Abstract
A plant growth-promoting bacterium, Kluyvera ascorbata SUD165, that contained high levels of heavy metals was isolated from soil collected near Sudbury, Ontario, Canada. The bacterium was resistant to the toxic effects of Ni2+, Pb2+, Zn2+, and CrO4−, produced a siderophore(s), and displayed 1-aminocyclopropane-1-carboxylic acid deaminase activity. Canola seeds inoculated with this bacterium and then grown under gnotobiotic conditions in the presence of high concentrations of nickel chloride were partially protected against nickel toxicity. In addition, protection by the bacterium against nickel toxicity was evident in pot experiments with canola and tomato seeds. The presence of K. ascorbata SUD165 had no measurable influence on the amount of nickel accumulated per milligram (dry weight) of either roots or shoots of canola plants. Therefore, the bacterial plant growth-promoting effect in the presence of nickel was probably not attributable to the reduction of nickel uptake by seedlings. Rather, it may reflect the ability of the bacterium to lower the level of stress ethylene induced by the nickel.
Pollution of the biosphere by toxic metals has accelerated dramatically since the beginning of the industrial revolution. The primary sources of this pollution include the burning of fossils fuels, mining and smelting of metalliferous ores, municipal wastes, fertilizers, pesticides, and sewage. Toxic-metal contamination of groundwater and soil, which poses a major environmental and human health problem, is currently in need of an effective and affordable technological solution. Moreover, unlike organic pollutants, metals cannot be degraded to harmless products, such as carbon dioxide, but instead persist indefinitely in the environment, complicating their remediation.
Living plants have the ability to accumulate heavy metals from soil and water, in particular heavy metals which are essential for their growth and development (3, 36). Certain plants also have the ability to accumulate heavy metals which have no known biological function (8). However, excessive accumulation of these metals can be toxic to most plants. Heavy metals ions, when present at an elevated level in the environment, are adsorbed by roots and translocated to different plant parts, leading to impaired metabolism and reduced growth (5, 16).
Phytoremediation, i.e., the use of green plants to remove, contain, or render harmless environmental contaminants, is considered to be an attractive alternative to the approaches that are currently in use for dealing with heavy metal contamination (6, 10, 11, 13, 41). Phytoremediation of metals might take one of several forms: phytoextraction, rhizofiltration, or phytostabilization. Phytoextraction refers to processes in which plants are used to concentrate metals from the soil into the roots and shoots of the plant; rhizofiltration is the use of plant roots to remove metals from effluents; and phytostabilization is the use of plants to reduce the mobility of heavy metals (and thereby reduce the spread of these metals in the environment). Recently, metal-tolerant plants have been used to vegetate and control soil erosion on metal mine tailings and waste piles, i.e., phytostabilization (13, 40). Moreover, there are a number of reports of using metal accumulating plants to remove toxic metals from soil, i.e., phytoextraction-also called phytodecontamination (2, 10, 11, 13, 32).
In the environment, the roots of plants interact with a large number of different microorganisms, and these interactions, together with the soil conditions, are major determinants of the extent to which plants grow and proliferate (20, 34). We previously reported that many plant growth-promoting bacteria, i.e., free-living soil bacteria that are involved in a beneficial association with plants, contain the enzyme 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase (19, 20, 30). It was hypothesized that this enzyme, which has no known function in bacteria, might be part of a hitherto undescribed mechanism used by certain bacteria to stimulate plant growth (21). This could occur by ACC deaminase modulating the level of ethylene in developing plants (18, 22, 23).
It is well documented that plants respond to a variety of different environmental stresses by synthesizing “stress” ethylene (1, 28). In fact, a significant portion of the damage to plants from environmental stress—such as infection with fungal phytopathogens—may occur as a direct result of the response of the plant to the increased level of stress ethylene (46). In the presence of fungal pathogens, not only does exogenous ethylene increase the severity of a fungal infection but also inhibitors of ethylene synthesis can significantly decrease the severity of infection. Since the enzyme ACC deaminase, when present in plant growth-promoting bacteria, can act to modulate the level of ethylene in a plant, we sought in the work reported here to test whether such bacteria might lower the stress placed on plants by the presence of heavy metals and therefore ameliorate some of the apparent toxicity of heavy metals to plants.
MATERIALS AND METHODS
Media.
The basic mineral medium used for isolation and growth of nickel-resistant bacteria was the Tris-buffered low-phosphate (TLP) medium described by Mergeay et al. (35) and supplemented with trace metals (1 ml of a stock solution of trace metals per liter, where the stock solution consists of [in grams per liter] FeSO4 · 7H2O, 0.2; ZnSO4 · 7H2O, 0.01; MnCl2 · 4H2O, 0.003; CoCl2 · 6H2O, 0.02; CuCl2 · 6H2O, 0.001; NiCl2 · 6H2O, Na2MoO4 · 2H2O, 0.5; H3BO3, 0.03) (7) and 0.2% sodium gluconate. For solid medium, Bacto Agar (Difco) was added at 2% (wt/vol). Stock solutions of nickel chloride (1 and 0.1 M) were autoclaved and added to the medium as required. Cell counts were determined by using nutrient broth agar (Difco). Utilization of carbon sources was investigated by supplementing M9 minimal medium (37) with various organic compounds (0.2%, wt/vol). For testing resistance to antibiotics and metals, stock solutions were filter sterilized and then added to sterile TLP medium.
Selection of nickel-resistant bacteria.
Soil samples were taken from metal-contaminated wetlands near Sudbury, Ontario, Canada, an area which had been exposed to heavy metal contamination from mines wastes following the discovery of a massive nickel-copper ore body (17). The soil sample from which the selected nickel-resistant plant growth-promoting bacterium was isolated contained a range of different metals including high levels of both nickel and copper (Table 1). From the data presented in this table, the nickel concentration in this soil is estimated to be approximately 4 mM.
TABLE 1.
Characteristics and heavy metal content of the soil sample used for the isolation of K. ascorbata SUD165
| Metal or characteristic | Value |
|---|---|
| Metal content (mg/kg [dry wt] of soil) | |
| Barium | 35.2 |
| Cadmium | 0.25 |
| Chromium | 36.0 |
| Cobalt | 57.9 |
| Copper | 219.0 |
| Lead | 15.1 |
| Nickel | 752.0 |
| Silver | 0.76 |
| Vanadium | 33.4 |
| Zinc | 94.5 |
| Loss on ignition (%) | 28.8 |
| Moisture content (%) | 67.3 |
| Total carbon (%) | 19.3 |
| Total inorganic carbon (%) | <0.01 |
| pH | 5.58 |
A 20-ml volume of TLP medium was added to 2 g of soil, and the suspension was incubated at 25°C for 2 h in a rotary shaker (400 rpm). The suspension was then allowed to stand for about 1 h before 0.3 ml of supernatant was spread over solid TLP medium containing 0.2% (wt/vol) gluconate, trace elements, and 1 mM NiCl2. The plates were incubated for 2 days at 30°C. Nickel-resistant colonies were purified on the same medium and then tested for the ability to grow on TLP medium with ACC as the only source of nitrogen.
Siderophore assay.
Following growth on King’s medium B without phosphate (38), siderophores secreted to the growth medium were detected and quantified by the “universal” method of Schwyn and Neilands (43). The siderophore desferal mesylate (Sigma) was used to produce a standard curve for 0.5 to 25 mM siderophore. The incubation period for the standard was 24 h at room temperature.
Root and shoot elongation assay.
Canola (Brassica campestris cv. Tobin) seeds were kindly provided by G. Brown (Agrium, Inc., Saskatoon, Sask., Canada). Seeds were surface sterilized by soaking for 10 min in 1.5% sodium hypochlorite and then thoroughly rinsed with sterile distilled water. The sterilized seeds were incubated for 1 h at room temperature either in sterile distilled water as a blank control or in a bacterial suspension in distilled water adjusted to an absorbance of 0.025 (∼3.0 × 107 CFU · ml−1) or 0.5 (∼7.4 × 108 CFU · ml−1) at 600 nm. Six canola seeds were placed in each seed-pack growth pouch (125 by 157 mm; Mega International) that had been filled with 10 ml of distilled water or 10 ml of 1 or 2 mM NiCl2 and autoclaved for 30 min at 121°C before the addition of the cells. Ten replicate pouches were used for each treatment. The pouches were incubated upright in a plastic tray partially filed with an amount of water sufficient to cover the bottom. The tray was covered with transparent Saran Wrap. The pouches were incubated at 25°C for 4 days in a growth chamber with a 12-h photoperiod and a light intensity of 12.9 μmol/m2/s. The growth-promoting effect of the bacterium was determined at a light intensity of 0.92 μmol/m2/s. At the end of 4 to 5 days of incubation, the pouches were opened and the seedling root and shoot length were measured. The results of these experiments are presented as the tolerance index, TI (49), where TI = RLm/RLc, where RLm is the root length of plants grown in the presence of a specific added metal and RLc is the root length of plants grown in the absence of that metal. The TI may also be expressed as the ratio of the shoot lengths of plants grown in the presence and absence of a specific added metal.
Pot experiments.
Plants were sown in plastic pots (top diameter, 77 mm; bottom diameter, 55 mm; height, 66 mm) filled with approximately 100 g of air-dried Pro-Mix ’BX’ general-purpose growth medium (General Horticulture, Inc., Red Hill, Pa.). Pro-Mix ’BX’ is a peat-based growth medium designed for cultivation of horticultural greenhouse plants; it contains 75 to 85% sphagnum peat moss, perlite, vermiculite, macronutrients (calcium, magnesium, nitrogen, phosphorus, potassium, and sulfur), micronutrients (boron, copper, iron, magnesium, molybdenum, and zinc), calcitic limestone, and a wetting agent. The plants were grown in a growth chamber at 25°C (canola) or at 20°C (tomato [Lycopersican esculentum Mill. cv Heinz 1439VF 402A; Stokes Seeds Ltd., St. Catherines, Ontario, Canada]) under a 12-h light/dark photoperiod with a light intensity of 12.9 μmol/m2/s. The pots were irrigated with either 250 ml distilled water or a solution of nickel chloride and incubated for 4 days (canola) or 10 days (tomato). The moisture content of the soil was maintained by placing each pot in a polyethylene bag. A total of 30 seeds were sown per pot, and 60 seeds were used per treatment.
For seed inoculation, bacteria were grown in TLP with 0.2% gluconate, 0.01% Casamino Acids, trace elements, and 0.1 mM NiCl2 at 25°C until late log phase. The cells were then pelleted by centrifugation at 25,000 × g for 10 min and washed twice with sterile distilled water. Bacterial suspensions in distilled water were adjusted to an absorbance at 600 nm of either 0.5 or 0.025 and then used for seed inoculation.
ACC deaminase determination.
The ACC deaminase activity of cell extracts was determined by estimating α-ketobutyrate production (in nanomoles per milligram of protein per hour) by the procedure of Honma and Shimomura (27).
NiCl2 uptake.
The seeds were sown in small pouches (60 by 40 mm) in the presence of 1 ml of 1 mM NiCl2 and 5 mCi of 63NiCl2 (Amersham) per ml. After a 4-day incubation at 25°C, shoots and roots were harvested separately and washed extensively, first in several changes of 0.01 M EDTA and then in distilled water to remove any nonspecifically bound radioactivity, dried overnight at 105°C, and weighed. The amount of 63Ni2+ accumulated by a plant root or shoot was determined with a liquid scintillation counter (Beckman LS1701). Each experimental point included three pouches with six seeds in each pouch.
Ethylene measurement.
The production of ethylene by canola seedlings was measured with a GOW-MAC model 69-750 gas chromatograph. Eight seeds were sown in each growth pouch (65 by 55 mm) containing 1 ml of 2 mM nickel chloride. Some seeds were pretreated with a bacterial suspension with an absorbance of 0.5 at 600 nm. After incubation for 4 days in a growth chamber at 25°C under a 12-h light/dark photoperiod with a light intensity of 12.9 μmol/m2/s, the growth pouches were placed in sealed 250-ml bottles for 18 h before samples were taken for gas chromatographic analysis.
Statistical analysis.
Pouch and pot experiments were set up in a randomized block design. Data were analyzed by means of analysis of variance (ANOVA), and treatment means were compared by Duncan’s multiple-range test.
RESULTS
Selection of a nickel-resistant bacterial strain.
Nickel is an essential micronutrient for many microorganisms (25). However, at millimolar concentrations, nickel inhibits the growth of most wild-type bacteria and is tolerated by only a minority of microorganisms (42). Nevertheless, we have isolated nickel-resistant bacteria from highly polluted nickel- and copper-containing soil (Table 1) by using a spread plate procedure with pH-neutral TLP medium. This medium is designed to avoid the precipitation of heavy metal salts at 1 mM. In total, there were approximately 4 × 103 nickel-resistant bacteria per g (dry weight) of soil, or about 1% of the total bacterial population culturable on TLP medium.
To isolate plant growth-promoting bacteria, all of the nickel-resistant isolates were tested for the ability to grow on minimal medium with ACC as the sole source of nitrogen (19). Approximately 7% of the nickel-tolerant strains also had the ACC+ phenotype.
Finally, nickel-resistant bacterial strains that were also able to grow on ACC were tested for the ability to produce siderophores. Based on the idea that bacterial siderophores might facilitate the uptake of nickel by plants, the best siderophore-producing strain (designated SUD165) was selected for subsequent study.
Identification and properties of strain SUD165.
The microorganism isolated was gram negative, motile, methyl red positive, indole positive, and citrate positive. On MacConkey agar, it fermented d-glucose, d-galactose, d-mannitol, d-mannose, d-sorbitol, l-arabinose, and sucrose but not lactose. SUD165 cells grew on M9 minimal medium with the above-mentioned sugars and salicin but not on M9 with lactose. On nutrient agar or solid TLP medium plus gluconate, the bacterium formed circular white colonies with an “entire” edge. On solid TLP medium plus gluconate, the bacterial colonies had a metallic blue sheen. These results suggest that the bacterium belongs to the family Enterobacteriaceae. Strain SUD165 was subsequently characterized by fatty acid analysis by Microbial ID, Inc. (Newark, Del.) as Kluyvera ascorbata (15).
K. ascorbata SUD165 was able to grow in nutrient broth (Difco) at temperatures from 5 to 37°C, with an optimal growth temperature at 20°C. On solid TLP medium with gluconate, the strain was resistant to 1 mM Ni2+, 1 mM Zn2+, 1 mM Pb2+, 0.1 mM CrO4−, 100 μg of penicillin G per ml, 25 μg of carbenicillin per ml, and 25 μg of ampicillin per ml. It was sensitive (i.e., no growth was observed) to 0.01 mM Hg2+, 0.25 mM Co2+, 0.25 mM Cd2+, 0.25 mM Cu2+, 50 μg of streptomycin per ml, 20 μg of tetracycline per ml, 50 μg of kanamycin per ml, and 25 μg of chloroamphenicol per ml.
Effect of nickel on K. ascorbata SUD165 growth.
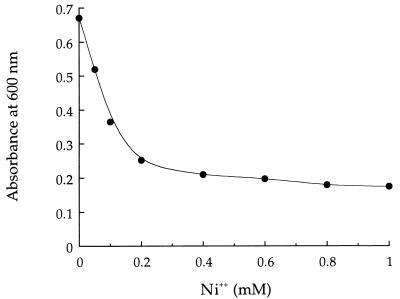
An overnight culture of K. ascorbata SUD165 grown in TLP medium without nickel was used to inoculate TLP medium containing different concentrations (0.1 to 1.0 mM) of nickel chloride. After 18 h of growth at 25°C in a rotary shaker (200 rpm), the absorbance at 600 nm of the cell suspensions was measured. At all of the concentrations of Ni2+ tested, cell growth was inhibited to some extent (Fig. 1). When added to a nongrowing bacterial suspension in distilled water, the metal did not decrease the number of CFU in the suspension after 5 days of incubation at 25°C (data not shown).
FIG. 1.
Influence of Ni2+ on the growth of K. ascorbata SUD165. Cells were grown for 18 h at 25°C in TLP medium plus gluconate.
Plant growth-promoting activity of K. ascorbata SUD165.
Since K. ascorbata was selected on the basis of its ability to utilize ACC as the sole source of nitrogen, it was reasonable to expect that it might contain ACC deaminase activity and, moreover, that it would be able to stimulate the growth of plant roots by hydrolyzing ACC from germinating seeds, thereby lowering the level of ACC and hence the level of ethylene in seeds. In fact, a cell extract of K. ascorbata SUD165 cells displayed a low level of ACC deaminase activity (26 nmol/mg/h, compared with 300 nmol/mg/h for the well-characterized plant growth-promoting bacterium Pseudomonas putida GR12-2) (44). Moreover, K. ascorbata SUD165 stimulated canola root elongation (Table 2) under conditions in which the light level was low, a condition often required to observe a significant root elongation effect (33). Furthermore, as expected, K. ascorbata SUD165 cells had the ability to bind to canola seed coats as visualized by scanning electron microscopy (data not shown).
TABLE 2.
Effect of K. ascorbata SUD165 on canola root elongation in gnotobiotic growth pouches
| Expt | SUD165 present | Root length, mm (mean ± SEM)a |
|---|---|---|
| 1 | No | 44.7 ± 3.8 |
| Yes | 59.2 ± 3.9 | |
| 2 | No | 36.1 ± 2.5 |
| Yes | 47.5 ± 3.0 | |
| 3 | No | 45.5 ± 3.1 |
| Yes | 55.7 ± 2.3 |
The values for the two treatments differ significantly (P < 0.001) in all three experiments. Data were analyzed by ANOVA, with 55 to 60 seeds tested for each value reported. The absorbance at 600 nm of the bacterial suspension was 0.025.
Effect of nickel on canola and tomato seedlings.
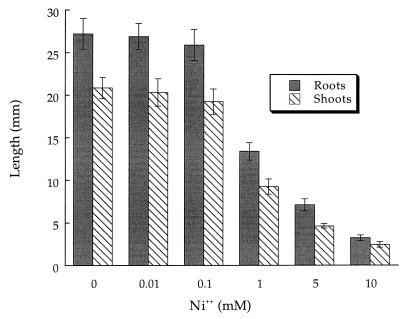
A series of experiments to study the sensitivity of canola to Ni2+ cations in growth pouches was undertaken (Fig. 2). These experiments revealed that canola seeds developed normally even in the presence of up to 0.1 mM nickel chloride. Above this concentration, plant root and shoot elongation was inhibited.
FIG. 2.
Influence of Ni2+ on canola seedling development in growth pouches. The error bars represent 1 SEM. Values differ significantly (P < 0.001) from those for the control group when [Ni2+] is 1 mM or greater. Data were analyzed by ANOVA with 50 to 60 seedlings in each treatment group. The results of a typical experiment are shown.
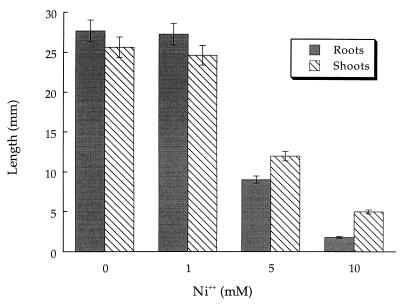
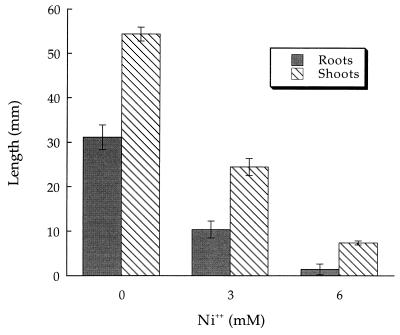
In pot experiments, i.e., in the presence of soil (Fig. 3), a higher concentration of Ni2+ was required to noticeably inhibit canola root and shoot length than in growth pouch experiments. The apparent lower level of toxicity of Ni2+ in soil most probably represents the binding of Ni2+ to soil particles, thereby making the cation unavailable to the developing seedlings. Tomato plants grown in pots were somewhat more sensitive to Ni2+ than were canola plants grown in pots (Fig. 4). With both tomatoes and canola grown in pots, the roots appeared more sensitive to the inhibitory effects of Ni2+ than did the shoots. With canola seedlings in growth pouches, the roots and shoots were equally sensitive to growth inhibition by Ni2+.
FIG. 3.
Influence of Ni2+ on canola seedling development in pots. The error bars represent 1 SEM. Values differ significantly (P < 0.001) from those for the control group when [Ni2+] is 5 mM or greater. Data were analyzed by ANOVA with 55 to 60 seedlings in each treatment group. The results of a typical experiment are shown.
FIG. 4.
Influence of Ni2+ on tomato seedling development in pots. The error bars represent 1 SEM. Values differ significantly (P < 0.001) from those for the control group at all Ni2+ concentrations. Data were analyzed by ANOVA with 55 to 60 seedlings in each treatment group. The results of a typical experiment are shown.
Effect of K. ascorbata SUD165 on the toxicity of nickel to canola and tomato seedlings.
The effect of adding K. ascorbata SUD165 to canola or tomato seeds before germinating the seeds, either in a growth pouch (canola only) or in pots (canola and tomato), in the presence of inhibitory concentrations of Ni2+ was examined (Table 3). The results of these experiments are presented as a TI, making it easier to compare the effects of different treatments. A TI of 1.0 indicates that the treatment was not inhibitory, while a TI of 0.1 indicates that the growth of treated plants was only 10% of the growth of the control. Examination of the data in Table 3 shows that at all concentrations of nickel tested (1 to 6 mM Ni2+), using both a low-level and a high-level bacterial cell treatment (cell suspension absorbance of 0.025 or 0.50, respectively), with both canola and tomato plants, with both roots and shoots, and in both gnotobiotic growth pouches and pots, the addition of K. ascorbata SUD165 significantly decreased the toxicity of the added nickel. The effect was highly reproducible in spite of variation in the root and shoot lengths. Moreover, the protective effect of K. ascorbata SUD165 increased as the density of the cell suspension increased.
TABLE 3.
Effect of K. ascorbata SUD165 on the toxicity of nickel to canola and tomato seedlings grown in gnotobiotic growth pouches or in soil in pots
| Expta | Organ | Ni2+ concn (mM) | TIb
|
||
|---|---|---|---|---|---|
| Ni2+ only | Ni2+ + cellsc | Ni2+ + cellsd | |||
| 1 | Shoots | 1 | 0.48 | 0.75 | NDe |
| Roots | 1 | 0.57 | 0.85 | ND | |
| 2 | Shoots | 2 | 0.22 | 0.49 | 0.71 |
| Roots | 2 | 0.11 | 0.34 | 0.54 | |
| 3 | Shoots | 2 | 0.22 | 0.47 | ND |
| Roots | 2 | 0.08 | 0.32 | ND | |
| 4 | Shoots | 1 | 0.44 | 0.71 | 0.81 |
| Roots | 1 | 0.49 | 0.73 | 0.87 | |
| 5 | Shoots | 6 | 0.12 | ND | 0.18 |
| Roots | 6 | 0.23 | ND | 0.33 | |
| 6 | Shoots | 6 | 0.18 | ND | 0.41 |
| Roots | 6 | 0.23 | ND | 0.36 | |
| 7 | Shoots | 3 | 0.54 | ND | 0.70 |
| Roots | 3 | 0.57 | ND | 0.80 | |
| 8 | Shoots | 4 | 0.42 | ND | 0.59 |
| Roots | 4 | 0.35 | ND | 0.56 | |
Experiments were performed with canola (experiments 1 to 6) and tomato (experiments 7 and 8) seedlings grown in gnotobiotic growth pouches (experiments 1 to 4) or in soil in pots (experiments 5 to 8).
The values for the different treatments differ significantly from the untreated control and from each other (P < 0.001). Each value (including the seeds grown in the absence of nickel, i.e., TI = 1.0) reflects the mean of the response of 50 to 60 seeds.
K. ascorbata SUD165 cells diluted to an absorbance at 600 nm of 0.025.
K. ascorbata SUD165 cells diluted to an absorbance at 600 nm of 0.50.
ND, not determined.
63Ni2+ accumulation by canola seedlings.
Since plants growing in metal-enriched environments take up nickel to different degrees in response to external and internal factors (40), it is important to assess whether the addition of K. ascorbata SUD165 affects the uptake of nickel by canola seedlings. In five independent experiments in which the total radioactivity incorporated into 10 to 15 roots or shoots was measured, the presence of K. ascorbata SUD165 did not change the amount of nickel taken up per milligram (dry weight) of either roots (1,430 ± 83.7 and 1,370 ± 380 pmol/mg in the absence and presence of K. ascorbata SUD165, respectively) or shoots (178 ± 42.3 and 174 ± 64.5 pmol/mg, respectively); results are mean ± standard error of the mean (SEM). By this measure, although K. ascorbata SUD165 decreased the toxicity of Ni2+ to canola, it had no influence on amount of Ni2+ accumulated by the plant.
Ethylene production by plants grown in the presence of nickel.
When a suspension of K. ascorbata SUD165 cells was used to treat canola seeds which were subsequently grown in gnotobiotic growth pouches in the presence of 2 mM Ni2+, the amount of ethylene that was evolved over 18 h decreased from 590 ± 182 nmol/mg (dry weight) in the absence of the bacterium to 275 ± 90 nmol/mg in the presence of the bacterium. The variation notwithstanding, ethylene levels were always higher in the absence of the bacterium each of the four times that this measurement was performed.
DISCUSSION
While the possibility of removing heavy metals from the environment by phytoextraction (10–13, 32, 41) is becoming increasingly attractive, heavy metals can be toxic, even for metal-accumulating and metal-tolerant plants, if the concentration of metals in the environment is too high.
One way to lessen the deleterious effects of heavy metals taken up from the environment on some plants might involve the use of plant growth-promoting bacteria or mycorrhizal fungi. In fact, it has been shown that the presence of ectomycorrhizal or vesicular-arbuscular fungi on the roots of plants decreased the uptake of metals by the plants and thereby increased plant biomass (8, 9, 14, 26, 31, 45). Similarly, chromium-resistant pseudomonads, isolated from paint industry effluents, were able to stimulate seed germination and growth of Triticum aestivus in the presence of potassium bichromate (24). In this case, the bacterial enhancement of seedling growth was associated with reduced chromium uptake.
In the present study, the newly isolated bacterium K. ascorbata SUD165 was highly effective at protecting plants from growth inhibition caused by the presence of high concentrations of nickel. However, on a dry-weight basis, the plant grown in the presence and absence of the bacterium took up approximately the same amount of nickel, so that it is unlikely that the bacterium is somehow limiting nickel uptake by the plant.
The most likely explanation of the data is that the bacterium protects the plant against the inhibitory effects of nickel-induced stress ethylene formation. In this regard, (i) heavy metals can induce ethylene production by plants (48), (ii) an excess of ethylene can inhibit plant development (29), and (iii) the direct promotion of plant root growth by a number of different soil bacteria is based on the ability of bacterial ACC deaminase to hydrolyze and decrease the amount of ACC, an ethylene precursor, in plants and, as a result, to decrease ethylene biosynthesis by plants (18, 21, 23). Moreover, with canola seedlings grown in the presence of high levels of nickel, it was observed that the addition of K. ascorbata SUD165 caused a significant decrease in ethylene production.
In the model that was previously proposed for the stimulation of plant growth by soil bacteria that contain the enzyme ACC deaminase, some of the plant ACC is exuded from roots or seeds and then taken up by the bacterium and cleaved by ACC deaminase to ammonia and α-ketobutyrate (21). To maintain the gradient between internal and external ACC levels, the plant must exude increasing amounts of ACC. The lowering of ACC levels within the plant results in a reduction in the amount of plant ethylene and a decreased extent of ethylene inhibition of plant seedling root elongation. This model may also be invoked to explain how plant growth-promoting bacteria lower the concentration of stress ethylene in plants. Evidence for this model includes the fact that the ability of a bacterium to promote root elongation is positively correlated with both the ACC deaminase activity of the bacterium and the ACC content (measured by high-pressure liquid chromatography) of the plant tissues. Mutants of plant growth-promoting bacteria that are devoid of ACC deaminase activity and therefore do not hydrolyze ACC are unable to promote the elongation of canola roots. In several different biological assays, the chemical ethylene synthesis inhibitor l-α-(aminoethoxyvinyl)glycine mimics the effect of the ACC deaminase-containing bacterium. Every bacterium that has so far been isolated on the basis of the ability to utilize ACC as a nitrogen source (and therefore to possess ACC deaminase activity) is capable of lowering plant ethylene levels and promoting root elongation.
Another possible explanation of the phenomenon described above is related to siderophore production by K. ascorbata SUD165. Thus, at least part of the toxic effects of some heavy metals, including nickel, in plants results from an induced iron deficiency, and there is evidence that increasing the supply of iron can reduce the severity of nickel toxicity (5, 7, 16, 50). Moreover, since bacterial siderophores can provide iron to various plants (4, 40, 47), siderophores produced by K. ascorbata SUD165 may reduce nickel toxicity by supplying the plant with iron and hence eliminating iron deficiency. However, it is likely that there is a sufficient amount of iron in seeds for the development of 4- to 10-day-old seedlings, so that mechanisms that involve providing iron to the plant do not need to be invoked here.
Regardless of the precise mechanism used by the bacterium to protect plants, the experiments with plant seedlings reported here suggest that certain bacteria may eventually find a use in the development of phytoremediation strategies. In this regard, heavy metals may be removed from polluted soil either by increasing the metal-accumulating ability of plants or by increasing the amount of plant biomass. In heavily contaminated soil where the metal content exceeds the limit of plant tolerance, it may be possible to treat plants with plant growth-promoting bacteria, increasing plant biomass and thereby stabilizing, revegetating, and remediating metal-polluted soils.
ACKNOWLEDGMENTS
We are grateful to The Waterloo Centre for Groundwater Research (Waterloo, Ontario, Canada); INCO, Inc. (Toronto, Ontario, Canada); and the Natural Sciences and Engineering Research Council of Canada for providing funds in support of this research.
We thank C. Wren, Ecological Services Group, Guelph, Ontario, Canada, for the soil analyses.
REFERENCES
- 1.Abeles F B, Morgan P W, Saltveit M E., Jr . Ethylene in plant biology. 2nd ed. San Diego, Calif: Academic Press, Inc.; 1992. Regulation of ethylene production by internal, environmental and stress factors; pp. 56–119. [Google Scholar]
- 2.Baker A J M, Reeves R D, McGrath S P. In situ decontamination of heavy metal polluted soil using crops of metal-accumulating plants. A feasibility study. In: Hinchee R E, Olfenbuttel R F, editors. In situ bioreclamation. Application and investigation for hydrocarbon and contaminated site remediation. London, England: Butterworth-Heineman; 1991. pp. 600–605. [Google Scholar]
- 3.Baker A J M, Walker P L. Ecophysiology of metal uptake by tolerant plants. In: Shaw A J, editor. Heavy metal tolerance in plants: evolutionary aspects. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 155–157. [Google Scholar]
- 4.Bar-Ness E, Chen Y, Hadar Y, Marschner H, Romheld V. Siderophores of Pseudomonas putida as an iron source for dicot and monocot plants. Plant Soil. 1991;130:231–241. [Google Scholar]
- 5.Bingham F T, Pereyea F J, Jarrell W M. Metal toxicity to agricultural crops. Metal Ions Biol Syst. 1986;20:119–156. [Google Scholar]
- 6.Bogardt A H, Hemmingsen B B. Enumeration of phenanthracene-degrading bacteria by an overlayer technique and its use in the evaluation of petroleum-contaminated sites. Appl Environ Microbiol. 1992;58:2579–2582. doi: 10.1128/aem.58.8.2579-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bollard E G. Involvement of unusual elements in plant growth and nutrition. In: Lauchli A, Bielsky R L, editors. Inorganic plant nutrition. Encyclopedia of plant physiology. 15B. Berlin, Germany: Springer-Verlag KG; 1983. pp. 695–744. [Google Scholar]
- 8.Bradley R, Burt A J, Read D J. The biology of mycorrhyza in the Ericaceae. VIII. The role of mycorrhyzal infection in heavy metal resistance. New Phytol. 1982;91:197–202. [Google Scholar]
- 9.Brown M T, Wilkins D A. Zinc tolerance of mycorrhyial Betula. New Phytol. 1985;99:101–106. [Google Scholar]
- 10.Cunningham S D, Berti W R. Remediation of contaminated soils with green plants: an overview. In Vitro Cell Dev Biol. 1993;29P:207–212. [Google Scholar]
- 11.Cunningham S D, Berti W R, Huang J W. Phytoremediation of contaminated soils. Trends Biotechnol. 1995;13:393–397. [Google Scholar]
- 12.Cunningham S D, Lee C R. Phytoremediation: plant-based remediation of contaminated soils and sediments. In: Skipper H D, Turco R F, editors. Bioremediation: science and application. Special publication 43. Madison, Wis: Soil Science Society of America; 1995. pp. 145–155. [Google Scholar]
- 13.Cunningham S D, Ow D W. Promises and prospects of phytoremediation. Plant Physiol. 1996;110:715–719. doi: 10.1104/pp.110.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dueck T A, Visser P, Ernest W H O, Schat H. Vesicular-arbuscular mycorrhyzae decrease zinc toxicity to grasses in zinc polluted soil. Soil Biol Biochem. 1986;18:331–333. [Google Scholar]
- 15.Farmer J J, Fanning G R, Huntley-Carter G P, Holmes B, Hickman F W, Richard C, Brenner D J. Kluyvera, a new (redefined) genus in the family Enterobacteriaceae: identification of Kluyvera ascorbata sp. nov. and Kluyvera cryocrescent sp. nov. in clinical specimens. J Clin Microbiol. 1981;13:919–933. doi: 10.1128/jcm.13.5.919-933.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foy C D, Chaney R L, White M C. The physiology of metal toxicity in plants. Annu Rev Plant Physiol. 1978;29:511–566. [Google Scholar]
- 17.Freedman B, Hutchison T C. Pollutant inputs from the atmosphere and accumulations in soils and vegetation near a nickel-copper smelter at Sudbury, Ontario, Canada. Can J Bot. 1980;58:108–132. [Google Scholar]
- 18.Glick B R, Jacobson C B, Schwarze M M K, Pasternak J J. 1-Aminocyclopropane-1-carboxylic acid deaminase mutants of the plant growth promoting rhyzobacterium Pseudomonas putida GR 12-2 do not stimulate canola root elongation. Can J Microbiol. 1994;40:911–915. [Google Scholar]
- 19.Glick B R, Karaturovic D M, Newell P C. A novel procedure for rapid isolation of plant growth promoting pseudomonads. Can J Microbiol. 1995;41:533–536. [Google Scholar]
- 20.Glick B R. The enhancement of plant growth by free-living bacteria. Can J Microbiol. 1995;41:109–117. [Google Scholar]
- 21.Glick B R, Penrose D M, Li J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol. 1998;190:63–68. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- 22.Glick B R, Liu C, Ghosh S, Dumbroff E B. Early development of canola seedlings in the presence of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. Soil Biol Biochem. 1997;29:1233–1239. [Google Scholar]
- 23.Hall J A, Peirson D, Ghosh S, Glick B R. Root elongation in various agronomic crops by the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Isr J Plant Sci. 1996;44:37–42. [Google Scholar]
- 24.Hasnain S, Sabri A N. Abstracts of the 7th International Symposium on Biological Nitrogen Fixation with Non-Legumes. 1996. Growth stimulation of Triticum aestivum seedlings under Cr- stresses by non rhizospheric pseudomonad strains; p. 36. [DOI] [PubMed] [Google Scholar]
- 25.Hausinger R P. Nickel utilization by microorganisms. Microbiol Rev. 1987;51:22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heggo A, Angle J S, Chaney R L. Effect of vesicular-arbuscular mycorrhyzae fungi on heavy metal uptake by soybeans. Soil Biol Biochem. 1990;22:865–869. [Google Scholar]
- 27.Honma M, Shimomura T. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem. 1978;42:1825–1831. [Google Scholar]
- 28.Hyodo H. Stress/wound ethylene. In: Mattoo A K, Suttle J C, editors. The plant hormone ethylene. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 65–80. [Google Scholar]
- 29.Jackson M R. Ethylene in root growth and development. In: Mattoo A K, Suttle J C, editors. The plant hormone ethylene. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 151–189. [Google Scholar]
- 30.Jacobson C B, Pasternak J J, Glick B R. Partial purification and characterization of the enzyme ACC deaminase from the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. Can J Microbiol. 1994;40:1019–1025. [Google Scholar]
- 31.Killham K, Firestone M K. Vesicular arbuscular mycorrhyzal mediation of grass response to acidic and heavy metal deposition. Plant Soil. 1983;72:39–48. [Google Scholar]
- 32.Kumar P B A N, Dushenkov V, Motto H, Raskin I. Phytoextraction: the use of plants to remove heavy metals from soil. Environ Sci Technol. 1995;29:1232–1238. doi: 10.1021/es00005a014. [DOI] [PubMed] [Google Scholar]
- 33.Lifshitz R, Kloepper J W, Kozlowski M, Simonson C, Carlson J, Tipping E M, Zaleska I. Growth promotion of canola (rapeseed) seedlings by a strain of Pseudomonas putida under gnotobiotic conditions. Can J Microbiol. 1987;33:390–395. [Google Scholar]
- 34.Lynch J M. The rhizosphere. Chichester, England: Wiley-Interscience; 1990. [Google Scholar]
- 35.Mergeau M, Nies D, Schlegel H G, Gerits J, Charles P, van Gijsegem F. Alcaligenes eutrophus CH34 is a facultative chemolitotroph with plasmid-bound resistance to heavy metals. J Bacteriol. 1985;162:328–334. doi: 10.1128/jb.162.1.328-334.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehra A, Farago M E. Metal ions and plant nutrition. In: Farago M E, editor. Plants and chemical elements. Biogeochemistry, uptake, tolerance and toxicity. Weinheim, Germany: VCH; 1994. pp. 32–36. [Google Scholar]
- 37.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 38.Raaska L, Viikari L, Mattila-Sandholm T. Detection of siderophores in growing cultures of Pseudomonas spp. J Ind Microbiol. 1993;11:181–189. [Google Scholar]
- 39.Reeves R D. Nickel and zinc accumulation by species of Thlaspi L., Cochlearia L., and other genera of the Brassicaceae. Taxon. 1988;37:309–318. [Google Scholar]
- 40.Reid C P P, Szaniszlo P J, Crowley D E. Siderophore involvement in plant iron nutrition. In: Swinburne T R, editor. Iron siderophores and plant diseases. New York, N.Y: Plenum Press; 1986. pp. 29–42. [Google Scholar]
- 41.Salt D E, Blaylock M, Kumar N P B A, Dushenkov V, Ensley B D, Chet I, Raskin I. Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Bio/Technology. 1995;13:468–474. doi: 10.1038/nbt0595-468. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt T, Schlegel H G. Nickel and cobalt resistance of various bacteria isolated from soil and highly polluted domestic and industrial wastes. FEMS Microbiol Ecol. 1989;62:315–328. [Google Scholar]
- 43.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 44.Shah S, Li J, Moffatt B, Glick B R. ACC deaminase genes from plant growth promoting rhizobacteria. In: Ogoshi A, Kobayashi K, Homma Y, Kodama F, Kondo N, Akino S, editors. Plant growth-promoting rhizobacteria—present status and future prospects. Paris, France: OECD; 1997. pp. 320–324. [Google Scholar]
- 45.Tam P C F. Heavy metal tolerance by ectomycorrhyzal fungi and metal amelioration by Pisolithus tinctorium. Mycorrhiza. 1995;5:181–187. [Google Scholar]
- 46.Van Loon L C. Regulation of pathogenesis and symptom expression in diseased plants by ethylene. In: Fuchs Y, Chalutz E, editors. Ethylene: biochemical, physiological and applied aspects. The Hague, The Netherlands: Martinus Nijhoff/Dr. W. Junk; 1984. pp. 171–180. [Google Scholar]
- 47.Wang Y, Brown H N, Crowley D E, Szaniszlo P J. Evidence for direct utilization of a siderophore, ferroxamine B, in axenically grown cucumber. Plant Cell Environ. 1993;16:579–585. [Google Scholar]
- 48.Weckx J, Vangronsveld J, Clijster H. Heavy metal induction of ethylene production and stress enzymes. I. Kinetics of response. In: Pech J C, Latche A, Balaque C, editors. Cellular and molecular aspects of the plant hormone ethylene. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 238–239. [Google Scholar]
- 49.Wilkins D A. The measurement of tolerance to edaphic factors by means of root growth. New Phytol. 1978;80:623–633. [Google Scholar]
- 50.Yang X, Baligar V C, Martens D C, Clark P B. Plant tolerance to nickel toxicity. II. Nickel effect on influx and transport of mineral nutrients in four plant species. J Plant Nutr. 1996;19:265–279. [Google Scholar]