreferred location in the remember-second task were characterized by a lower firing rate, though this difference did not reach statistical significance (two-tailed paired t test, t149 = 1.78, P = 0.078).
Importantly, the reduced firing rate in error trials involving the presentation of the cue in the neuron’s receptive field was specific for neurons with persistent activity. Analysis of neurons that did not exhibit significantly elevated activity in the first delay period revealed generally higher firing rates in error than in correct trials, across all conditions (Fig. 4).
Figure 4.

A: averaged PSTH of neuronal spike discharges from the PFC neurons that did not show significantly elevated cue delay activity (n = 130) in the remember-first task when the cue appeared in the preferred location of the neurons. Conventions are the same as in Fig. 3. B: as in A, for the remember-first task when the cue appeared in the diametric position. C: as in A, for the remember-second task when the cue appeared at the preferred location. D: as in A, for the remember-second task when the cue appeared at the diametric location. PFC, prefrontal cortex; PSTH, averaged peristimulus time histogram.
The difference between correct and error trials was smaller among neurons that generated persistent activity in the posterior parietal cortex (Fig. 5). The firing rate for correct and error trials for the neurons’ best location in Fig. 5A did not reach statistical significance (two-tailed paired t test, t176 = 0.01, P = 0.9). A number of previous studies have suggested that activity of posterior parietal neurons is less able to resist the effect of distractors (22) and firing rate differences have less influence on behavior.
Figure 5.

A: averaged PSTH of neuronal spike discharges from the PPC neurons that showed significantly elevated cue delay activity (n = 85) in the remember-first task when the cue appeared in the preferred location of the neurons. Conventions are the same in as in Fig. 3. B: as in A, for the remember-first task when the cue appeared in the diametric position. C: as in A, for remember-second task when the cue appeared at the preferred location. D: as in C, for remember-second task when the cue appeared at the diametric location. PPC, posterior parietal cortex; PSTH, averaged peristimulus time histogram.
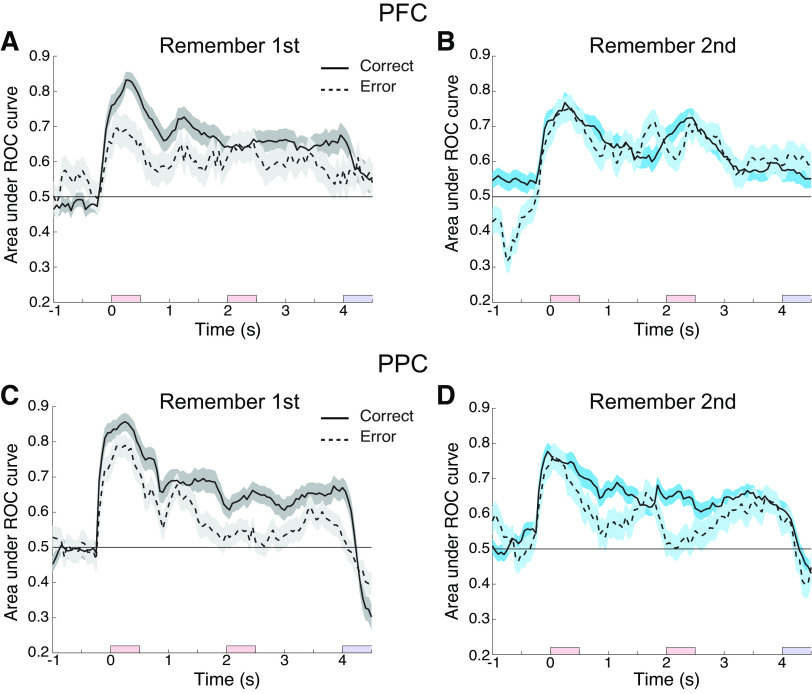
Based on these results, we calculated the area under the ROC curve comparing firing rate distributions involving presentation of the cue stimulus in the neuron’s preferred location versus the diametric location, for neurons that generated persistent activity (Fig. 6). This analysis revealed that both PFC (Fig. 6, A and B) and PPC (Fig. 6, C and D) represented spatial information robustly, during the delay periods of the task. This was true for both the remember-first (Fig. 6, A and C) and remember-second tasks (Fig. 6, B and D).
Figure 6.
A: averaged area under the ROC curve for neurons with significant delay activity recorded from PFC (n = 75 neurons for correct trials and n = 73 for error trials) for the remember-first task, plotted as a function of time across the trial. The solid line represents ROC value comparing the distribution of trials for the preferred and diametric cue condition in correct trials and the dotted line for the error trials. The shaded area represents SE. The three colored bars along the x-axis indicate presentation of first stimulus, second stimulus, and response period, respectively. B: as in A, for the remember-second task (n = 75 neurons for correct trials and n = 72 for error trials). C: averaged area under the ROC curve for neurons with significant delay activity recorded from the PPC (n = 85 neurons for both correct and error trials) for the remember-first task. D: as in C, for the remember-second task (n = 85 neurons for correct trials and n = 67 for error trials). PFC, prefrontal cortex; PPC, posterior parietal cortex; ROC, receiver operating characteristic.
We proceeded to calculate “choice probability” for neurons that generated persistent activity, defined as the area under the ROC curve involving identical stimulus conditions but contrasting trials that resulted in correct and error trials (Fig. 7). This is the conventional method of determining the relationship between firing rate and probability of correct choice (19, 20). Results were consistent with the analysis of firing rate, as presented in the preceding paragraphs of this section. Choice probability for the prefrontal cortex exceeded chance values (>0.5) in the delay interval following presentation of the cue in the neurons’ preferred location for the remember-first task (Fig. 7A). Such an effect was not present for the posterior parietal cortex (Fig. 7C).
Figure 7.

A: averaged area under the ROC curve for neurons with significant delay activity recorded from PFC (n = 75 neurons for best and n = 73 for diametric cue condition) for the remember-first task, plotted as a function of time across the trial. The solid line represents ROC value comparing the distribution of correct and error trials from the preferred cue condition (known as choice probability). The dashed line represents choice probability for the diametric cue condition. The shaded area represents SE. B: as in A, for the remember-second task (n = 74 neurons for best and n = 73 for diametric cue condition). C: averaged area under the ROC curve for neurons with significant delay activity recorded from the PPC (n = 85 neurons for both best and diametric cue condition) for the remember-first task. D: as in C, for the remember-second task (n = 69 for best and n = 83 for diametric cue condition). PFC, prefrontal cortex; PPC, posterior parietal cortex; ROC, receiver operating characteristic.
Similarly, in the remember-second task, choice probability for the prefrontal cortex exceeded chance values in the delay interval following presentation of the cue in the neuron’s nonpreferred location (Fig. 7B). When a stimulus elicits a stronger than average response, this is more difficult to be suppressed and the trial may result in an error. However, when the stimulus is out of the receptive field, spurious higher firing rate of the neuron ends up reinforcing the spatial signal in the opposite direction, where the second stimulus is more likely to appear and more effectively negating the influence of the first stimulus that is to be ignored (dotted line in Fig. 7B). This choice probability value for the diametric location was significantly higher than chance levels (two-tailed, one-sample, t test, t72 = 3.206, P = 0.002).
Population Measures in Correct and Error Trials
These results are broadly consistent with previous studies that identified changes in firing rate of neurons that generate persistent activity as accounting for behavior in working memory tasks. Recent work has revealed changes in population activity that are not always visible at the level of single neurons or neuron averages, leading to appreciation that the dynamics of population activity can represent cognitive processes and task contexts (13). We therefore used dimensionality reduction methods to identify population dynamics in the collective activity of neurons and determine how these relate to firing rate changes. Principal component analysis (PCA) involving all neurons from the dlPFC and (separately) for the PPC is shown in Fig. 8. Responses are grouped in trajectories with blue or red hue, depending on the location of the cue, at the neurons’ preferred location (blue-hue traces in Fig. 8A), or diametric (red-hue traces in Fig. 8A). The cue was followed by a second stimulus at varying locations (as illustrated in Fig. 1), corresponding to trajectories with slightly different hues. This analysis revealed that activity was relatively stable during the first delay period (gray highlighted rectangle in Fig. 8). Conditions that involved appearance of a stimulus in the neurons’ preferred location were clearly separable from conditions that involved appearance of the stimulus in the diametric location.
Figure 8.

A: PCA trajectories for correct trials using neurons recorded from dlPFC in the remember-first task (n = 104 neurons). The rectangular area highlights the delay period. Vertical black lines indicate times of the cue and second stimulus presentations. The blue hue lines indicate the trials with preferred cue condition and red hue lines represent the diametric cue conditioned classes. Different hues represent different second stimulus locations relative to the cue (0°–180° plus null condition, for which no second stimulus was presented). B: averaged correct (solid lines) and error (dotted lines) trial trajectories from dlPFC in the remember-first task (n = 104 neurons). The shaded rectangular area again highlights the cue delay period. The blue lines indicate the trials with preferred cue condition and red lines represent the diametric cue conditioned classes. C: as in A, for PFC neurons in the remember-second task (n = 104 neurons). D: as in B, for PFC neurons in the remember-second task (n = 104 neurons). E: as in A, for PPC neurons in the remember-first task (n = 100 neurons). F: same as in B, for PPC neurons in the remember-first task (n = 87 neurons). G: as in C, for PPC neurons in the remember-second task (n = 100 neurons). H: same as in D, for PPC neurons in the remember-second task (n = 87 neurons). dlPFC, dorsolateral prefrontal cortex; PCA, principal component analysis; PFC, prefrontal cortex; PPC, posterior parietal cortex.
This analysis also captured the well-known ability of the prefrontal cortex to better resist distractor stimuli (22, 23). The dark blue line represents the condition involving the first stimulus at the preferred location of the neuron and the second at the diametric location. For the PPC in the remember-first task (Fig. 8E), appearance of the second stimulus at the diametric location elicits a response essentially identical to that of the first stimulus at the diametric location, which is represented by the red-hue lines. This is why the dark blue line merges with the red-hue lines in this plot. In contrast, for the PFC, the second, diametric stimulus produces much less such deviation (Fig. 8A); PFC neurons continue to represent mostly the first stimulus in the remember-first task and resist the distractor, as firing rate analysis also indicates. In the remember-second task, the deviation is much greater for both PFC and PPC, because this is the stimulus that needs to be remembered, and the initial response to the first stimulus is effectively overwritten.
Error trials in the remember-first task generated overall similar trajectories with those of correct trials. However, the separation of preferred and nonpreferred trajectories was now reduced. This difference was evident when we projected delay-period trajectories to the plane defined by the first two principal components (Fig. 9A). The difference in mean distance was significantly reduced (paired t test, t99 = 24.2, P < 0.001, illustrated in Fig. 9C). It was also notable that PPC better reflected the error at the end of the trial; correct and error trajectories diverged greatly in PPC (Fig. 8F, around the 4 s time point), whereas such a difference was not present in PFC (Fig. 8B).
Figure 9.
A: distance between correct (solid lines) and error (dashed lines) trials when mean points of delay period trajectories were projected to the state-space plane defined by the first two PCs for PFC neurons (n = 104 neurons). Here, black lines represent the remember-first task and blue lines indicate the remember-second task. The star shapes indicate the position of the preferred cue and diamond shapes represent diametric cue conditions. B: same as in A, for PPC neurons (n = 87 neurons). C: box plots showing distribution of average distances for the delay period (as defined earlier) in correct (blue) and error (red) for PFC neurons (n = 104 neurons) in both remember-first and remember-second tasks. D: same as in C, for PPC neurons (n = 87 neurons). PFC, prefrontal cortex; PPC, posterior parietal cortex.
Importantly, no such decrease in distance between trajectories of population activity in state space was present for the remember-second task (Fig. 8D), and in fact, a significant increase was evident (paired t test, t99 = −33.2, P < 0.001, illustrated in Fig. 9, A–C). This finding mirrors the change in firing rate discussed earlier; as the first stimulus in this task was to be ignored, increases in firing rate following its presentation were more likely to lead to errors. The opposite trend was present in the PPC for the remember-first task (Fig. 8F), as the distance between trajectories increased significantly between correct and error trials (paired t test, t99 = −8.7, P < 0.001, Fig. 9, B–D). The distance between stimuli again decreased significantly for error trials in the remember-second task (paired t test, t99 = 3.13, P = 0.002, Fig. 9, B–D).
The population analysis produced an additional finding that was not evident in mean firing rates. The representation of the best and worst stimulus location was shown to rotate between remember-first and remember-second tasks in the neuronal activity state space, both for the PFC and PPC (Fig. 9, A and B). Although the conditions represented by the black and blue traces involved identical stimuli, the best and diametric location appeared at different positions in the first two principal component planes. This result suggests that different populations of neurons responded to the same stimuli during the delay period of the remember-first and remember-second tasks.
DISCUSSION
The role of persistent activity in working memory has been reevaluated recently. Although strong evidence links persistent discharges of neurons in the prefrontal cortex with behavioral responses in spatial working memory tasks (24), objections have been raised about its significance (11, 25, 26). At the same time, it has been increasingly appreciated that the collective activity of ensembles of neurons represents more information than is typically evident in the responses of single neurons (13, 14), or average of firing rate across multiple neurons, as persistent activity is typically defined. The results presented here explain how measures of single neuron and population firing are related to working memory behavior.
Comparison of Single Neuron and Population Measures of Activity
Our analysis revealed findings of neuronal firing rate consistent with previous studies, including that persistent activity is generated in the dorsolateral prefrontal and posterior parietal cortex during spatial working memory tasks (27); that activity of neurons that generate persistent activity better predicts working memory recall compared with activity of neurons without persistent activity (10); and that prefrontal neurons better predict performance than posterior parietal neurons, at least in more challenging working memory tasks (28). At the level of mean firing rate, the best predictor of whether a trial might result in an error was whether the delay-period firing of prefrontal neurons generating persistent activity was decreased. Error trials are likely to include lapses, over which firing rate is expected to be generally lower (29). However, the pattern we observed depended specifically on whether the remembered stimulus appeared at the neurons’ preferred location, inside their receptive field. That was the condition best predictive of erroneous responses. Although we emphasize the difference between correct and error trials, it is important to note that a substantial amount of spatial information survived in error trials (for example, the difference between correct and error trials is modest in Fig. 3A). This suggests that many errors were due to nonspatial signals (for example, having to do with engagement with the task) or that subtle differences in firing rate could sway behavioral responses.
Analysis of the same neuronal datasets using dimensionality reduction methods provided a reflection of these findings at the population level. Population activity was largely captured by a low-dimensional manifold, in which different stimuli were represented at separable loci during working memory, and at a stable subspace even though neuronal activity exhibited considerable dynamics (30). Smaller trajectory deviations of the prefrontal population from their baseline state were more likely to result in errors.
This analysis also revealed changes that were nonobservable in neuron measures such as average firing rate. Most importantly, we identified rotation of stimulus representations in correct trials being associated with different task conditions (whether to remember the first stimulus or the second one). The finding suggests that activation of different ensembles of neurons is needed to maintain stimulus in different working memory tasks. Stimulus rotations have been associated with cognitive operations (31–34) and our findings add to the range of phenomena that suggest that behavior relies on such transformations. Other variables beyond the mean firing rate have also been described that affect behavior, including the variability of neuronal responses from trial to trial (35, 36) and the pair-wise correlation of discharge rates across neurons (37, 38).
Some limitations still apply to this analysis. Population measures were obtained from neurons recorded in different sessions but treated as if they were recorded simultaneously (pseudo-populations). It is understood, however, that correlated neuronal firing is modulated by task factors and bounds the information that can be decoded from populations of neurons (38, 39).
Contributions of Prefrontal and Posterior Parietal Cortex in Working Memory
Neurons in the dorsolateral prefrontal and posterior parietal cortex are coactivated during visuospatial working memory tasks, and activity in either area can be predictive of behavior, particularly in simpler tasks that require maintenance of single stimulus in memory (27, 40). Prefrontal neurons do exhibit some specialization, however. Posterior parietal neurons are generally less able to resist distracting stimuli that the subject has to ignore (22, 23, 41), though what is being represented in the activity of each area depends on the specific task a subject is performing (16, 42). These differences, in turn, have been attributed to distinct anatomical and functional properties of prefrontal circuits (7, 43, 44).
These findings were generally evident in the analysis we performed here. In the posterior parietal cortex, firing rate differences after the presentation of the first stimulus of the remember-first task were generally not well predictive of correct and error trials, precisely because this activity was disrupted by the second stimulus, even though the latter was not relevant for the task. The posterior parietal cortex exhibited the opposite trend compared with the prefrontal cortex in the population analysis. Although the rotation depending on task condition was also evident in posterior parietal populations, the shrinkage or expansion of trajectories moved in the opposite direction than prefrontal ones. These results demonstrate specialization of cortical processes related to working memory and emphasize the role of the prefrontal cortex in the maintenance of working memory (45).
In evaluating the role of the prefrontal and posterior parietal cortex, it is also important to consider the variability of error sources. The most straightforward types of errors involved incorrect response to the second stimulus when the first needed to be remembered, presumably because the second stimulus overwrote the representation of the first in working memory (16). As discussed earlier, errors are likely to include lapses, when the monkey disengages with the task or makes a random response (29). It is also possible that information was correctly maintained in working memory but failed to be transmitted correctly to a motor command. This could explain why a lot of stimulus information was maintained in error trials, even if diminished relative to correct trials (e.g., visible in Fig. 8). Across these multiple types of errors, it appears that prefrontal activity ultimately plays a greater role in determining the outcome of a trial.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
The research reported in this paper was supported by the NIH National Eye Institute under award numbers R01 EY017077 and P30 EY008126 and by the National Institute of Mental Health under award number R01 MH116675.
DISCLOSURES
C. Constantinidis is an editor of Journal of Neurophysiology and was not involved and did not have access to information regarding the peer-review process or final disposition of this article. An alternate editor oversaw the peer-review and decision-making process for this article. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
C.C. conceived and designed research; C.C. performed experiments; R.M. and C.C. analyzed data; R.M. and C.C. interpreted results of experiments; R.M. and C.C. prepared figures; R.M. and C.C. drafted manuscript; R.M. and C.C. edited and revised manuscript; R.M. and C.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Chrissy Suell for technical help and Wenhao Dang, Zhengyang Wang, and Rye Jaffe for helpful comments on the manuscript.
REFERENCES
- 1. Baddeley A. Working memory: theories, models, and controversies. Annu Rev Psychol 63: 1–29, 2012. doi: 10.1146/annurev-psych-120710-100422. [DOI] [PubMed] [Google Scholar]
- 2. Jaffe RJ, Constantinidis C. Working memory: from neural activity to the sentient mind. Compr Physiol 11: 2547–2587, 2021. doi: 10.1002/cphy.c210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science 173: 652–654, 1971. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- 4. Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349, 1989. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 5. Constantinidis C, Klingberg T. The neuroscience of working memory capacity and training. Nat Rev Neurosci 17: 438–449, 2016. doi: 10.1038/nrn.2016.43. [DOI] [PubMed] [Google Scholar]
- 6. Kamiński J, Sullivan S, Chung JM, Ross IB, Mamelak AN, Rutishauser U. Persistently active neurons in human medial frontal and medial temporal lobe support working memory. Nat Neurosci 20: 590–601, 2017. [Erratum in Nat Neurosci 20: 1189, 2017]. doi: 10.1038/nn.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hart E, Huk AC. Recurrent circuit dynamics underlie persistent activity in the macaque frontoparietal network. eLife 9: e52460, 2020. doi: 10.7554/eLife.52460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex 10: 910–923, 2000. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- 9. Seung HS, Lee DD, Reis BY, Tank DW. Stability of the memory of eye position in a recurrent network of conductance-based model neurons. Neuron 26: 259–271, 2000. doi: 10.1016/s0896-6273(00)81155-1. [DOI] [PubMed] [Google Scholar]
- 10. Wimmer K, Nykamp DQ, Constantinidis C, Compte A. Bump attractor dynamics in prefrontal cortex explains behavioral precision in spatial working memory. Nat Neurosci 17: 431–439, 2014. doi: 10.1038/nn.3645. [DOI] [PubMed] [Google Scholar]
- 11. Stokes MG. ‘Activity-silent' working memory in prefrontal cortex: a dynamic coding framework. Trends Cogn Sci 19: 394–405, 2015. doi: 10.1016/j.tics.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stokes MG, Kusunoki M, Sigala N, Nili H, Gaffan D, Duncan J. Dynamic coding for cognitive control in prefrontal cortex. Neuron 78: 364–375, 2013. doi: 10.1016/j.neuron.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ebitz RB, Hayden BY. The population doctrine in cognitive neuroscience. Neuron 109: 3055–3068, 2021. doi: 10.1016/j.neuron.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kobak D, Brendel W, Constantinidis C, Feierstein CE, Kepecs A, Mainen ZF, Qi X-L, Romo R, Uchida N, Machens CK. Demixed principal component analysis of neural population data. eLife 5: e10989, 2016. doi: 10.7554/eLife.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Constantinidis C, Franowicz MN, Goldman-Rakic PS. The sensory nature of mnemonic representation in the primate prefrontal cortex. Nat Neurosci 4: 311–316, 2001. doi: 10.1038/85179. [DOI] [PubMed] [Google Scholar]
- 16. Qi XL, Elworthy AC, Lambert BC, Constantinidis C. Representation of remembered stimuli and task information in the monkey dorsolateral prefrontal and posterior parietal cortex. J Neurophysiol 113: 44–57, 2015. doi: 10.1152/jn.00413.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meyer T, Constantinidis C. A software solution for the control of visual behavioral experimentation. J Neurosci Methods 142: 27–34, 2005. doi: 10.1016/j.jneumeth.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 18. Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsaki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol 84: 401–414, 2000. doi: 10.1152/jn.2000.84.1.401. [DOI] [PubMed] [Google Scholar]
- 19. Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci 13: 87–100, 1996. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- 20. Mendoza-Halliday D, Torres S, Martinez-Trujillo JC. Sharp emergence of feature-selective sustained activity along the dorsal visual pathway. Nat Neurosci 17: 1255–1262, 2014. doi: 10.1038/nn.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou X, Zhu D, Qi XL, Lees CJ, Bennett AJ, Salinas E, Stanford TR, Constantinidis C. Working memory performance and neural activity in the prefrontal cortex of peri-pubertal monkeys. J Neurophysiol 110: 2648–2660, 2013. doi: 10.1152/jn.00370.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qi X-L, Katsuki F, Meyer T, Rawley JB, Zhou X, Douglas KL, Constantinidis C. Comparison of neural activity related to working memory in primate dorsolateral prefrontal and posterior parietal cortex. Front Syst Neurosci 4: 12, 2010. doi: 10.3389/fnsys.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suzuki M, Gottlieb J. Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nat Neurosci 16: 98–104, 2013. doi: 10.1038/nn.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Constantinidis C, Funahashi S, Lee D, Murray JD, Qi X-L, Wang M, Arnsten AFT. Persistent spiking activity underlies working memory. J Neurosci 38: 7020–7028, 2018. doi: 10.1523/JNEUROSCI.2486-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buschman TJ, Miller EK. Working memory is complex and dynamic, like your thoughts. J Cogn Neurosci 35: 17–23, 2022. doi: 10.1162/jocn_a_01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. D'Esposito M, Postle BR. The cognitive neuroscience of working memory. Annu Rev Psychol 66: 115–142, 2015. doi: 10.1146/annurev-psych-010814-015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol 79: 2919–2940, 1998. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- 28. Masse NY, Hodnefield JM, Freedman DJ. Mnemonic encoding and cortical organization in parietal and prefrontal cortices. J Neurosci 37: 6098–6112, 2017. doi: 10.1523/JNEUROSCI.3903-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Astrand E, Wardak C, Baraduc P, Ben Hamed S. Direct two-dimensional access to the spatial location of covert attention in macaque prefrontal cortex. Curr Biol 26: 1699–1704, 2016. doi: 10.1016/j.cub.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 30. Murray JD, Bernacchia A, Roy NA, Constantinidis C, Romo R, Wang X-J. Stable population coding for working memory coexists with heterogeneous neural dynamics in prefrontal cortex. Proc Natl Acad Sci USA 114: 394–399, 2017. doi: 10.1073/pnas.1619449114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang C, Herikstad R, Parthasarathy A, Libedinsky C, Yen S-C. Minimally dependent activity subspaces for working memory and motor preparation in the lateral prefrontal cortex. eLife 9: e58154, 2020. doi: 10.7554/eLife.58154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okazawa G, Hatch CE, Mancoo A, Machens CK, Kiani R. Representational geometry of perceptual decisions in the monkey parietal cortex. Cell 184: 3748–3761.e18, 2021. doi: 10.1016/j.cell.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Libby A, Buschman TJ. Rotational dynamics reduce interference between sensory and memory representations. Nat Neurosci 24: 715–726, 2021. doi: 10.1038/s41593-021-00821-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Minxha J, Adolphs R, Fusi S, Mamelak AN, Rutishauser U. Flexible recruitment of memory-based choice representations by the human medial frontal cortex. Science 368: eaba3313, 2020. doi: 10.1126/science.aba3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hussar C, Pasternak T. Trial-to-trial variability of the prefrontal neurons reveals the nature of their engagement in a motion discrimination task. Proc Natl Acad Sci USA 107: 21842–21847, 2010. doi: 10.1073/pnas.1009956107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qi X-L, Constantinidis C. Variability of prefrontal neuronal discharges before and after training in a working memory task. PLoS One 7: e41053, 2012. doi: 10.1371/journal.pone.0041053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ni AM, Bowes BS, Ruff DA, Cohen MR. Methylphenidate as a causal test of translational and basic neural coding hypotheses. Proc Natl Acad Sci USA 119: e2120529119, 2022. doi: 10.1073/pnas.2120529119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qi X-L, Constantinidis C. Correlated discharges in the primate prefrontal cortex before and after working memory training. Eur J Neurosci 36: 3538–3548, 2012. doi: 10.1111/j.1460-9568.2012.08267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bartolo R, Saunders RC, Mitz AR, Averbeck BB. Information-limiting correlations in large neural populations. J Neurosci 40: 1668–1678, 2020. doi: 10.1523/JNEUROSCI.2072-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li S, Constantinidis C, Qi X-L. Drifts in prefrontal and parietal neuronal activity influence working memory judgments. Cereb Cortex 31: 3650–3664, 2021. doi: 10.1093/cercor/bhab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Constantinidis C, Steinmetz MA. Neuronal activity in posterior parietal area 7a during the delay periods of a spatial memory task. J Neurophysiol 76: 1352–1355, 1996. doi: 10.1152/jn.1996.76.2.1352. [DOI] [PubMed] [Google Scholar]
- 42. Jacob SN, Nieder A. Complementary roles for primate frontal and parietal cortex in guarding working memory from distractor stimuli. Neuron 83: 226–237, 2014. doi: 10.1016/j.neuron.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 43. Zhou X, Katsuki F, Qi X-L, Constantinidis C. Neurons with inverted tuning during the delay periods of working memory tasks in the dorsal prefrontal and posterior parietal cortex. J Neurophysiol 108: 31–38, 2012. doi: 10.1152/jn.01151.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Katsuki F, Qi X-L, Meyer T, Kostelic PM, Salinas E, Constantinidis C. Differences in intrinsic functional organization between dorsolateral prefrontal and posterior parietal cortex. Cereb Cortex 24: 2334–2349, 2014. doi: 10.1093/cercor/bht087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Riley MR, Constantinidis C. Role of prefrontal persistent activity in working memory. Front Syst Neurosci 9: 181, 2015. doi: 10.3389/fnsys.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.