Abstract
Summary: Neuroimaging has important applications in the diagnosis and treatment of patients with seizures and epilepsy. Having replaced computed tomography (CT) in many situations, MRI is the preferred imaging technique for patients with epilepsy. Advances in radionuclide-based techniques such as single-photon emission CT/positron emission tomography and electromagnetic source imaging with magnetoencephalography are providing new insights into the pathophysiology of epilepsy. In addition, techniques such as magnetic resonance spectroscopy are beginning to impact treatment. In this review, I discuss how these techniques are used in clinical practice but more importantly, how imaging findings play an increasing role in neurotherapeutics.
Keywords: Epilepsy, MRI, PET, SPECT, functional MRI, magnetoencephalography, MR spectroscopy
INTRODUCTION
Seizures may occur in up to 10% of the population, whereas epilepsy is a chronic disease characterized by recurrent seizures that may affect 2% of the population. Although primarily defined by EEG abnormalities, it is presently recognized that epilepsy is often associated with gross or subtle structural or metabolic lesions of the brain. Modern neuroimaging is useful in the diagnosis of the abnormalities underlying the epilepsies, but the information provided by imaging techniques can also contribute to the proper classification of certain epileptic disorders and can delineate the genetics underlying some syndromes. Neuroimaging is even more important for those patients who have medically intractable seizures.1 Advances in technology to localize focal epileptogenic substrates, especially that of high-resolution structural imaging with magnetic resonance imaging (MRI),2 have substantially improved the success of surgical treatment.3 This review compares available imaging modalities, their specific role in patients with epilepsy, and practical applications of imaging data in the management of patients with epilepsy.
IMAGING MODALITIES
Computed tomography
Computed tomography (CT) uses ionizing radiation and can generate excellent hard tissue imaging contrast with moderately good soft tissue resolution. CT has a number of advantages, and those include lower cost, scan speed, ready accessibility, and easy use, which provide a relatively reliable imaging modality for most patients.4 In addition, last-generation CT scans can generate images of the brain in seconds.
Although the use of CT for patients with epilepsy has been greatly diminished by MRI, CT is still the technique of choice for the investigation of patients with seizures and epilepsy under certain conditions. In the neonate and young infant, CT is often of secondary or adjunctive importance, but it serves as a significant backup role to ultrasound.5,6 CT can accurately detect hemorrhage, infarctions, gross malformations, ventricular system pathologies, and lesions with underlying calcification. In older children and adults, CT is the technique of choice in the perioperative state because it can rapidly detect recent hemorrhage, hydrocephalus, and major structural changes.
One should recognize that the sensitivity of CT in patients with epilepsy is not higher than 30% in unselected populations.7 CT has overall a low sensitivity because of poor resolution in the temporal fossa, and thus it is not surprising, that CT is of no use in detecting mesial temporal sclerosis, the most common pathology in intractable temporal lobe epilepsy.
The International League against Epilepsy (ILAE) guidelines for neuroimaging studies suggest that a CT can be the diagnostic imaging of choice in patients with epilepsy if an MRI is not available. This recommendation should be weighed against the fact that studies have shown that CT may fail to detect abnormalities in up to 50% of patients with epileptogenic structural lesions such as small tumors and vascular malformations. The ILAE also recommends that patients who have intractable seizures have an MRI study if a CT is normal. Presently, in the U.S., an MRI is considered the standard of care for patients with epilepsy.
CT scan and epilepsy management.
As stated above, CT has a reduced role in the management of patients with epilepsy. However, CT is still crucial in emergency situations particularly in the acute situation and the perioperative period.
In an acute seizure scenario, CT can accurately detect major pathologies such as trauma, hemorrhage, ischemic stroke, hydrocephalus, tumors, arterio-venous malformations, etc. In patients with any of the above pathologies, therapy will be directed by neurosurgical intervention or supportive care. For most patients who present with a first seizure in the context of a normal neurologic examination, the CT scan is unrevealing. Most patients with chronic epilepsy who have recurrent seizures with a previous normal imaging study have a normal CT scan. However, some patients may sustain cranial trauma during seizures, and CT scan can be helpful in ruling out hemorrhage, subdural, epidural collections, or hematomas. The role of CT in patients with acute seizures secondary to alcohol is reflective of the overall sensitivity of CT in the management of patients in the ER. In a large study, Earnest et al.8 showed that CT was diagnostic in 6% of patients with first time alcohol seizure. For perioperative patients, CT scan is also the imaging technique of choice since it can detect hemorrhage, hydrocephalus, and infarction and can be useful to assess electrode placement.
MRI
MRI is the imaging procedure of choice in the investigation of patients with epilepsy. The advantages of MRI over CT are numerous and do not need further elaboration. Increasingly, imaging studies in epilepsy are based on algorithms that are weighted by what is found on MRI and other investigations. As is the case with EEG, MRI is often not completed with one study. In MRI, multiple sessions addressing different issues may be needed to acquire all of the relevant imaging information for that patient.
The sensitivity of MRI in detecting abnormalities in patients with epilepsy is in part associated to the pathologies underlying epilepsy and by the MRI techniques and experience of the interpreting physician. Mesial temporal sclerosis (MTS), small tumors, and trauma are more common in adults.9 In contrast, developmental malformations constitute the most common underlying pathology in infants and young children with epilepsy.10–12 MRI epilepsy protocols have been established in may centers with the intention of improving sensitivity and specificity (Table 1).
TABLE 1.
Epilepsy Protocols and Analysis Techniques
| Routine MRI Protocol for Epilepsy |
| FLAIR coronal |
| T-1W <2 mm volume acquisition (spoiled gradient echo) |
| Fast spin-echo axial |
| Inversion recovery axial or coronal |
| Specialized MRI Protocol for Epilepsy |
| Surface coils |
| Diffusion tensor imaging/magnetization transfer contrast |
| fMRI |
| MR spectroscopy |
| Special Analysis Techniques |
| Volumetry |
| Cortical reformatting |
| Gray/white matter segmentation |
| Texture analysis |
| Statistical parametric mapping |
The most common pathologic entity encountered in patients with intractable temporal lobe epilepsy is MTS. MTS is pathologically characterized by the presence of a firm, atrophic hippocampus and the presence on histology of neuronal loss and gliosis in CA1, CA3, and CA4 of the hippocampal subfields.13 The MRI features of hippocampal sclerosis include 1) hippocampal atrophy; 2) increased signal on T2-weighted images or FLAIR (fluid-attenuated inversion recovery); and 3) decreased signal on inversion recovery sequences.14–16 Volumetric measurements may also aid in the diagnosis, but the gains in sensitivity should be balanced by the requirements and experience in performing quantitative studies (FIG. 1). 17
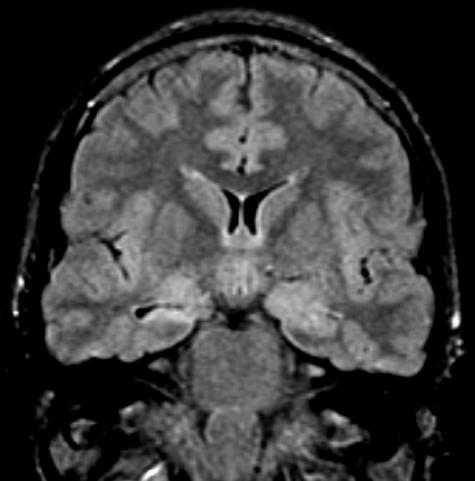
FIG. 1.
Hippocampal Sclerosis. T2-W coronal MRI shows evidence of right hippocampal sclerosis. Note signal changes and atrophy from right hippocampus. Surgical outcome: seizure free for 3 years.
The other major group of pathologies in which MRI has made enormous contributions to epilepsy is in malformations of cortical development (MCDs). MCDs are common in children and should be sought in children with epilepsy.18 MRI can accurately define diffuse malformations such as lissencephaly, band heterotopia, and periventricular nodular heterotopia (FIG. 2). It can also define hemimegalencephaly, schizencephaly, and focal subcortical heterotopia. Focal lesions such as focal cortical dysplasia (FCD) are the most common developmental pathologies in children with extratemporal lobe seizures and recognition of these lesions can have an important bearing on the management and prognosis (please see the next section).18 The MRI features consist of an abnormal cortical mantle with disturbed gray-white matter architecture and thick cortex. Because these lesions can be small in size, the MRI examination should be targeted to the clinically suspected regions using both three-dimensional volume techniques and inversion recovery sequences. Other common pathologies in children with epilepsy include Sturge-Weber syndrome, ischemia and porencephaly, developmental tumors (ganglioglial), vascular lesions, and less common neurocutaneous syndromes (incontinentia pigmenti, NF I, etc.). In general, these disorders are diagnosed on the basis of other features and will not be discussed here.
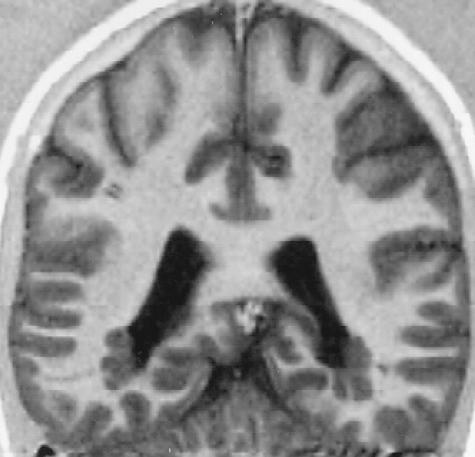
FIG. 2.
Periventricular band heterotopia due to Filamin 1 (FLM) mutation. Note periventricular gray matter nodules.
MRI and epilepsy management.
Not all patients with epilepsy are in need of neuroimaging studies, in particular MRI. Neuroimaging studies may not be necessary in patients with well-defined idiopathic generalized epilepsies such as typical childhood absence epilepsy. In addition, patients with typical idiopathic benign partial epilepsy with centro-temporal spikes may not require an imaging study. However, reports of patients with apparent generalized epilepsies or benign partial seizures in which structural abnormalities are seen on MRI have surfaced. In general, the clinical course and the atypical features of such patients will identify those that require imaging studies. Similarly, children with uncomplicated febrile convulsions and a normal neurological exam do not require imaging studies. Despite the knowledge that many idiopathic epilepsies are benign, for practical and medico-legal reasons almost all patients with epilepsy in the U.S. end up having a brain MRI.
Conversely, all patients with symptomatic generalized or focal seizures should have a structural neuroimaging study. Because MRI is far better than CT in the detection of structural lesions, it is suggested that MRI should be the imaging procedure of choice when evaluating patients with seizures, especially if focal features are present on the neurologic exam or EEG. In addition, MRI is indicated if seizures persist in the presence of a previously normal CT scan or when there are progressive neurologic changes. A repeated MRI is also indicated at 2–5 years in the context of a previously normal MRI in a patient with persistent seizures.
The meaning of a lesion on MRI and treatment implications.
Finding an MRI abnormality in a patient with epilepsy does not automatically mean that the lesion is the culprit. Some lesions are epileptogenic, whereas others are not. The distinction is difficult and often impossible without other information such as electrophysiology and clinical data. However, it is useful to analyze lesion type and location to estimate the likelihood of a lesion being epileptogenic. Mesial temporal lesions and cortical malformations are more likely to be epileptogenic, whereas white matter cystic lesions are less likely to be epileptogenic.
The first basic example in which MRI has a major impact on treatment is in patients with new onset seizures. Traditionally, the treatment of the first seizure has been a contentious issue among neurologists. When the suspected diagnosis is partial onset seizures, treatment may be initiated if the MRI demonstrates a lesion likely to be causative in nature because the recurrence rate among lesional epilepsy patients reaches almost 80% by 2 years.19 If the lesion is not clearly associated to the onset of seizures such as white matter abnormalities, demyelinating disease, etc., the recurrence rate may be much lower. The second most common and illustrative example in which pathologic findings affect management in a patient with epilepsy is MTS. Although MTS may be found in some rare families and may be in some cases associated with a benign course,20 it is not in most cases. MTS is rarely found at seizure onset, except after status epilepticus21 and it may be strongly predictive of seizure recurrence.22 Most commonly, the presence of MTS in the context of temporal lobe epilepsy is a strong prognostic indicator for seizure intractability. Semah et al.22 reported that, among patients with MTS followed at a tertiary center, only 11% were seizure free on anticonvulsant treatment. The available data suggest that detection of MTS in a given patient is likely to be associated with failure to antiepileptic drugs (AEDs) and may lead to intractability. Similarly, many studies have shown that MTS is highly predictive of surgical success although no randomized control study has been done.23,24 Long-term studies seem to support the above findings.25 In practical terms this means that recognition of typical MTS coupled with failure to two consecutive AEDs should lead to surgical evaluation and treatment.
Management of patients with bilateral MTS or MTS associated with other pathologies (MTS +) is more challenging. Although patients with bilateral MTS may have bilateral ictal origin, about 30–40% may benefit from surgical treatment if at least 70% of seizures originate in one lobe. Similarly, patients with MTS + may be more difficult because the lesion location, proximity to MTS and language and memory dominance may impact management.
Other symptomatic epilepsies may include those associated with tumors and MCD. In cases of tumors, the decision-making process is expedited by the imaging features. Any lesion that has contrast enhancement raises the possibility of a neoplasm and thus, surgical biopsy or resection independent of the epilepsy may take precedent. Conversely, lesions with low likelihood for neoplastic transformation may require close observation. Infectious diseases are becoming more frequent particularly in certain areas of the U.S. Neurocysticercosis can be localized in the CNS in different regions including the brain parenchyma, meninges, and intraventricular space, and may have more than one location in some patients. Almost half of the patients have intraventricular cysts without associated parenchyma cysts. Parenchyma cysts are most common and involve the gray-white matter junction. Acute and chronic changes can be observed with MRI, including treatment-related improvement. MRI with conventional imaging sequences may be less sensitive than CT scan in the detection of calcification in cysticercosis. However, using appropriate sequences, microcalcifications can be detected.
The presence of a MCD raises a number of important management issues that include treatment modalities, prognosis, and genetic counseling. From a hierarchical level, the diagnosis of a MCD on a given patient with new onset seizures usually means that medical treatment is necessary. Although no control data on the natural history of untreated patients is available, most patients with a recognizable MCD have high recurrence rates following AED withdrawal. Although for the most part, any AED can be of use in these patients, some specific syndromes may respond better to certain AEDs. For example, tuberous sclerosis children with infantile spasms are particularly sensitive to Vigabatrin. The MRI findings in patients with MCDs are critical for the surgical decision-making process. For example, patients with MCDs that are generalized in nature, often have limited surgical treatment options. Conversely, a patient with intractable seizures and a focal MCD that is amenable to surgical surgery has the best chance for a cure. Furthermore, multiple correlative studies have indicated that surgical outcome correlates to completeness of lesion resection. Thus, MRI findings are crucial for surgical planning and outcome.
The MRI findings are also of particular help in patients with MCD for prognosis and genetic counseling. For prognostic purposes, certain imaging features have considerable weight. In cases of band heterotopia, the thickness of the subcortical band is directly correlated to motor, psychological, and seizure outcome. In schizencephaly, cleft size and bilaterality directly correlates with intellectual and motor outcome. As a rule, the more widespread the MCD, the worse developmental outcome is expected. For genetic counseling, MRI is becoming extremely important. The imaging findings can often lead to the correct molecular diagnosis. Recent studies have shown that certain imaging findings can define specific MCD syndromes and in turn, help identify the molecular defect. The best example is that of lissencephaly/band heterotopia syndrome in which the MRI pattern correlates with the genotype (FIG. 3). Pilz et al.26 showed that patients with predominantly parietooccipital agyria have LIS1 mutations, whereas frontal lobe malformations are likely to be secondary to double cortin (DCX) mutations Similarly, patients with familial periventricular nodular heterotopia have a higher likelihood of having Filamin (FLM) mutations than patients without a family history. Thus, imaging phenotype can define genotype and assist in the genetic counseling of these patients
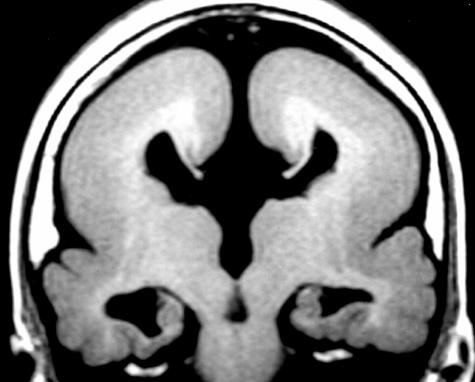
FIG. 3.
MRI of a patient with lisencephaly due to DCX mutation. Note thick gray matter smooth cortex. Genetic counseling and testing indicated in family.
Single-photon emission CT
Single-photon emission CT (SPECT) is a nuclear medicine imaging method that allows for the quantitative and qualitative evaluation of regional cerebral perfusion.27 SPECT is more readily available than positron emission tomography (PET) and is considerably less expensive. SPECT is not indicated for the majority of patients with epilepsy but has an important role in the investigation of surgical candidates. The use of SPECT in epilepsy stems from the known association of seizures with increased ictal regional cerebral perfusion or interictal decreases in perfusion.28 Numerous studies using dynamic and static SPECT have demonstrated interictal temporal hypoperfusion in up to 50% of patients with well-documented temporal lobe epilepsy. However, 5–10% of patients may demonstrate hypoperfusion in the contralateral temporal region raising the possibility of false lateralization.29 Although interictal studies may confirm lateralization in patients with well-defined MRI and EEG abnormalities, the studies tend to be redundant. However, interictal scans may be useful as baseline studies for comparison with ictal or postictal SPECT studies in selected patients.
In contrast to interictal studies, ictal or postictal SPECT studies in temporal lobe and extratemporal lobe epilepsy are accurate in the localization of the epileptogenic focus. Numerous studies have shown focal increased perfusion in up to 90% of patients.30 Ictal SPECT in extratemporal lobe epilepsy can also be useful for localization of ictal onset with sensitivity ranging between 60% and 90%.31 The yield of postictal studies decreases with the time of injection as demonstrated in various studies. The practice of combining an ictal with an interictal study is useful.31–35 More recently, it has been shown that the accuracy of this method may be enhanced by subtraction of the interictal from the ictal SPECT and then coregistration of the resulting images onto MRI [substraction ictal SPECT coregistered MRI (SISCOM)].36
SPECT and epilepsy management.
Interictal or ictal SPECT has limited value beyond the localization of ictal onset and the study of ictal propagation patterns. From the point of view of therapy and intervention, ictal SPECT has no clear role. The presence of interictal hypoperfusion does not correlate with response to AED treatment neither is predictive in terms of seizure outcome. However, certain ictal SPECT patterns have been shown to be predictive of surgical outcome in patients with subtypes of temporal and extratemporal lobe epilepsy according to one study. These findings have not been replicated, however, in large-scale populations. However, ictal SPECT studies appear to correlate with outcome when there is close concordance between the area of hyperperfusion and the resected area and vice versa with surgical failure when there is poor concordance.
PET
PET neuroimaging provides a wide array of functional and metabolic information to help understand mechanisms of neurologic diseases and guide therapeutic approaches.37 PET is most useful in patients with epilepsy who are candidates for surgery.38 Most studies have used 2-deoxy-2 (18F) fluoro-d-glucose (FDG) in the interictal state. In temporal lobe epilepsy, interictal studies show hypometabolic areas in the epileptogenic regions in approximately 70–80% of patients.39 The changes, however, are more extensive than the structural and EEG abnormalities and may involve the ipsilateral suprasylvian and parietal regions. When MRI is normal, PET may be indicated to aid in localization.
In extratemporal lobe epilepsy, interictal PET-FDG is not as sensitive, especially if the MRI is normal and the scalp EEG is nonfocal. However, PET has been reported to be more sensitive in neonates and infants with focal seizures because it is likely that, in those cases, a developmental malformation is present. When a single region of metabolic abnormality corresponding to the EEG abnormality is detected, surgical treatment is effective in controlling seizures and improving developmental outcome.
In contrast to FDG PET, ligand/neuroreceptor PET studies can improve sensitivity and specificity for temporal versus extratemporal lobe epilepsy and for particular clinical conditions. Unlike FDG, which shows decreased uptake in the epileptogenic region interictally, neuroreceptor tracers show increased or decreased uptake in epileptogenic brain regions in the interictal state. This represents a clear advantage over other imaging studies, especially when multiple structural lesions are present and potentially confound detection of the epileptogenic zone. The development of AMT ([11C]methyl-l-tryptophan) PET has had an impact on the differentiation between epileptogenic and nonepileptogenic lesions in the interictal state in children with tuberous sclerosis.40 Recent studies in nontuberous sclerosis patients have shown that AMT PET can occasionally detect epileptic cortex in patients with normal MRI,41 although further studies are needed. In addition, benzodiazepine-labeling studies with 11C-flumazenil, a central benzodiazepine receptor antagonist, have shown reduced binding in the epileptogenic focus. Flumazenil-labeled PET studies are more specific than FDG studies,42 but the findings may be modified by ictal/interictal interactions. Lastly, opiate, histamine, and N-methyl-d-aspartate receptor studies have been applied to epilepsy with preliminary results being difficult to estimate at this point. [11C]carfentanil studies, a μ-opiate agonist, have shown a reduction in binding in temporal lobe epilepsy (TLE). However, other opiate ligands such as [11C]methylnaltrindole show different patterns of activation.43
PET and epilepsy management.
Similar to SPECT, PET data either using FDG or receptor ligands have not been used in the pharmacological management of patients. PET remains a diagnostic modality for presurgical localization but potentially can be helpful in cases where there are diagnostic uncertainties. For example, the presence of focal hypermetabolism in the absence of EEG abnormalities may suggest ongoing epileptic activity and may indicate the need for intervention. Because PET is sensitive to certain AEDs (phenobarbital can cause diffuse hypometabolism), it is likely that the PET findings can be dynamically coupled to multiple factors making therapeutic decisions more difficult. It is unclear at the present time, whether receptor PET can provide information that may modify pharmacologic decisions.
NEW IMAGING TECHNIQUES AND THERAPEUTIC IMPLICATIONS
New imaging techniques include magnetic resonance spectroscopy (MRS), functional magnetic resonance imaging (fMRI), and magnetic source imaging (MSI). It should be noted, however, that clinical therapeutic data on these techniques are preliminary at present.
MRS
MRS can provide noninvasive biochemical measurements of specific brain metabolites.44 31P MRS or phosphorous spectroscopy is designed to measure phospholipid metabolism and high-energy phosphate compounds. Studies have demonstrated a consistent abnormality in the epileptogenic region characterized by abnormal phosphocreatine/inorganic phosphate ratios. 31P, however, is less sensitive than proton studies for localization, but whole brain studies are possible, albeit at a lower resolution, and therefore, 31P MRS can be potentially useful for therapy.45
1H spectroscopy has demonstrated abnormalities of N-acetyl-aspartate (NAA) a mitochondrial neuronal compound, creatine (Cr) and choline (Cho) in patients with epilepsy.46–49 The abnormalities typically consist of reduced NAA signal and increased choline, creatine and myoinositol signals.50 These MRS findings are consistent with the histopathological characteristics of reduced neuron cell counts with neuronal dysfunction and increased glial cellularity. In contrast, MRS shows normal metabolic function in primary generalized epilepsies.51
Clinical correlative studies suggest that proton MRS may be extremely sensitive in detecting metabolic changes in dysfunctional epileptogenic regions. In a large series of consecutive patients with TLE, Cendes et al.52 reported abnormal NAA/Cr values in at least one temporal lobe in all but one patient and were low bilaterally in 54%. Another study by Kuzniecky et al.53 showed similar findings with a high degree of lateralization and low level of negative findings patients who had pathologic confirmation of MTS.
MRS and epilepsy management.
Proton MRS has proven to be a sensitive measure to detect metabolic dysfunction in patients with TLE. However, it remains to be clarified whether this additional information adds to the overall management of the patients. With its high sensitivity, metabolite abnormalities can be detected in brain regions distinct from the seizure focus and it remains difficult to disentangle which abnormalities cause seizures or are their consequences. Some indication can be taken from a large study performed in 82 patients with refractory TLE that found in both the ipsilateral and contralateral temporal lobe a low NAA/Cr ratio that was negatively correlated with the duration of epilepsy.54 Patients with frequent generalized tonic-clonic seizures had lower NA/Cr than patients with no or rare generalized tonic-clonic seizures. Independent but supportive are the findings of reversibility of metabolic dysfunction in homotopic brain areas after successful surgery.55 This suggests that ongoing seizures may induce additional neuronal damage, which will progress in parallel to the duration of the epilepsy. If this is the case, MRS may be important in providing a metabolic marker for disease progression. One can speculate whether, in future years, MRS evidence of disease progression may modify treatment.
Another area with potential therapeutic impact is that of neurotransmitter MRS studies. Glutamate and γ-amino-butyric-acid (GABA) can be measured using MRS editing techniques. Research suggests that intracellular glutamate concentrations are elevated in the epileptogenic human hippocampus and neocortex.56 The high glutamate content may contribute to the epileptic state by increasing cellular excitability.
Studies have also shown that cellular glutamate content is abnormal in patients treated with antiepileptic drugs. MRS measurements have shown that cellular glutamate levels measured in occipital cortex are increased in patients with refractory complex partial seizures.57–59 Occipital lobe glutamate levels are below normal in over 40% of patients treated with carbamazepine, phenytoin, or valproate. Whether a decrease in brain glutamate is associated with improved seizure control requires serial measurements in a larger sample of patients.
GABA is the major inhibitory neurotransmitter in human cortex. MRS development has resulted in techniques for the measurement of intracellular GABA and other metabolites such as homocarnosine noninvasively, and safely in the brain of healthy human subjects.60–64
Studies support a key role for GABA levels and, in turn, GABA release in the regulation of cortical excitability and epilepsy. Primate models of photosensitive epilepsy have low GABA levels and seizures improve with GABAergic drugs.65,66 Similarly, AEDs that increase GABA or enhance GABAergic inhibition block the photoparoxysmal response in photosensitivity epilepsies.67,68 Petroff et al.59 showed that up to two-thirds of patients with refractory focal epilepsy treated with traditional AEDs have below normal occipital lobe GABA levels. The use of the traditional AEDs do not appear to have a major effect on intracellular GABA levels measured in the visual cortex.58,59,69 In patients with refractory localization-related epilepsies treated with carbamazepine, phenytoin, phenobarbital, primidone, valproate, or combinations of these drugs, cortical GABA levels are low. However, vigabatrin, topiramate, gabapentin, or zonisamide appears to be associated with increased cellular GABA. Topiramate and gabapentin clearly raise GABA in human brain but have no clear-cut effect in rodent models.70–73
In healthy controls without epilepsy, topiramate, gabapentin, and lamotrigine may increase intracellular brain GABA within 3 h of the first oral dose.72,74 In patients with refractory localization-related epilepsies, acute oral doses of gabapentin, topiramate, or vigabatrin increase intracellular GABA levels in the visual cortex within 1–2 h.70,71,75 Using GABA and glutamate imaging may help tailor AED selection to individualize therapy for the neurotransmitter/neurometabolic condition of the patient. An epilepsy patient with low GABA levels may benefit from an AED that increases cellular GABA or perhaps lowers cellular glutamate. Thus, it is possible that MRS may provide biochemical information useful when treating epilepsy in the near future.
fMRI
fMRI utilizes very rapid scanning techniques that theoretically can demonstrate alterations in blood oxygenation.2,76 fMRI has also been applied to the study of patients with epilepsy.76,77 The technique has been applied to the study of patients with epilepsy such as before cranial surgery to map with high accuracy functional areas such as language, motor, and visual cortices. A large number of fMRI studies have demonstrated primary sensorimotor cortex activation along the central sulcus during movement,77 including demonstration of the somatotopic organization of this region.
The clinical utility of such maps is in functional localization before surgery in this region for tumor or seizure focus resection. When the lesion is in close proximity to primary sensorimotor cortex along the central sulcus, precise localization of the activated region relative to the lesion can help predict whether a sensorimotor deficit is likely to occur from lesion resection. fMRI information is particularly useful when anatomical structures are distorted by mass effects, making it difficult to ascertain the location of the central sulcus with certainty.
fMRI can be of use in localizing language functions preoperatively to minimize postoperative language deficits.78 Several studies have found good correlation between Wada test and fMRI.79–81 Recent experience suggests that with simple paradigms and training, even children down to age 5–7 years are able to perform reliable task for fMRI. More recently, fMRI has been applied to study memory using improved stimulating paradigms and better imaging techniques. However, the technique has not been validated in a prospective manner.
fMRI and epilepsy management.
As stated above, fMRI has a role in the pre-surgical investigation of patients with epilepsy and particularly in those with lesions. There is no clear role for fMRI in the medical management of patients with epilepsy.
MSI
Magnetoencephalography (MEG), also commonly referred to as MSI when it is combined with structural imaging, provides a new noninvasive tool for epilepsy localization.82 MEG is similar to EEG, however, unlike electrical potentials measured with EEG, which are attenuated in strength and spatially blurred by tissues between brain and scalp surface, magnetic fields are minimally affected by intervening tissue layers.83 The potential advantage of MEG over EEG is based on greater accuracy of the observed signal at the scalp such that it allows cerebral sources to be modeled more simply; and this in turn allows for more clinically usable and reliable localization of brain activity.84 The clinical applications of MEG depend mostly on the ability to estimate the dipole source, which provides noninvasive information on the normal or abnormal function of discrete cortical areas. MSI is useful for presurgical localization of epilepsy and for localization of stimulus-induced normal neuronal function. This latter application is used for mapping the location of somatosensory and motor function in the presurgical planning of tumor or other lesion resection. Mapping of language and other cognitive functions is in development and preliminary results appear quite promising.
MEG is reliable for localization of spike sources in patients with no lesion visible on MRI, cystic lesions (post-traumatic encephalomalacia with prior surgical resection) in which MRI localization is ambiguous, and in patients with lesions of undetermined significance. The most important difference between MEG and EEG is the accuracy of source localization. Because MEG is more accurate than EEG for source localization, it may be useful in patients with bilateral synchronous spikes or those with multifocal spike discharges on EEG. The detection of cryptogenic lesions is one of the main goals of functional epilepsy imaging with PET or SPECT. MEG may have a similar, but even more important role by directly revealing the source of the epileptiform disturbance in relation to the cryptic pathology, tying together the epileptogenic significance of focal abnormalities on PET or SPECT. A recent large study85 demonstrated a sensitivity of 89% for lobar localization with critical information in 10% of surgical candidates.
MSI and epilepsy management.
MSI is increasingly useful in surgical planning for tumors or other epileptogenic lesions, such that costly invasive procedures and postoperative neurological deficits can be minimized. The management and outcome of a patient with a lesion near eloquent cortex is mostly dependent on the totality of resection of the epileptogenic area. MSI can provide noninvasively vital information about the possibility for complete resection. This information in conjunction with the location of the epileptogenic region can be critical to predict outcome. A critical question is whether MSI is predictive of outcome in the presence of a lesion. Recent data suggest that epilepsy outcome may be better if the identified dipole fit area is resected regardless of the lesion.86 Genow reported that, in five patients, four were seizure free when resection included the dipole area. Further studies are indicated.
Acknowledgments
Research was supported by FACES (Finding a Cure for Epilepsy and Seizures) and The CURE Foundation.
REFERENCES
- 1.Hauser W. Epilepsy: frequency, causes and consequences. New York: Demos Press, 1990.
- 2.Bammer R, Skare S, Newbould R, Liu C, Thijs V, Ropele S, Clayton DB, Krueger G, Moseley ME, Glover GH. Foundations of advanced magnetic resonance imaging. NeuroRx 2: 167–196, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bronen RA, Fulbright RK, Spencer SS, Spencer DD, Kim JH, Lange RC. Economic impact of replacing CT with MR imaging for refractory epilepsy. Magn Reson Imaging 15: 857–862, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Sá de Camargo EC, Koroshetz WJ. Neuroimaging of ischemia and infarction. NeuroRx 2: 265–276, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hankey G, Davies L, Gubbay SS. Long term survival with early childhood intracerebral tumours. J Neurol Neurosurg Psychiatry 52: 778–781, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyllie E, Rothner AD, Luders H. Partial seizures in children: clinical features, medical treatment, and surgical considerations. Pediatr Clin North Am 36: 343–364, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Gastaut H, Gastaut JL. Computerized transverse axial tomography in epilepsy. Epilepsia 17: 325–336, 1976. [DOI] [PubMed] [Google Scholar]
- 8.Earnest M, Feldman H, Marx J, Harris J, Biletch M, Sullivan E: Intracranial lesions shown by CT scans in 259 first alcohol related seizures. Neurology 38: 1561–1565, 1988. [DOI] [PubMed] [Google Scholar]
- 9.Mathieson G. Pathology of temporal lobe foci. In: Complex partial seizures and their treatment (Penry JK, Daly DD, eds), pp 163–185. New York: Raven Press, 1975. [PubMed]
- 10.Kuzniecky R, Murro A, King D, Morawetz R, Smith J, Powers R, et al. Magnetic resonance imaging in childhood intractable partial epilepsies: pathologic correlations. Neurology 43: 681–687, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Kuzniecky R. Magnetic resonance imaging in developmental disorders of the cerebral cortex. Epilepsia 35: S44–S56, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Kuzniecky R, Garcia JH, Faught E, Morawetz RB. Cortical dysplasia in TLE: MRI correlations. Ann Neurol 29: 293–298, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Babb TL, Pretorius JK. Pathological substrates of epilepsy. In: The treatment of epilepsy: principles and practice (Wylie E, ed), pp 55–70. Philadelphia: Lea & Febiger, 1993.
- 14.Cascino GD, Jack CR Jr, Parisi JE, Sharbrough FW, Hirschorn KA, Meyer FB, et al. Magnetic resonance imaging- based volume studies in temporal lobe epilepsy: pathological correlations. Ann Neurol 30: 31–36, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Cendes F, Andermann F, Gloor P, Evans A, Jones-Gotman M, Watson C, et al. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology 43: 719–725, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Kuzniecky R, de la Sayette V, Ethier R, Melanson D, Andermann F, Berkovic S, et al. Magnetic resonance imaging in temporal lobe epilepsy: pathological correlations. Ann Neurol 22: 341–347, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Jack C. MRI-based hippocampal volume measuremnts in epilepsy. Epilepsia 35: 14–19, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Barkovich A, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. Classification system for malformations of cortical development: Update 2001. Neurology 57: 2168–2178, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology 41: 965–972, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi E, D′Agostino MD, Lopes-Cendes I, Berkovic SF, Li ML, Andermann E, et al. Hippocampal atrophy and T2-weighted signal changes in familial mesial temporal lobe epilepsy. Neurology 60: 405–409, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Scott RC, Gadian DG, King MD, Chong WK, Cox TC, Neville BG, et al. Magnetic resonance imaging findings within 5 days of status epilepticus in childhood. Brain 125: 1951–1959, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Semah F, Lamy C, Demeret S. Hippocampal sclerosis and other hippocampal abnormalities in the early identification of candidates for epilepsy surgery. Arch Neurol 59: 1042–1043, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Kuzniecky R, Burgard S, Faught E, Morawetz R, Bartolucci A. Predictive value of magnetic resonance imaging in temporal lobe epilepsy surgery. Arch Neurol 50: 65–69, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Berkovic SF, McIntosh AM, Kalnins RM, Jackson GD, Fabinyi GC, Brazenor GA, et al. Preoperative MRI predicts outcome of temporal lobectomy: an actuarial analysis. Neurology 45: 1358–1363, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Paglioli E, Palmini A, Paglioli E, da Costa JC, Portuguez M, Martinez JV, et al. Survival analysis of the surgical outcome of temporal lobe epilepsy due to hippocampal sclerosis. Epilepsia 45: 1383–1391, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Pilz DT, Matsumoto N, Minnerath S, Mills P, Gleeson JG, Allen KM, W, et al. LIS1 and XLIS (DCX) mutations cause most classical lissencephaly, but different patterns of malformation. Hum Mol Genet 7: 2029–2037, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Devous MD Sr. Single-photon emission computed tomography in neurotherapeutics. NeuroRx 2: 237–249, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horsely V. An address on the origin and seat of epileptic disturbance. Br Med J 1: 693–696, 1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krausz Y, Cohen D, Konstantini S, Meiner Z, Yaffe S, Atlan H. Brain SPECT imaging in temporal lobe epilepsy. Neuroradiology 33: 274–276, 1991. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien TJ. SPECT: methodology. Adv Neurol 83: 11–32, 2000. [PubMed] [Google Scholar]
- 31.Laich E, Kuzniecky R, Mountz J, Liu HG, Gilliam F, Bebin M, et al. Supplementary sensorimotor area epilepsy: seizure localization, cortical propagation and subcortical activation pathways using ictal SPECT. Brain 120: 855–864, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Harvey A, Hopkins IJ, Bowe JM, Cook DJ, Shield LK, Berkovic SF. Frontal lobe epilepsy: clinical seizure characteristics and localization with ictal 99mTc-HMPAO SPECT. Neurology 43: 1966–1980, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Marks DA, Katz A, Hoffer P, Spencer SS. Localization of extratemporal epileptic foci during ictal single photon emission computed tomography. Ann Neurol 31: 250–255, 1992. [DOI] [PubMed] [Google Scholar]
- 34.O′Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Bohnen NI, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology 50: 445–454, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Spanaki MV, Spencer SS, Corsi M, MacMullan J, Seibyl J, Zubal IG. Sensitivity and specificity of quantitative difference SPECT analysis in seizure localization. J Nucl Med 40: 730–736, 1999. [PubMed] [Google Scholar]
- 36.Zubal IG, Spencer SS, Imam K, Seibyl J, Smith EO, Wisniewski G, Hoffer PB. Difference images calculated from ictal and interictal technetium-99m-HMPAO SPECT scans of epilepsy. J Nucl Med 36: 684–689, 1995. [PubMed] [Google Scholar]
- 37.Brooks DJ. Positron emission tomography and single-photon emission computed tomography in central nervous system drug development. NeuroRx 2: 226–236, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chugani DC, Chugani HT. New directions in PET neuroimaging for neocortical epilepsy. Adv Neurol 84: 447–456, 2000. [PubMed] [Google Scholar]
- 39.Engel JJ, Brown WJ, Kuhl DE, Phelps ME, Mazziotta JC, Crandall PH. Pathological findings underlying focal temporal lobe hypometabolism in partial epilepsy. Ann Neurol 12: 518–528, 1982. [DOI] [PubMed] [Google Scholar]
- 40.Juhasz C, Chugani DC, Muzik O, Shah A, Asano E, Mangner TJ, et al. α-Methyl-L-tryptophan PET detects epileptogenic cortex in children with intractable epilepsy. Neurology 60: 960–968, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Fedi M, Reutens D, Okazawa H, Andermann F, Boling W, Dubeau F, et al. Localizing value of α-methyl-l-tryptophan PET in intractable epilepsy of neocortical origin. Neurology 57: 1629–1636, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Koepp MJ, Hammers A, Labbe C, Woermann FG, Brooks DJ, Duncan JS. 11C-flumazenil PET in patients with refractory temporal lobe epilepsy and normal MRI. Neurology 54: 332–339, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Madar I, Lesser RP, Krauss G, Zubieta JK, Lever JR, Kinter CM, et al. Imaging of δ- and μ-opioid receptors in temporal lobe epilepsy by positron emission tomography. Ann Neurol 41: 358–367, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Lin A, Ross BD, Harris K, Wong W. Efficacy of proton magnetic resonance spectroscopy in neurological diagnosis and neurotherapeutic decision making. NeuroRx 2: 197–214, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuzniecky R, Elgavish GA, Hetherington HP, Evanochko WT, Pohost GM. In vivo 31P nuclear magnetic resonance spectroscopy of human temporal lobe epilepsy. Neurology 42: 1586–1590, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Cendes F, Andermann F, Preul PC, Arnold DL. Lateralization of temporal lobe epilepsy based on regional metabolic abnormalities in proton MRS. Ann Neurol 35: 211–216, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Hetherington H, Kuzniecky R, Pan J, Mason G, Morawetz R, Harris C, et al. Proton nuclear magnetic resonance spectroscopic imaging of human temporal lobe epilepsy. Ann Neurol 38: 396–404, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Hugg JW, Laxer KD, Matson GB, Maudsley AA, Weiner MW. Neuron loss localizes human temporal lobe epilepsy by in vivo proton magnetic resonance spectroscopic imaging. Ann Neurol 34: 788–794, 1993. [DOI] [PubMed] [Google Scholar]
- 49.Li LM, Caramanos Z, Cendes F, Andermann F, Antel SB, Dubeau F, et al. Lateralization of temporal lobe epilepsy (TLE) and discrimination of TLE from extra-TLE using pattern analysis of magnetic resonance spectroscopic and volumetric data. Epilepsia 41: 832–842, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Maton BM, Kuzniecky RI. Proton MRS: N-acetyl aspartate, creatine, and choline. Adv Neurol 83: 253–259, 2000. [PubMed] [Google Scholar]
- 51.Cendes F, Stanley J, Dubeau F, Anderamnn F, Arnold DL. MR spectroscopy differentiates lactate in complex partial versus absence seizures. Ann Neurol 41: 74–81, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Cendes F, Caramanos Z, Andermann F, Dubeau F, Arnold D. Proton MRSI and MRI volumetry in the lateralization of temporal lobe epilepsy: a series of 100 patients. Ann Neurol 42: 737–746, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Kuzniecky R, Hugg JW, Hetherington H, Butterworth E, Bilir E, Faught E, et al. Relative utility of 1H spectroscopic imaging and hippocampal volumetry in the lateralization of mesial temporal lobe epilepsy. Neurology 51: 66–71, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Bernasconi A, Tasch E, Cendes F, Li LM, Arnold DL. Proton magnetic resonance spectroscopic imaging suggests progressive neuronal damage in human temporal lobe epilepsy. Prog Brain Res 135: 297–304, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Hugg JW, Kuzniecky RI, Gilliam FG, Morawetz RB, Fraught RE, Hetherington HP. Normalization of contralateral metabolic function following temporal lobectomy demonstrated by 1H magnetic resonance spectroscopic imaging. Ann Neurol 40: 236–239, 1996. [DOI] [PubMed] [Google Scholar]
- 56.Petroff OA. GABA and glutamate in the human brain. Neuroscientist 8: 562–573, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Petroff OA, Rothman DL, Behar KL, Mattson RH. Initial observations on effect of vigabatrin on in vivo 1H spectroscopic measurements of gamma-aminobutyric acid, glutamate, and glutamine in human brain. Epilepsia 36: 457–464, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Petroff OA, Rothman DL, Behar KL, Hyder F, Mattson RH. Effects of valproate and other antiepileptic drugs on brain glutamate, glutamine, and GABA in patients with refractory complex partial seizures. Seizure 8: 120–127, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Petroff OA, Mattson RH, Rothman DL. Proton MRS: GABA and glutamate. Adv Neurol 83: 261–271, 2000. [PubMed] [Google Scholar]
- 60.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of γ-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci USA 90: 5662–5666, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hyder F, Petroff OA, Mattson RH, Rothman DL. Localized 1H NMR measurements of 2-pyrrolidinone in human brain in vivo. Magn Reson Med 41: 889–896, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Shen J, Rothman DL, Brown P. In vivo GABA editing using a novel doubly selective multiple quantum filter. Magn Reson Med 47: 447–454, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Hanstock CC, Coupland NJ, Allen PS. GABA X2 multiplet measured pre- and post-administration of vigabatrin in human brain. Magn Reson Med 48: 617–623, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Terpstra M, Ugurbil K, Gruetter R. Direct in vivo measurement of human cerebral GABA concentration using MEGA-editing at 7 Tesla. Magn Reson Med 47: 1009–1012, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Menini C, Silva-Barrat C. The photosensitive epilepsy of the baboon. A model of generalized reflex epilepsy. Adv Neurol 75: 29–47, 1998. [PubMed] [Google Scholar]
- 66.Lloyd KG, Scatton B, Voltz C, Bryere P, Valin A, Naquet R. Cerebrospinal fluid amino acid and monoamine metabolite levels of Papio papio: correlation with photosensitivity. Brain Res 363: 390–394, 1986. [DOI] [PubMed] [Google Scholar]
- 67.Kasteleijn-Nolst Trenite DG. Reflex seizures induced by intermittent light stimulation. Adv Neurol 75: 99–121, 1998. [PubMed] [Google Scholar]
- 68.Rimmer EM, Milligan NM, Richens A. A comparison of the acute effect of single doses of vigabatrin and sodium valproate on photosensitivity in epileptic patients. Epilepsy Res 1: 339–346, 1987. [DOI] [PubMed] [Google Scholar]
- 69.Petroff OA, Behar KL, Rothman DL. New NMR measurements in epilepsy. Measuring brain GABA in patients with complex partial seizures. Adv Neurol 79: 939–945, 1999. [PubMed] [Google Scholar]
- 70.Petroff OA, Hyder F, Rothman DL, Mattson RH. Effects of gabapentin on brain GABA, homocarnosine, and pyrrolidinone in epilepsy patients. Epilepsia 41: 675–680, 2000. [DOI] [PubMed] [Google Scholar]
- 71.Petroff OA, Hyder F, Rothman DL, Mattson RH. Topiramate rapidly raises brain GABA in epilepsy patients. Epilepsia 42: 543–548, 2001. [DOI] [PubMed] [Google Scholar]
- 72.Kuzniecky R, Ho S, Pan J, Martin R, Gilliam F, Faught E, et al. Modulation of cerebral GABA by topiramate, lamotrigine, and gabapentin in healthy adults. Neurology 58: 368–372, 2002. [DOI] [PubMed] [Google Scholar]
- 73.Errante LD, Williamson A, Spencer DD, Petroff OA. Gabapentin and vigabatrin increase GABA in the human neocortical slice. Epilepsy Res 49: 203–210, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Kuzniecky R, Hetherington H, Ho S, Pan J, Martin R, Gilliam F, et al. Topiramate increases cerebral GABA in healthy humans. Neurology 51: 627–629, 1998. [DOI] [PubMed] [Google Scholar]
- 75.Petroff OA, Hyder F, Collins T, Mattson RH, Rothman DL. Acute effects of vigabatrin on brain GABA and homocarnosine in patients with complex partial seizures. Epilepsia 40: 958–964, 1999. [DOI] [PubMed] [Google Scholar]
- 76.Detre JA, Sirven JI, Alsop DC, O’Connor MJ, French JA. Localization of subclinical ictal activity by functional magnetic resonance imaging: correlation with invasive monitoring. Ann Neurol 38: 618–624, 1995. [DOI] [PubMed] [Google Scholar]
- 77.Detre JA, Floyd TF. Functional MRI and its applications to the clinical neurosciences. Neuroscientist 7: 64–79, 2001. [DOI] [PubMed] [Google Scholar]
- 78.Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology 46: 978–984, 1996. [DOI] [PubMed] [Google Scholar]
- 79.Sabbah P, Chassoux F, Leveque C, Landre E, Baudoin-Chial S, Devaux B, et al. Functional MR imaging in assessment of language dominance in epileptic patients. Neuroimage 18: 460–467, 2003. [DOI] [PubMed] [Google Scholar]
- 80.Rutten GJ, Ramsey NF, van Rijen PC, Alpherts WC, van Veelen CW. FMRI-determined language lateralization in patients with unilateral or mixed language dominance according to the Wada test. Neuroimage 17: 447–460, 2002. [DOI] [PubMed] [Google Scholar]
- 81.Gao X, Jiang C, Lu C, Shen T. Determination of the dominant language hemisphere by functional MRI in patients with temporal lobe epilepsy. Chin Med J (Engl) 114: 711–713, 2001. [PubMed] [Google Scholar]
- 82.Gallen CC, Hirschkoff EC, Buchanan DS. Magnetoencephalography and magnetic source imaging. Capabilities and limitations. Neuroimaging Clin N Am 5: 227–249, 1995. [PubMed] [Google Scholar]
- 83.Ricci GB, Romani GL, Salustri C, Pizzella V, Torrioli G, Buonomo S, et al. Study of focal epilepsy by multichannel neuromagnetic measurements. Electroencephalogr Clin Neurophysiol 66: 358–368, 1987. [DOI] [PubMed] [Google Scholar]
- 84.Ebersole JS. Magnetoencephalography/magnetic source imaging in the assessment of patients with epilepsy. Epilepsia 38: S1–S5, 1997. [DOI] [PubMed] [Google Scholar]
- 85.Stefan H, Hummel C, Scheler G, Genow A, druschky K, Tilz C, et al. Magnetic brain source imaging of focal epileptic activity: a synopsis of 455 cases. Brain 126: 2396–2405, 2003. [DOI] [PubMed] [Google Scholar]
- 86.Genow A, Hummel C, Scheler G, Hopfengartner R, Kaltenhauser M, Buchfelder M, et al. Epilepsy surgery, resection volume and MSI localization in lesional frontal lobe epilepsy. Neuroimage 21: 444–449, 2004. [DOI] [PubMed] [Google Scholar]