Abstract
This review addresses the pathophysiology and treatment of hemorrhagic shock – a condition produced by rapid and significant loss of intravascular volume, which may lead sequentially to hemodynamic instability, decreases in oxygen delivery, decreased tissue perfusion, cellular hypoxia, organ damage, and death. Hemorrhagic shock can be rapidly fatal. The primary goals are to stop the bleeding and to restore circulating blood volume. Resuscitation may well depend on the estimated severity of hemorrhage. It now appears that patients with moderate hypotension from bleeding may benefit by delaying massive fluid resuscitation until they reach a definitive care facility. On the other hand, the use of intravenous fluids, crystalloids or colloids, and blood products can be life saving in those patients who are in severe hemorrhagic shock. The optimal method of resuscitation has not been clearly established. A hemoglobin level of 7–8 g/dl appears to be an appropriate threshold for transfusion in critically ill patients with no evidence of tissue hypoxia. However, maintaining a higher hemoglobin level of 10 g/dl is a reasonable goal in actively bleeding patients, the elderly, or individuals who are at risk for myocardial infarction. Moreover, hemoglobin concentration should not be the only therapeutic guide in actively bleeding patients. Instead, therapy should be aimed at restoring intravascular volume and adequate hemodynamic parameters.
Keywords: blood loss, estimated blood volume, hemorrhage, oxygen consumption, oxygen delivery, shock, transfusion
Introduction
Life-threatening decreases in blood pressure often are associated with a state of shock – a condition in which tissue perfusion is not capable of sustaining aerobic metabolism. Shock can be produced by decreases in cardiac output (cardiogenic), by sepsis (distributive), or by decreases in intravascular volume (hypovolemic). The latter may be caused by dehydration from vomiting or diarrhea, by severe environmental fluid losses, or by rapid and substantial loss of blood. A less common form of shock (cytopathic) may occur when the mitochondria are incapable of producing the energy required to sustain cellular function [1]. Agents that interfere with oxidative phosphorylation, such as cyanide, carbon monoxide and rotenone, can produce this type of shock.
Hemorrhage is a medical emergency that is frequently encountered by physicians in emergency rooms, operating rooms, and intensive care units. Significant loss of intravascular volume may lead sequentially to hemodynamic instability, decreased tissue perfusion, cellular hypoxia, organ damage, and death. This review addresses the pathophysiology and treatment of hypovolemic shock produced by hemorrhage, which is also known as hemorrhagic shock.
Physiologic considerations in hemorrhagic shock
Estimating blood loss
The average adult blood volume represents 7% of body weight (or 70 ml/kg of body weight) [2]. Estimated blood volume (EBV) for a 70 kg person is approximately 5 l. Blood volume varies with age and physiologic state. When indexed to body weight, older individuals have a smaller blood volume. Children have EBVs of 8–9% of body weight, with infants having an EBV as high as 9–10% of their total body weight [3].
Estimating blood loss is complicated by several factors, including urinary losses and the development of tissue edema. To help guide volume replacement, hemorrhage can be divided into four classes (Table 1). Class I is a nonshock state, such as occurs when donating a unit of blood, whereas class IV is a preterminal event requiring immediate therapy [4]. Massive hemorrhage may be defined as loss of total EBV within a 24-hour period, or loss of half of the EBV in a 3-hour period.
Table 1.
Classification of hemorrhage
| Class | ||||
| Parameter | I | II | III | IV |
| Blood loss (ml) | <750 | 750–1500 | 1500–2000 | >2000 |
| Blood loss (%) | <15% | 15–30% | 30–40% | >40% |
| Pulse rate (beats/min) | <100 | >100 | >120 | >140 |
| Blood pressure | Normal | Decreased | Decreased | Decreased |
| Respiratory rate (breaths/min) | 14–20 | 20–30 | 30–40 | >35 |
| Urine output (ml/hour) | >30 | 20–30 | 5–15 | Negligible |
| CNS symptoms | Normal | Anxious | Confused | Lethargic |
Modified from Committee on Trauma [4]. CNS = central nervous system.
A relatively simple way to estimate acute blood loss is by considering the intravascular space as a single compartment, in which hemoglobin changes according to the degree of blood loss and fluid replacement (Fig. 1). When volume losses are not replaced during hemorrhage, hemoglobin concentration will remain constant. In that condition a rough estimate of blood loss may be obtained using the classification provided in Table 1. Conversely, when blood losses are sequentially replaced by isovolemic fluid infusion, the estimated blood loss may be obtained as follows [5]:
Figure 1.
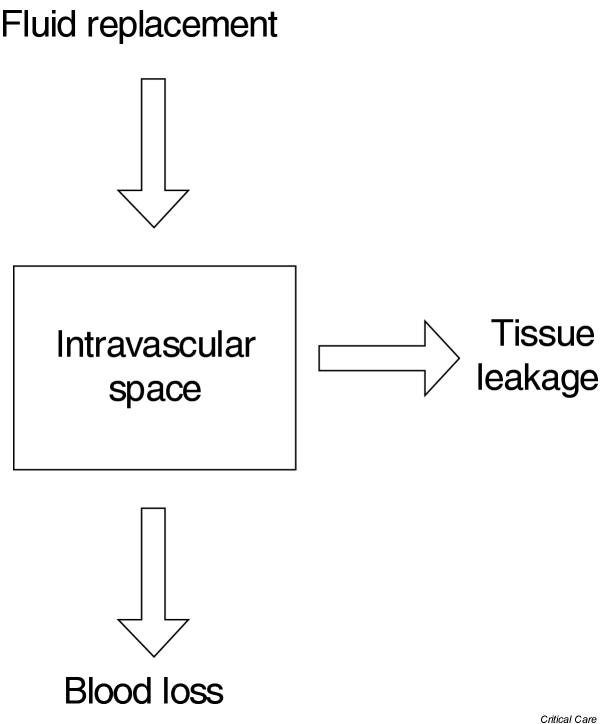
One compartment model of the vascular space.
EBL = EBV × ln(Hi/Hf)
Where Hi and Hf denote the initial and final hematocrit. Implicit in this equation is the absence of significant urinary losses or the leakage of intravascular fluid into the tissues. For example, a decrease in hematocrit from 40% to 26% with complete fluid replacement of blood losses corresponds to an estimated blood loss of 2.1 l.
Intravenous fluid infusion in the absence of bleeding also will lower hemoglobin concentration. Using the one-compartment model, a first approximation to hemodilution with intravenous fluids is as follows:
Hf = EBV × Hi/(EBV + volume infused)
This is the lowest possible estimate of Hf, because fluid administration and expansion of intravascular fluid volume will trigger compensatory mechanisms to increase glomerular filtration rate and decrease plasma volume.
Transfusing packed red cells in a person who is not actively bleeding will increase hemoglobin concentration by 1 g/dl (or 3% hematocrit) per unit of packed red blood cell transfused. It is impossible to estimate the effect of blood transfusion on volume or hemoglobin concentration in actively bleeding individuals. Measures of central venous or, preferably, pulmonary artery pressures are needed to estimate the degree of fluid replacement that may be required.
Alterations in systemic oxygen delivery during hemorrhagic shock
Decreases in circulating blood volume during severe hemorrhage can depress cardiac output and lower organ perfusion pressure. Severe hemorrhage impairs the delivery of oxygen and nutrients to the tissues and produces a state of shock. A clearer understanding of the pathophysiology of hemorrhagic shock may be obtained by defining the process of oxygen delivery and utilization by the tissues. Total oxygen delivery (DO2 [mlO2/min per m2]) is the product of cardiac index (l/min per m2) and arterial oxygen content (CaO2 [mlO2/l blood]). CaO2 is calculated as 13.4 × [Hb] × SaO2 + 0.03 PaO2, where [Hb] represents the concentration of hemoglobin in blood (g/dl), SaO2 is the hemoglobin oxygen saturation and PaO2 is the partial pressure of oxygen in arterial blood.
Under normal aerobic conditions, systemic oxygen consumption (VO2) is proportional to the metabolic rate and varies according to the body's energy needs. VO2 may be calculated using Fick's principle as the difference between the rates of oxygen delivered and oxygen leaving the tissues: VO2 = cardiac index × (CaO2 - CmvO2), where CmvO2 is the oxygen content of mixed venous blood. Calculation of VO2 using Fick's equation does not account for pulmonary oxygen consumption, which may be substantial during acute lung injury [6].
Another useful parameter when defining tissue oxygenation is the fraction of oxygen consumed to oxygen delivered to the tissues, termed the oxygen extraction ratio and calculated as (CaO2 - CmvO2)/CaO2.
The relationship of oxygen delivery to oxygen consumption during hemorrhagic shock
Rapid decreases in blood volume may lead to decreases in cardiac output and in DO2 with little change in VO2, because blood flow is preferentially distributed to tissues with greater metabolic requirements. Increased efficiency in oxygen utilization during hypoxia is reflected by a rise in oxygen extraction ratio [7]. Lowering regional vascular resistance by adenosine, prostaglandins, and nitric oxide induces hypoxic redistribution of blood flow [8,9]. In spite of this organ-specific microvascular response, all organs, with the possible exception of the heart, experience decreases in blood flow during severe hypovolemia [10].
Another targeted response to hemorrhage is an increase in the number of open capillaries in organs that are capable of this. For example, in skeletal muscle only a fraction of capillaries are usually open to accommodate the passage of erythrocytes whereas the remaining capillaries allow only passage of plasma [11]. During hemorrhage the number of open capillaries increases in proportion to the degree of tissue hypoxia [12]. Capillary recruitment shortens the diffusion distance from red blood cells to the surrounding tissue [13] and increases the capillary surface area available for oxygen diffusion [14]. The overall effect of capillary recruitment is the maintenance of tissue oxygen flux at a lower capillary oxygen tension, which is a vital response in organs on the edge of hypoxia.
Severe and sustained decreases in DO2 eventually overwhelm the microvascular responses to hypoxia. As tissue oxygen flux falters, mitochondria cannot sustain aerobic metabolism and VO2 decreases. The rate of DO2 associated with the initial decline in VO2 is defined as the critical DO2 (DO2crit) [15]. Animal experiments show that DO2crit is a remarkably constant parameter regardless of the method used to decrease DO2, be it anemia, hypoxemia, or hypovolemia [16].
Hypovolemia and isovolemic anemia
Patients with massive hemorrhage may experience conditions ranging from severe hypovolemia, in which blood volume decreases with no changes in hemoglobin concentration, to isovolemic anemia, in which extreme decreases in hemoglobin concentration occur with normal or even increased blood volume.
Hypovolemia occurs in rapidly bleeding individuals who are not receiving intravenous fluids. The importance of circulating blood volume has been demonstrated in animals subjected to the sequential removal of blood aliquots from a central vein [17]. These experiments show that VO2 remains constant as the circulating blood volume decreases. VO2 falls precipitously and death rapidly ensues below a DO2crit of 8–10 mlO2/min per kg. At this critical juncture, decreases in blood volume approach 50% with no changes in hemoglobin concentration. Hypovolemia is associated with substantial decreases in cardiac output and mixed venous oxygen tension.
Aggressive fluid replacement may produce the condition of isovolemic anemia, which is characterized by adequate blood volume but decreased hemoglobin concentration and low oxygen carrying capacity. Isovolemic anemia occurs when blood for transfusion is not readily available or in individuals who are bleeding but refuse to accept blood products. Experimental isovolemic anemia is produced by drawing blood aliquots from a central vein and replacing the exact amount of blood removed with a colloidal solution such as albumin. Animals subjected to progressive isovolemic anemia also exhibit a DO2crit in the neighborhood of 10 mlO2/min per kg [18]. DO2crit is reached at a hemoglobin concentration of approximately 4.0 g/dl (corresponding to a hematocrit <8%). Isovolemic anemia is associated with increased cardiac output and greater mixed venous oxygen tensions than those noted for hypovolemia or hypoxemia [19].
Individuals with chronic isovolemic anemia, such as those with renal failure, tolerate decreases in hemoglobin to levels of 6–7 g/dl. In fact, acute hemodilution did not produce tissue hypoxia in healthy human volunteers who had their blood hemoglobin concentration reduced to 5.0 g/dl [20]. Acute isovolemic hemodilution decreased systemic vascular resistance and increased heart rate, stroke volume, and cardiac index, but there were no changes in VO2 or in plasma lactate. In a subsequent study conducted in resting volunteers, hemoglobin concentration was lowered by isovolemic anemia to 4.8 g/dl, decreasing DO2 to 7.3 mlO2/min per kg without evidence of inadequate systemic oxygenation [21].
Cellular responses to acute blood loss
Compensated shock occurs when systemic DO2 decreases below DO2crit and the tissues turn to anaerobic sources of energy. Under these conditions, cellular function is maintained as long as the combined yield of aerobic and anaerobic sources of energy provides sufficient ATP for protein synthesis and contractile processes. Some tissues are more resistant to hypoxia than others. Skeletal and smooth muscles are highly resistant to hypoxia [22,23] and irreversible damage does not occur in isolated hepatocytes until 2.5 hours of ischemia [24]. Conversely, brain cells sustain permanent damage after only a few minutes of hypoxia [25]. The gut appears to be particularly sensitive to decreases in perfusion. The intestinal and gastric mucosa show evidence of anaerobic metabolism before decreases in systemic VO2 are detected [26].
Uncompensated shock resulting in irreversible tissue damage occurs when the combined aerobic and anaerobic supplies of ATP are not sufficient to maintain cellular function (Fig. 2). Failure of membrane-associated ion transport pumps, in particular those associated with the regulation of calcium and sodium, results in the loss of membrane integrity and in cellular swelling [27,28]. Among other mechanisms that lead to irreversible cellular injury during hypoxia are depletion of cellular energy, cellular acidosis, oxygen free radical generation, and loss of adenine nucleotides from the cell [29].
Figure 2.
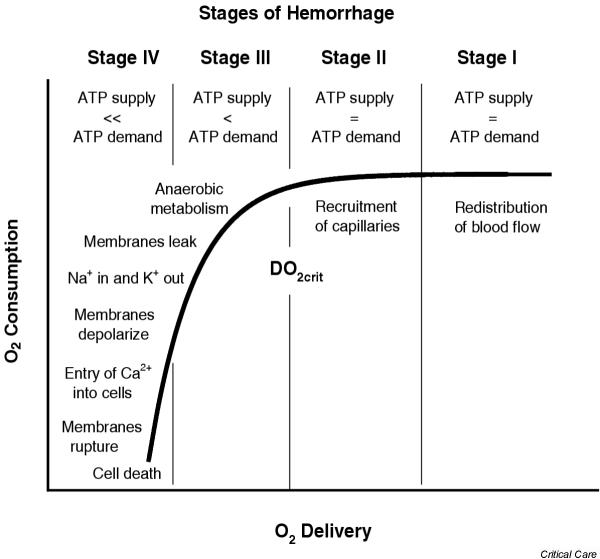
Changes in oxygen consumption shown as a function of oxygen delivery. Also shown are the hypothetical relationships of these parameters to the stages of hemorrhage (Table 1) and changes in cellular membrane integrity. DO2crit, critical oxygen delivery.
Systemic responses to acute blood loss
The first response to blood loss is an attempt to form a clot at the local site of hemorrhage. As hemorrhage progresses, catecholamines, antidiuretic hormone, and atrial natriuretic receptors respond to the perceived loss of volume by vasoconstriction of arterioles and muscular arteries and by increasing the heart rate. The aim of these compensatory mechanisms is to increase cardiac output and maintain perfusion pressure. Urine output drops somewhat and thirst is stimulated to maintain circulating blood volume.
Anxiety may be related to the release of catecholamines and to mild decreases in cerebral blood flow. A person who is bleeding briskly also may develop tachypnea and hypotension. As hypovolemia worsens and tissue hypoxia ensues, increases in ventilation compensate for the metabolic acidosis produced by increased carbon dioxide production. Compensatory mechanisms are eventually overwhelmed by volume losses, and blood flow to the renal and splanchnic vasculature decreases and systolic blood pressure declines. The loss of coronary perfusion pressure adversely affects myocardial contractility; cerebral blood flow decreases, resulting in the loss of consciousness, coma, and eventually death.
Clinical considerations in hemorrhagic shock
The therapeutic goals for hemorrhagic shock are to stop bleeding and to restore intravascular volume. This review does not address methods of stopping hemorrhage, but rather deals with the physiologic and pathologic derangements produced by severe hemorrhage and how best to treat them.
Clinical manifestations
Shock is a state of hypoperfusion associated with hemodynamic abnormalities leading to the collapse of homeostasis, or as poetically stated by John Collins Warren, a 'momentary pause in the act of death' [30]. The etiology of shock in traumatized patients is likely to be massive blood loss but other causes of shock must be considered. These include blunt myocardial damage, spinal cord injury, tension pneumothorax, or pericardial tamponade.
Not all trauma patients with tissue hypoperfusion as the result of massive hemorrhage arrive at the emergency department with signs of shock. The lack of a specific diagnosis should not delay resuscitation from severe hypovolemia when hemorrhage is suggested by history, physical examination, or laboratory findings.
A rapid assessment of the possible source of bleeding is essential when acute hemorrhage is the suspected cause for hemodynamic instability, and a thorough physical examination should be performed. Emergency personnel may give an estimate of blood loss at the scene, but one should always be wary of such estimates because they are notoriously inaccurate. In general, young patients who present with tachycardia and mild hypotension are in danger of losing their compensatory mechanisms and may well slip into profound shock unless vigorous therapy is initiated. Reliance on systolic blood pressure alone may delay recognition of the shock state. Most practitioners can palpate a carotid pulse in an adult. This is equivalent to a systolic pressure of 60 mmHg. A femoral pulse is produced by a systolic pressure of 60–70 mmHg. A palpable radial pulse usually requires slightly higher pressures.
Gastrointestinal bleeding and trauma are the most common causes of hemorrhage. Other causes of hemorrhagic shock include ruptured abdominal aortic aneurysms, spontaneous bleeding from anticoagulation, and postpartum bleeding secondary to a placenta previa or placenta abruption (Table 2). A ruptured ectopic pregnancy or a ruptured ovarian cyst also can cause hemorrhagic shock without an obvious source of blood loss [31]. The evaluation of shock in a woman of childbearing age should include a pregnancy test and possibly a culdocentesis. Stopping the bleeding, as well as replacing the blood volume, is the treatment for shock resulting from postpartum hemorrhage.
Table 2.
Common causes of hemorrhagic shock
| Cause | Examples (where applicable) |
| Antithrombotic therapy | |
| Coagulopathies | |
| Gastrointestinal bleeding | Esophageal varices |
| Esophagogastric mucosal tear (Mallory–Weiss) | |
| Gastritis | |
| Gastric and duodenal ulcerations | |
| Gastric and esophageal cancer | |
| Colon cancer | |
| Colonic diverticula | |
| Obstetric/gynecologic | Placenta previa |
| Abruptio placentae | |
| Ruptured ectopic pregnancy | |
| Ruptured ovarian cyst | |
| Pulmonary | Pulmonary embolus |
| Lung cancer | |
| Cavitary lung disease: tuberculosis, aspergillosis | |
| Goodpasture's syndrome | |
| Ruptured aneurysms | |
| Retroperitoneal bleeding | |
| Trauma | Lacerations |
| Penetrating wounds to the abdomen and chest | |
| Ruptured major vessels |
Blood losses from external lacerations are difficult to estimate but usually respond to direct pressure and volume resuscitation. Intrathoracic injuries, especially to the lung, heart, or the great vessels, can result in the loss of several liters of blood into the thorax without external evidence of hemorrhage. Intra-abdominal injuries to solid organs (spleen and liver) and great vessels (ruptured aneurysm, penetrating injury to intra-abdominal vessels) can cause rapid loss of the entire blood volume into the abdomen. Massive bleeding into the gastrointestinal tract from ulcers or intestinal diverticuli can likewise cause shock, but the patient usually manifests either hematochezia or hematemesis when blood loss is rapid and acute.
Fractures of the pelvis can hide massive amounts of bleeding with little external evidence [32]. An unstable pelvis on physical examination always raises the possibility of significant blood loss. Spontaneous bleeding into the retroperitoneum can also cause shock without significant physical findings. Fractures of the lower extremities, especially closed femur fractures, can easily hide 2–3 units of blood, whereas open fractures can lacerate major vessels and cause significant blood loss. Head injury is rarely a cause of hypotension and is never the cause of massive blood loss, unless there is external bleeding.
Treatment of hemorrhagic shock
The main goals of resuscitation are to stop the source of hemorrhage and to restore circulating blood volume. Actively bleeding patients should have their intravascular fluid replaced because tissue oxygenation will not be compromised, even at low hemoglobin concentrations, as long as circulating volume is maintained. Hemoglobin concentration in an actively bleeding individual has dubious diagnostic value because it takes time for the various intravascular compartments to equilibrate. Rather, therapy should be guided by the rate of bleeding and changes in hemodynamic parameters, such as blood pressure, heart rate, cardiac output, central venous pressure, pulmonary artery wedge pressure, and mixed venous saturation.
Restoration of the intravascular fluid volume
Since the time of World War II, the accepted therapeutic dogma has been to restore blood volume rapidly and achieve normal physiologic parameters. Generations of physicians have been trained to reverse shock within the 'golden hour' in order to preserve organ function and prevent death.
As early as 1918, however, Cannon and coworkers [33] questioned the feasibility of restoring blood pressure back to normal in the face of active hemorrhage. Wiggers [34] proposed the concept of 'irreversible shock' after showing that reinfusing blood into a profoundly shocked animal was not sufficient to prevent mortality and morbidity. Subsequently, Shires and coworkers [35] demonstrated in experimental preparations that crystalloid fluids were needed in addition to blood to restore perfusion. They were able to demonstrate failure of the sodium–potassium pump, resulting in the ingress of sodium and water into the cells. The awareness of 'third space losses' into the interstitium and tissues resulted in the 'three-to-one' rule for resuscitation: that is, 3 ml of crystalloid (Ringers lactate or normal saline) for every 1 ml of blood loss replaced.
Four issues should be considered when treating hemorrhagic shock: type of fluid to give, how much, how fast, and what the therapeutic end-points are. The ideal fluid for resuscitation has not been established. The three-to-one rule has been applied to the classification of hemorrhage to establish a baseline for guiding therapy [36], and use of crystalloid (Ringers lactate or normal saline) is recommended by the American College of Surgeons [4]. Although resuscitative end-points are similar when using Ringers lactate or normal saline, metabolic hyperchloremic acidosis has been reported when infusing large volumes of normal saline (>10 l) [35].
Colloidal solutions, such as albumin and hetastarch (6% hydroxyethyl starch in 0.9% NaCl), can be administered to increase circulatory volume rapidly. Although it is beyond the scope of this review to enter the crystalloid versus colloid fray, we should note that the use of albumin solutions in the initial resuscitation stages has not proven to be more effective than crystalloid [37-39]. A meta-analysis of 26 prospective randomized trials (including a total of 1622 patients) revealed an increased absolute risk for death of 4% when colloids were used for resuscitation [40]. The results of this meta-analysis sparked a great deal of controversy on the use of albumin as a replacement fluid. The conclusions of these analyses should be viewed with caution because the inclusion criteria for the various studies included in the meta-analyses differed. It should be noted, however, that the American College of Surgeons does recommend the use of albumin as a resuscitative fluid [4].
Hypertonic saline
There is continuing interest in the role of hypertonic saline during resuscitation from hypovolemic shock. There is some evidence that the use of hypertonic saline in traumatized patients with closed head injury may be efficacious [41], but this is controversial and the US Food and Drug Administration has not given approval for its use during the resuscitation of patients. A prospective, randomized study comparing hypertonic saline with dextran found no difference in survival between the hypertonic saline group and the dextran-treated group [42]. Small volume hypertonic saline does hold some promise in cases of penetrating trauma [43].
Blood substitutes
Blood substitutes have been tried in many forms [44]. A report by Gould and colleagues [45] on the effect of massive doses of hemoglobin solutions in hemorrhagic trauma patients demonstrated a possible benefit when compared with infusion of crystalloids. In that study, 171 patients received rapid infusion of 1–20 units of poly-HEME (Sigma, St. Louis, MO, USA; human polymerized hemoglobin) in lieu of human blood. Mortality was 25%, as compared with 64% for historical matched control individuals. On the other hand, the sobering results of a randomized, prospective, multicenter study conducted by Sloan and coworkers [46], in which traumatic hemorrhagic shock patients were treated with diaspirin cross-linked hemoglobin, will remain an impediment to further research in this area for many years to come. At 28 days, 24 (46%) of the 52 patients infused with diaspirin cross-linked hemoglobin died compared with eight (17%) of the 46 patients infused with a saline solution (P = 0.003).
When to transfuse
The use of blood and blood products is necessary when the estimated blood loss from hemorrhage exceeds 30% of the blood volume (class III hemorrhage). Determining this point has been extremely difficult during an acute hemorrhage because of hemodilution produced by fluid resuscitation. As mentioned previously, whereas formulas have been proposed to estimate blood losses, the use of blood as a resuscitative fluid is empirical [5,47].
Presently, a hypotensive patient who fails to respond to 2 l crystalloid in the face of probable hemorrhage should be treated with blood and blood products. O-negative blood for women and O-positive for men is infused if type and cross-matched blood is not easily available. Blood transfusions have several negative side effects and have been associated with worse outcome in patients with trauma [48]. Among the complications of blood transfusion are decreased immunity and increased rate of infection, as well as problems associated with transmissible diseases and improper administration [49,50].
Transfusion in the critically ill patient
Several national organizations in the USA and Canada have issued guidelines for blood transfusion. These include the consensus conferences of the National Institutes of Health [51], the American College of Physicians [52], the American Society of Anesthesiology [53], and the Canadian Medical Association [54]. These guidelines recommend a hemoglobin level between 6 and 8 g/dl as a threshold for transfusion in patients without known risk factors. They also agree in their disapproval of prophylactic blood transfusion, because patients with hemoglobin levels greater than 10 g/dl are unlikely to benefit from blood transfusion. These guidelines have rapidly been incorporated into the everyday practice of medicine, leading some to question whether blood transfusion is now under-used [55].
When it comes to high risk or critically ill patients, clinical evidence in support of transfusion guidelines is more difficult to obtain and therapy has often been guided by clinical judgment. A study of transfusion practices in Canada noted that 28% of patients admitted to tertiary level intensive care units received red cell transfusions [56]. The most frequent reason for administering red cells was not the patient's hemoglobin concentration. Instead, blood transfusions were ordered if patients were acutely bleeding (35% of patients transfused) or in order to increase DO2 (25% of patients transfused).
A multi-institutional, prospective, randomized study was conducted to determine whether a restrictive strategy of red cell transfusion and a liberal strategy produced equivalent results in critically ill patients [57]. Patients were enrolled in the study within 72 hours of admission to the intensive care unit if their hemoglobin concentrations was below 9 g/dl. Patients were randomly assigned either to a liberal strategy of transfusion (n = 420), in which hemoglobin values were maintained at a level between 10 and 12 g/dl, or to a restrictive strategy of transfusion, in which hemoglobin values were maintained between 7 and 9 g/dl (n = 418). Mortality at 30 days was similar for the two groups (19% versus 23%). Subgroup analysis showed that mortality rates were lower with the restrictive transfusion strategy among less acutely ill patients and among those under 55 years old. Furthermore, the mortality rate during hospitalization was significantly lower in the restrictive strategy group (22% versus 28%). These data suggest that a restrictive strategy of red cell transfusion in critically ill patients is at least as effective as a liberal transfusion strategy. Moreover, a prospective observational study of 1136 patients conducted in Europe showed an association between transfusions and decreased organ function and increased mortality [58].
Transfusion in elderly patients
Tolerance of anemia is dependent on the recruitment of physiologic reserve, mainly by increasing cardiac output. Low levels of hemoglobin that are tolerated by younger patients may be deleterious in the elderly. Reserve mechanisms in the elderly may be blunted with advanced age and the presence of coronary artery stenosis. This also may explain why elderly patients with acute myocardial infarction are at extremely high risk for death despite having infarct sizes similar to those in younger patients.
A study conducted by Wu and coworkers [59] indicated that a substantial number of people who present to the hospital with acute myocardial infarction and a hematocrit of 24% or lower may benefit from blood transfusion. In a retrospective analysis of data from 78,974 patients aged 65 years or older and who were hospitalized with acute myocardial infarction, those with lower hematocrit values (<24%) on admission had higher 30-day mortality rates. Blood transfusion was associated with a reduction in 30-day mortality among patients whose hematocrit on admission was in the 5–24% range. Blood transfusion did not improve survival among those whose hematocrit values fell in the higher ranges.
Delayed versus immediate resuscitation
Recent data question the practice of initial aggressive resuscitation of hemorrhagic shock. Cannon and coworkers [33] raised the concern that raising blood pressure in a bleeding patient would eliminate the clot and increase bleeding. This theory was replaced in World War II and in Vietnam by the concept that restoration of blood volume as soon as possible was the key to survival. The concept of the 'golden hour' as the time period allowed for medical personnel to reverse shock and prevent organ system damage has dominated the thinking of trauma surgeons for a generation.
Bickell and coworkers [60] challenged this approach when they performed a randomized prospective study of patients with penetrating truncal injuries who were hypotensive in the field (systolic blood pressure <90 mmHg). Patients were randomized according to the day of the month to receive either standard resuscitation with Ringers lactate or placement of intravenous catheters without intravenous fluid administration. Patients were excluded if they had cardiopulmonary collapse in the field, severe head injury, or did not need surgical intervention. A total of 598 matched control patients were included in the study group. The immediate resuscitation group received an average of 900 ml fluid before hospitalization compared with 100 ml fluid in the delayed resuscitation group. Of the delayed resuscitation group 70% were discharged, as compared with 62% of the immediate fluid group (P = 0.04), and the delayed group trended to have fewer complications.
Animal data demonstrate a reduced risk for death with fluid resuscitation in severe hemorrhage. On the other hand, a systematic review of the animal studies also showed an increased risk for death from aggressive resuscitation in animals with less severe hemorrhage [61]. This finding suggests that excessive fluid resuscitation can be lethal when severe hemorrhage is not present. Another study [62] found no differences in survival in patients presenting in hemorrhagic shock treated with two fluid replacement protocols, one that required fluid replacement to a systolic blood pressure in excess of 100 mmHg (conventional) and another that required fluid replacement to a systolic blood pressure in excess of 70 mmHg.
Whether or not one fully resuscitates a bleeding patient depends on the rate of bleeding, the ability to control the bleeding, and the presence of coagulopathy. It may be that excessive fluid resuscitation before surgical hemostasis will be accompanied by increased bleeding that may ultimately affect mortality. Although some actively bleeding patients will exsanguinate immediately, others will stop bleeding spontaneously. Fluid resuscitation should be focused on injuries that will not undergo spontaneous hemostasis [63]. The challenge lies in identifying those patients.
End-points in resuscitation
Defining the end-points of resuscitation is also a difficult area of study. Up to 85% of patients are under-resuscitated when using blood pressure and urine output as the sole guides to fluid replacement [64]. The problem may be 'compensated shock', in which cellular perfusion lags behind gross physiologic parameters. Other end-points, such as oxygen transport variables, DO2, cardiac index, VO2, lactate, base deficit, and mucosal gastric pH, are all more sensitive endpoints of cellular resuscitation [65]. Recent data on tissue oxygen parameters also suggest that these measures are promising markers of adequate restoration of perfusion [66]. The use of super normal delivery of oxygen has been proposed but a study conducted by McKinley and coworkers [67] demonstrated that levels of DO2 greater than 600 ml/min per m2 are not warranted.
Conclusion
Hemorrhagic shock can be rapidly fatal. The primary goal is to stop the bleeding. Resuscitation may well depend on estimated severity of hemorrhage. It now appears that patients who have moderate hypotension from moderate bleeding may well benefit from a delay in massive resuscitation in order to reach a definitive care facility. On the other hand, when patients are obviously in severe hemorrhagic shock, the use of intravenous crystalloids or colloids and blood products when available can be life saving. Uncertainties remain regarding the best method for resuscitation, what type of fluid, how much, when, and how fast [68].
A hemoglobin level of 7–8 g/dl is an appropriate threshold for transfusion in critically ill patients with no risk factors for tissue hypoxia. Maintaining a hemoglobin level of 10 g/dl is a reasonable goal for patients who are actively bleeding, the elderly, or individuals at risk for a myocardial infarction. Moreover, hemoglobin concentration should not be the only therapeutic guide in actively bleeding patients. Instead, therapy should be aimed at restoring intravascular volume and adequate hemodynamic parameters.
Competing interests
None declared.
Abbreviations
CaO2 = arterial oxygen content; DO2 = oxygen delivery; EBV = estimated blood volume; VO2 = oxygen consumption.
See related letter, http://ccforum.com/content/8/5/396
References
- Fink MP. Bench-to-bedside review: cytopathic hypoxia. Crit Care. 2002;6:491–499. doi: 10.1186/cc1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya H, Onda H, Yoneyama T, Sasaki T, Hori T. Bedside monitoring of circulating blood volume after subarachnoid hemorrhage. Stroke. 2003;34:956–960. doi: 10.1161/01.STR.0000064321.10700.63. [DOI] [PubMed] [Google Scholar]
- Cropp GJ. Changes in blood and plasma volumes during growth. J Pediatr. 1971;78:220–229. doi: 10.1016/s0022-3476(71)80004-5. [DOI] [PubMed] [Google Scholar]
- Committee on Trauma . Advanced Trauma Life Support Manual. Chicago: American College of Surgeons; 1997. pp. 103–112. [Google Scholar]
- Bourke DL, Smith TC. Estimating allowable hemodilution. Anesthesiology. 1974;41:609–612. doi: 10.1097/00000542-197412000-00015. [DOI] [PubMed] [Google Scholar]
- Jolliet P, Thorens JB, Nicod L, Pichard C, Kyle U, Chevrolet JC. Relationship between pulmonary oxygen consumption, lung inflammation, and calculated venous admixture in patients with acute lung injury. Intensive Care Med. 1996;22:277–285. doi: 10.1007/s001340050081. [DOI] [PubMed] [Google Scholar]
- Adachi H, Strauss W, Ochi H, Wagner NH. The effect of hypoxia on the regional distribution of cardiac output in the dog. Circ Res. 1976;39:314–319. doi: 10.1161/01.res.39.3.314. [DOI] [PubMed] [Google Scholar]
- Ray CJ, Abbas MR, Coney AM, Marshall JM. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol. 2002;544:195–209. doi: 10.1113/jphysiol.2002.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds NJ, Marshall JM. Vasodilatation, oxygen delivery and oxygen consumption in rat hindlimb during systemic hypoxia: roles of nitric oxide. J Physiol. 2001;532:251–259. doi: 10.1111/j.1469-7793.2001.0251g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichtig R, Kramer DJ, Pinsky MR. Flow distribution during progressive hemorrhage is a determinat of critical O2 delivery. J Appl Physiol. 1991;70:169–178. doi: 10.1152/jappl.1991.70.1.169. [DOI] [PubMed] [Google Scholar]
- Vetterlein F, Schmidt G. Effects of propranolol and epinephrine on density of capillaries in rat heart. Am J Physiol. 1984;246:H189–H196. doi: 10.1152/ajpheart.1984.246.2.H189. [DOI] [PubMed] [Google Scholar]
- Krolo I, Hudetz AG. Hypoxemia alters erythrocyte perfusion pattern in the cerebral capillary network. Microvasc Res. 2000;59:72–79. doi: 10.1006/mvre.1999.2185. [DOI] [PubMed] [Google Scholar]
- Parthasarathi K, Lipowsky HH. Capillary recruitment in response to tissue hypoxia and its dependence on red blood cell deformability. Am J Physiol. 1999;277:H2145–H2157. doi: 10.1152/ajpheart.1999.277.6.H2145. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Hogan MC, Stary C, Bebout DE, Mathieu-Costello O, Wagner PD. Structural basis of muscle O2 diffusing capacity: evidence from muscle function in situ. J Appl Physiol. 2000;88:560–566. doi: 10.1152/jappl.2000.88.2.560. [DOI] [PubMed] [Google Scholar]
- Cain SM. Peripheral oxygen uptake and delivery in health and disease. Clin Chest Med. 1983;4:139–148. [PubMed] [Google Scholar]
- Schwartz S, Frantz RA, Shoemaker WC. Sequential hemodynamic and oxygen transport responses in hypovolemia, anemia, and hypoxia. Am J Physiol. 1981;241:H864–H871. doi: 10.1152/ajpheart.1981.241.6.H864. [DOI] [PubMed] [Google Scholar]
- Nelson DP, King CE, Dodd SL, Schumacker PT, Cain SM. Systemic and intestinal limits of O2 extraction in the dog. J Appl Physiol. 1987;63:387–394. doi: 10.1152/jappl.1987.63.1.387. [DOI] [PubMed] [Google Scholar]
- Chapler CK, Cain SM. Circulatory adjustments to anemic hypoxia. Adv Exp Med Biol. 1988;227:103–115. doi: 10.1007/978-1-4684-5481-9_9. [DOI] [PubMed] [Google Scholar]
- Gutierrez G, Marini C, Acero AL, Lund N. Skeletal muscle PO2 during hypoxemia and isovolemic anemia. J Appl Physiol. 1990;68:2047–2053. doi: 10.1152/jappl.1990.68.5.2047. [DOI] [PubMed] [Google Scholar]
- Weiskopf RB, Viele MK, Feiner J, Kelley S, Lieberman J, Noorani M, Leung JM, Fisher DM, Murray WR, Toy P, Moore MA. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–221. doi: 10.1001/jama.279.3.217. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Weiskopf RB, Kelley SD, Feiner J, Noorani M, Leung J, Toy P, Viele MK. Critical oxygen delivery in conscious humans is less than 7.3 ml O2 × kg-1 × min-1. Anesthesiology. 2000;92:407–413. doi: 10.1097/00000542-200002000-00022. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Vogt M. Muscle tissue adaptations to hypoxia. J Exp Biol. 2001;204:3133–3139. doi: 10.1242/jeb.204.18.3133. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Dreja K, Sward K, Hellstrand P. Effects of oxygen tension on energetics of cultured vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2002;283:H110–117. doi: 10.1152/ajpheart.00040.2001. [DOI] [PubMed] [Google Scholar]
- Schumacker PT, Chandel N, Agusti AGN. Oxygen conformance of cellular respiration in hepatocytes. Am J Physiol Lung Cell Mol Physiol. 1993;265:L395–L402. doi: 10.1152/ajplung.1993.265.4.L395. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. Tissue oxygenation and brain sensitivity to hypoxia. Respir Physiol. 2001;128:263–276. doi: 10.1016/S0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- Dubin A, Estensoro E, Murias G, Canales H, Sottile P, Badie J, Barán M, Pálizas F, Laporte M, Rivas Díaz M. Effects of hemorrhage on gastrointestinal oxygenation. Intensive Care Med. 2001;27:1931–1936. doi: 10.1007/s00134-001-1138-9. [DOI] [PubMed] [Google Scholar]
- Oakes SA, Opferman JT, Pozzan T, Korsmeyer SJ, Scorrano L. Regulation of endoplasmic reticulum Ca2+ dynamics by proapoptotic BCL-2 family members. Biochem Pharmacol. 2003;66:1335–40. doi: 10.1016/S0006-2952(03)00482-9. [DOI] [PubMed] [Google Scholar]
- Boutilier RG. Mechanisms of cell survival in hypoxia and hypothermia. J Exp Biol. 2001;204:3171–3181. doi: 10.1242/jeb.204.18.3171. [DOI] [PubMed] [Google Scholar]
- Gutierrez G. Cellular effects. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editor. The Lung: Scientific Foundations. 2. New York: Raven Press Ltd; 1996. pp. 1969–1979. [Google Scholar]
- Warren JC. Surgical Pathology and Therapeutics. Philadelphia: Lea & Febiger; 1895. [Google Scholar]
- Shevell T, Malone FD. Management of obstetric hemorrhage. Semin Perinatol. 2003;27:86–104. doi: 10.1053/sper.2003.50006. [DOI] [PubMed] [Google Scholar]
- Wolinsky PR. Assessment and management of pelvic fracture in the hemodynamically unstable patient. Orthop Clin North Am. 1997;28:321–329. doi: 10.1016/s0030-5898(05)70291-1. [DOI] [PubMed] [Google Scholar]
- Cannon WB, Fraser J, Cowell EM. The preventive treatment of wound shock. JAMA. 1918;70:618–621. [Google Scholar]
- Wiggers CJ. Physiology of Shock. New York: Commonwealth Fund; 1950. Irreversible shock; pp. 121–146. [Google Scholar]
- Shires T, Coln D, Carrico J, Lightfoot S. Fluid therapy in hemorrhagic shock. Arch Surg. 1964;8:688–693. doi: 10.1001/archsurg.1964.01310220178027. [DOI] [PubMed] [Google Scholar]
- Healey MA, Davis RE, Liu FC, Loomis WH, Hoyt DB. Lactated Ringers is superior to normal saline in a model of massive hemorrhage and resuscitation. J Trauma. 1998;45:894–898. doi: 10.1097/00005373-199811000-00010. [DOI] [PubMed] [Google Scholar]
- Cochrane Injuries Group Albumin Reviewers Human albumin administration in critically ill patients:systematic review of randomized controlled trials. BMJ. 1998;317:235–240. doi: 10.1136/bmj.317.7153.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi PTL, Yip G, Quinonez LG, Cook DJ. Crystalloids vs. colloids in fluid resuscitation: a systematic review. Crit Care Med. 1999;27:200–210. doi: 10.1097/00003246-199901000-00053. [DOI] [PubMed] [Google Scholar]
- Hoyt D. Fluid resuscitation: the target from an analysis of trauma systems and patient survival. J Trauma. 2003;Suppl:S31–S35. doi: 10.1097/01.TA.0000047221.49816.0C. [DOI] [PubMed] [Google Scholar]
- Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: systematic review of randomised controlled trials. BMJ. 1998;316:961–964. doi: 10.1136/bmj.316.7136.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CE, Grady JJ, Kramer GC, Younes RN, Gehlsen K, Holcroft JW. Individual patient cohort analysis of the efficacy of HSD in patients with traumatic brain injury and hypotension. J Trauma. 1997;Suppl:S61–S65. doi: 10.1097/00005373-199705001-00011. [DOI] [PubMed] [Google Scholar]
- Mattox KL, Maningas PA, Moore EE, Mateer JR, Marx JA, Aprahamian C, Burch JM, Pepe PE. Prehospital hypertonic saline/ dextran infusion for post-traumatic hypotension. The U.S.A. Multicenter Trial. Ann Surg. 1991;213:482–491. doi: 10.1097/00000658-199105000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CE, Grady JJ, Kramer GC. Efficacy of hypertonic saline dextran fluid resuscitation for patients with hypotension from penetrating trauma. J Trauma. 2003;Suppl:S144–148. doi: 10.1097/01.TA.0000047223.62617.AB. [DOI] [PubMed] [Google Scholar]
- Creteur J, Sibbald W, Vincent JL. Hemoglobin solutions: not just red blood cell substitutes. Crit Care Med. 2000;28:3025–3034. doi: 10.1097/00003246-200008000-00058. [DOI] [PubMed] [Google Scholar]
- Gould SA, Moore EE, Hoyt DB, Burch JM, Haenel JB, Garcia J, DeWoskin R, Moss GS. The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergency surgery. J Am Coll Surg. 1998;187:113–120. doi: 10.1016/S1072-7515(98)00095-7. [DOI] [PubMed] [Google Scholar]
- Sloan EP, Koenigsberg M, Gens D, Cipolle M, Runge J, Mallory MN, Rodman G., Jr Diaspirin cross-linked hemoglobin (DCLHb) in the treatment of severe traumatic hemorrhagic shock: a randomized controlled efficacy trial. JAMA. 1999;282:1857–1864. doi: 10.1001/jama.282.19.1857. [DOI] [PubMed] [Google Scholar]
- Singbartl K, Innerhofer P, Radvan J, Westphalen B, Fries D, Stogbauer R, Van Aken H. Hemostasis and hemodilution: a quantitative mathematical guide for clinical practice. Anesth Analg. 2003;96:929–935. doi: 10.1213/01.ANE.0000052711.68903.5D. [DOI] [PubMed] [Google Scholar]
- Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napoliltano LM. Blood transfusion, independent of shock severity is associated with worse outcome in trauma. J Trauma. 2003;54:898–907. doi: 10.1097/01.TA.0000060261.10597.5C. [DOI] [PubMed] [Google Scholar]
- Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion medicine. First of two parts–blood transfusion. N Engl J Med. 1999;340:438–447. doi: 10.1056/NEJM199902113400606. [DOI] [PubMed] [Google Scholar]
- Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion medicine. Second of two parts: blood conservation. N Engl J Med. 1999;340:525–533. doi: 10.1056/NEJM199902183400706. [DOI] [PubMed] [Google Scholar]
- Anonymous Consensus conference: perioperative red blood cell transfusion. JAMA. 1988;260:2700–2703. [PubMed] [Google Scholar]
- American College of Physicians Practice strategies for elective red blood cell transfusion. Ann Intern Med. 1992;116:403–406. doi: 10.7326/0003-4819-116-5-403. [DOI] [PubMed] [Google Scholar]
- Anonymous Practice guidelines for blood component therapy: a report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology. 1996;84:732–747. [PubMed] [Google Scholar]
- Expert Working Group Guidelines for red blood cell and plasma transfusions for adults and children. CMAJ. 1997;Suppl 11:S1–S25. [Google Scholar]
- Lenfant C. Transfusion practice should be audited for both undertransfusion and overtransfusion. Transfusion. 1992;32:873–874. doi: 10.1046/j.1537-2995.1992.32993110764.x. [DOI] [PubMed] [Google Scholar]
- Khanna MP, Hebert PC, Fergusson DA. Review of the clinical practice literature on patient characteristics associated with allogeneic redblood cell transfusion. Transfus Med Rev. 2003;17:110–119. doi: 10.1053/tmrv.2003.50008. [DOI] [PubMed] [Google Scholar]
- Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D, ABC Investigators Anemia and Blood Transfusion in Critically Ill Patients. JAMA. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- Wu W-C, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- Bickell WH, Wall MJ, Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- Mapstone J, Roberts I, Evans P. Fluid resuscitation strategies: a systematic review of animal trials. J Trauma. 2003;55:571–589. doi: 10.1097/01.TA.0000062968.69867.6F. [DOI] [PubMed] [Google Scholar]
- Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma. 2002;52:1141–1146. doi: 10.1097/00005373-200206000-00020. [DOI] [PubMed] [Google Scholar]
- Innerhofer P, Fries D, Margreiter J, Klingler A, Kühbacher G, Wachter B, Oswald E, Salner E, Frischhut B, Schobersberger W. The effects of perioperatively administered colloids and crystalloids on primary platelet-mediated hemostasis and clot formation. Anesth Analg. 2002;95:858–865. doi: 10.1097/00000539-200210000-00012. [DOI] [PubMed] [Google Scholar]
- Porter JM, Ivatury RR. In search of the optimal end points of resuscitation in trauma patients: a review. J Trauma. 1998;44:908–914. doi: 10.1097/00005373-199805000-00028. [DOI] [PubMed] [Google Scholar]
- Gutierrez G, Taylor D. Gastrointestinal tonometry: basic principles and recent advances in monitoring regional CO2 metabolism. Semin Respir Crit Care Med. 1999;20:17–27. [Google Scholar]
- McKinley BA, Parmley CL, Butler BD. Skeletal muscle pO2, pCO2, and pH in hemorrhage, shock and resuscitation in dogs. J Trauma. 1998;44:119–127. doi: 10.1097/00005373-199801000-00015. [DOI] [PubMed] [Google Scholar]
- McKinley BA, Kozar RA, Cocanour CS, Valdivia A, Sailors RM, Ware DN, Moore FA. Normal versus supranormal oxygen delivery goals in shock resuscitation: the response is the dame. J Trauma. 2002;53:825–832. doi: 10.1097/00005373-200211000-00004. [DOI] [PubMed] [Google Scholar]
- Kwan I, Bunn F, Roberts I, WHO Pre-Hospital Trauma Care Steering Committee Timing and volume of fluid administration for patients with bleeding. Cochrane Database Syst Rev. 2003;3:CD002245. doi: 10.1002/14651858.CD002245. [DOI] [PubMed] [Google Scholar]


