Abstract
Summary: Neuroimaging plays a crucial role in the diagnosis and therapeutic decision making in infectious diseases of the nervous system. The review summarizes imaging findings and recent advances in the diagnosis of pyogenic brain abscess, ventriculitis, viral disease including exotic and emergent viruses, and opportunistic disease. For each condition, the ensuing therapeutic steps are presented. In cases of uncomplicated meningitis, cranial computed tomography (CT) appears to be sufficient for clinical management to exclude acute brain edema, hydrocephalus, and pathology of the base of skull. Magnetic resonance imaging (MRI) is superior in depicting complications like sub-/epidural empyema and vasculitic complications notably on FLAIR (fluid-attenuated inversion recovery)-weighted images. The newer technique of diffusion-weighted imaging (DWI) shows early parenchymal complications of meningitis earlier and with more clarity and is of help in differentiation of pyogenic abscess (PA) from ring enhancing lesions of other etiology. Proton magnetic resonance spectroscopy (PMRS) seems to produce specific peak patterns in cases of abscess. The presence of lactate cytosolic amino acids and absence of choline seems to indicate PA. Also in cases of suspected opportunistic infection due to toxoplasma DWI may be of help in the differentiation from lymphoma, showing no restriction of water diffusion. In patients with herpes simplex and more exotic viruses like West Nile and Murray Valley virus DWI allows earlier lesion detection and therapeutic intervention with virustatic drugs.
Keywords: Neuroimaging, infections, therapy, MRI, diffusion-weighted imaging
INTRODUCTION
Infections of the nervous system and adjacent structures are often life-threatening conditions. The prognosis mainly depends on rapid identification of the site of inflammation and pathogen to install effective antimicrobial treatment as early as possible. Whereas analysis of CSF, biopsy, and laboratory analysis remain the gold standard to identify the infectious agent for instance in meningitis, neuroimaging is crucial in clearly depicting inflammatory lesions of brain and spine. Visualization of typical lesion patterns often allows a rapid diagnosis and subsequent therapeutic decisions. Notably, in opportunistic disease neuroimaging has a pivotal role not only in diagnosis but also in monitoring therapeutic response. The following review summarizes recent findings in neuroimaging of CNS infections such as bacterial meningoencephalitis, ventriculitis, infectious disease of the spinal cord as well as viral and opportunistic disease of the CNS.
MENINGITIS
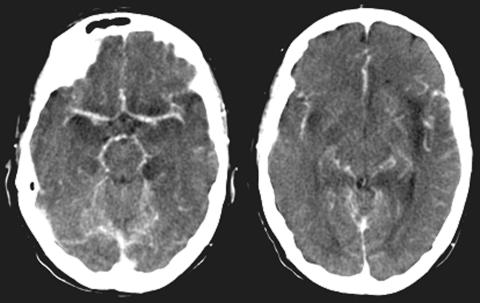
In cases of suspected bacterial meningitis with clouded consciousness, an immediate cranial computed tomography (CT) is recommended before lumbar puncture to rule out causes for swelling that might lead to herniation. However, it has to be noted that empirical antimicrobial treatment has to be started before CT scan and/or lumbar puncture is performed. In the early phase of meningitis, the CT findings are mostly normal. Contrast-enhanced CT may show beginning meningeal enhancement, which becomes more accentuated in later stages of disease. Parenchymal lesions are not easily visualized, except for areas of ischemia due to secondary vasculitis, a complication in up to 20% of cases ( FIG. 1). CT is important and sufficient to define pathology of the base of skull that may be causative and require rapid therapeutic intervention and surgical consultation. Potential sources of infection include fractures of the paranasal sinus or petrous bone as well as inner ear infection and mastoiditis. CT venography is an excellent tool to diagnose complicating thrombosis of the transverse and sagittal sinus, necessitating therapeutic anticoagulation with intravenous heparin. In later stages, persistent drowsiness and meningeal signs should be regarded as an indication for repeat CT to rule out a resorptive hydrocephalus. If external ventricular drainage is required, further CT studies to check on ventricular size will help in timing of the operation and later cessation of this measure. Often subdural effusions are noted, which usually resolve spontaneously without specific therapeutic intervention. An abnormal parenchymal scan correlates with neurological signs and a worse prognosis.1
FIG. 1.
Enhanced CT of a patient with tuberculous meningitis showing perivascular inflammatory changes and temporal infarction due to vasculitis.
Magnetic resonance imaging (MRI) is not routinely required in cases of uncomplicated bacterial meningitis. It helps to visualize meningeal enhancement more clearly. Sometimes not only the meninges around brain and spinal cord show enhancement after administration of gadolinium (Gd)-DTPA, but also the CSF, as reported in cases of spirochaetal meningitis.2 Recently, magnetization transfer MRI has been proposed as a useful tool in the diagnosis of tuberculous meningitis. Visibility of the meninges on precontrast T1-weighted magnetization transfer images may be considered highly suggestive of tuberculous meningitis.3 It is important to institute tuberculostatic triple therapy as early as possible because morbidity and mortality is still high. A recent study of adjunctive dexamethasone for the treatment of tuberculous meningitis in adolescents and adults demonstrated an improvement in survival but no prevention of disability.4
In complicated cases with seizures and evolving focal signs, MRI is superior to CT in demonstrating parenchymal lesions due to meningoencephalitis or vasculitic complications on FLAIR (fluid-attenuated inversion recovery) sequences. In Lyme disease, multifocal nonenhancing patchy lesions on T2 WI can be seen. Together with a suspect history and pathologic CSF, immediate intravenous therapy with ceftriaxone for 21 days is mandatory. Additional information can be gleaned from diffusion-weighted imaging (DWI). Acute inflammatory lesions, including encephalitis, cerebritis, and tuberculosis, are hyperintense. Neurocysticercosis shows hypointense lesions on DWI. The diagnosis of neurocysticercosis can be readily made neuroradiologically. Open brain or stereotaxic biopsy is usually not necessary. The lesions resolve after treatment with praziquantel or albendazole. The appearance of toxoplasmosis is variable on DWI. Treatment should be instituted immediately, and the treatment response monitored with a follow-up scan after 4 weeks.
Some pathogens have a predilection for brain stem involvement, readily visualized on MRI. Notably, the finding of rhombencephalitis points to listeria monocytogenes as the causative agent, necessitating appropriate choice of antibiotics including ampicillin.5 Neurobrucellosis shows a wide spectrum of imaging findings from normal to nonspecific findings of inflammation of CNS and nerve roots or vascular complications.6 Therapy remains empirical.
Vascular complications must be suspected in patients with rapid deterioration despite therapy. In these cases, the sensitivity of DWI has higher sensitivity than conventional MRI in depicting small cortical or deep white matter infarcts due to septic vasculitis. Magnetic resonance angiography can exclude or confirm vasculitis, which is of clinical help in the decision to use high-dose steroid therapy. Recent studies have also advocated the adjunct use of steroids immediately after the diagnosis of bacterial meningitis before antibiotic therapy due to an improved outcome without increased risk of gastrointestinal bleeding.7
Pyogenic ventriculitis is an uncommon but very severe intracranial infection requiring rapid diagnosis and therapy because of its high mortality. Neuroimaging is the only tool to reliably diagnose this life-threatening condition. MRI is more sensitive and shows periventricular high signal on FLAIR images, ependymal enhancement and in most cases also pial or dura-arachnoid pathology. Irregular intraventricular debris seems to be the most specific abnormality. MRI is indispensable in diagnosing intraventricular rupture of pyogenic abscess.8 High-dose intravenous antibiotics must be given over a protracted period over weeks. In case of worsening despite intravenous therapy, intraventricular administration via an Ommaya reservoir must be considered.
SUBDURAL AND EPIDURAL EMPYEMA
Extraaxial bacterial empyema is most reliably diagnosed by MRI. CT often leaves doubt as to the nature of the lesion and its exact location. These fluid collections can be found over the convexities or interhemispherically. They are mildly hyperintense relative to CSF and hypointense to white matter on T1WI and hyperintense relative to CSF and white matter on T2WI allowing distinction from sterile effusions and most chronic hematomas. In contrast to subdural empyemas, epidural empyemas show a hypointense rim representing displaced dura depicted at the interface between lesion and brain. Often inflammation-induced parenchymal abnormalities like edema, mass effect, and reversible cortical hyperintensity can be seen. DWI can be used to confirm that extra-axial collections represent empyemas and differentiate them further. Subdural empyema usually show high signal, whereas epidural empyema tend to be of low or mixed signal intensity.9 Neurosurgical evacuation is the therapy of choice.
PYOGENIC ABSCESS
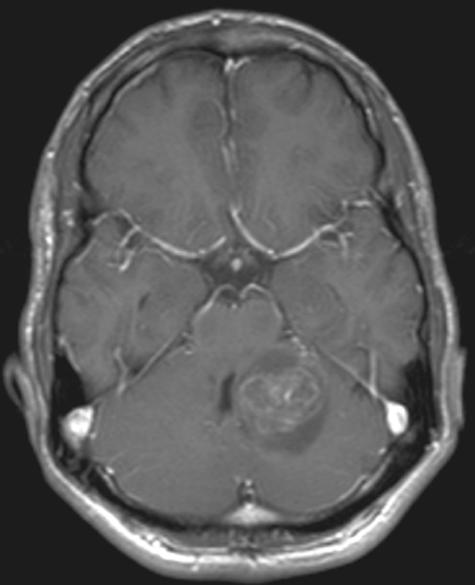
The diagnosis of pyogenic brain abscesses remains challenging. The clinician faces a diagnostic and therapeutic dilemma mostly in those cases where a single ring-enhancing lesion with perifocal edema has been identified on CT giving rise to the differential diagnosis of abscess versus necrotic tumor (glioblastoma) or metastasis ( FIG. 2). Gd-enhanced MRI is of help to identify multiple small additional lesions indicating metastatic disease. In cases of single lesions on MRI, stereotactic biopsy is the next diagnostic step. Because abscesses should be preferentially centrally aspirated whereas necrotic tumors should have biopsy from the cavity wall, further information to optimize stereotactic surgical planning is required. DWI has been proposed as the method of choice. In several studies, almost all pyogenic abscesses had markedly hyperintense signal on DWI and reduced calculated apparent diffusion coefficient (ADC) indicating restricted water diffusion opposite to nonpyogenic lesions that showed hypointense or mixed signal. Only chordoma and epidermoid can show markedly elevated DWI signal.10,11 Other authors have stressed that the calculated ADC values alone do not allow a reliable differentiation due to a large overlap.12,13 Although the method is helpful, it does not solve the diagnostic dilemma or obviate the need for biopsy. In unclear cases, additional information can be gathered from proton magnetic resonance spectroscopy (PMRS). This method is not routinely available, but some authors have found the results promising. The presence of lactate cytosolic amino acids with/without succinate, acetate, alanin, and glycine can be regarded as a marker for abscess, lactate and choline for nonabscess cases.14 Further confirmatory studies are needed. Although some authors have reported on the possibility of differentiating aerobic from anaerobic or sterile brain abscesses, this should be regarded with caution.15 The contribution of this technique and of PET to differentiate infection from tumors is further discussed in other articles of this issue, dealing with PMRS and with the imaging of brain tumors.
FIG. 2.
Axial post-gadolinium T11WI showing ring-enhancing lesion with mass effect in a patient with pyogenic brain abscess.
TOXOPLASMOSIS
This is the most frequent opportunistic infection in immunosuppressed patients.16 Prenatal infection may give rise to later seizures, prompting the use of neuroimaging. Notably, in third world countries multiple small calcified lesions may be detected. Acute infection in patients with acquired immune deficiency syndrome (AIDS) or after bone marrow transplantation causes lesions that are typically multiple, with ring enhancement or solid. MRI delineates them most clearly, sometimes revealing hemorrhagic zones.17 In cases with typical lesion appearance therapy with pyrimethamin 50–100 mg/day and sulfadiazine 4 g/day should be started promptly. In cases of sulfa allergy, the patient can alternatively be put on clindamycin 600 mg q.id. Not infrequently, neuroimaging reveals toxoplasma lesions with mass effect and marked perifocal edema. In these cases, in the first 7days dexamethasone 4 mg q.i.d should be given additionally. Further progression of edema requires osmodiuretics. In about 80% of patients, radiological improvement can be seen in about 1 week, supporting the diagnosis. Should the lesions be unchanged or progressive, the diagnosis has to be reconsidered and the therapeutic strategy reevaluated. Unfortunately, in cases of severe immunosuppression, the MR appearance can be completely atypical, misleading radiologist and clinician. Notably in fulminate encephalitic variants of the disease, lesions are widespread on T2WI and are completely devoid of enhancement.18 In these cases, antitoxoplasma therapy should be started until the diagnosis has been clarified further. Also in cases of atypical large solitary toxoplasma lesions showing marked enhancement resembling lymphoma, the clinician must look for additional diagnostic modalities other than conventional imaging while treating the patient for toxoplasmosis. DWI mostly shows no restriction of water diffusion in the core tissue, perfusion studies demonstrate an extremely hypovascular lesion.19 Spectroscopy is characterized by a predominant lipid peak. A widely available method is thallium-201 brain SPECT, of particular help in the differentiation from lymphoma, which shows marked uptake in contrast to toxoplasma abscess. Tuberculosis abscess may also show uptake.20
SPINAL INFECTIONS
Affections of bony structures, discs, ligaments, and soft tissue
Traditionally the radiological diagnosis of a spinal infection used to be hampered by the lack of specificity and sensitivity of plain x-rays. Only frank bony erosions and destruction of vertebrae could be seen. Thus, only the clinical differential diagnosis of a compressive myelopathy due to vertebral fracture or destruction can be clarified.
Although spinal CT has a higher sensitivity notably after contrast enhancement and may demonstrate spondylitis, it is insufficient for the detection of early discitis or epidural abscess. In cases of bacterial and tuberculous spondylitis, enhanced CT shows erosion and destruction of vertebral bodies with marked contrast enhancement in the disc space and inflammatory changes in the paravertebral regions. The diagnosis of spinal epidural abscess cannot be reliably excluded by CT. CT myelography can at least show the site of cord compression, although it still leaves doubt as to the exact etiology. In sum, it can only be recommended in an emergency setting in the absence of MRI.
Since the advent of MRI, nuclear medicine studies are no longer applied routinely. MRI is the method of choice in the diagnosis of suspected spondylodiscitis. T1-weighted images demonstrate signal loss of vertebral bodies, destruction of cortical margins and interruption of cortical continuity. T2-weighted images show high signal in the affected bony and disc structures. Application of gadolinium is mandatory and facilitates diagnosis. Contrast enhancement can be seen as the earliest sign in the acute phase where changes on T1/T2WI can be subtle.21 The most common pathogen is Staphylococcus aureus, but other bacteria including rare cases of Brucellar spondylitis have been reported.22 Several features have been identified appearing helpful in the differentiation of tuberculous from pyogenic spondylitis. Tuberculous spondylitis more often shows a well-defined paraspinal abnormal signal, a thin smooth abscess wall, subligamentous spread to three or more vertebral bodies and involvement of multiple, mostly thoracic vertebral bodies.23 It is important to bear in mind that rare cases of fungal vertebral osteomyelitis, mostly due to aspergillus and rarely cryptococcus can show the same MRI findings as bacterial spondylitis.24,25 Spinal mucormycosis has been reported in some patients being treated for leukemia.
Treatment is mostly conservative with antibiotics after CT guided aspiration for culture and percutaneous drainage. If radiological improvement is seen in 2 weeks, conservative therapy is sufficient. Only instability and intraspinal abscess require neurosurgical intervention. MRI is crucial for follow up to monitor the course under treatment. Even after clinical response and in the absence of systemic inflammation, gadolinium enhancement can persist for months. Additional courses of antibiotic treatment are not indicated in this setting.
Spinal epidural abscess requires a high level of clinical awareness. Notably in patients after paravertebral injections, early scanning must be considered when there is localized and increasing back pain, elevated sedimentation rate, and leukocytosis. MRI depicts epidural abscess clearly as a hyperintense and mixed signal mass with marked Gd enhancement on T1WE. Images in the axial and sagittal planes facilitate preoperative planning. Therapy with emergent surgical decompression and drainage is necessary in cases with compression of neural structures.26 Cases without frank spinal cord compression by the abscess but severe neurological signs can be a diagnostic pitfall. In these cases spinal cord ischemia due to thrombosis of leptomeningeal vessels or compression of spinal arteries must be suspected as the underlying mechanism.27 Neuroimaging thus clarifies the etiology and prevents further unnecessary therapeutic surgical interventions.
Involvement of the spinal cord and meninges
Plain radiographs and CT are not helpful. Only MRI can show inflammatory spinal cord pathology. In bacterial disease, inflammatory changes in the spinal cord are mostly due to secondary changes in intraspinal abscesses. MRI shows high signal changes compatible with inflammation and edema on T2WI. Today, spirochetal infections are mostly due to Lyme disease caused by Borrelia burgdorferi. In early stages of Neuroborreliosis, spinal leptomeningitis and root inflammation on Gd-enhanced studies may be the first radiologic feature before the signal changes of myelitis become apparent, allowing early antibiotic treatment.28 Rarely tuberculosis can manifest with intramedullary tuberculoma that is visible on Gd-enhanced T1WI. Gadolinium-enhanced studies are helpful in demonstrating spinal arachnoiditis, which is a disabling complication of tuberculous meningitis.29 Additional steroid therapy is recommended for this complication.
Myelitis can be a frequent complication of viral infections. In a large number of cases, the virus remains unidentified. Cases due to herpesviridae such as Varicella zoster virus, cytomegalovirus, and Epstein-Barr virus (EBV) have frequently been described, often in immunocompromised patients.30 Because these cases often present with ascending paraparesis, differentiation from inflammatory polyradiculitis is essential to rapidly decide on the necessity of therapy with antiviral agents or high-dose steroids versus intravenous immunoglobulins (IVIG). MRI shows high signal changes in the cord with variable edema and Gd enhancement, additionally in lumbosacral roots in EBV infection.31 Coxsackie and ECHO virus can lead to transverse myelitis.32 Recently reports have drawn attention to spinal complications of West Nile virus infection.33 MRI changes include parenchymal spinal cord abnormalities and cauda equina enhancement. In early stages of HIV infection, a myelitis can occur that resembles autoimmune-mediated myelitis. In later stages, the typical MRI features allowing a rapid diagnosis are tract pallor and vacuolar myelopathy showing mixed signal intramedullary lesions, sometimes with marked cystic appearance. Sometimes Gd enhancement is found.34 Steroid treatment usually is not beneficial in these cases. Conversely, in cases of tropical spastic paraparesis due to HTLV-2 myelopathy the MRI is normal and only rarely shows atrophy.35
VIRAL MENINGOENCEPHALITIS
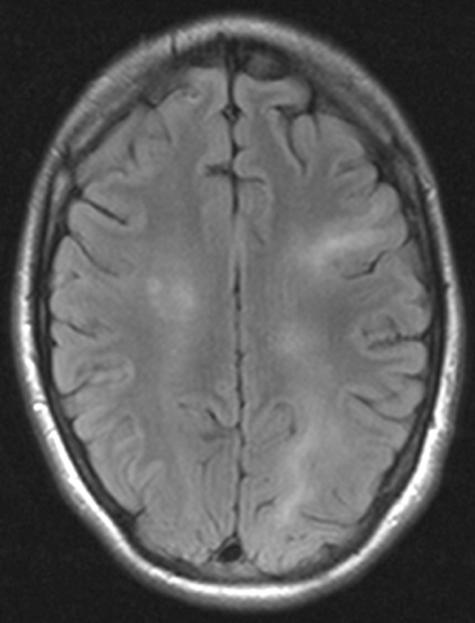
Herpes simplex virus (HSV) I remains the most common cause of viral encephalitis.36,37 However, the recently observed epidemic of the West Nile virus, newly recognized viruses such as the Nipah virus and the previously unnoticed association of viruses like the human herpes viruses 6 or 7 (HHV 6, HHV 7) or enterovirus 71 with CNS infections underline that agents other than HSV I have to be considered in acute encephalitis. Even in immunocompetent adults, HHV 6 can cause a chronic encephalitis ( FIG. 3). In immunocompromised patients the spectrum of possible causative agents is even broader.
FIG. 3.
Axial FLAIR images of a patient with chronic HHV 6 encephalitis showing patchy signal hyperintensities in white matter and cortex.
Detection of HSV DNA in the CSF by PCR remains the mainstay for the diagnosis of HSV encephalitis,38 although results of this laboratory test may be false negative or may arrive belatedly. Thus, results of imaging studies are important to decide whether antiviral treatment has to be started in patients with suspected HSV encephalitis. Cranial MRI is superior to CT in early detection of signs of this necrotizing encephalitis, which can be demonstrated within the first 48 h on T2-weighted (T2WI) or FLAIR images.39 In infants and neonates, DWI appears to be more sensitive than T2WI or FLAIR imaging in early detection of the cytotoxic cortical edema.40 Recently, this finding could be confirmed in adults.41 Interestingly, repeated MRIs performed in the same study demonstrated that diffusion abnormalities disappear within 14 days after symptom onset, whereas hyperintensities on T2WI persist. Further studies are warranted to see whether resolution of these changes on DWI is related to treatment with antiviral substances and whether the persistence of these changes reflects cortical damage and poorer outcome in patients with HSV encephalitis.
The Nipah virus, a new paramyxovirus closely related to the Hendra virus (an equine mobillivirus), has recently been shown to cause severe acute encephalitis.42 Radiological features usually consist of multiple small hyperintense lesions within the white matter on T2WI.43 T2WI may also demonstrate transient hyperintense punctate lesions in the brainstem and cortex. Interestingly, T2WI of asymptomatic seropositive individuals may show small hyperintense lesions similar to those found in encephalitis patients suggesting that a mild subclinical variant of Nipah virus encephalitis exists.44
Enterovirus 71 (EV71), an enterovirus of the family Picornaviridae, may lead to a polio-like brainstem encephalitis and acute flaccid paralysis. MRI of EV71 encephalitis typically shows hyperintense lesions on T2WI located within the brainstem and dentate nuclei of the cerebellum.45 In some patients, lesions may expand to the spinal cord, thalamus, and putamen. In some patients, DWI is able to demonstrate hyperintense changes in the posterior medulla without other brain abnormalities on T1WI or T2WI on the first day of neurological deterioration, underlining the superiority of DWI in early detection of infectious CNS disease compared with results of T2WI or contrast enhanced T1WI.46
Japanese encephalitis (JE) affects approximately 50,000 people per year, of whom approximately 10,000 will die. As with other infectious CNS diseases, cranial MRI appears more sensitive than CT in detection of JE-related brain abnormalities.39 Typical MRI features consist of either mixed intensity or hypointense lesions on T1WI and hyperintense or mixed intensity lesions on T2WI predominantly in the thalami, but also in the basal ganglia, brainstem, cerebellum, and cortical areas.47 A recently published study found that cranial CT was abnormal in around 38%, whereas MRI revealed pathological changes in 90.6–95.5%.48 Thalamic abnormalities on T2WI were found in 87.5% both in children and adults, in 40.6–54.2% in the basal ganglia, in 28.1–45.8% in the midbrain and in 21.9–25% in cortical areas.
The West Nile virus (WNV) has caused encephalitis outbreaks in Southern Europe, Russia, and the United States, with the last large encephalitis outbreak in 2002. Clinical, laboratory, and neuroimaging features were described in a recent study evaluating WNV seropositive patients.49 Five patients presented with meningitis, eight with encephalitis and three with polio-like acute flaccid paralysis. T2WI and DWI revealed focal hyperintense lesions within the basal ganglia, thalamus and pons in only two of the eight encephalitic patients, whereas CT remained normal in all patients. In patients with acute flaccid paralysis, MRI demonstrated enhancement of the cauda equina and nerve root clumping. In some patients, the virus affects substantia nigra as suggested by hyperintensities on T2WI in this region.50 Similar to HSV and EV71 encephalitis DWI seems to be more sensitive to detect signal abnormalities especially in the initial phase of WNV infection of the brain.
Murray Valley encephalitis (MVE) belongs to the Japanese encephalitis antigenic complex and is endemic to Australia and Papua New Guinea. MRI demonstrates abnormalities very similar to those in Japanese encephalitis. As it has recently been reported, T2WI shows hyperintense changes within the thalami, red nucleus, substantia nigra, and cervical spinal cord.51 Thus, the similarities in MRI appearance of Japanese encephalitis, West Nile encephalitis, and Murray Valley encephalitis do not allow discrimination of these CNS infections only from imaging features.
Acute measles virus encephalitis and subacute sclerosing panencephalitis (SSPE) Infection of the CNS with the measles virus (MV) may result in 1) acute postinfectious encephalitis, 2) acute progressive encephalitis, and 3) SSPE. Data about imaging findings in acute measles encephalitis are sparse. T2WI may reveal cortical edema and bilateral symmetric hyperintense lesions within the putamen and caudate nuclei as well as within the centrum semiovale.52 Sometimes patients also present bilateral thalamic lesions and signal abnormalities within the corpus callosum. The value of DWI in detection of early acute measles encephalitis has not been evaluated. Contrast enhancement may appear in cortical areas and leptomeninges in some patients. SSPE is a rare progressive CNS disease that usually occurs in childhood and early adolescence but may also be present in older adults.53,54 Differences in appearance of early and late stage SSPE on MRI are not very well defined. A recent study compared MR spectroscopy and conventional MRI in children with early stage and children with late stage SSPE.55 Conventional MRI revealed no abnormalities in early stage SSPE, but disclosed widespread periventricular hyperintense changes on T2WI in late stage SSPE. In contrast, MR spectroscopy demonstrated an increase in choline/creatinine ratios in the frontal and parieto-occipital white matter in all patients suggestive of inflammation also in early stages of SSPE. N-acetylasparate/creatine ratios were normal in the early stage probably reflecting an absence of neuronal loss, which could be detected in the late stage of SSPE.
FUNGAL INFECTIONS
Fungal infections of the CNS are very rare in the general population. Except for people with longstanding diabetes, they are most frequently encountered in immunocompromised patients such as those with AIDS or after organ transplantation. Due to the lack of inflammatory response, neuroradiological findings are often nonspecific. Although almost any fungus may cause encephalitis, cryptococcal meningoencephalitis is most frequently seen, followed by aspergillosis and more rarely candidasis. Cerebral candidasis is usually preceded by a systemic candida infection and is frequently catheter related. In immunocompetent patients, it can manifest as solid or abscess-like lesions giving rise to the differential diagnosis of a pyogenic abscess. In immunosuppressed patients, the neuroradiological findings are often difficult to interpret. MRI shows punctuate or patchy signal hyperintensities on T2WI, gadolinium enhancement is frequently absent.56 These findings alone do not allow a specific diagnosis, so that treatment decisions must be based on clinical parameters and CSF findings.
In cryptococcal meningoencephalitis, diffuse meningeal enhancement and also ventriculitis can be seen on MRI. Typical findings are multiple punctuate lesions, often in the basal ganglia. These are characteristic cystic lesions due to cryptococcal invasion of the Virchow-Robin-spaces. They are termed “soap bubble lesions” and allow the quick provisional diagnosis leading to rapid antifungal treatment. In nonimmunodeficient patients or patients with AIDS under highly active antiretroviral treatment, who develop an immune reconstitution syndrome the lesions can become ring enhancing. Even with intensive treatment (amphotericin B and 5-flucytosine), outcome is often poor and mortality as high as 70%.57
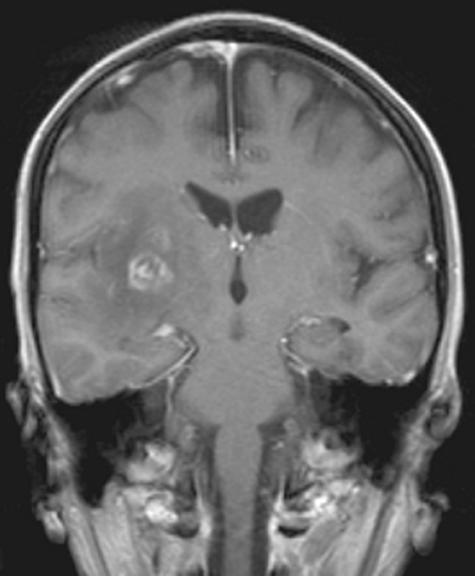
Rarely in patients with AIDS and more frequently in patients who have had bone marrow transplantation (BMT), aspergillus is an agent for opportunistic infection of the CNS.58 Mortality is high in these patients, and early diagnosis is mandatory if survival is to be achieved.59 Laboratory findings do not always confirm the diagnosis of fungal infection so that neuroimaging is crucial in establishing the diagnosis. CT findings may be nonspecific and the diagnosis of fungal infection is often made retrospectively at autopsy. The appearance of aspergillus infection of the CNS is extremely varied. Using MRI, several patterns of cerebral aspergillosis have been reported: edematous lesions, hemorrhagic lesions, solid enhancing lesions referred to as aspergilloma or “tumoral form,” abscess-like ring-like enhancing lesions ( FIG. 4), and infarction-like lesions. Dural enhancement is usually seen in lesions adjacent to infected paranasal sinuses. On MRI, lesions may show areas of isointense or low signal intensity on T2WI, which is attributed to fungal hyphen containing paramagnetic elements like manganese, iron, and magnesium, but may be also related to blood breakdown products.60 Cortical and subcortical infarction with or without hemorrhage is a common finding in aspergillus infection explained by fungal infiltration of the vessel wall and thrombosis.61 Recognition of these radiological patterns in patients with cerebral aspergillosis is helpful in establishing an early diagnosis. Patients with AIDS and after BMT, who are severely immunoincompetent, often do not show any enhancement or perifocal edema.62
FIG. 4.
Coronal T1WI after gadolinium enhancement. Patient after bone marrow transplantation with aspergillus encephalitis. Ring-enhancing lesion with perifocal edema and mass effect compressing the lateral ventricle.
REFERENCES
- 1.Tuncer O, Caksen H, Arslan S, Atas B, Uner A, Oner AF, et al. Cranial computed tomography in purulent meningitis of childhood. Int J Neurosci 114: 167–174, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Good CD, Jager HR. Contrast enhancement of the cerebrospinal fluid on MRI in two cases of spirochaetal meningitis. Neuroradiology 42: 448–450, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Kamra P, Azad R, Prasad KN, Jha S, Pradhan S, Gupta RK. Infectious meningitis: prospective evaluation with magnetisation transfer MRI. Br J Radiol 77: 387–394, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Thwaites GE, Nguyen DB, Nguyen HD, Hoang TW, Do TT. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 351: 1741–1751, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Soulie D, Meyer P, Raynaud M, Berge J, Dousset V. MRI and Listeria monocytogenes rhombencephalitis. J Radiol 77: 489–496, 1996. [PubMed] [Google Scholar]
- 6.Al-Sous MW, Bohlega S, Al-Kawi MZ, Alwatban J, McLean DR. Neurobrucellosis: clinical and neuroimaging correlation. Am J Neuroradiol 25: 395–401, 2004. [PMC free article] [PubMed] [Google Scholar]
- 7.De Gans J, Van den Beek D. European dexamethasone in adulthood—Bacterial Meningitis Study Investigators. N Engl J Med 347: 1549–1556, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Fukui MB, Williams RL, Mudigonda S. CT and MR imaging features of pyogenic ventriculitis. Am J Neuroradiol 22: 1510–1516, 2001. [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuchiya K, Osawa A, Katase S, Fujikawa A, Hachiya J, Aoki S. Diffusion-weighted MRI of subdural and epidural empyemas. Neuroradiology 45: 220–223, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Guzman R, Barth A, Lovblad KO, El-Koussy, M. Weis J, Schroth G et al. Use of diffusion-weighted magnetic resonance imaging in differentiating purulent brain processes from cystic brain tumors. J Neurosurg 97: 1101–1107, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Leuthardt EC, Wippold FJ 2nd, Oswood MC, Rich KM. Diffusion-weighted MR imaging in the preoperative assessment of brain abscesses. Surg Neurol 58: 395–402, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Dorenbeck U, Butz B, Schlaier J, Bretschneider T, Schuierer G, Feuerbach S. Diffusion-weighted echo-planar MRI of the brain with calculated ADC—a useful tool in the differential diagnosis of tumor necrosis from abscess. Neuroimaging 13: 330–338, 2003. [PubMed] [Google Scholar]
- 13.Tung GA, Evangelista P, Rogg JM, Duncan JA 3rd. Diffusion-weighted MR imaging of rim-enhancing brain masses: is markedly decreased water diffusion specific for brain abscess? Am J Roentgenol. 177: 709–712, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Mishra AM, Gupta RK, Jaggi RS, Reddy JS, Jha DK, Husain N, et al. Role of diffusion-weighted imaging and in vivo proton magnetic resonance spectroscopy in the differential diagnosis of ring-enhancing intracranial cystic mass lesions. J Comput Assist Tomogr 28: 540–547, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Garg M, Gupta RK, Husain M, Chawla S, Chawla J, Kumar R, et al. Brain abscesses: etiologic categorization with in vivo proton MR spectroscopy. Radiology 230: 519–527, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Maschke M, Dietrich U, Prumbaum M, Kastrup O, Turowski B, Schaefer UW, et al. Opportunistic CNS infection after bone marrow transplantation. Bone Marrow Transplant 23: 1167–1176, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich U, Maschke M, Dorfler A, Prumbaum M, Forsting M. MRI of intracranial toxoplasmosis after bone marrow transplantation. Neuroradiology 42: 14–18, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Ionita C, Wasay M, Balos L, Bakshi R. MR imaging in toxoplasmosis encephalitis after bone marrow transplantation: paucity of enhancement despite fulminant disease. Am J Neuroradiol 25: 270–273, 2004. [PMC free article] [PubMed] [Google Scholar]
- 19.Chong-Han CH, Cortez SC, Tung GA. Diffusion-weighted MRI of cerebral toxoplasma abscess. Am J Roentgenol 181: 1711–1714, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Skiest DJ, Erdman W, Chang WE, Oz OK, Ware A, Fleckenstein J SPECT thallium-210 combined with toxoplasma serology fort the presumptive diagnosis of focal central nervous system mass lesions in patients with AIDS. J Infect 40: 274–281, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Tali ET. Spinal infections. Eur J Radiol 50: 120–133, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Tur BS, Suldur N, Ataman S, Ozturk EA, Bingol A, Atay MB. Brucellar spondylitis: a rare cause of spinal cord compression. Spinal Cord 42: 321–324, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Jung NY, Jee WH, Ha KY, Park CK, Byun JY. Discrimination of tuberculous spondylitis from pyogenic spondylitis by MRI. Am J Roentgenol 182: 1405–1410, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Gupta SK, Chhabra R, Sharma BS, Das A, Khosla VK. Vertebral cryptococcosis simulating tuberculosis. Br J Neurosurg 17: 556–559, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Vaishya S, Sharma MS. Spinal aspergillus vertebral osteomyelitis with extradural abscess: case report and review of the literature. Surg Neurol 61: 551–555, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Bluman EM, Palumbo MA, Lucas PR. Spinal epidural abscess in adults. Am Acad Orthop Surg 12: 155–163, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Van de Warrenber BP, Wesseling P, Leyten QH, Boermann RH. Myelopathy due to spinal epidural abscess without cord compression. Clin Neuropathol 23: 102–106, 2004. [PubMed] [Google Scholar]
- 28.Tullmann, MJ, Delman BN, Lublin FD, Weinberger J Magnetic resonance imaging of meningoradiculomyelitis in early disseminated Lyme disease. Neuroimaging 13: 264–268, 2003. [PubMed] [Google Scholar]
- 29.Srivastava T, Kochar DK. Asymptomatic spinal arachnoiditis in patients with tuberculous meningitis. Neuroradiology 45: 727–729, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Karacostas D, Christodoulou C, Drevelengas A, Paschalidou M, Ioannides P, Constantinou A, et al. Cytomegalovirus-associated transverse myelitis in a non-immunocompromised patient. Spinal Cord 40: 145–149, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Majid A, Galetta SL, Sweeney CJ, Robinson C, Mahalingam R, Smith J, et al. Epstein-Barr virus myeloradiculitis and encephalomyeloradiculitis. Brain 125: 159–165, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Minami K, Tsuda Y, Maeda H, Yanagawa T, Izumi G, Yoshikawa N. Acute transverse myelitis caused by Coxsackie virus B5 infection. Paediatr Child Health 40: 66–68, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Jeha LE, Sila CA, Lederman RJ, Prayson RA, Isada CM, Gordon SM. West Nile virus infection: a new acute paralytic illness. Neurology 8: 55–59, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Thurnher MM, Post MJ, Jinkins JR. MRI of infections and neoplasms of the spine and spinal cord in55 patients with AIDS. Neuroradiology 42: 551–563, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Oomman A, Madhusoodanan M. Tropical spastic paraparesis in Kerala. Neurol India 51: 493–496, 2003. [PubMed] [Google Scholar]
- 36.Schmutzhard E. Viral infections of the CNS with special emphasis on herpes simplex infections. J Neurol 248: 469–477, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinson VK, Tyor WR. Update on viral encephalitis. Curr Opin Neurol 14: 369–374, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Cinque P, Cleator GM, Weber T, Monteyne P, Sindic CJ, van Loon AM. The role of laboratory investigation in the diagnosis and management of patients with suspected herpes simplex encephalitis: a consensus report. J Neurol Neurosurg Psychiatry 61: 339–345, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maschke M, Kastrup O, Forsting M, Diener HC. Update on neuroimaging in infectious central nervous system disease. Curr Opin Neurol 17: 475–480, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Teixeira J, Zimmerman RA, Haselgrove JC, Bilaniuk LT, Hunter JV. Diffusion imaging in pediatric central nervous system infections. Neuroradiology 43: 1031–1039, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Kuker W, Nagele T, Schmidt F, Heckl S, Herrlinger U. Diffusion-weighted MRI in herpes simplex encephalitis: a report of three cases. Neuroradiology 46: 122–125, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Chua KB, Goh KJ, Wong KT, Kamarulzaman A, Tan PS, Ksiazek TG, Zaki SR, Paul G, Lam SK, Tan CT. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet 354: 1257–1259, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Lim CC, Lee KE, Lee WL, Tambyah PA, Lee CC, Sitoh YY, Auchus AP, Lin BK, Hui F. Nipah virus encephalitis: serial MR study of an emerging disease. Radiology 222: 219–226, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Lim CC, Lee WL, Leo YS, Lee KE, Chan KP, Ling AE, Oh H, Auchus AP, Paton NI, Hui F, Tambyah PA. Late clinical and magnetic resonance imaging follow up of Nipah virus infection. J Neurol Neurosurg Psychiatry 74: 131–133, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen WC, Chiu HH, Chow KC, Tsai CH. MR imaging findings of enteroviral encephaloymelitis: an outbreak in Taiwan. Am J Neuroradiol 20: 1889–1895, 1999. [PMC free article] [PubMed] [Google Scholar]
- 46.Nolan MA, Craig ME, Lahra MM, Rawlinson WD, Prager PC, Williams GD, Bye AM, Andrews PI. Survival after pulmonary edema due to enterovirus 71 encephalitis. Neurology 60: 1651–1656, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Kalita J, Misra UK. Comparison of CT scan and MRI findings in the diagnosis of Japanese encephalitis. J Neurol Sci 174: 3–8, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Kalita J, Misra UK, Pandey S, Dhole TN. A comparison of clinical and radiological findings in adults and children with Japanese encephalitis. Arch Neurol 60: 1760–1764, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin AA, Van Gerpen JA, Fleischauer A, Leis AA, Stokic DS, Petersen LR. Neurologic manifestations and outcome of West Nile virus infection. JAMA 290: 511–515, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Bosanko CM, Gilroy J, Wang AM, Sanders W, Dulai M, Wilson J, Blum K. West Nile virus encephalitis involving the substantia nigra: neuroimaging and pathologic findings with literature review. Arch Neurol 60: 1448–1452, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Einsiedel L, Kat E, Ravindran J, Slavotinek J, Gordon DL. MR findings in Murray Valley encephalitis. AJNR Am J Neuroradiol 24: 1379–1382, 2003. [PMC free article] [PubMed] [Google Scholar]
- 52.Lee KY, Cho WH, Kim SH, Kim HD, Kim IO. Acute encephalitis associated with measles: MRI features. Neuroradiology 45: 100–106, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Frings M, Blaeser I, Kastrup O. Adult-onset subacute sclerosing panencephalitis presenting as a degenerative dementia syndrome. J Neurol 249: 942–943, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Gagnon A, Bouchard RW. Fulminating adult-onset subacute sclerosing panencephalitis in a 49-year-old man. Arch Neurol 60: 1160–1161, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Alkan A, Sarac K, Kutlu R, Yakinci C, Sigirci A, Aslan M, Baysal T. Early- and late-state subacute sclerosing panencephalitis: chemical shift imaging and single-voxel MR spectroscopy. Am J Neuroradiol 24: 501–506, 2003. [PMC free article] [PubMed] [Google Scholar]
- 56.Burgert SJ, Classen DC, Burke JP, Blatter DD. Candidal brain abscess associated with vascular invasion: a devastating complication of vascular catheter-related candidemia. Clin Infect Dis 21: 202–205, 1995. [DOI] [PubMed] [Google Scholar]
- 57.Narai H, Manabe Y, Deguchi K, Iwatsuki K, Sakai K, Abe K. Serial MRI findings in patients with chronic Cryptococcus meningoencephalitis. Neurol Res 23: 810–812, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Coley SC, Jäger HR, Szydlo RM, Goldman JM. CT and MRI manifestations of central nervous system infection following allogeneic bone marrow transplantation. Clin Radiol 54: 390–397, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Shuper A, Levitsky HI, Cornblath DR. Early invasive CNS aspergillosis. An easily missed diagnosis. Neuroradiology 33: 183–185, 1991. [DOI] [PubMed] [Google Scholar]
- 60.Ashdown BC, Tien RD, Felsberg GJ. Aspergillosis of the brain and paranasal sinuses in immunocompromised patients: CT and MR imaging findings. Am J Radiol 162: 155–159, 1994. [DOI] [PubMed] [Google Scholar]
- 61.Dietrich U, Hettmann M, Maschke M, Dörfler A, Schwechheimer K, Forsting M. Cerebral aspergillosis: comparison of radiological and neuropathological findings in patients with bone marrow transplantation. Eur Radiol 11: 1242–1249, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Yuh WTC, Nguyen HD, Gao F, Tali ET, Fisher DJ, Mayr NA, et al. Brain parenchymal infection in bone marrow transplantation patients. CT and MR findings. Am J Radiol 162: 425–430, 1994. [DOI] [PubMed] [Google Scholar]