Abstract
Summary: In this review, we discuss the role of neuroimaging in assessing treatment options for movement disorders, particularly Parkinson's disease (PD). Imaging methods to assess dopaminergic function have recently been applied in trials of potential neuroprotective agents. Other imaging methods using regional metabolism and/or cerebral perfusion have been recently introduced to quantify the modulation of network activity as an objective marker of the treatment response. Both imaging strategies have provided novel insights into the mechanisms underlying a variety of pharmacological and stereotaxic surgical treatment strategies for PD and other movement disorders.
Keywords: Movement disorders, Parkinson's disease, Huntington's disease, Tourette's syndrome, dystonia, positron emission tomography (PET), magnetic resonance imaging (MRI)
INTRODUCTION
Movement disorders are a group of syndromes characterized by an impairment of the regulation of voluntary motor activity without deficits of force, cerebellar function, or sensation. This class of neurological diseases includes hypokinetic disorders associated with a slowing of movements such as Parkinson's disease (PD), as well as hyperkinetic disorders characterized by involuntary abnormal movements such as Huntington's disease (HD), torsion dystonia, and tic disorders. Generally, the clinical manifestations of movement disorders result from dysfunction of the basal ganglia. Although histopathologic studies reveal specific neurodegenerative changes in some of these diseases (e.g., PD and HD), the pathologic basis for many movement disorders remains unknown. Various neuroimaging techniques have been used to visualize pathological changes in these disorders. Radiotracer imaging techniques using positron emission tomography (PET) and single-photon emission computed tomography (SPECT) imaging can be used to evaluate and quantify changes in specific neurochemical systems.1, 2 Alternatively, disease-related changes in local brain function can be assessed with generalized tracers for regional cerebral metabolism and blood flow.3, 4
Brain imaging in movement disorders was originally introduced to visualize the pathological changes associated with different clinical syndromes.5 Subsequently, these techniques have been used in longitudinal studies designed to assess disease progression6, 7 and the effects of potential neuroprotective strategies.8–10 Lately, functional imaging has also been applied in the objective assessment of symptomatic treatment responses.11–14 In addition to providing an objective descriptor of the treatment response, brain imaging can also link clinical outcome to alterations in regional brain function.15
In this review, we describe functional neuroimaging strategies to evaluate and monitor therapeutic interventions for movement disorders. Because PD is the most common and broadly studied of these disorders, this review will focus on the use of imaging to assess treatment options for this condition.
DOPAMINERGIC IMAGING IN PARKINSON'S DISEASE: NEUROPROTECTION TRIALS
PET and SPECT assessments of nigrostriatal dopaminergic function have been a traditional focus of imaging studies in parkinsonism and other movement disorders. Presynaptic dopaminergic imaging can be conducted using the following approaches: 1. [18F]-fluorodopa (FDOPA) PET to evaluate the uptake and conversion from fluorodopa to fluorodopamine by the aromatic acid decarboxylase (AADC); 2. [11C]-dihydrotetrabenazine (DTBZ) PET to assess the density of monoamine-containing synaptic vesicles; and 3. a variety of radiolabeled cocaine derivatives [e.g., [123I]-2β-carbomethyl-3β-(4-iodophenyl) tropane (β-CIT)] to quantify the expression of the dopamine transporter (DAT), which facilitates the release and reabsorption of dopamine in the nigrostriatal intersynaptic cleft. The relative merits of these agents have been summarized elsewhere.1, 2
Radiotracer imaging of the presynaptic nigrostriatal dopaminergic system has been used to assess the rate of disease progression deterioration in PD. FDOPA PET and β-CIT SPECT show a 4% to 13% yearly reduction in baseline putamen uptake compared with 0–2.5% in healthy controls in longitudinal studies.6, 7 This technique has also been used to estimate the duration of the preclinical period of PD using a linear regression analysis.16 Striatal FDOPA measurements correlate with dopamine cell counts measured in postmortem specimens17, 18 and striatal DAT binding decreases with age in healthy volunteers and PD patients.19–21
The precise determination of neuroprotective treatment effects represents a major challenge in current movement disorders therapeutics. Although well established in clinical trials for PD, clinical rating scales like the Unified Parkinson's Disease Rating Scale (UPDRS) 22 may not be sufficiently sensitive to detect subtle changes in rates of progression, as are likely to be encountered in neuroprotective trials. Moreover, clinical ratings reflect long-term symptomatic effects, which are apt to persist following protracted medication washout.9
Imaging assessment of presynaptic nigrostriatal function has been used for evaluation of disease progression in PD patients (see above) and may therefore provide a useful adjunct to clinical assessment in assessing disease progression in pharmacologic therapeutic trials of potential neuroprotective agents.2 Two large randomized, blinded studies have employed imaging to investigate disease progression in patients receiving dopamine agonists relative to those treated with levodopa. In both studies, imaging based descriptors of neuroprotection diverged from the clinical outcome measures. The CALM-PD study investigated early treatment with levodopa versus pramipexole.10 It was found that PD patients receiving levodopa did better clinically, although they experienced more motor complications. The REAL-PET study disclosed similar results in a comparison of levodopa with ropinirole. By contrast, presynaptic dopaminergic imaging with β-CIT SPECT (CALM-PD) and FDOPA PET (REAL-PET) revealed slower declines with agonist treatment. Similarly, in the subsequent ELLDOPA trial,9 subjects treated with high-dose levodopa had the best clinical outcome even following up to 4 weeks of medication washout, despite a more rapid decline in putamen DAT binding as measured by β-CIT SPECT.9
These trials reveal discrepancies between the clinical assessment and radiotracer-based imaging of the presynaptic dopaminergic system, and raise the question of comparability of these measures as neuroprotection outcome variables. Even though most imaging descriptors of nigrostriatal dopaminergic function correlate with independent disease severity measures (see Ravina et al.2 for review), these techniques do not directly assess the number or density of nigral dopaminergic neurons. Moreover, these radiotracer-based imaging methods require simplifying assumptions for the acquisition and analysis of data. Thus, the results of these imaging studies may be affected by factors other than the primary biological process under study.
A critical issue in this regard is the occurrence of possible temporal up- and downregulation of neuropeptides and receptors that can affect the results of imaging studies. A study comparing FDOPA, DTBZ, and a DAT ligand, in the same PD subjects revealed a relative upregulation of AADC and downregulation of DAT.23 This suggests that surviving neurons may synthesize more dopamine but perhaps also take up less from the synaptic cleft. Dopaminergic treatment can change the amount of available dopamine at the synaptic level, which may differentially influence the regulation of AADC and DAT. Although a number of studies have not revealed an effect of dopaminergic treatment on presynaptic dopaminergic imaging measures,8, 24, 25 other evidence suggests that dopaminergic treatment can affect these parameters in PD patients.26, 27 Specifically, Guttman et al.27 reported a decline in DAT binding after treatment with levodopa, which was not significant after pramipexole treatment. Similar treatment effects in the CALM-PD study could have contributed to the differences in imaging measures that were observed with the pramipexole and levodopa treatment groups. Further investigations with larger numbers of patients will be required to characterize time-dependent changes in the regulation of neuropeptides or receptors that might occur with treatment. Indeed, the optimal duration of medication washout before imaging will need to be determined before further studies of this sort can be pursued effectively.
Lastly, the evaluation of disease progression in PD with radiotracer imaging has relied upon the notion that pathology is limited to the nigrostriatal neurons. This assumption may be overly restrictive given that other neural pathways are likely to be involved with disease progression, even at early clinical phases of the illness.
Dopaminergic imaging with PET has also been used to assess the efficacy of embryonic cell transplantation for PD. The presence of increases in putamen FDOPA uptake after transplantation is consistent with graft survival.28, 29 These localized changes may underlie the development of post-transplantation dyskinesia in some patients.30 Additionally, graft function has been directly demonstrated using PET imaging and 11C-raclopride (RAC) displacement methods.31 Nonetheless, the meaning of these post-transplantation changes on PET is unclear given that motor performance does not necessarily improve in these patients (e.g.,32). Whereas the ultimate clinical role of embryonic dopamine cell transplantation for PD is unclear, imaging methodologies are likely to provide useful biomarkers in future trials of cell-based therapies.33
METABOLIC BRAIN NETWORKS IN PARKINSON'S DISEASE
PD patients exhibit characteristic changes in regional glucose utilization on PET studies conducted with [18F]-fluorodeoxyglucose (FDG) in the resting state.3, 34 Formal multiregion, multisubject network approaches using principal components analysis (PCA)3, 35 have been employed to identify patterns of regional metabolism that are associated with PD3, 36 and other movement disorders (e.g., Huntington's Disease,37 torsion dystonia,38, 39 Tourette syndrome).40 PCA extracts multiple, statistically independent components that account, singly or in combination, for the majority of the variability in the regional PET data. The technique also quantifies the expression of these patterns in individual subjects.41, 42
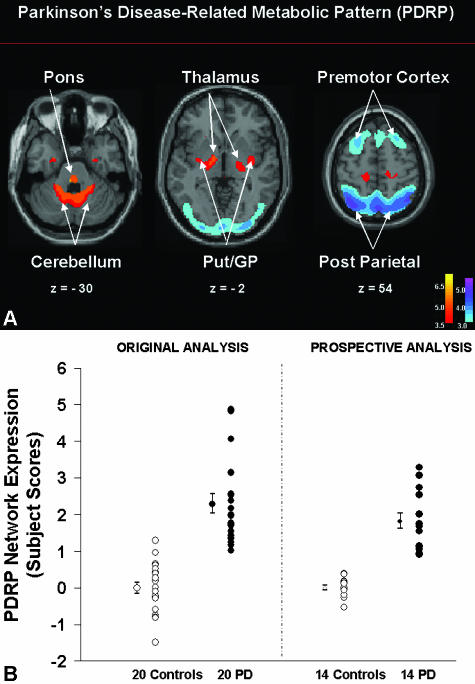
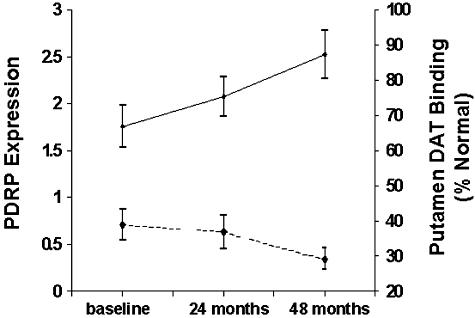
Specific criteria have been developed to determine whether one or more patterns are “disease-related,” i.e., have significantly different expression in patients relative to controls.36, 38 For instance, employing this mathematical-statistical approach, we identified a specific regional metabolic network in PD patients scanned in the resting state with FDG PET.3 This PD-related covariance pattern (PDRP) was characterized by increased glucose metabolism of the lentiform nucleus, thalamus, and brainstem as well as decreased glucose metabolism of the lateral premotor cortex and the supplementary motor area (SMA). This characteristic pattern (FIG. 1A) 1) has been validated in multiple populations of unmedicated PD patients4, 36, 43; 2) can be detected early in the disease course34; and 3) correlates consistently with disease severity and duration.3, 44 We also found that PDRP activity correlates with nigrostriatal dopamine deficiency as determined by [18F]-fluorodopa PET34, 45 and with internal pallidal (GPi) single unit activity recorded during surgery.46 Blinded prospective PDRP calculations show that this pattern not only discriminates PD patients from normal subjects (FIG. 1B), 36 but can also distinguish between patients with PD and atypical parkinsonian syndromes.3, 4, 34 Additionally, preliminary results from a longitudinal PET study of early stage PD conducted at our center suggest that PDRP expression may also be sensitive to disease progression (FIG. 2). These results suggest that network imaging with FDG PET may have certain advantages in clinical trial settings: 1) FDG PET is becoming commonplace in North American, European, and Japanese medical centers. Although the technology has focused upon oncology uses, brain imaging of neurodegenerative conditions can be performed conveniently employing the same imaging platforms. Indeed, pattern analysis can be performed remotely on images transferred electronically from remote sites.13, 14 2) Prospective quantification of network activity can be performed on a case by case basis,41 even using scans of cerebral perfusion obtained using less expensive imaging devices such as SPECT.4 This methodology may ultimately be applicable to perfusion-based magnetic resonance imaging (MRI)47, thereby totally eliminating the need for tracer injections and radiation exposure. 3) Network approaches may be useful in quantifying the effects of therapy by scanning subjects before and after treatment (see below). Given the capacity of metabolic imaging to assess multiple networks in single subjects, this technique may be able to parse out motor and nonmotor treatment effects on brain function.43, 48
FIG. 1.
A: Parkinson's disease-related metabolic pattern. This pattern of regional metabolic covariation was identified by network analysis of [18F]-fluorodeoxyglucose (FDG) PET scans from 20 PD patients and 20 age-matched normal volunteer subjects.14, 123 This PDRP (representing the first principal component, which accounted for 20.7% of the subject × voxel variation) was characterized by pallidal, thalamic, pontine, and cerebellar hypermetabolism associated with metabolic decrements in the lateral premotor and posterior parietal areas. The display represents voxels that contribute significantly to the network at p = 0.001, and that were demonstrated to be reliable by bootstrap estimation (p < 0.0001). [Voxels with positive region weights (metabolic increases) are color coded from red to yellow; those with negative region weights (metabolic decreases) are color coded from blue to purple. The numbers under each slice are in millimeters, relative to the anterior-posterior commissure line.] B: Prospectively computed PDRP scores accurately discriminate subjects by blinded network analysis. Left: PDRP expression (subject scores) in the 20 PD patients (filled circles) and 20 control subjects (open circles) described above. Network activity was significantly increased in the PD cohort (p < 0.00001). Right: In a prospective individual case analysis (see text), we computed the expression of the PDRP (see panel A) in 14 subsequent PD patients (filled circles) and 14 control subjects (open circles). These computations were conducted using an automated routine that was blind to diagnostic category.38, 41 As in the original analysis, prospectively computed PDRP scores were significantly elevated in the disease group (p < 0.00001).
FIG. 2.
Longitudinal changes in early stage PD: dopaminergic loss and network evolution. Top: Mean expression of the PD-related metabolic covariance pattern (PDRP, see FIG. 1A) at baseline, and at the second (24 months) and third (36 months) time points as part of our longitudinal FDG PET study of early stage Parkinson's disease (solid line). PDRP values increased significantly over time (p < 0.005; repeated measures ANOVA). Bottom: Mean putamen DAT binding measured by [18F]-fluoropropyl βCIT PET in the same PD patients scanned longitudinally at the three time points (dashed line). DAT binding was expressed as percentage of the normal mean value for 15 age-matched control subjects. Unlike the longitudinal increases that occurred with PDRP expression, DAT binding declined over time (p < 0.04, reading a minimum of 30% normal for the putamen.
Network modulation by therapy
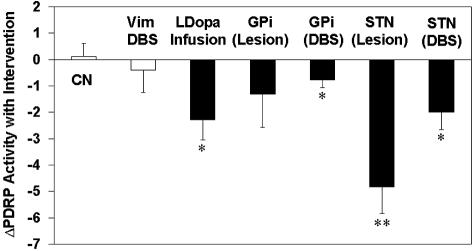
Network quantification during therapeutic interventions for PD may provide an objective means of assessing treatment effects on brain function. This imaging approach may be particularly useful in screening new agents for symptomatic therapy in that network modulation during treatment can be detected with sample sizes as small as seven subjects. Initial assessments of treatment responses employing this network approach were conducted in patients undergoing stereotaxic surgical interventions. Significant declines in PDRP activity were first observed following unilateral pallidotomy.11 Subsequent studies investigating the effects of deep brain stimulation (DBS) of the internal segment of the globus pallidus (GPi),12 ablation of the subthalamic nucleus (STN),13, 14 and STN DBS,15 revealed significant suppression of PDRP network activity as a common feature of these interventions (FIG. 3). Indeed, unilateral surgery resulted in significant reductions in PDRP expression in the ipsilateral hemisphere, and network modulation was not present contralateral to intervention. In most cases, the degree of network reduction correlated significantly with improvement in standardized motor ratings,12, 13 suggesting the potential role of this approach in the objective assessment of treatment effects in a blinded trials setting. Even though all these interventions suppressed the PDRP, there were differences in magnitude of PDRP reduction, depending upon the surgical target (GPi, STN, or Vim thalamic) or mode of treatment (ablation or DBS). In agreement with the general clinical impression that STN is superior to GPi as a treatment target,49, 50 the magnitude of PDRP suppression was higher for interventions involving the former structure. The finding that PDRP expression is not altered by Vim DBS for severe PD tremor is also consistent with prior studies suggesting different mechanisms underlying the akinetic-rigid and tremulous manifestation of the disease51, 52 in PD patients undergoing Vim DBS for intractable PD tremor.53 Significant reductions in PDRP expression have also been observed following pharmacological intervention. Comparison of FDG PET scans before and during levodopa infusion revealed a significant reduction of PDRP expression during treatment.54 As in the surgical interventions, reductions in PDRP scores correlated with clinical improvement measured in standardized motor rating scales. Interestingly, the degree of PDRP suppression observed during levodopa infusion was similar in magnitude to that encountered with STN DBS15 (FIG. 3). This observation is consistent with human and animal studies suggesting mechanistic similarities between the two forms of treatment.55 In aggregate, these results indicate that the expression of disease-related brain networks may constitute a useful biomarker for objectively assessing outcomes in trials of new treatment approaches for PD and other movement disorders.
FIG. 3.
Network modulation with antiparkinsonian interventions. Bar graph illustrating relative changes in the expression of the PD-related metabolic covariance pattern (Δ PDRP; see text), during antiparkinsonian therapy with levodopa infusion,54 and unilateral ventral pallidotomy,11 pallidal and STN DBS,12, 15 and subthalamotomy13 (filled bars). For the surgical interventions, Δ PDRP reflects changes in network activity in the operated hemispheres. With levodopa infusion, the PDRP changes were averaged across hemispheres. [The control data (open bars) represent: 1) Δ PDRP values in the unoperated contralateral hemispheres (CN) of the surgical patients scanned in the unmedicated state; and 2) PDRP changes with unilateral Vim thalamic stimulation for tremor-predominant PD53]. [Asterisks represent p values with respect to the untreated condition (paired Student's t test). *: p < 0.01; **: p < 0.005].
FDG PET has been used in other investigations to assess regional changes in glucose utilization occurring with levodopa treatment56 as well as with STN DBS.57 Rather than network analysis, these studies used statistical parametric mapping (SPM) to localize mean treatment effects subsequent to the two interventions. Although useful in identifying brain regions affected by therapy, this approach does not use regional covariation to quantify the network-wide changes that might occur during treatment. Comprehensive imaging investigations using PET in conjunction with different analytical tools will be of value in assessing the comparative efficacy of new and established treatments for PD.
Network analysis of imaging data can also be used to identify specific metabolic topographies associated with different manifestations of disease such as tremor and akinesia.51 Additionally, we used PCA to characterize patterns associated with visuospatial and memory function in PD,43, 48 as well as affective symptoms. We found48 that mnemonic functioning correlated with a specific metabolic pattern involving parietal decrements, covarying with increases in temporal cortex, pons, and cerebellum. By contrast, dysphoria in PD patients was associated with metabolic decrements in dorsolateral prefrontal, orbitofrontal, and anterior cingulate cortex. A subsequent FDG PET study43 used network analysis to demonstrate that brain metabolism in PD could be segregated into two discrete (orthogonal) networks, relating respectively to motor and nonmotor disease manifestations. The first pattern correlated with clinical ratings for bradykinesia and was topographically similar to the previously characterized PDRP. The second pattern was characterized by relative ventromedial frontal, hippocampal, and striatal hypometabolism, as well as mediodorsal thalamic hypermetabolism. Scores for this pattern correlated significantly with indices of executive function.43 These studies emphasize the versatility of the network approach in clinically applied brain imaging. By quantifying the activity of multiple networks in single resting state images, this method can allow investigators to assess the differential effects of treatment on parallel neural systems using simple, widely available, and potentially automated scanning routines.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of parkinsonian syndromes on clinical grounds, even if considered to be “gold standard,” may be limited, especially at early disease stages.58–62 It may be argued that the inadvertent inclusion of atypical “look alikes” may not impact the results of large-scale randomized blind studies. However, given that up to 30% of patients enrolled may not have classical PD63 even with rigid inclusion criteria in early disease stages,61 one can only assume that the assessment of treatment effects would be improved by eliminating heterogenous cohorts of variant patients from all treatment arms. Correct diagnosis early in disease is also important for the patients themselves because prognosis64–66 and treatment options67–72 can differ substantially between patients with PD and patients with atypical parkinsonian syndromes.
Various imaging techniques for differential diagnosis have been employed.5 These include imaging of glucose metabolism using FDG PET; imaging of presynaptic dopaminergic function with presynaptic ligands;34, 73–76 imaging of the postsynaptic dopaminergic D2-receptor binding with [123I]-(S)-2-hydroxy-3-iodo-6-methoxy-[(1-ethyl-2-pyrrolidinyl)methyl] benzamide (IBZM) SPECT and raclopride PET;77–79 imaging of cardiac sympathetic denervation;80, 81 as well as various MRI techniques.82–84
Examples of the use of these methods have appeared in the literature of the past decade. Presynaptic dopaminergic imaging has been shown to differentiate between patients with parkinsonian syndromes on one side, and healthy control subjects and essential tremor patients on the other.34, 73–76 Postsynaptic dopamine D2-receptor imaging may help in distinguishing between PD and atypical parkinsonian syndromes.77–79 Imaging of the cardiac sympathetic system reveals denervation in PD patients, even in early disease stages, and may differentiate between patients with multiple system atrophy (MSA) and PD.80, 81 However, these techniques are not generally able to discriminate between the various atypical parkinsonian syndromes.
Several MRI techniques have been employed for differential diagnosis in parkinsonian syndromes. Even though routine MRI shows characteristic changes as an atrophic putamen and a hyperintensive rim of the putamen in MSA patients, these changes are observed in only half of subjects suspected as having this diagnosis.82 Recent MRI studies have revealed decreases of the apparent diffusion coefficient of the putamen in MSA patients83 as well as decreases in the magnetization transfer ratio in regions of neuronal degeneration in MSA and progressive supernuclear palsy (PSP) patients.84 These noninvasive methods may ultimately prove helpful in differentiating these syndromes from PD.
Evaluation of regional glucose utilization with FDG PET has revealed characteristic metabolic patterns for the different parkinsonian syndromes. As described above, clinical PD is characterized by a distinctive metabolic pattern involving increased activity in the putamen/globus pallidus, thalamus, cerebellum, and brainstem, as well as relative reductions in cortical motor regions.3, 34, 85, 86 Characteristic patterns have been associated with other parkinsonian syndromes. MSA is characterized by metabolic decreases in the lentiform nucleus and cerebellum.87–92 By contrast, PSP is associated with decreased metabolism in midline frontal cortical areas and the brainstem.74, 93–95 Corticobasal ganglionic degeneration (CBGD) is associated with relatively reduced glucose metabolism in many cortical areas, including the insula and in the basal ganglia of the hemisphere contralateral to the most affected side.96–98
Results of an ongoing study99 have shown that these characteristic patterns can prospectively distinguish between patients with PD, MSA, PSP, CBGD, and healthy age-matched control subjects. In a large cohort of patients who underwent FDG PET imaging for purposes of differential diagnosis of parkinsonian disorders at North Shore University Hospital between 1995 and 2001, a probable/likely clinical diagnosis was achieved at follow-up by blind review undertaken independently by two movement disorder specialists. Blind reading of the scans by either visual inspection or a single subject statistical parametric mapping (SPM) approach were compared in terms of their ability to conform with an ultimate diagnosis achieved at follow-up. Overall, correct imaging diagnosis was obtained in about 90% of all subjects using the SPM approach and in about 84% by blind visual inspection of the FDG scans.99 These results emphasize the excellent potential of FDG PET imaging to augment clinical assessments in the evaluation of subjects for potential enrollment in pharmaceutical trials for PD.
METABOLIC NETWORKS IN OTHER MOVEMENT DISEASES: POTENTIAL SURROGATE MARKERS FOR CLINICAL TRIALS
In contrast to PD where PET and SPECT techniques can provide quantitative descriptors of dopaminergic dysfunction, other movement disorders are not associated with specific, localized neurochemical abnormalities that can be measured in vivo. However, the quantification of disease-related patterns of glucose metabolism may provide unique data regarding diagnosis and treatment effects in situations in which other imaging approaches are lacking or insufficient. Indeed, we have used FDG PET to describe unique metabolic networks associated with Huntington's disease,37, 100 idiopathic torsion dystonia,38, 39 and Tourette syndrome.40, 101
Huntington disease
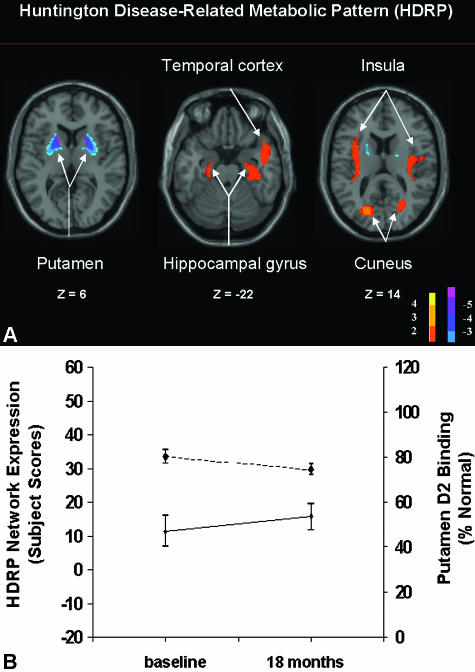
Huntington's disease (HD) is a hereditary neurodegenerative disorder characterized by progressively worsening abnormalities of movement and cognition. While investigating the glucose metabolism in HD patients, we identified an HD-related pattern (HDRP). HDRP is characterized by caudate and putaminal hypometabolism but also includes mediotemporal metabolic reductions as well as relative metabolic increases in the occipital cortex (FIG. 4A). Assessment of presymptomatic HD gene carriers revealed that HDRP expression is not only elevated before the development of clinical symptoms, but may also be increased significantly at a time when striatal D2 receptor binding is still normal.37 Results of an ongoing longitudinal study show that HDRP expression is also a valuable measure for disease progression in HD patients (FIG. 4B).100 It is currently not known whether HDRP expression is more sensitive to advancing neurodegeneration in the preclinical period than either striatal D2 binding102 or MRI-based measures of local atrophy.103 Quite possibly, the most accurate assessment of disease progression in the preclinical period of HD will be achieved with multiple imaging approaches in combination.104
FIG. 4.
A: Huntington's disease-related metabolic pattern (HDRP). This pattern of regional metabolic covariation was identified by network analysis of [18F]-fluorodeoxyglucose (FDG) PET scans from 10 presymptomatic HD gene carriers and 20 age-matched normal volunteer subjects. This HDRP (representing the first principal component, which accounted for 18% of the subject × voxel variation) discriminated carriers from controls (p < 0.0001) and was characterized by relative metabolic decreases in the striatum, associated with increases in the temporal cortex, insula, and occipital association cortex. [The display represents voxels that contribute significantly to the network at p = 0.01. Voxels with positive region weights (metabolic increases) are color coded from red to yellow; those with negative region weights (metabolic decreases) are color coded from blue to purple. The numbers under each slice are in millimeters, relative to the anterior-posterior commissure line.] B: Preclinical progression of HDRP: dopaminergic loss and network evolution. Bottom: Mean expression of the HD-related metabolic covariance pattern (HDRP, see panel A) in preclinical gene carriers scanned at baseline and at 18 months as part of our longitudinal FDG PET study (solid line). HDRP values increased significantly over this time period (p < 0.03; paired t test). Top: Mean RAC binding in the same preclinical HD cohort scanned at baseline and at 18 months (dashed line). Striatal D2 receptor binding was abnormally reduced at both time points (p < 0.001 relative to gene negative controls). RAC binding declined significantly between the two time points (p < 0.05; paired t test).
Torsion dystonia
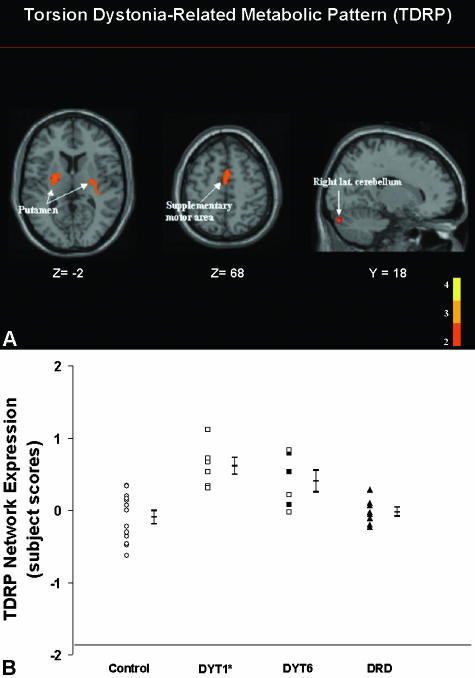
Torsion dystonia is a movement disorder characterized by sustained muscle contractions with twisting and repetitive movements or abnormal postures. Although primary torsion dystonia (PTD) is linked to several genetic mutations,105 there is no consistent histopathology associated with this disorder. Nonetheless, our FDG PET studies revealed a reproducible pattern associated with primary dystonia.38, 39, 106 This torsion dystonia-related pattern (TDRP) is characterized by hypermetabolism of the basal ganglia, cerebellum, and SMA (FIG. 5A). 38 Whereas this characteristic pattern was initially identified in affected PTD patients,106 its presence was subsequently confirmed in genotyped subjects, even without clinical manifestations.38, 39, 107 Further studies revealed subtle behavioral impairments in nonmanifesting DYT1 carriers,108 as well as abnormalities in brain activation responses during movement and nonmotor learning. It is therefore possible that TDRP expression can represent an endophenotype for dystonia, which may be useful in gene identification in at risk populations (FIG. 5B). The clinical spectrum of dystonia may indeed be broader than previously suspected,109 with separate structural/functional abnormalities underlying the motor and nonmotor manifestations of the disorder.110, 111 How genotypic and phenotypic patterns are altered by the treatment of dystonia, particularly DBS, is a topic of ongoing investigation.
FIG. 5.
A: Torsion dystonia-related metabolic pattern (TDRP). This pattern of regional metabolic covariation was identified by network analysis of [18F]-fluorodeoxyglucose (FDG) PET scans from nonmanifesting DYT1 gene carriers and control subjects (see text). This TDRP was characterized by bilateral covarying metabolic increases in the putamen, extending into the globus pallidus (GP), the SMA, and the cerebellar hemisphere. Subject scores for this pattern discriminated the DYT1 carriers from controls (p < 0.002). The display represents voxels that contribute significantly to the network at p = 0.001. Voxels with positive region weights (metabolic increases) are color coded red. B: TDRP activity in dystonia genotypes (prospective FDG/PET study). Scatter diagram of torsion dystonia-related pattern subject scores computed prospectively in six new nonaffected DYT1 gene carriers, six DYT6 gene carriers, seven dopa-responsive dystonia (DRD) patients, and 13 control subjects. Subject scores were abnormally elevated in DYT1 (p < 0.001) and DYT6 carriers (p < 0.007), but not in DRD patients (p = 0.4). The error bars indicate subgroups standard errors of the mean. Circles, normal controls; squares, subjects with genotypes associated with primary torsion dystonia; triangles, DRD patients; open symbols, clinically nonmanifesting subjects; filled symbols, affected dystonia patients.
Tourette syndrome
Gilles de la Tourette's syndrome (TS) is a movement disorder characterized by multiple motor and vocal tics of varying intensity. Despite its well-known clinical presentation, the histopathology and the underlying physiologic mechanisms mediating the clinical manifestations of TS are unknown. An early network analysis of FDG PET data in TS40 revealed the disease-related metabolic patterns. One pattern was characterized by metabolic increases in the lateral premotor and supplementary motor association cortices and the midbrain, perhaps secondary abnormal spontaneous movements occurring during PET imaging. The second pattern was characterized by metabolic decreases in the caudate and thalamus, with less pronounced decrements in the lentiform nucleus and the hippocampus. This network correlated significantly with Tourette Syndrome Global Scale (TSGS) ratings. Importantly, this TS-related topography has recently been identified in a subsequent group of patients scanned on a modern tomograph. A voxel-based network analysis of these FDG PET data101 confirmed that this pattern predicted TSGS ratings, indicating its potential as a surrogate marker for this condition in future clinical trials. This may be particularly relevant in movement disorders such as TS in which fully objective treatment markers are not available.
OTHER IMAGING APPROACHES POSSIBLY APPLICABLE TO MONITOR TREATMENT
Investigations have been conducted to assess changes in brain activation responses during therapy. These imaging studies have been conducted with 15O-water (H215O) PET and functional MRI methods during a variety of interventions such as dopaminergic therapy (apomorphine,112 levodopa113, 114) and DBS.115 These studies have been reviewed recently.116, 117 Despite the elegance of this approach, the demands of experimental control of task performance during dynamic imaging make this strategy less desirable for the study of treatment effects in large populations. The assessment of brain activation responses with treatment requires the performance of specified motor and/or cognitive tasks in different treatment and, thus, changes of brain perfusion or BOLD signal during task performance are dependent on factors such as movement frequency and force118, 119 as well as cognitive load.120 These parameters are particularly difficult to control in a motorically impaired patient population. Moreover, task equivalence within subjects across treatment conditions is necessary if the activation comparisons are truly to reflect the inherent effects of therapy on brain function. Nonetheless, carefully controlled activation studies may shed light on the mechanisms by which different therapeutic strategies modulate neural circuitry during motor performance and learning.121, 122 Indeed, network analysis of these data has revealed reproducible patterns of regional activation that may be used to assess the effects of therapy on higher order motor functioning.123
FUTURE DIRECTIONS
New imaging methodologies are emerging124 that may be useful in the quantitative assessment of potential neuroprotective agents. Recent MRI studies in parkinsonian syndromes have revealed changes in the apparent diffusion coefficient83 and the magnetization transfer ratio,84 perhaps reflecting aspects of the underlying pathological features of these conditions. Such techniques may provide interesting biomarkers for neurodegenerative processes that may be suitable for neuroprotective trials. Diffusion tensor imaging124 has revealed microstructural changes in the white matter of DYT1 gene carrier in the subgyral white matter of the sensorimotor cortex.110 This suggests that abnormal anatomical connectivity in cortical-subcortical pathways may be important in determining whether carriers are more or less likely to develop clinical signs of disease, and may be the basis for variable penetrance in some inherited neurological conditions.107 Similar mechanisms may be involved in other movement disorders in which conventional methods have failed to reveal consistent functional or anatomical abnormalities.
Perfusion-weighted MR imaging124 represents another emerging imaging method with potential promise in the assessment of disease progression and treatment effects. Because cerebral blood flow and metabolism are generally coupled in neurodegenerative disorders, changes in cerebral perfusion may be used as a simple noninvasive, radiation-free alternative to PET and SPECT for network quantification in the resting state (e.g.,4). Although appealing, more research is needed to determine the ultimate utility of these methods in the context of clinical trials.
SUMMARY
Functional neuroimaging has contributed greatly to our understanding of the pathophysiology and natural history of the movement disorders. Recently, these methodologies have generated a variety of imaging-based biomarkers for the assessment of both symptomatic and neuroprotective therapies for this class of neurological disease. Although the quantification of dopaminergic function with imaging has shown great promise in clinical trials for PD, this approach may be less appropriate for other movement disorders in which other neurotransmitter systems may be involved. In this review, we demonstrate the use of alternative metabolic imaging strategies for the objective measurement of treatment effects in PD. In addition to providing novel imaging biomarkers for motor and nonmotor manifestations of PD, this general approach may be of value in the determination of treatment outcomes in other movement disorders in which a specific neurochemical pathology is not precisely known.
REFERENCES
- 1.Brooks DJ. Positron emission tomography and single-photon emission computed tomography in central nervous system drug development. NeuroRx 2: 226–236, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravina B, Eidelberg D, Ahlskog JE, Albin R, Brooks DJ, Carbon M, et al. The role of radiotracer imaging in Parkinson's disease. Neurology 64: 208–215, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, et al. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab 14: 783–801, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Feigin A, Antonini A, Fukuda M, De Notaris R, Benti R, Pezzoli G, et al. Tc-99m ethylene cysteinate dimer SPECT in the differential diagnosis of parkinsonism. Mov Disord 17: 1265–1270, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Eckert T, Eidelberg D. The role of functional neuroimaging in the differential diagnosis of idiopathic Parkinson's disease and multiple system atrophy. Clin Auton Res 14: 84–91, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Brooks DJ. Imaging end points for monitoring neuroprotection in Parkinson's disease. Ann Neurol 53: S110–S118, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Marek K, Jennings D, Seibyl J. Dopamine agonists and Parkinson's disease progression: what can we learn from neuroimaging studies. Ann Neurol 53: S160–166, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Parkinson-Study-Group. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA 287: 1653–1661, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K; Parkinson Study Group. Levodopa and the progression of Parkinson’s disease. N Engl J Med 351: 2498–2508, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Whone AL, Watts RL, Stoessl AJ, Davis M, Reske S, Nahmias C, et al. Slower progression of Parkinson's disease with ropinirole versus levodopa: the REAL-PET study. Ann Neurol 54: 93–101, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Silbersweig D, et al. Regional metabolic correlates of surgical outcome following unilateral pallidotomy for Parkinson's disease. Ann Neurol 39: 450–459, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda M, Mentis MJ, Ma Y, Dhawan V, Antonini A, Lang AE, et al. Networks mediating the clinical effects of pallidal brain stimulation for Parkinson's disease: a PET study of resting-state glucose metabolism. Brain 124: 1601–1609, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Su PC, Ma Y, Fukuda M, Mentis MJ, Tseng HM, Yen RF, et al. Metabolic changes following subthalamotomy for advanced Parkinson's disease. Ann Neurol 50: 514–520, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Trošt M, Su PC, Barnes A, Su SL, Yen RF, Tseng HM, et al. Evolving metabolic changes during the first postoperative year after subthalamotomy. J Neurosurg 99: 872–878, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Devous MD Sr. Single-photon emission computed tomography in neurotherapeutics. NeuroRx 2: 237–249, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrish PK, Rakshi JS, Bailey DL, Sawle GV, Brooks DJ. Measuring the rate of progression and estimating the preclinical period of Parkinson's disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry 64: 314–319, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pate BD, Kawamata T, Yamada T, McGeer EG, Hewitt KA, Snow BJ, et al. Correlation of striatal fluorodopa uptake in the MPTP monkey with dopaminergic indices. Ann Neurol 34: 331–338, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Snow B, Tooyama I, McGeer E, Yamada T, Calne D, Takahashi H, et al. Human positron emission tomographic [18F]fluorodopa studies correlate with dopamine cell counts and levels. Ann Neurol 34: 324–330, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Tissingh G, Bergmans P, Booij J, Winogrodzka A, Stoof JC, Wolters EC, et al. [123I]β-CIT single-photon emission tomography in Parkinson's disease reveals a smaller decline in dopamine transporters with age than in controls. Eur J Nucl Med 24: 1171–1174, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Kazumata K, Dhawan V, Chaly T, Antonini A, Margouleff C, Belakhlef A, et al. Dopamine transporter imaging with fluorine-18-FPCIT and PET. J Nucl Med 39: 1521–1530, 1998. [PubMed] [Google Scholar]
- 21.Dhawan V, Eidelberg D. SPECT imaging in Parkinson's disease. Adv Neurol 86: 205–213, 2001. [PubMed] [Google Scholar]
- 22.Fahn S, Elton R, UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne D, eds. Recent developments in Parkinson's disease. New York: MacMillan, pp 153–163, 1987.
- 23.Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, et al. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson's disease. Ann Neurol 47: 493–503, 2000. [PubMed] [Google Scholar]
- 24.Innis RB, Marek KL, Sheff K, Zoghbi S, Castronuovo J, Feigin A, et al. Effect of treatment with L-dopa/carbidopa or L-selegiline on striatal dopamine transporter SPECT imaging with [123I]beta-CIT. Mov Disord 14: 436–442, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Nurmi E, Bergman J, Eskola O, Solin O, Hinkka SM, Sonninen P, et al. Reproducibility and effect of levodopa on dopamine transporter function measurements: a [18F]CFT PET study. J Cereb Blood Flow Metab 20: 1604–1609, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Ahlskog JE, Uitti RJ, O'Connor MK, Maraganore DM, Matsumoto JY, Stark KF, et al. The effect of dopamine agonist therapy on dopamine transporter imaging in Parkinson's disease. Mov Disord 14: 940–946, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Guttman M, Stewart D, Hussey D, Wilson A, Houle S, Kish S. Influence of L-dopa and pramipexole on striatal dopamine transporter in early PD. Neurology 56: 1559–1564, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Dhawan V, Chaly T, Fukuda M, Ma Y, Breeze R, et al. Blinded positron emission tomography study of dopamine cell implantation for Parkinson's disease. Ann Neurol 50: 181–187, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol 54: 403–414, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Ma Y, Feigin A, Dhawan V, Fukuda M, Shi Q, Greene P, et al. Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann Neurol 52: 628–634, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Piccini P, Brooks DJ, Bjorklund A, Gunn RN, Grasby PM, Rimoldi O, et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat Neurosci 2: 1137–1140, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med 344: 710–719, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Lindvall O. Stem cells for cell therapy in Parkinson's disease. Pharmacol Res 47: 279–287, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Chaly T, et al. Early differential diagnosis of Parkinson's disease with 18F-fluorodeoxyglucose and positron emission tomography. Neurology 45: 1995–2004, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Alexander GE, Moeller JR. Application of the scaled subprofile model to functional imaging in neuropsychiatric disorders: a principal component approach to modeling brain function in disease. Hum Brain Mapp 2: 1–16, 1994. [Google Scholar]
- 36.Moeller JR, Nakamura T, Mentis MJ, Dhawan V, Spetsieres P, Antonini A, et al. Reproducibility of regional metabolic covariance patterns: comparison of four populations. J Nucl Med 40: 1264–1269, 1999. [PubMed] [Google Scholar]
- 37.Feigin A, Leenders KL, Moeller JR, Missimer J, Kuenig G, Spetsieris P, et al. Metabolic network abnormalities in early Huntington's disease: an [(18)F]FDG PET study. J Nucl Med 42: 1591–1595, 2001. [PubMed] [Google Scholar]
- 38.Eidelberg D, Moeller JR, Antonini A, Kazumata K, Nakamura T, Dhawan V, et al. Functional brain networks in DYT1 dystonia. Ann Neurol 44: 303–312, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Trošt M, Carbon M, Edwards C, Ma Y, Raymond D, Mentis MJ, et al. Primary dystonia: is abnormal functional brain architecture linked to genotype? Ann Neurol 52: 853–856, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Eidelberg D, Moeller JR, Antonini A, Kazumata K, Dhawan V, Budman C, et al. The metabolic anatomy of Tourette's syndrome. Neurology 48: 927–934, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Chaly T, et al. Assessment of disease severity in parkinsonism with fluorine-18-fluorodeoxyglucose and PET. J Nucl Med 36: 378–383, 1995. [PubMed] [Google Scholar]
- 42.Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Mandel F, Alexander GE, et al. The metabolic topography of normal aging. J Cereb Blood Flow Metab 16: 385–398, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Lozza C, Baron JC, Eidelberg D, Mentis MJ, Carbon M, Marie RM. Executive processes in Parkinson's disease: FDG-PET and network analysis. Hum Brain Mapp 22: 236–245, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moeller JR, Eidelberg D. Divergent expression of regional metabolic topographies in Parkinson's disease and normal ageing. Brain 120: 2197–2206, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Eidelberg D, Moeller JR, Dhawan V, Sidtis JJ, Ginos JZ, Strother SC, et al. The metabolic anatomy of Parkinson's disease: complementary [18F]fluorodeoxyglucose and [18F]fluorodopa positron emission tomographic studies. Mov Disord 5: 203–213, 1990. [DOI] [PubMed] [Google Scholar]
- 46.Eidelberg D, Moeller JR, Kazumata K, Antonini A, Sterio D, Dhawan V, et al. Metabolic correlates of pallidal neuronal activity in Parkinson's disease. Brain 120: 1315–1324, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Alsop DC, Casement M, Press D. Increased hippocampal perfusion in early Alzheimer’s disease. Proc Intl Soc Mag Reson Med 11: A178, 2003. [Google Scholar]
- 48.Mentis MJ, McIntosh AR, Perrine K, Dhawan V, Berlin B, Feigin A, et al. Relationships among the metabolic patterns that correlate with mnemonic, visuospatial, and mood symptoms in Parkinson's disease. Am J Psychiatry 159: 746–754, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM. The subthalamic nucleus in the context of movement disorders. Brain 127: 4–20, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Volkmann J Deep brain stimulation for the treatment of Parkinson's disease. J Clin Neurophysiol 21: 6–17, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Antonini A, Moeller JR, Nakamura T, Spetsieris P, Dhawan V, Eidelberg D. The metabolic anatomy of tremor in Parkinson's disease. Neurology 51: 803–810, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Fukuda M, Barnes A, Simon ES, Holmes A, Dhawan V, Giladi N, et al. Thalamic stimulation for parkinsonian tremor: correlation between regional cerebral blood flow and physiological tremor characteristics. Neuroimage 21: 608–615, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Trošt M, Simon ES, Dhawan V, Okulski J, Fodstad H, Eidelberg D. Clinical and metabolic brain changes in tremor predominant Parkinson's disease patients treated with Vim deep brain stimulation. Mov Disord 199: S383, 2004. [Google Scholar]
- 54.Feigin A, Fukuda M, Dhawan V, Przedborski S, Jackson-Lewis V, Mentis MJ, et al. Metabolic correlates of levodopa response in Parkinson's disease. Neurology 57: 2083–2088, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Brown RG, Dowsey PL, Brown P, Jahanshahi M, Pollak P, Benabid AL, et al. Impact of deep brain stimulation on upper limb akinesia in Parkinson's disease. Ann Neurol 45: 473–488, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Berding G, Odin P, Brooks DJ, Nikkhah G, Matthies C, Peschel T, et al. Resting regional cerebral glucose metabolism in advanced Parkinson's disease studied in the off and on conditions with [(18)F]FDG-PET. Mov Disord 16: 1014–1022, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Hilker R, Voges J, Weisenbach S, Kalbe E, Burghaus L, Ghaemi M, et al. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson's disease. J Cereb Blood Flow Metab 24: 7–16, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Litvan I, Agid Y, Goetz C, Jankovic J, Wenning GK, Brandel JP, et al. Accuracy of the clinical diagnosis of corticobasal degeneration: a clinicopathologic study. Neurology 48: 119–125, 1997. [DOI] [PubMed] [Google Scholar]
- 59.Litvan I, Goetz CG, Jankovic J, Wenning GK, Booth V, Bartko JJ, et al. What is the accuracy of the clinical diagnosis of multiple system atrophy? A clinicopathologic study. Arch Neurol 54: 937–944, 1997. [DOI] [PubMed] [Google Scholar]
- 60.Litvan I, Booth V, Wenning GK, Bartko JJ, Goetz CG, McKee A, et al. Retrospective application of a set of clinical diagnostic criteria for the diagnosis of multiple system atrophy. J Neural Transm 105: 217–227, 1998. [DOI] [PubMed] [Google Scholar]
- 61.Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125: 861–870, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Osaki Y, Wenning GK, Daniel SE, Hughes A, Lees AJ, Mathias CJ, et al. Do published criteria improve clinical diagnostic accuracy in multiple system atrophy? Neurology 59: 1486–1491, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Hughes AJ, Daniel SE, Blankson S, Lees A. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol 50: 140–148, 1993. [DOI] [PubMed] [Google Scholar]
- 64.Diamond SG, Markham CH, Hoehn MM, McDowell FH, Muenter MD. Multi-center study of Parkinson mortality with early versus later dopa treatment. Ann Neurol 22: 8–12, 1987. [DOI] [PubMed] [Google Scholar]
- 65.Golbe LI, Davis PH, Schoenberg BS, Duvoisin RC. Prevalence and natural history of progressive supranuclear palsy. Neurology 38: 1031–1034, 1988. [DOI] [PubMed] [Google Scholar]
- 66.Wenning GK, Tison F, Ben-Shlomo Y, Daniel SE, Quinn N. Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord 12: 133–147, 1997. [DOI] [PubMed] [Google Scholar]
- 67.Krack P, Pollak P, Limousin P, Hoffmann D, Benazzouz A, Le Bas JF, et al. Opposite motor effects of pallidal stimulation in Parkinson's disease. Ann Neurol 43: 180–192, 1998. [DOI] [PubMed] [Google Scholar]
- 68.Litvan I, Chase TN, eds. Traditional and experimental therapeutic approaches. New York: Oxford University Press, 1992.
- 69.Wenning GK, Pramstaller PP, Ransmayr G, Poewe W. [A typical Parkinson syndrome.] Nervenarzt 68: 102–115, 1997. [DOI] [PubMed] [Google Scholar]
- 70.Tarsy D, Apetauerova D, Ryan P, Norregaard T. Adverse effects of subthalamic nucleus DBS in a patient with multiple system atrophy. Neurology 61: 247–249, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Visser-Vandewalle V, Temel Y, Colle H, van der Linden C. Bilateral high-frequency stimulation of the subthalamic nucleus in patients with multiple system atrophy—parkinsonism. Report of four cases. J Neurosurg 98: 882–887, 2003. [DOI] [PubMed] [Google Scholar]
- 72.Lezcano E, Gomez-Esteban JC, Zarranz JJ, Alcaraz R, Atares B, Bilbao G, et al. Parkinson's disease-like presentation of multiple system atrophy with poor response to STN stimulation: a clinicopathological case report. Mov Disord 19: 973–977, 2004. [DOI] [PubMed] [Google Scholar]
- 73.Schwarz J, Linke R, Kerner M, Mozley PD, Trenkwalder C, Gasser T, et al. Striatal dopamine transporter binding assessed by [I-123]IPT and single photon emission computed tomography in patients with early Parkinson's disease: implications for a preclinical diagnosis. Arch Neurol 57: 205–208, 2000. [DOI] [PubMed] [Google Scholar]
- 74.Piccini P, de Yebenez J, Lees AJ, Ceravolo R, Turjanski N, Pramstaller P, et al. Familial progressive supranuclear palsy: detection of subclinical cases using 18F-dopa and 18fluorodeoxyglucose positron emission tomography. Arch Neurol 58: 1846–1851, 2001. [DOI] [PubMed] [Google Scholar]
- 75.Dhawan V, Ma Y, Pillai V, Spetsieris P, Chaly T, Belakhlef A, et al. Comparative analysis of striatal FDOPA uptake in Parkinson's disease: ratio method versus graphical approach. J Nucl Med 43: 1324–1330, 2002. [PubMed] [Google Scholar]
- 76.Ma Y, Dhawan V, Mentis M, Chaly T, Spetsieris PG, Eidelberg D. Parametric mapping of [18F]FPCIT binding in early stage Parkinson's disease: a PET study. Synapse 45: 125–133, 2002. [DOI] [PubMed] [Google Scholar]
- 77.Antonini A, Vontobel P, Psylla M, Gunther I, Maguire PR, Missimer J, et al. Complementary positron emission tomographic studies of the striatal dopaminergic system in Parkinson's disease. Arch Neurol 52: 1183–1190, 1995. [DOI] [PubMed] [Google Scholar]
- 78.Antonini A, Leenders KL, Vontobel P, Maguire RP, Missimer J, Psylla M, et al. Complementary PET studies of striatal neuronal function in the differential diagnosis between multiple system atrophy and Parkinson's disease. Brain 120: 2187–2195, 1997. [DOI] [PubMed] [Google Scholar]
- 79.Ghaemi M, Hilker R, Rudolf J, Sobesky J, Heiss WD. Differentiating multiple system atrophy from Parkinson's disease: contribution of striatal and midbrain MRI volumetry and multi-tracer PET imaging. J Neurol Neurosurg Psychiatry 73: 517–523, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Braune S, Reinhardt M, Schnitzer R, Riedel A, Lucking CH. Cardiac uptake of [123I]MIBG separates Parkinson's disease from multiple system atrophy. Neurology 53: 1020–1025, 1999. [DOI] [PubMed] [Google Scholar]
- 81.Druschky A, Hilz MJ, Platsch G, Radespiel-Troger M, Druschky K, Kuwert T, et al. Differentiation of Parkinson's disease and multiple system atrophy in early disease stages by means of I-123-MIBG-SPECT. J Neurol Sci 175: 3–12, 2000. [DOI] [PubMed] [Google Scholar]
- 82.Schrag A, Good CD, Miszkiel K, Morris HR, Mathias CJ, Lees AJ, et al. Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology 54: 697–702, 2000. [DOI] [PubMed] [Google Scholar]
- 83.Seppi K, Schocke MF, Esterhammer R, Kremser C, Brenneis C, Mueller J, et al. Diffusion-weighted imaging discriminates progressive supranuclear palsy from PD, but not from the parkinson variant of multiple system atrophy. Neurology 60: 922–927, 2003. [DOI] [PubMed] [Google Scholar]
- 84.Eckert T, Sailer M, Kaufmann J, Schrader C, Peschel T, Bodammer N, et al. Differentiation of idiopathic Parkinson's disease, multiple system atrophy, progressive supranuclear palsy, and healthy controls using magnetization transfer imaging. Neuroimage 21: 229–235, 2004. [DOI] [PubMed] [Google Scholar]
- 85.Martin WR, Beckman JH, Calne DB, Adam MJ, Harrop R, Rogers JG, et al. Cerebral glucose metabolism in Parkinson's disease. Can J Neurol Sci 11: 169–173, 1984. [DOI] [PubMed] [Google Scholar]
- 86.Wolfson LI, Leenders KL, Brown LL, Jones T. Alterations of regional cerebral blood flow and oxygen metabolism in Parkinson's disease. Neurology 35: 1399–1405, 1985. [DOI] [PubMed] [Google Scholar]
- 87.Gilman S, Markel DS, Koeppe RA, Junck L, Kluin KJ, Gebarski SS, et al. Cerebellar and brainstem hypometabolism in olivopontocerebellar atrophy detected with positron emission tomography. Ann Neurol 23: 223–230, 1988. [DOI] [PubMed] [Google Scholar]
- 88.De Volder AG, Francart J, Laterre C, Dooms G, Bol A, Michel C, et al. Decreased glucose utilization in the striatum and frontal lobe in probable striatonigral degeneration. Ann Neurol 26: 239–247, 1989. [DOI] [PubMed] [Google Scholar]
- 89.Eidelberg D, Takikawa S, Moeller JR, Dhawan V, Redington K, Chaly T, et al. Striatal hypometabolism distinguishes striatonigral degeneration from Parkinson's disease. Ann Neurol 33: 518–527, 1993. [DOI] [PubMed] [Google Scholar]
- 90.Otsuka M, Ichiya Y, Kuwabara Y, Hosokawa S, Sasaki M, Yoshida T, et al. Glucose metabolism in the cortical and subcortical brain structures in multiple system atrophy and Parkinson's disease: a positron emission tomographic study. J Neurol Sci 144: 77–83, 1996. [DOI] [PubMed] [Google Scholar]
- 91.Antonini A, Kazumata K, Feigin A, Mandel F, Dhawan V, Margouleff C, et al. Differential diagnosis of parkinsonism with [18F]fluorodeoxyglucose and PET. Mov Disord 13: 268–274, 1998. [DOI] [PubMed] [Google Scholar]
- 92.Taniwaki T, Nakagawa M, Yamada T, Yoshida T, Ohyagi Y, Sasaki M, et al. Cerebral metabolic changes in early multiple system atrophy: a PET study. J Neurol Sci 200: 79–84, 2002. [DOI] [PubMed] [Google Scholar]
- 93.Foster NL, Gilman S, Berent S, Morin EM, Brown MB, Koeppe RA. Cerebral hypometabolism in progressive supranuclear palsy studied with positron emission tomography. Ann Neurol 24: 399–406, 1988. [DOI] [PubMed] [Google Scholar]
- 94.Leenders KL, Frackowiak RS, Lees AJ. Steele-Richardson-Olszewski syndrome. Brain energy metabolism, blood flow and fluorodopa uptake measured by positron emission tomography. Brain 111: 615–630, 1988. [DOI] [PubMed] [Google Scholar]
- 95.Blin J, Baron JC, Dubois B, Pillon B, Cambon H, Cambier J, et al. Positron emission tomography study in progressive supranuclear palsy. Brain hypometabolic pattern and clinicometabolic correlations. Arch Neurol 47: 747–752, 1990. [DOI] [PubMed] [Google Scholar]
- 96.Blin J, Vidailhet MJ, Pillon B, Dubois B, Feve JR, Agid Y. Corticobasal degeneration: decreased and asymmetrical glucose consumption as studied with PET. Mov Disord 7: 348–354, 1992. [DOI] [PubMed] [Google Scholar]
- 97.Eidelberg D, Dhawan V, Moeller JR, Sidtis JJ, Ginos JZ, Strother SC, et al. The metabolic landscape of cortico-basal ganglionic degeneration: regional asymmetries studied with positron emission tomography. J Neurol Neurosurg Psychiatry 54: 856–862, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laureys S, Salmon E, Garraux G, Peigneux P, Lemaire C, Degueldre C, et al. Fluorodopa uptake and glucose metabolism in early stages of corticobasal degeneration. J Neurol 246: 1151–1158, 1999. [DOI] [PubMed] [Google Scholar]
- 99.Eckert T, Barnes A, Frucht S, Dhawan V, Feigin A, Eidelberg D. Differential diagnosis of parkinsonian disorders: the diagnostic value of FDG PET. Mov Disord 19: S376, 2004. [Google Scholar]
- 100.Feigin A, Ma Y, Zgaljardic D, Carbon M, Dhawan V, Eidelberg D. PET measures of longitudinal progression in presymptomatic Huntington's disease. Neurology 60(Suppl 1): A246, 2003. [Google Scholar]
- 101.Feigin A, Budman C, Zgaljardic D, Dhawan V, Eidelberg D. Metabolic brain networks in Tourette syndrome. Mov Disord 17(Suppl 5): S339, 2002. [Google Scholar]
- 102.Pavese N, Andrews TC, Brooks DJ, Ho AK, Rosser AE, Barker RA, et al. Progressive striatal and cortical dopamine receptor dysfunction in Huntington's disease: a PET study. Brain 126: 1127–1135, 2003. [DOI] [PubMed] [Google Scholar]
- 103.Aylward EH, Sparks BF, Field KM, Yallapragada V, Shpritz BD, Rosenblatt A, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology 63: 66–72, 2004. [DOI] [PubMed] [Google Scholar]
- 104.Antonini A, Leenders KL, Spiegel R, Meier D, Vontobel P, Weigell-Weber M, et al. Striatal glucose metabolism and dopamine D2 receptor binding in asymptomatic gene carriers and patients with Huntington's disease. Brain 119: 2085–2095, 1996. [DOI] [PubMed] [Google Scholar]
- 105.Klein C, Breakefield XO, Ozelius LJ. Genetics of primary dystonia. Semin Neurol 19: 271–280, 1999. [DOI] [PubMed] [Google Scholar]
- 106.Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Przedborski S, et al. The metabolic topography of idiopathic torsion dystonia. Brain 118: 1473–1484, 1995. [DOI] [PubMed] [Google Scholar]
- 107.Eidelberg D. Brain networks and clinical penetrance: lessons from hyperkinetic movement disorders. Curr Opin Neurol 16: 471–474, 2003. [DOI] [PubMed] [Google Scholar]
- 108.Ghilardi MF, Carbon M, Silvestri G, Dhawan V, Tagliati M, Bressman S, et al. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Ann Neurol 54: 102–109, 2003. [DOI] [PubMed] [Google Scholar]
- 109.Bressman SB. Dystonia genotypes, phenotypes, and classification. Adv Neurol 94: 101–107, 2004. [PubMed] [Google Scholar]
- 110.Carbon M, Kingsley PB, Su S, Smith GS, Spetsieris P, Bressman S, et al. Microstructural white matter changes in carriers of the DYT1 gene mutation. Ann Neurol 56: 283–286, 2004. [DOI] [PubMed] [Google Scholar]
- 111.Carbon M, Su S, Dhawan V, Raymond D, Bressman S, Eidelberg D. Regional metabolism in primary torsion dystonia: effects of penetrance and genotype. Neurology 62: 1384–1390, 2004. [DOI] [PubMed] [Google Scholar]
- 112.Jenkins IH, Fernandez W, Playford ED, Lees AJ, Frackowiak RS, Passingham RE, et al. Impaired activation of the supplementary motor area in Parkinson's disease is reversed when akinesia is treated with apomorphine. Ann Neurol 32: 749–757, 1992. [DOI] [PubMed] [Google Scholar]
- 113.Feigin A, Ghilardi MF, Fukuda M, Mentis MJ, Dhawan V, Barnes A, et al. Effects of levodopa infusion on motor activation responses in Parkinson's disease. Neurology 59: 220–226, 2002. [DOI] [PubMed] [Google Scholar]
- 114.Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, et al. Event-related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain 124: 558–570, 2001. [DOI] [PubMed] [Google Scholar]
- 115.Fukuda M, Mentis M, Ghilardi MF, Dhawan V, Antonini A, Hammerstad J, et al. Functional correlates of pallidal stimulation for Parkinson's disease. Ann Neurol 49: 155–164, 2001. [DOI] [PubMed] [Google Scholar]
- 116.Ceballos-Baumann AO. Functional imaging in Parkinson's disease: activation studies with PET, fMRI and SPECT. J Neurol 250: I15–23, 2003. [DOI] [PubMed] [Google Scholar]
- 117.Thobois S, Jahanshahi M, Pinto S, Frackowiak R, Limousin-Dowsey P. PET and SPECT functional imaging studies in Parkinsonian syndromes: from the lesion to its consequences. Neuroimage 23: 1–16, 2004. [DOI] [PubMed] [Google Scholar]
- 118.Sadato N, Ibanez V, Deiber MP, Campbell G, Leonardo M, Hallett M. Frequency-dependent changes of regional cerebral blood flow during finger movements. J Cereb Blood Flow Metab 16: 23–33, 1996. [DOI] [PubMed] [Google Scholar]
- 119.Dai TH, Liu JZ, Sahgal V, Brown RW, Yue GH. Relationship between muscle output and functional MRI-measured brain activation. Exp Brain Res 140: 290–300, 2001. [DOI] [PubMed] [Google Scholar]
- 120.Mentis MJ, Dhawan V, Nakamura T, Ghilardi MF, Feigin A, Edwards C, et al. Enhancement of brain activation during trial-and-error sequence learning in early PD. Neurology 60: 612–619, 2003. [DOI] [PubMed] [Google Scholar]
- 121.Fukuda M, Ghilardi MF, Carbon M, Dhawan V, Ma Y, Feigin A, et al. Pallidal stimulation for parkinsonism: improved brain activation during sequence learning. Ann Neurol 52: 144–152, 2002. [DOI] [PubMed] [Google Scholar]
- 122.Feigin A, Ghilardi MF, Carbon M, Edwards C, Fukuda M, Dhawan V, et al. Effects of levodopa on motor sequence learning in Parkinson's disease. Neurology 60: 1744–1749, 2003. [DOI] [PubMed] [Google Scholar]
- 123.Carbon M, Ghilardi MF, Feigin A, Fukuda M, Silvestri G, Mentis MJ, et al. Learning networks in health and Parkinson's disease: reproducibility and treatment effects. Hum Brain Mapp 19: 197–211, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bammer R, Skare S, Newbould R, Liu C, Thijs V, Ropele S, Clayton DB, Krueger G, Moseley ME, Glover GH. Foundations of advanced magnetic resonance imaging. NeuroRx 2: 167–196, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]