Abstract
Objective
Electrode patch position may not be critical for success when cardioverting atrial fibrillation (AF), but the relevance of applied electrical energy is unclarified. Our objective was to perform a meta-analysis of randomised trials to examine the dose–response relation between energy level and cardioversion success by electrode position in elective cardioversion.
Methods
We searched PubMed, Embase, The Cochrane Library, Google Scholar and Scopus Citations. Inclusion criteria were randomised controlled trials using biphasic shock waves and self-adhesive patches, and publication date from 2000 to 2023. We used random-effects dose-response models to meta-analyse the relation between energy level and cardioversion success by anterolateral and anteroposterior position. Random-effects models estimated pooled risk ratios (RR) for cardioversion success after the first and the final shocks between the two electrode positions.
Results
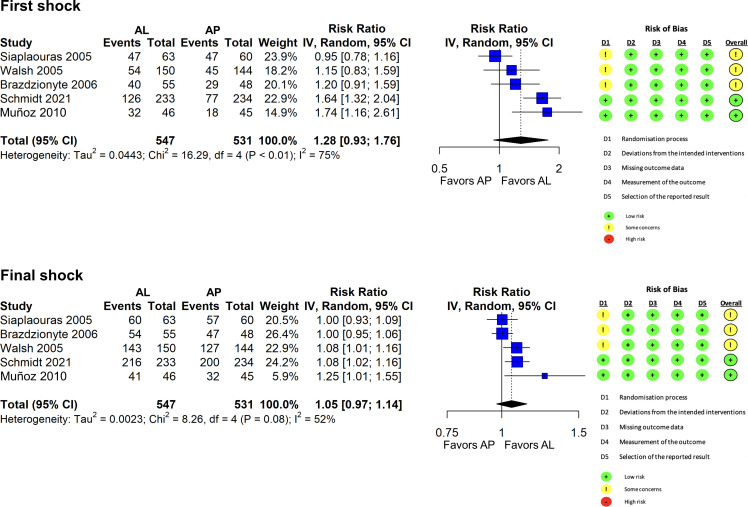
We included five randomised controlled trials (N=1078). After the first low-energy shock, the electrode position was not significantly associated with the likelihood of successful cardioversion (pooled RR anterolateral vs anteroposterior placement 1.28, 95% CI 0.93 to 1.76, with considerable heterogeneity). After a high-energy final shock, there was no evidence of an association between the electrode position and the cumulative chance of cardioversion success (pooled RR anterolateral vs anteroposterior 1.05, 95% CI 0.97 to 1.14). Regardless of electrode position, cardioversion success was significantly less likely with shock energy levels < 200J compared with 200J.
Conclusion
Evidence from contemporary randomised trials suggests that higher level of electrical energy is associated with higher conversion rate when cardioverting AF with a biphasic shockwave. Positioning of electrodes can be based on convenience.
Keywords: Atrial Fibrillation, Meta-Analysis, Electrophysiology
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Several systematic reviews with meta-analyses of randomised controlled trials that examined the optimal electrode position for cardioverting atrial fibrillation have shown conflicting results.
Attention has increasingly been focused on the electrical energy delivered and the success rate of electrical cardioversion, but this has been analysed sparsely in meta-analysis.
WHAT THIS STUDY ADDS
Applied energy levels below 200J were significantly associated with lower chances of success compared with energy applied at 200J, while there was no evidence of differential cardioversion success between energy levels above 200J.
There were no substantial differences between the two electrode positions, although with considerable heterogeneity.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This systematic review with meta-analysis of biphasic shock and self-adhesive electrodes suggests that shock energy is more important for effective electrical cardioversion than the electrode position.
The electrode placement may depend on the physician’s discretion.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical practice, with a lifetime risk of one in three.1 In contemporary practice, patients with incident AF lose on average 2 years of expected lifetime over 10 years of follow-up.2 Rhythm control constitutes a central part of symptom management in the Atrial fibrillation Better Care pathway for integrated care.3 Elective electrical cardioversion is often used for rhythm control of AF and has a high immediate success rate for restoring normal sinus rhythm, but the intermediate-term and long-term recurrence rates are high.4 5
The current European Society of Cardiology (ESC) guidelines include no specific guidance on the electrode position.3 Yet, more systematic reviews with meta-analyses of randomised controlled trials (RCTs) have recently examined the optimal electrode position for cardioverting AF, but the results are conflicting.6–11 Lately, attention has increasingly been focused on the electrical energy delivered and the success rate of electrical cardioversion.12 Systematic reviews with meta-analysis have sparsely analysed the association between energy dose and effect,7 9 and the ESC guidelines for AF do not state any recommendations on the most effective strategy.3
In this study, we performed a systematic review and meta-analysis of the dose–response relation between applied energy and cardioversion rates. As patch position may influence the success rate of electrical cardioversion, we included RCTs that examined self-adhesive patches positioned anterior lateral (AL) or anterior–posterior. The review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement.13
Methods
Eligibility criteria
We selected RCTs that used a step-up protocol using increasing electrical current and compared anterolateral vs anteroposterior electrode placement for elective cardioversion of AF among adults (>18 years of age) published from 2000 to 2023. Eligible RCTs were required to use self-adhesive patch electrodes and biphasic shockwave to reflect contemporary practice. Trials that reported use of monophasic shockwaves or manual hand-held paddles were not eligible for the reason of not being a standard procedure in contemporary medical practice. The RACE 7 study reported that in patients presenting to the emergency department with recent-onset and symptomatic AF, a wait-and-see approach was non-inferior to early cardioversion in achieving a return to sinus rhythm at 4 weeks.14 Therefore, we did not include studies reporting on acute cardioversions in this systematic review. We intended to select articles published in English, Spanish, Portuguese, Danish, Norwegian or Swedish. This review was not registered.
Search strategy and information sources
We searched PubMed, Embase and the Cochrane Library from 2000 up to and including 2023. The final search was performed on 18 July 2023. The search strategy is reported in online supplemental table 1. We developed the search strategy with the help of a professional healthcare librarian. To account for publications not indexed in the three databases, Scopus Citations and Google Scholar were searched. All potential records were manually screened for eligibility. Two reviewers assessed each record for inclusion based on titles and abstracts. Eligible articles were retrieved as full-text and read by two independent reviewers (LF and NV). Discrepancies were resolved by formal consensus. Finally, we screened the list of references of selected studies.
openhrt-2023-002456supp001.pdf (645.4KB, pdf)
Data collection process
Two reviewers extracted all data independently and in duplicate. We evaluated study characteristics, including trial location, whether patients with recent-onset or persistent AF were included, inclusion and exclusion criteria, and the shock protocol (energy level of shocks and step-up protocol). We also assessed patient characteristics, including mean age, mean body mass index, proportion of men and proportion of patients with hypertension.
We examined restoration of sinus rhythm by delivered energy (dose–response evaluation). The outcomes of interest were restoration of sinus rhythm after the first shock in a step-up protocol using increasing electrical current, and restoration of sinus rhythm after the final shock. The final shock was either the shock that led to restoration of sinus rhythm or the final shock in the study-specific shock protocol. From each study, we collected the total number of patients and the number of patients with restored sinus rhythm after the first shock and after all consecutive shocks. We extracted the outcome data for success as defined according to each study-specific protocol (table 1). Some RCTs used a step-up protocol, which included sequential shocks with increasing energy and a final cross-over of electrodes (switching from the initial allocated position to the alternate position on cardioversion failure). In studies with cross-over protocols, we considered the final shock as the last one in the allocated electrode position and not after cross-over. Trial investigators were contacted to obtain missing data. In Muñoz-Martínez et al’s study,15 we used the first of two shocks with 200J for the dose–response analysis.
Table 1.
Study characteristics
| Reference | Location | Sample size | Recent onset or persistent AF |
Inclusion criteria | Exclusion criteria | Shock protocol suggests | Successful cardioversion |
| Walsh et al 200525 | England | 294 | Persistent | Elective cardioversion of stable AF | Other arrhythmias, electrolyte imbalances or age <18 years | 70, 100, 150, 200J | Restoration of sinus rhythm for at least 30 s after a shock |
| Siaplaouras et al 200523 | Germany | 123 | Persistent | Elective cardioversion of stable AF | Other arrhythmias, pacemaker, potassium <3.5 or >5 mmol/L | 120, 150, 200J | Termination of AF with at least two consecutive sinus beats after a shock |
| Brazdzionyte et al 200624 | Lithuania | 103 | Recent onset/persistent | Elective cardioversion of stable AF | Age <18 years. Inadequate anticoagulation therapy* |
100, 150, 200, 300J+crossover | Presence of at least one clearly visible P wave within 30 s after the administration of the shock |
| Muñoz-Martínez et al 201015 | Spain | 91 | Persistent | Elective cardioversion of persistent and stable AF | Age <18 years, acute myocardial infarction, pregnancy, high risk of thromboembolism† | 150, 200, 200J+crossover‡ | After the final shock all patients were observed for 15 min at the intensive care unit and then moved back to the department and discharged with sinus rhythm after 2 hours if the patient was stable. |
| Schmidt et al 202126 | Denmark | 467 | Recent onset/persistent | Elective cardioversion of stable AF | Age <18 years, other arrhythmias, ICD, pregnancy or previous enrollment in protocol | 100, 150, 200, 360J | Sinus rhythm 1 min after first shock or 1 min after final shock. |
*International normalised ratio >2 for at least 3 weeks before cardioversion.
†Inadequate anticoagulation therapy or verified auricular thrombus in echocardiography.
‡As the energy level for second and third shock were similar, we counted the second shock as the final.
AF, atrial fibrillation; ICD, implantable cardioverter-defibrillator.
Assessment of risk of bias
Reviewers assessed within-study biases with the RoB 2 Tool (version 2 of the Cochrane risk-of-bias tool for randomised trials).16 This tool provided a framework for the assessment of the overall risk of bias by evaluating: (1) the randomisation process, (2) deviations from the intended procedure, (3) missing outcome data, (4) measurement of outcome and (5) selective reporting. To assess small-study effects, we created funnel plots for the first and final shock conversion success outcomes.
Synthesis methods
We calculated the cumulative proportions of patients with restored sinus rhythm by cumulative shocks and estimated risk ratios (RRs). For example, when counting patients with restored sinus rhythm after the second shock, patients with restored sinus rhythm after both the first and second shock were included in the numerator and all patients were included in the denominator. The RRs were pooled across eligible studies by using inverse-variance random-effects models. We used the Paule-Mandel procedure to estimate the between-trial variance with the Knapp-Hartung adjustment procedure. To quantify heterogeneity between studies, we also estimated the I2 statistic. To examine the effect of shock energy, we conducted a dose–response meta-analysis to assess the association of shock energy with the probability of restored sinus rhythm, for each electrode position separately. In each study, we extracted the cumulative number of patients with restored sinus rhythm at each increasing energy level; we assumed that patients with success at lower energy levels would have success at a given energy level. We fitted a one-stage random-effects dose–response model using restricted cubic splines with three knots for each electrode position.17 As all studies delivered a 200J shock, this amount of energy was used as the reference level in this analysis.
All analyses were performed in R V.4.0.3., with the ‘meta’ package, except the dose–response model that we fitted with Stata V.15.1 by using the command drmeta.
Results
Literature search
Online supplemental figure 1 shows the flow of study selection. The initial search yielded 331 results. We retrieved 10 full-text records of which five studies were excluded because of monophasic shock use and/or use of hand-held defibrillator paddles.18–22 We included five RCTs,15 23–26 with a total of 1078 patients. Of the patients, 547 were allocated to the AL electrode placement, and 531 patients were allocated to the anterior–posterior electrode placement.
Trial and baseline patient characteristics
The five trials included ‘recent-onset’ (AF present <48 hours) or persistent AF. The mean age of participants ranged from 55 to 69 years, the proportion of men ranged from 63% to 82.5%, and the prevalence of hypertension from 32% to 64.5% (table 2).
Table 2.
Baseline characteristics of study populations in included trials
| Trial | Sample size | Age (years) | Sex, male | Body mass index (kg/m2) | Hypertension | |||||
| AL | AP | AL | AP | AL | AP | AL | AP | AL | AP | |
| Siaplaouras et al23 2005 | 63 | 60 | 66±10 | 67±10 | 47 (75) | 40 (67) | 28±5 | 28±4 | 18 (28) | 26 (44) |
| Walsh et al25 2005 | 150 | 144 | 67±10 | 66±14 | 95 (63) | 100 (64) | 28±5 | 29±5 | 57 (38) | 81 (52) |
| Brazdzionyte et al24 2006 | 55 | 48 | 63±12 | 62±10 | 36 (66) | 29 (60) | 30±5 | 30±5 | 20 (36) | 19 (39) |
| Muñoz-Martínez et al15 2010 | 46 | 45 | 63±9 | 55±13 | 40 (87) | 35 (78) | NA | NA | 15 (33) | 14 (31) |
| Schmidt et al26 2021 | 233 | 234 | 69±10 | 69±9 | 156 (67) | 158 (67) | 29±6 | 29±5 | 149 (64) | 151 (65) |
Data are mean ±SD or n (%).
AL, anterior lateral; AP, anterior–posterior; NA, not available.
The trials used different step-up protocols with sequential shocks of increasing energy (tables 1 and 3). Two trials included a cross-over shock with high energy.15 24 The anterior–posterior electrode position differed between trials, with some placing the electrodes in the left infraclavicular and left infrascapular region,15 24 26 while others used the right infraclavicular and left infrascapular region,25 and one trial placed the electrodes in the midsternal and interscapular region.23
Table 3.
Dose–response evaluation of cumulated cardioversion proportion of biphasic electrical cardioversion of atrial fibrillation using self-adhesive patches in anterolateral versus anteroposterior positions
| Energy (J) | Trial | Anterolateral cardioversion proportion | Anteroposterior cardioversion proportion |
| 70 | Walsh et al25 2005 | 54/150 | 45/144 |
| 100 | Walsh et al25 2005 | 99/150 | 74/144 |
| Brazdzionyte et al24 2006 | 40/55 | 29/48 | |
| Schmidt et al26 2021 | 126/233 | 77/234 | |
| 120 | Siaplaouras et al23 2005 | 47/63 | 47/60 |
| 150 | Siaplaouras et al23 2005 | 55/63 | 54/60 |
| Walsh et al25 2005 | 123/150 | 109/144 | |
| Brazdzionyte et al24 2006 | 52/55 | 41/48 | |
| Muñoz-Martínez et al15 2010* | 32/46 | 18/45 | |
| Schmidt et al26 2021 | 175/233 | 125/234 | |
| 200 | Siaplaouras et al23 2005 | 60/63 | 57/60 |
| Walsh et al25 2005 | 143/150 | 127/144 | |
| Brazdzionyte et al24 2006 | 53/55 | 46/48 | |
| Muñoz-Martínez et al15 2010, first shock* | 39/46 | 27/45 | |
| Muñoz-Martínez et al15 2010, second shock | 41/46 | 32/45 | |
| Schmidt et al26 2021 | 200/233 | 162/234 | |
| 300 | Brazdzionyte et al24 2006 | 54/55 | 47/48 |
| 360 | Schmidt et al26 2021 | 216/233 | 200/234 |
*Data shared by the author. In the dose–response analyses, we used the first of the two shocks.
In all RCTs, clinicians and patients were not blinded, and only one study used blinded adjudication of the outcome.26 The outcome definition differed between trials, one trial defined successful cardioversion as two consecutive P waves within 1 min after the procedure,23 another trial defined cardioversion as restoration of sinus rhythm for a least 30 s,25 one trial defined success as at least one clearly visible P wave within 30 s after the shock,24 and another trial defined the outcome as sinus rhythm 1 min after the final shock.26 One trial did not specify the time window for the adjudication of successful cardioversion.15
Effect of energy level by electrode position
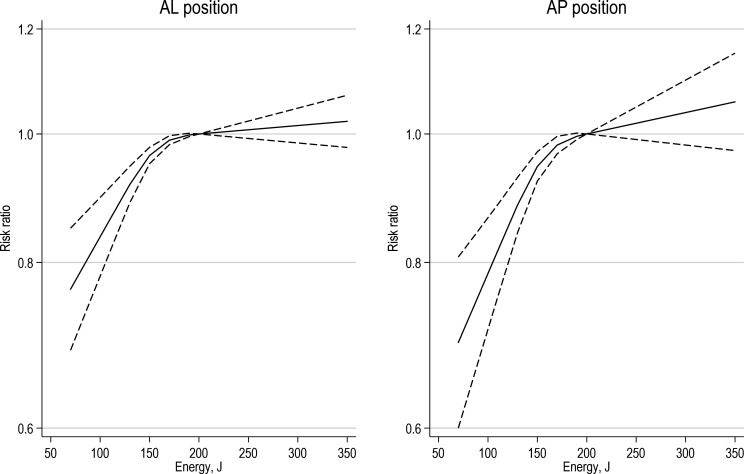
After a first low-energy shock, the electrode position was not significantly associated with the likelihood of successful cardioversion (pooled RR for anterolateral vs anteroposterior position: 1.28, 95% CI 0.93 to 1.76, with considerable heterogeneity across trials, figure 1). After a high-energy final shock as per protocol, we found no evidence of a difference between the two electrode positions (pooled RR for anterolateral vs anteroposterior electrode position: 1.05, 95% CI 0.97 to 1.14). We found a dose–response association so that an increasing shock energy was associated with a higher probability of cardioversion success to sinus rhythm (figure 2). Applied energy levels below 200J were significantly associated with lower chances of success compared with energy applied at 200J. Conversely, there was no evidence of differential cardioversion success between energy levels above 200J and at 200J. We found no substantial differences between the two electrode positions.
Figure 1.
Meta-analysis comparing electrode pad position at first shock and final shock. AL, anterior lateral; AP, anterior–posterior; IV, inverse variance.
Figure 2.
Dose–response relationship between biphasic shock energy (Joule) and successful cardioversion using anterolateral and anteroposterior self-adhesive patch position. For AL and AP separately, each figure shows risk ratios for restored sinus rhythm between the shock energy level on the horizontal axis and 200 J (as a reference), together with 95% CIs. AL, anterior lateral; AP, anterior–posterior.
Figure 1 shows the risk of bias assessment across trials. Two studies had some concerns, and no study was deemed at high risk of bias. We did not find any indication of small-study effects on either outcome after the first or final shock, although the number of studies was small for interpreting the funnel plots (online supplemental figure 1).
Discussion
This comprehensive systematic review that included a meta-analysis and dose–response evaluation presents an up-to-date analysis of RCTs that compared anterolateral versus anteroposterior electrode positioning in cardioversion of AF using biphasic shock and self-adhesive electrodes. The results showed that applied energy levels below 200J were significantly associated with lower chances of success compared with energy applied at 200J, while there was no evidence of differential cardioversion success between energy levels above 200J. We found no substantial differences between the two electrode positions, although with considerable heterogeneity.
Recently, a systematic review with meta-analyses by Nguyen et al examined cardioversion success, electrode position and energy level, respectively.7 We aimed to examine the relationship between energy level and cumulative cardioversion success. In contrast, Nguyen et al aimed to compare the cumulative cardioversion success between high-energy shocks (minimum 200J) and escalating energy protocols. Our prespecified eligibility criteria focused on trials that randomised electrode placement and that used biphasic shock waves while trials of acute cardioversion or those involving monophasic shockwaves or manual hand-held paddles that no longer reflect standard contemporary practice were not eligible. Our meta-analysis included five randomised trials, none of which overlapped with the four randomised trials selected by Nguyen et al. Nguyen et al reported that high-energy shocks did not significantly improve cumulative cardioversion success compared with an escalating energy protocol. A subgroup analysis suggested a larger effect with anteroposterior pad positioning compared with anteroapical or anterolateral positioning. In contrast, we found a dose–response relationship with increasing levels of energy leading to larger cardioversion success rate up to 200J. Beyond 200J, we found no evidence of improved cardioversion success. Additionally, we found no evidence of difference between anteroposterior and anterolateral pad positioning.
Assessment of risk of bias of the included trials showed some concerns in two trials. Four out of five trials did not report any method for allocation concealment, which may increase the risk of selection bias. Due to the nature of the studies, blinding of personnel and trialists was impossible, though the open-label design was not thought to influence the outcome assessment. Only one trial had blinded outcome adjudication,26 and only two trials were rated as having low risk of bias.
Participants in the included studies differed with respect to age, weight, comorbidities, duration of AF and use of antiarrhythmics drugs. For example, the user rate of amiodarone varied from 9% to 10% in one trial25 to from 40% to 50% in another trial.24 Such factors are known to influence the cardioversion rate. Selected studies used slightly different methods to ascertain success as well as different step-up protocols with initial low-energy shock, the latter not being in line with contemporary practice. Furthermore, electrode placement, specifically the anteroposterior electrode placement, was slightly different (eg, right infraclavicular or left infraclavicular) between the trials. Differences in anteroposterior position could, theoretically, target the atrium differently and therefore be of significance in cardioversion success. Only 4% of the transthoracic shock traverses the heart while the rest of the current is shunted around the heart and through the thoracic cage and lungs.27 Therefore, a successful cardioversion could be more dependent on the total energy delivery rather than electrode placement, a trend that was observed in all RCTs.
Schmidt et al showed that adverse events, for example, arrhythmias and transient bradycardia, were rare.26 Other adverse effects such as skin discomfort was rarely experienced.26 A recent study by Lobo et al examined the effect of high-energy biphasic shock cardioversion on high-sensitive troponin release, and even when delivering 360J shocks, an increase in troponin release could not be detected; hence, cardioversion should be considered a safe procedure, even at high energy shock delivery.28 One randomised trial demonstrated that maximum fixed energy shocks of 360J had higher conversion rates compared with sequential and escalating shock energies.12 However, considering the high conversion rates at 200J, independent of electrode position, it can be speculated that electrode position becomes irrelevant when delivering high energy shocks (≥200J).
Limitations
This meta-analysis included five relatively small studies and has, therefore, limited statistical power and precision. There was considerable clinical diversity across studies, which resulted in large statistical heterogeneity. Studies were non-blinded, and outcome assessment was blinded in only one study. Results from our dose–response analyses should be interpreted with caution given the risk of ecological bias and considerable clinical diversity across studies. Moreover, we made the assumption that success at a lower energy level would have been observed at a higher energy level.
Conclusions
This systematic review and meta-analysis of contemporary RCTs suggest that an initial shock energy of 200J, possibly followed by higher energy shocks up to 360J, has a higher success than previously used lower energy shocks when elective cardioverting AF. We did not find evidence of differential success between anterolateral and anteroposterior electrode positions, although with considerable heterogeneity across trials. These findings suggest that the electrode position is not a critical factor compared with shock energy, and the placement may depend on the physician’s discretion, with possible preference for AL placement.
Footnotes
Twitter: @nicklas_vinter
Contributors: NV, LF, MZBH-H and LT: conceptualisation, methodology and writing-original draft preparation. LF and LT: supervision and investigation. NV, LF and MZBH-H performed the searches and extracted data from the eligible studies. NV and MZBH-H assessed the quality of the studies, and NV conducted the statistical analysis with critical appraisal from LT. NV led the drafting of the manuscript, with review and contributions from LT, LF, MZBH-H, SPJ and GYHL. All authors contributed to the article and approved the submitted version. NV and LF are responsible for the overall content as guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: NV: Grants from the Danish Cardiovascular Academy, the Health Research Foundation of Central Denmark Region, and the Danish Heart Foundation. Advisory board member for Astra Zeneca. No fees were received personally. MZBH-H: none declared SPJ: Consultant and speaker for BMS/Pfizer. Research grants from BMS/Pfizer and Novo Nordisk. GYHL: Consultancy and speaker fees from BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo outside the submitted work. No fees received personally. LF: Supported by the Health Research Foundation of Central Denmark Region. Consultant for BMS and Pfizer. LT: Received funding from the American Heart Association (18SFRN34150007)
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Staerk L, Wang B, Preis SR, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the framingham heart study. BMJ 2018;361:k1453. 10.1136/bmj.k1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinter N, Huang Q, Fenger-Grøn M, et al. Trends in excess mortality associated with atrial fibrillation over 45 years (Framingham heart study): community based cohort study. BMJ 2020;370:m2724. 10.1136/bmj.m2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hindricks G, Potpara T, Dagres N, et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2020;42:373–498. 10.1093/eurheartj/ehaa945 [DOI] [PubMed] [Google Scholar]
- 4.Brandes A, Crijns H, Rienstra M, et al. Cardioversion of atrial fibrillation and atrial flutter revisited: current evidence and practical guidance for a common procedure. Europace 2020;22:1149–61. 10.1093/europace/euaa057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinter N, Frederiksen AS, Albertsen AE, et al. Role for machine learning in sex-specific prediction of successful electrical cardioversion in atrial fibrillation? Open Heart 2020;7:e001297. 10.1136/openhrt-2020-001297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motawea KR, Mostafa MR, Aboelenein M, et al. Anteriolateral versus anterior-posterior electrodes in external cardioversion of atrial fibrillation: a systematic review and meta-analysis of clinical trials. Clin Cardiol 2023;46:359–75. 10.1002/clc.23987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen ST, Belley-Côté EP, Ibrahim O, et al. Techniques improving electrical cardioversion success for patients with atrial fibrillation: a systematic review and meta-analysis. Europace 2023;25:318–30. 10.1093/europace/euac199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eid M, Abu Jazar D, Medhekar A, et al. Anterior-posterior versus anterior-lateral electrodes position for electrical cardioversion of atrial fibrillation: a meta-analysis of randomized controlled trials. Int J Cardiol Heart Vasc 2022;43:101129. 10.1016/j.ijcha.2022.101129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Virk SA, Rubenis I, Brieger D, et al. Anteroposterior versus anterolateral electrode position for direct current cardioversion of atrial fibrillation: a meta-analysis of randomised controlled trials. Heart Lung Circ 2022;31:1640–8. 10.1016/j.hlc.2022.08.016 [DOI] [PubMed] [Google Scholar]
- 10.Salah HM, Devabhaktuni SR, Shah SD, et al. Meta-analysis comparing anterior-lateral versus anterior-posterior electrode position for biphasic cardioversion in atrial fibrillation. Am J Cardiol 2022;169:164–5. 10.1016/j.amjcard.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 11.Kirkland S, Stiell I, AlShawabkeh T, et al. The efficacy of pad placement for electrical cardioversion of atrial fibrillation/flutter: a systematic review. Acad Emerg Med 2014;21:717–26. 10.1111/acem.12407 [DOI] [PubMed] [Google Scholar]
- 12.Schmidt AS, Lauridsen KG, Torp P, et al. Maximum-fixed energy shocks for cardioverting atrial fibrillation. Eur Heart J 2020;41:626–31. 10.1093/eurheartj/ehz585 [DOI] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pluymaekers N, Dudink E, Crijns H. Early or delayed cardioversion in recent-onset atrial fibrillation. reply. N Engl J Med 2019;381:387–8. 10.1056/NEJMc1906729 [DOI] [PubMed] [Google Scholar]
- 15.Muñoz-Martínez T, Castañeda-Saiz A, Vinuesa-Lozano C, et al. Electrode position in elective electrical cardioversion of atrial fibrillation. a randomized study. Med Intensiva 2010;34:225–30. 10.1016/j.medin.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 16.Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 17.Shim SR, Lee J. Dose-response meta-analysis: application and practice using the R software. Epidemiol Health 2019;41:e2019006. 10.4178/epih.e2019006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirchhof P, Eckardt L, Loh P, et al. Anterior-posterior versus anterior-lateral electrode positions for external cardioversion of atrial fibrillation: a randomised trial. Lancet 2002;360:1275–9. 10.1016/s0140-6736(02)11315-8 [DOI] [PubMed] [Google Scholar]
- 19.Chen C-J, Guo G-F. External cardioversion in patients with persistent atrial fibrillation: a reappraisal of the effects of electrode pad position and transthoracic impedance on cardioversion success. Jpn Heart J 2003;44:921–32. 10.1536/jhj.44.921 [DOI] [PubMed] [Google Scholar]
- 20.Alp NJ, Rahman S, Bell JA, et al. Randomised comparison of antero-lateral versus antero-posterior paddle positions for DC cardioversion of persistent atrial fibrillation. Int J Cardiol 2000;75:211–6. 10.1016/s0167-5273(00)00326-0 [DOI] [PubMed] [Google Scholar]
- 21.Vogiatzis IA, Sachpekidis V, Vogiatzis IM, et al. External cardioversion of atrial fibrillation: the role of electrode position on cardioversion success. Int J Cardiol 2009;137:e8–10. 10.1016/j.ijcard.2008.05.038 [DOI] [PubMed] [Google Scholar]
- 22.Stanaitiene G, Babarskiene RM. Impact of electrical shock waveform and paddle positions on efficacy of direct current cardioversion for atrial fibrillation. Medicina (Kaunas) 2008;44:665–72. [PubMed] [Google Scholar]
- 23.Siaplaouras S, Buob A, Rötter C, et al. Randomized comparison of anterolateral versus anteroposterior electrode position for biphasic external cardioversion of atrial fibrillation. Am Heart J 2005;150:150–2. 10.1016/j.ahj.2004.08.009 [DOI] [PubMed] [Google Scholar]
- 24.Brazdzionyte J, Babarskiene RM, Stanaitiene G. Anterior-posterior versus anterior-lateral electrode position for biphasic cardioversion of atrial fibrillation. Medicina (Kaunas) 2006;42:994–8. [PubMed] [Google Scholar]
- 25.Walsh SJ, McCarty D, McClelland AJJ, et al. Impedance compensated biphasic waveforms for transthoracic cardioversion of atrial fibrillation: a multi-centre comparison of antero-apical and Antero-posterior pad positions. Eur Heart J 2005;26:1298–302. 10.1093/eurheartj/ehi196 [DOI] [PubMed] [Google Scholar]
- 26.Schmidt AS, Lauridsen KG, Møller DS, et al. Anterior-lateral versus anterior-posterior electrode position for cardioverting atrial fibrillation. Circulation 2021;144:1995–2003. 10.1161/CIRCULATIONAHA.121.056301 [DOI] [PubMed] [Google Scholar]
- 27.Lerman BB, Deale OC. Relation between transcardiac and transthoracic current during defibrillation in humans. Circ Res 1990;67:1420–6. 10.1161/01.res.67.6.1420 [DOI] [PubMed] [Google Scholar]
- 28.Lobo R, Jaffe AS, Cahill C, et al. Significance of high-sensitivity troponin T after elective external direct current cardioversion for atrial fibrillation or atrial flutter. Am J Cardiol 2018;121:188–92. 10.1016/j.amjcard.2017.10.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2023-002456supp001.pdf (645.4KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.