Abstract
Anthracnose diseases, caused by Colletotrichum spp., are considered to be among the most destructive diseases that have a significant impact on the global production of strawberries. These diseases alone can cause up to 70% yield loss in North America. Colletotrichum spp. causes several disease symptoms on strawberry plants, including root, fruit, and crown rot, lesions on petioles and runners, and irregular black spots on the leaf. In many cases, a lower level of infection on foliage remains non-symptomatic (quiescent), posing a challenge to growers as these plants can be a significant source of inoculum for the fruiting field. Reliable detection methods for quiescent infection should play an important role in preventing infected plants’ entry into the production system or guiding growers to take appropriate preventative measures to control the disease. This review aims to examine both conventional and emerging approaches for detecting anthracnose disease in the early stages of the disease cycle, with a focus on newly emerging techniques such as remote sensing, especially using unmanned aerial vehicles (UAV) equipped with multispectral sensors. Further, we focused on the acutatum species complex, including the latest taxonomy, the complex life cycle, and the epidemiology of the disease. Additionally, we highlighted the extensive spectrum of management techniques against anthracnose diseases on strawberries and their challenges, with a special focus on new emerging sustainable management techniques that can be utilized in organic strawberry systems.
Keywords: anthracnose fruit rot, Colletotrichum acutatum, diagnostics, crop protection, sustainable management techniques, strawberry
1. Introduction
Strawberry (Fragaria ananassa Duchesne), a major small fruit across the world, has an attractive flavor and taste and a high content of essential nutrients that benefit human health [1]. The Food and Agriculture Organization of the United Nations (FAO) reports that strawberries were planted on 389,665 ha and produced about 9,175,384.43 metric tons globally in 2021 [2]. The United States of America is among the countries with the highest strawberry production and plants approximately 23,500 ha of strawberries, with a value of US $2.4 billion [3]. In addition, the south-Atlantic region of the United States produces 947 ha of strawberries, with an average yield of 15,668 kg/ha and a total farm gate value of $47,158,000 [4]. Strawberry consumption in the United States has grown over the last two decades, from 0.9 kg per capita in 1980 to 3.6 kg in 2013 [5]. However, diseases and pests significantly lower the quality and yield of strawberry fruit, incurring significant financial losses for growers.
The mid-Atlantic region of the United States, including the Commonwealth of Virginia, ranks third in the production of fresh market strawberries after California and Florida, and most growers are using the annual hill plasticulture (AHP) production system, likely due to the disease risk from the buildup of inoculum in a perennial system [4]. Field preparation begins with debris removal, disking, and tilling the soil, followed by bedding, plastic covering, drip tape installation, and overhead watering in September. In most cases, the raised soil beds are fumigated to control nematodes, fungi, and weeds. However, due to new regulations, fumigation with synthetic chemicals is becoming difficult for small growers and farms close to public places. Then, the transplants are planted and overhead watered for establishment between mid-September and early October, when temperatures couldreach above 30 °C [6,7]. Strawberry fruits are harvested two to three times each week on average when the berries are fully ripe, from mid-April until late June. The strawberry plant can be infected by different organisms, including many arthropods, nematodes, fungi, bacteria, viruses, and other pests [8]. Further, the strawberry plant is highly susceptible to a large variety of soilborne pathogens, including the genera Verticillium, Phytophthora, and Colletotrichum, which are considered the most damaging pathogens on this crop in the United States strawberry production system [8,9]. In general, anthracnose diseases are caused by fungal pathogens belonging to multiple species under the acutatum species complex and the gloeosporioides species complex of the genus Colletotrichum. All of these pathogens can cause infection on any part of a strawberry; however, the acutatum species complex tends to be more destructive as fruit rot pathogens, and the gloeosporioides species complex is more damaging as crown rot pathogens. Considering the overall importance and frequency of occurrence of U.S. strawberries, this review highlights the acutatum species complex.
In the strawberry industry, strawberry transplants are grown mainly by using starter plant material like runner tips that come from mother plants located in northern latitudes, such as Canada, or mother plants that are located in higher altitudes in the U.S., such as in the mountain regions of North Carolina and California, which have relatively cooler temperatures. Low temperatures may slow disease progression but do not eliminate the pathogen from strawberry transplants [10]. These tips are then rooted in propagation houses in July through September to generate strawberry plug plants at various locations in the U.S. Pathogens such as those under the C. acutatum species complex may be transferred to freshly formed plants since transplants are propagated vegetatively [11]. The warm temperatures and humidity in the propagation house create a favorable environment for the moderate-to-high temperature-loving pathogen to thrive. When the infected transplants arrive in fruiting fields, plants are still subjected to ideal environmental conditions for anthracnose disease development, such as extended wetness periods and temperatures between 20 and 30 °C, as overhead irrigation is commonly practiced to aid in the establishment of transplants [12]. Therefore, new technology for detecting the acutatum species complex in asymptomatic nursery plants is a critical need in the North American Strawberry Nursery System to minimize the potential of selling transplants latently infected with Colletotrichum spp. to fruit growers [13].
2. Anthracnose Diseases in Strawberry
Anthracnose, described as a disease that shows as black, sunken lesions on stems, runners, or fruit and is caused by fungi that generate asexual spores in acervuli, is derived from the Greek roots “anthrak-” (coal) and “-nosos” (disease). Anthracnose fruit rot (AFR) disease on strawberries is caused by multiple strains belonging to the acutatum species complex, which includes 44 species of Colletotrichum [14,15,16,17,18]. These diseases are among the most dangerous, causing significant crop losses of up to 70% in commercial production fields planted with susceptible strawberry cultivars [12,19]. Colletotrichum spp. has a wide host range and causes several disease symptoms in strawberry plants, including root, fruit, and crown rot, lesions on petioles and runners, and irregular spots on the leaf [20]. C. acutatum can infect all parts of the strawberry plant (leaves, petioles, flowers, crowns, and roots) along with the fruit, and lesions may expand and entirely cover the surface of the fruit under favorable conditions (high temperature and humidity), especially on susceptible varieties [21,22].
3. Taxonomy of C. acutatum
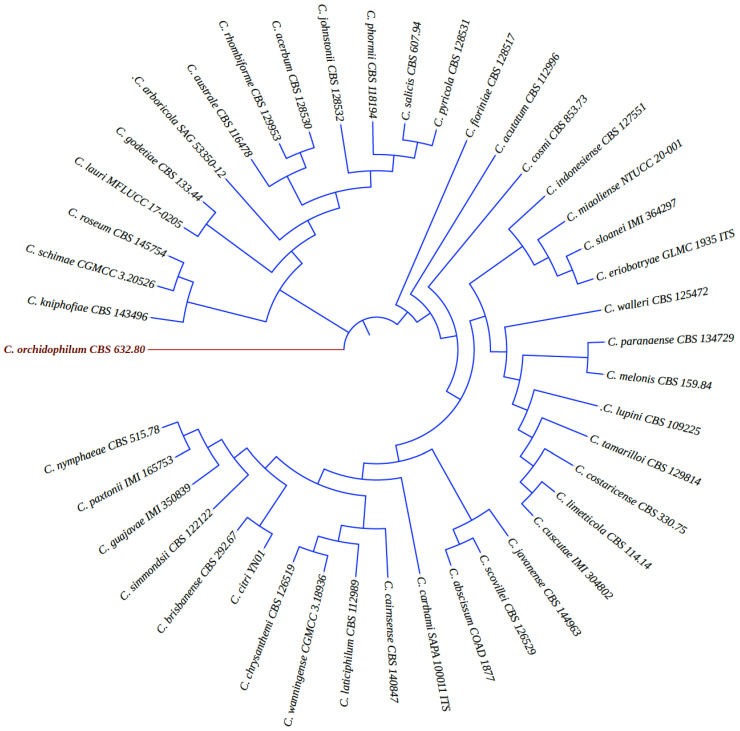
The latest classification of species within the genus Colletotrichum is based on their extensive molecular differences, their extensive host ranges, and their diverse lifestyles. The current classification system for the genus Colletotrichum consists of more than 280 species, including 16 species complexes (including the acutatum species complex) and 15 singleton species [18]. The fungus Colletotrichum acutatum J.H. Simmonds taxonomically belongs to: Fungi, Ascomycota, Pezizomycotina, Sordariomycetes, Hypocreomycetidae, Glomerellales, Glomerellaceae, Colletotrichum acutatum, as reported by Simmonds (1965), who described it as a distinct species in Queensland, Australia, in a pathogen survey of fruit rot [23]. The pathogen was previously identified as a species of Cladosporium that affected strawberries in Australia, causing mature fruit rot and lesions on the stolon, petiole, and peduncle [24]. In this review, a neighbor-joining phylogenetic tree was generated using the nuclear ribosomal internal transcribed spacer (ITS) region that was retrieved from GenBank of the most updated lists of Colletotrichum species that were accepted as members of the acutatum species complex (Figure 1) [18,25]. The majority of the species within the acutatum species complex are recognized as destructive plant pathogens on a global scale [18,26]. The acutatum species complex has been linked to 171 plant species, which are distributed across 129 genera. The majority of these plant species (90.9%) are dicots, while only a small proportion of them are monocots and gymnosperms, accounting for 5.3% and 1.6% of the total, respectively [27]. The acutatum species complex has seven species that were sorted as strawberry pathogens, including C. acutatum s.s., C. fioriniae, C. godetiae, C. miaoliense, C. nymphaeae, C. salicis, and C. simmondsii [28].
Figure 1.
Phylogenetic analysis was generated using a neighbor-joining tree based on 42 strains of Colletotrichum spp. that were accepted as members of the acutatum species complex [18,25]. The analysis of sequences of internal transcribed spacers (ITS) were retrieved from GenBank. Colletotrichum orchidophilum was employed as an outgroup strain and is highlighted in red color.
4. Epidemiology and Lifestyle of C. acutatum
Colletotrichum acutatum inoculum in annual strawberry fields is derived from symptomless infected transplants from nurseries or plug plant production facilities where overhead irrigation might have resulted in conidial dissemination [29]. Under warm temperatures and high humidity, this pathogen rapidly produces conidia, which can spread to flowers and fruit by rain/overhead irrigation, splashing water, and harvesting operations, and consequently show the symptoms of the infections caused by the pathogen, mostly at the fruiting stage. Several studies showed that conidia dispersal occurs within a 25 cm radius of the inoculum source and may vary depending on rainfall intensity and ground cover [30]. This fungus is considered to be hemibiotrophic, where the fungus initially enters a biotrophic phase and then switches to a necrotrophic phase [31]. The pathogen penetrates the cuticle via a specialized cell called an appressorium and grows within the cuticle and cell walls of epidermal, subepidermal, and subtending cells. Then, the fungus produces acervuli as a stroma immediately under the outer periclinal epidermal walls when the cortical tissue is substantially disturbed, and the conidia are released from these acervuli. Although C. acutatum may produce quiescent infections on strawberry plants, according to several studies, the epidemiological significance of latent infection before the colonization of fruits and senescent foliage has not been investigated [32].
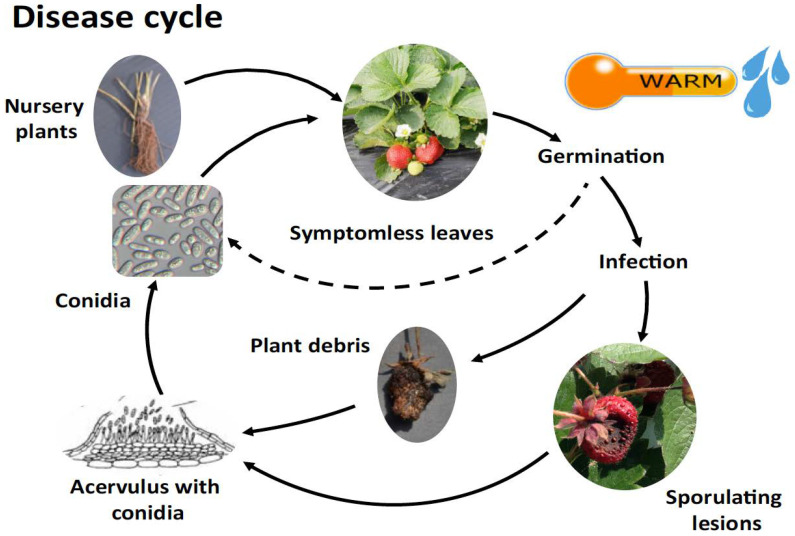
The quiescent stage (the latent period) is considered to be the time between the fungal infection of the host and the first symptom appearance [33]. The latent period depends on temperature and is between 2–3 days at 25 °C and 6–17 days at 5 °C [34]. The germination of C. acutatum conidia, the development of appressoria, latency on non-targeted vegetative organs, including leaves, and serving as a source of overwintering inoculum (Figure 2). In comparison to C. theobromicola or C. gloeosporioides, C. acutatum can produce more conidia at lower temperatures and show the shortest latent period among the three species at 5 and 10 °C. The concentration of inoculum present as latent infections on strawberry plants determines the beginning of disease symptoms. Knowing the minimum inoculum concentration for the start of a disease is informative, particularly in the case of strawberry AFR, as the disease begins as infected transplants from nurseries in commercial fields [35]. Therefore, the development of new tools for the detection of the latent infection at the transplant stage in nurseries is a key factor in controlling AFR diseases on strawberries in fruiting fields.
Figure 2.
The disease cycle of anthracnose fruit rot caused by (AFR) C. acutatum [36].
The primary source of inoculum for fruit infections may be C. acutatum appressoria and secondary conidia, which are formed on symptomless leaves and help keep inoculum available throughout the growing season. Wetness for more than 4 h is required for secondary conidia production and appressoria formation [31]. In general, Colletotrichum spp. conidia are transmitted from plant to plant in the field primarily by rain splash. Conidia of C. acutatum are disseminated over short distances on low-growing crops such as strawberries, and using straw as a ground cover can drastically reduce conidia dispersal [37,38]. Further, the conidia of the three Colletotrichum spp. were evaluated with water splash, and it was discovered that the conidia of C. theobromicola dispersed over the shortest distance while those of C. acutatum spread over the longest distance due to the high production of spores [39]. Although C. acutatum can survive in soil and on inanimate surfaces for various lengths of time, depending on the conditions, it appears to compete poorly as a saprophyte [40]. The fungus can survive in the soil for at least two winters with temperatures below 0 °C, causing anthracnose to grow in the following years [41]. Therefore, the application of appropriate disease treatment is required not just for the current year’s crop but also for subsequent seasons. Additionally, it has been proven that weeds host the strawberry infection caused by C. acutatum. Nevertheless, a multicrop study conducted in Florida found that C. acutatum isolates can be host-specific and offer little threat to other crops [42].
5. Detection: Morphological, Molecular, and Remote Sensing
Transplants infected with Colletotrichum spp. can spread pathogens from the nursery to the field, and the best management option to avoid the disease is to begin with disease-free planting materials [43]. However, there is no reliable diagnostic method-based protocol to detect latent infection of anthracnose diseases in the early stages of the production cycle in a large production area. Currently, the production of disease-free transplants in nurseries relies on scouting for symptomatic plants and the identification of diseases using colony and spore morphology, which is time-consuming, error-prone, and sometimes inaccurate [41,44]. Molecular techniques such as polymerase chain reaction (PCR), real-time PCR, and ELISA using DNA have become a robust detection and diagnostic tool for plant pathogens [45,46]. However, the polymerase chain reaction (PCR) has some challenges, such as the presence of PCR inhibitors in plant tissues, failure to amplify low DNA concentrations, and the detection of dead pathogens, which can give false positives. Further, all molecular techniques for the diagnosis of plant pathogens are costly, time-consuming, and require the use of highly skilled human resources [47].
Visual inspection, along with field sampling of plant material, is the conventional way of detecting infections in the field, but it is tedious and requires specialized skills [47,48]. The traditional methods cannot detect the latent infection in the early stages of infection. Other laboratory analyses, such as microscopy, molecular, biochemical, and microbiological methods, have been applied for the detection of crop diseases; however, these techniques have disadvantages, as the sampling process is destructive and offers limited diagnostic points, and it is not field-scalable or may not represent accurate field variability [47,49]. Therefore, precise, high-throughput, non-invasive, and field-scalable approaches are required [47,50]. As an alternative in recent years, non-destructive methods such as spectral vegetation indexing, multispectral imaging (MSI), or hyperspectral imaging (HSI) based on ground, aerial, and satellite platforms have emerged that are capable of crop disease diagnostics at high accuracy and on high spatial scales (from leaf to plant to field). Additionally, MSI and HSI could also offer early detection, even before the visual symptoms develop. Such detection can help with proactive management of anthracnose, thereby improving productivity [51].
5.1. Remote Sensing of Anthracnose
Remote sensing (RS) with MSI and HSI systems has demonstrated its ability for spatiotemporal vegetation monitoring, including the detection of crop diseases in the early stages [52]. During the infection process to cause disease symptoms, MSI captures light reflected from the surface of the target object, such as a leaf, which is dependent on the changes in both physiological and biological status due to the infection process and subsequent growth stages, e.g., alternations in plant pigmentations such as chlorophyll and carotenoids [51]. Since anthracnose causes physiological, morphological, and plant pigmentation variations, the MSI and HSI techniques could be useful in estimating its incidence. [51]. Remote sensing, such as HSI, was used to detect anthracnose diseases on tea plants with a detection accuracy of 98% for identifying the disease at the leaf level and 94% at the pixel level, where they identified disease-sensitive bands at 542, 686, and 754 nm, which were used to create two disease indices, including the Tea Anthracnose Ratio Index (TARI) and the Tea Anthracnose Normalized Index (TANI) [53].
MSI imaging corresponds to imaging within 3–10 bands of the electromagnetic spectrum in the optical range of 350–1000 nm. Each pixel in the image is represented by a vector, referred to as the spectral signature or fingerprint region of the spectrum [51]. Each fingerprint region of the spectrum has complex absorption sequences due to various bending vibrations within molecules of the plant tissue, and a slight alteration in a compound’s molecular structure will result in a significant change in spectral absorption [54]. The spectral data are helpful, although it may be redundant for adjacent wavelengths. To minimize data size and enhance data utilization efficiency, only significant wavelengths with essential information should be selected for the application of MSI to reduce expenses and increase the speed of plant disease detection [49]. In the field, the sheath blight (ShB) disease on rice, caused by Rhizoctonia solani, has been detected with high efficiency using MSI, where five vegetation indices were then calculated from the multispectral images, including the Normalized Difference Vegetation Index (NDVI), Ration Vegetation Index (RVI), Difference Vegetation Index (DVI), Normalized Difference Water Index (NDWI), and Red Edge (RE) [55]. The MSI technique was used to detect the light leaf spot infection with 92% accuracy on oilseed rape (Brassica napus) within 13 days before the beginning of visible symptoms, and they used false color mapping of spectral vegetation indices to quantify disease severity and its distribution within the plant canopy in the field [51]. A non-destructive model for the evaluation of firmness, total soluble solids (TSS) content, and ripeness stage in strawberry fruit was established with 100% accuracy using the MSI [56]. Hyperspectral imaging (HIS) has been used to detect anthracnose on strawberry plants, and the spectra of disease in symptomless and symptomatic sections of leaves vary significantly at wavelengths ranging from 540–570 nm to 750–310 nm in the laboratory [57]. Several studies used ultraviolet fluorescence (440–740 nm), multispectral (green [540 nm], red [660 nm], and near-infrared [800 nm]), RGB, and hyperspectral (900–1700 nm) imaging techniques for the detection of crop diseases such as powdery mildew (Erysiphales) on the grapevine (Vitis vinifera) [58]. Six machine learning-assisted techniques were devised utilizing the chosen spectral fingerprint characteristics to enable early detection of anthracnose and gray mold diseases on strawberries through the use of a hyperspectral imaging system. The majority of the classification models demonstrated a high level of accuracy (100%) and consistent performance, successfully identifying asymptomatic fungal infections prior to the manifestation of overt disease symptoms, particularly in the strawberry crop [59]. A study investigated the potential of employing hyperspectral imaging (HSI) in conjunction with spectral features, vegetation indices (VIs), and textural features (TFs) to effectively detect gray mold on strawberry leaves. The integration of these combined features in the detection process significantly enhances the accuracy of recognizing strawberry gray mold, enabling the precise identification of infected leaves during the initial stages of infection [60]. A recent study investigated the potential of integrating hyperspectral technology with machine learning and deep learning techniques for the detection of asymptomatic strawberry anthracnose crown rot (ACR), which is caused by Colletotrichum gloeosporioides. The accuracy rates of the model test set for healthy, asymptomatic, and symptomatic samples were 99.1%, 93.5%, and 94.5%, respectively [61].
5.2. Unmanned Aerial Vehicle (UAV) Platform
Unmanned aerial vehicles (UAVs), typically referred to as drones, have had extensive applications in the past decade for managing crop production operations. A particularly promising use of UAV is in the monitoring of crop health since it may enhance conventional crop monitoring methods, including visual observation to assist in rapid detection, which in turn can have a significant positive effect on crop yield and quality [62]. UAVs are used with MSI or HSI systems to provide high spatial resolution images at flexible flight schedules and short data-acquisition timeframes [52]. Additionally, the imaging data technology based on UAVs has been implemented effectively in diverse applications, including the rapid evaluation of crop vigor and soil characteristics, crop water requirements, disease infestation, and yield prediction [63]. Three platforms, including UAV, sentinel 2, and planet-scope satellite platforms with multispectral (MS) imagery systems, were evaluated based on the analysis of the spatial resolution using soil-adjusted vegetation index (SAVI) to monitor onion crops in the field, and the best result was achieved with the images provided by the UAV platform, which could give more detailed images at critical moments in the crop cycle [64]. Small unmanned aerial systems (UAS) equipped with high-resolution visible (red, green, and blue [RGB]) and multispectral imaging techniques were used to detect powdery mildew (PM) in apple orchards with 77% accuracy [65]. Using a mobile platform, three algorithms, including Stepwise discriminant analysis (SDA), Fisher discriminant analysis (FDA), and K-nearest neighbor (KNN) methods with 32 spectral vegetation indices, were applied to train the model to detect anthracnose diseases at different infection stages on strawberries in both indoor and field trails, and the three models’ classification accuracies were 71.3%, 70.5%, and 73.6%, respectively [48]. To our knowledge, there has been no research on the detection of latent infection of anthracnose diseases on strawberries in the field by using MSI technology with UAV. However, this technological breakthrough for the detection of Colletotrichum quiescent infection in strawberries is very promising.
6. Management: Chemical, Resistance Breeding, Biological and Biorational
Integrated Pest Management (IPM) programs can help producers by combining techniques that focus on long-term disease and pest management, including using pesticides when required, excellent sanitation practices, planting disease-free plants, and modified cultural practices [66]. Several cultural control methods were used to reduce the C. acutatum infection, such as the removal of runners from mother plants. Another cultural practice is the reduction of leaf wetness hours by using drip irrigation instead of overhead irrigation, which helps reduce the movement of the conidia from one plant to another through water splash. Further, hot water therapy has been used for a long time to eradicate pest and disease issues, including cyclamen mites (Phytonemus pallidus ssp. fragariae Zimmerman) and endoparasite nematodes, in dormant strawberry stock. Runner cuttings were collected from mother plants that had been inoculated with C. acutatum, and they were immersed for 7 min at 35 °C, followed by 2 or 3 min at 50 °C. Both treatments of cuttings successfully reduced C. acutatum infections from over 80% in the controls to between 6% and 17%, respectively [67].
Chemical methods, such as the usage of fungicides, are a common method among growers to control AFR and other strawberry diseases and frequently rely on a calendar schedule of weekly application [68]. Early in the season, between November and December, inoculum levels are low, and typically the environment is unfavorable for C. acutatum; therefore, infected plants do not exhibit symptoms. During this time, the first step in chemical management includes the use of low-label rates of broad-spectrum protectant fungicides like Captan. Then, the inoculum levels increase, and the environment will reach the ideal condition for AFR development from January to March; therefore, higher label rates of broad-spectrum fungicides must be applied weekly depending on the detection of latent infections [68]. In the past, mancozeb, carbendazim, prochloraz, and Tecto 60 have all been used as synthetic fungicides to control the Colletotrichum spp. that causes anthracnose in fruits, including strawberries [69,70]. In the last update of the 2023 southeast regional strawberry integrated pest management guide focused on plasticulture production, many fungicides are labeled as “excellent” for controlling the AFR of strawberry plants, such as Merivon (fluxapyroxad + pyraclostrobin), Pristine (pyraclostrobin + boscalid), Luna Sensation (fluopyram + trifloxystrobin), Quadris Top (azoxystrobin + difenoconazole), Quilt Xcel (azoxystrobin + propiconazole), Cabrio (pyraclostrobin), Abound (azoxystrobin), Flint Extra (trifloxystrobin), and Miravis Prime (pydiflumetofen + fludioxonil). On the other hand, other common fungicides such as captan and thiram were rated “good” and "fair,” respectively [71]. However, to avoid the development of resistance strains of C. acutatum, all fungicides must be used carefully and in rotation, using different active chemical ingredients following the fungicide resistance action group (FRAC) guide.
The anthracnoseresistance in strawberry cultivars has long been a focus in the scientific community around the world, as the use of resistant cultivars is an excellent management technique against anthracnose diseases. However, growers still plant highly susceptible strawberry cultivars such as “Chandler” and “Camarosa” due to their high fruit quality features, despite suffering significant yield losses due to anthracnose diseases [13,72]. The anthracnose-resistance screening procedure implemented by the United States Department of Agriculture (USDA) has proven to be useful in identifying genotypes that are resistant to the disease in seedling progenies derived from the breeding program of North Carolina State University. Notably, a total of over 32,000 strawberry seedlings that exhibited resistance to anthracnose were identified between the years 1998 and 1999 [73]. Field trials were conducted to evaluate several strawberry cultivars against AFR, and high resistance levels were observed on “Sweet Charlie”, “Ruby Gem”, “Florida Elyana”, and “Florida Radiance” cultivars; intermediate susceptibility was observed in “Strawberry Festival” and advanced selection 99–117; conversely, “Albion”, “Camarosa”, “Camino Real”, “Ventana”, “Candonga”, and “Treasure” were evaluated as susceptible or highly susceptible [22]. Recently, commercial strawberry cultivars including “Flavorfest”, “Florida Belle”, “Florida Elyana”, “Pelican”, “Prado”, “Sweet Sensation”, and “Winter Dawn” were labeled as resistant cultivars in the 2023 Southeast Regional Strawberry Integrated Pest Management Guide [71]. In addition, “Dover”, “Florida Radiance”, and “Winterstar” were graded as medium resistance, and some commercial cultivars such as “Carmine”, “Florida Brilliance”, “Ovation”, “Ruby Gem”, and “Sweet Charlie” were evaluated as medium resistance [71].
Strawberry breeders and plant pathologists are currently engaged in the development of strawberry germplasm that is resistant to AFR and ACR diseases [74,75,76,77]. A recent study demonstrated that the ectopic expression of FvChi-14 in Arabidopsis thaliana conferred enhanced resistance against Colletotrichum higginsianum by regulating the expression of key genes involved in the salicylic acid (SA) and jasmonic acid (JA) signaling pathways, namely AtPR1, AtICS1, AtPDF1.2, and AtLOX3, that could introduce fresh prospects for the development of disease-resistant strawberry cultivars [78]. Further, another study indicated that the transgenic octoploid strawberries that exhibited overexpression of FaMBL1 demonstrated a reduced susceptibility to the fungal diseases anthracnose and grey mold [77]. However, there are several key factors, such as Colletotrichum species, isolate, or race, inoculation method, resistance evaluation method, plant tissue evaluation, and environmental conditions, that can influence the susceptibility or resistance of strawberry genotypes to Colletotrichum species during evaluation steps [79].
Many biological control methods and biofungicides have been evaluated on fruit crops against Colletotrichum spp., but none have consistently demonstrated field efficacy. Several bacterial biocontrol agents, such as Bacillus spp., were summarized and evaluated against Colletotrichum spp., such as C. acutatum, C. gloeosporioides, and C. truncatum clades, and they exhibit some inhibitory activities due to the production of antifungal activity via secretion of antifungal metabolites and enzymes or induction of disease resistance in fruits under low disease pressure [80]. B. subtilis, P. polymyxa, and B. amyloliquefaciens have generally been the best agents for managing C. acutatum. According to reports, Paenibacillus polymyxa secretes antifungal enzymes that can break down chitin, amylase, cellulose, and proteins [81]. The conidial germination of C. acutatum was decreased by more than 60% by using Bacillus spp., which was isolated from the apple phylloplane, due to the production of fixed and volatile compounds [82]. Prestop (Gliocladium catenulatum) and PlantShield (Trichoderma harzianum), two commercial fungal biocontrol agents, significantly decreased anthracnose development by up to 45% when sprayed three times onto plants between blooming and fruit ripening [83]. Six isolates of yeast (Saccharomyces cerevisiae) successfully controlled C. acutatum on citrus plants during preharvest due to several actions, including the production of antifungal compounds, competition for nutrients, inhibition of pathogen germination, and the production of killer activity and hydrolytic enzymes when in contact with the fungus wall [84].
7. Anthracnose Diseases Management Challenges
Commercial strawberry growers depend on several management strategies, such as disease-free plants, proper irrigation, mulching, good sanitation practices, pesticides, crop rotation, and disease-resistant cultivars, but none of these have achieved effective control. To control different phytopathogenic fungi, including Colletotrichum spp., growers rely on the use of expensive fungicide input in strawberry production systems in the Northeast and Mid-Atlantic; however, the usage of agrochemicals in the management of Anthracnose fruit rot (AFR) and crown rot (ACR) diseases in the strawberry system faces many challenges, including (i) The fumigant methyl bromide (MeBr), which has been banned since 2005 in many countries, including the United States, because of its ozone-depleting properties. (ii) In some cases, fungicide applications failed to control anthracnose disease epidemics due to several reasons, including fungicide-resistant fungi [10]. (iii) In the fresh strawberry fruit market, pesticide usage is less desirable to consumers, and disease-free transplants in the field are a good starting point to achieve that [85]. (iv) A few fungicides were effective against diseases caused by Colletotrichum spp. on strawberry plants due to the variability of fungicide sensitivity [86]. In addition, AFR is difficult to control since there is no effective protocol to detect non-symptomatic but infected plants to discard those or take appropriate measures, and the pathogen may build up to large levels in the field without being detected, creating the perfect environment for severe epidemics on ripening fruit under disease-favorable weather conditions. All these considerations highlight the importance of viable biologically-based options in strawberry production systems for the management of soilborne diseases and pests, including anthracnose, that can sometimes affect the crowns and roots of strawberries.
8. Alternative and Sustainable Integrated Pest Management Strategies for Soilborne Diseases
Sustainable integrated pest management strategies are needed to meet the global demand for food production for an ever-increasing population [87]. Many alternative soil fumigation methods with synthetic chemicals, such as glucosinolate containing Brassica spp., are known to release volatile isothiocyanates (ITCs), which are lethal to different soilborne plant pathogens [88]. Several studies have reported that bio-fumigation with ITC-producing plants is effective against some soilborne plant pathogens, including Rhizoctonia, Verticillium, Fusarium, Pythium, and Phytophthora spp. [89]. However, this fumigation method is not consistent due to the variable concentration of ITCs in different mustard varieties. From the grower’s perspective, the efficacy of biofumigation was investigated on different plants, and the level of adoption was low. The low efficacy of the treatment has been attributed to many factors, including variations in soil texture, moisture, temperature, soil microbial community, and pH [90].
8.1. Overview of Anaerobic Soil Disinfestation (ASD)
Anaerobic soil disinfestation (ASD), a preplant soil disinfestation strategy, is another soil bio-rational method that shows promise to control a wide range of soilborne pathogens and plant-parasitic nematodes. This strategy has not been well experimented with in the Northeast U.S. [91,92]. The ASD process depends on adding carbon (C) sources to stimulate microbially driven anaerobic soil conditions in moist soils covered with polyethylene mulch, which is supposed to convert organic material into other organic compounds that should be lethal to soilborne pathogens [93]. This technique causes changes in soil physical and chemical characteristics, such as the formation of volatile fatty acids, a decrease in soil pH, a rise in soil moisture, and changes in soil nutrients as a result of organic matter addition [94]. Another mechanism of ASD against soilborne pathogens is lowering the redox potential below the critical redox potential (about +200 mV), which can reduce the survival stage of soilborne pathogens [95]. In soils treated with ASD, the inoculum of soilborne pathogens such as V. dahliae was reduced by 80–100% compared with the nontreated control and produced marketable fruit yields equivalent to fumigation [96]. In addition, root rot disease complexes on tomato plants, caused by some pathogens including Colletotrichum spp., Verticillium dahliae, and Meloidogyne spp., were significantly reduced in ASD-treated soils in high tunnels compared with plants grown in control soils [97]. In a recent study, the application of 20 t/ha of rice bran to ASD-treated soils resulted in a significant decrease in disease severity after 25 and 60 days of incubation in the strawberry production system. However, the application of a 13.5 t/ha dose did not yield the same reduction in disease severity [98]. ASD treatments with brewer’s spent grain (BSG) and carbon sources considerably reduced the seed viability of all weed species and the Pythium irregulare inoculum in a greenhouse trial [99]. The present study has revealed that the application of organic materials at varying dose rates has resulted in significant efficacy against soil-borne pathogens, namely Fusarium spp. and Phytophthora spp., with a range of 69–99% and 63–98%, respectively. Furthermore, the implementation of ASD has led to a notable increase in the levels of soil organic matter and ammonium nitrogen [100]. However, this technique needs to be optimized in terms of engaging beneficial microorganisms to control different soilborne pathogens and enhance plant vigor and productivity at the same time [101]. The mechanisms of ASD are not fully understood; they may be due to the toxic by-products of anaerobic decomposition, volatile compounds, biocontrol by anaerobic soil microorganisms, or oxygen deficiency [91].
8.2. Optimizing Anaerobic Soil Disinfestation (ASD) with Endophytic Bacteria
In general, several factors influence the efficacy of ASD, such as the carbon source, the addition of beneficial microorganisms, and environmental conditions including soil types, pH, and temperature. Based on our previous work, BSG was used effectively as a carbon source to support soil microbial growth in ASD applications in field trials [102]. Engaging beneficial microorganisms such as endophytic bacteria that are used as biofertilizers or biostimulants with the ASD technique could generate a powerful tool to control soilborne pathogens and improve the growth and yield of strawberries, which may play a crucial role in sustainable crop production in the future [103]. Beneficial microorganisms can improve plant nutrition, support plant development under natural or stressed conditions, and increase the yield and quality of many important crops [104]. In the interaction between beneficial microorganisms and plants, these organisms act as nutrient suppliers, phytohormone producers, plant growth enhancers, biocontrol agents of phytopathogens, and improvers of soil structure [105,106]. Root dipping of seedlings (plug plants), followed by spray treatments of both probiotic bacteria, including Bacillus amylolequefaciens (BChi1) and Paraburkholderia fungorum (BRRh-4) on leaves in the field, dramatically enhanced the fruit yield of strawberry plants by 48% compared to the untreated controls [107]. In the greenhouse, three strains of Bacillus velezensis, an endophyte bacterium, significantly suppressed strawberry pathogen growth (C. gloeosporioides) and increased marketable fruit yields in the field [108]. The gray mold disease in strawberry plants, caused by Botrytis cinerea, was controlled by five different isolates of Bacillus spp. via the production of diffusible and volatile antifungal chemicals [109]. The severity of Rhizoctonia root rot disease on Viburnum plants (Viburnum odoratissimum) was reduced on both greenhouse and field trials using the TerraGrow product, which is a complex of five Bacillus strains, including B. licheniformis, B. subtilis, B. pumilus, B. amylolquefiens, and B. megaterium [110]. In a perennial strawberry production system, the combination of beneficial microbes and ASD enhanced plant vigor and fruit yield and suppressed the weed population and pathogenic microbes compared with untreated plants [111].
9. Future Perspectives
Strawberry production has increased around the world in the past few years due to rising demand. Cutting-edge research programs are ongoing to solve problems that threaten strawberry production while also enhancing fruit quality to meet customer demands. There is an urgent need for the adoption of sustainable alternative disease management measures that pose little threat to human health and the ecological system [111]. Integrated Pest Management (IPM) systems, which combine biological, cultural, and chemical tools with other supporting technologies, are effective, efficient, and sustainable. The combination of ASD with beneficial microbes is being introduced lately in agricultural practices in place of fungicides and soil fumigants due to their economic viability and environmental friendliness [111]. It appears that ASD with different C sources is a viable approach to controlling diseases, increasing yield, and improving soil, especially in limited sources, organic farming, and smallholder farming. Furthermore, integrating ASD with beneficial microbes could reduce the initial investment for ASD treatments alone and create a powerful tool for pest management, including fungi, bacteria, nematodes, and weeds in the strawberry production system. Future research should concentrate on understanding how to incorporate suitable beneficial microbes to control specific pathogens, as well as better understanding which mechanism(s) are responsible for disease control in different situations.
A precise detection method for pathogens is a requirement for the application of the appropriate disease control techniques. The current review may also highlight the need for a rapid, non-destructive, and accurate method for anthracnose fruit rot (AFR) at the early stage of the infection (latent period). Further, strawberry growers benefit from early incubation stage identification because it allows them to immediately remove contaminated plants before the disease spreads and causes further damage. In recent years, a combination of small unmanned aerial systems (UAS) equipped with multispectral imaging (MSI) sensors, which integrate spectral and image data, has demonstrated considerable benefits for non-destructive inspections, plant disease identification, and the safety of agricultural products. We believe this review of using remote sensing to diagnose anthracnose fruit rot (AFR) will provide novel thoughts and encourage the development of appropriate theories, methods, and tools to monitor strawberry transplants in nurseries, which are considered the main source of inoculum for the production farms.
Author Contributions
Conceptualization M.R.; methodology B.D.A., M.R. and J.B.S., formal analysis B.D.A.; investigation B.D.A.; resources, J.B.S. and M.R.; writing—original draft preparation B.D.A.; writing—review and editing J.B.S., M.R. and B.D.A.; visualization M.R., B.D.A. and J.B.S.; supervision J.B.S. and M.R.; funding acquisition M.R. and J.B.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
We are grateful for funding from the North East Sustainable Agriculture Research (NE-SARE), USDA research and education grant (#LNE20-401-34268-FE) program that enabled us to complete this article.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Giampieri F., Tulipani S., Alvarez-Suarez J.M., Quiles J.L., Mezzetti B., Battino M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition. 2012;28:9–19. doi: 10.1016/j.nut.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 2.FAO. FAOSTAT. Agriculture Organization of the United . FAOSTAT Statistics Database. FAO; Rome, Italy: 2021. [Google Scholar]
- 3.Wu F., Guan Z., Whidden A.J. An Overview of the US and Mexico Strawberry Industries. EDIS. 2020;2016:1–4. doi: 10.32473/edis-fe971-2015. [DOI] [Google Scholar]
- 4.Samtani J.B., Rom C.R., Friedrich H., Fennimore S.A., Finn C.E., Petran A., Wallace R.W., Pritts M.P., Fernandez G., Chase C.A. The status and future of the strawberry industry in the United States. HortTechnology. 2019;29:11–24. doi: 10.21273/HORTTECH04135-18. [DOI] [Google Scholar]
- 5.U.S. Department of Agriculture (USDA) U.S. Strawberry Consumption Continues to Grow. [(accessed on 26 October 2023)]; Available online: https://www.ers.usda.gov/data-products/chart-gallery/gallery/chart-detail/?chartId=77884.
- 6.Brown M. Florida strawberry production and marketing. In: Childers N.F., editor. The Strawberry: A Book for Growers. Dr. Norman N. Childers Publications; Gainesville, FL, USA: 2003. pp. 31–42. [Google Scholar]
- 7.Christman J., Samtani J.B. A Survey of Strawberry Production Practices in Virginia. Virginia Cooperative Extension; Blacksburg, VA, USA: 2019. [Google Scholar]
- 8.Garrido C., Carbú M., Fernández-Acero F.J., González-Rodríguez V.E., Cantoral J.M. New insights in the study of strawberry fungal pathogens. Genes Genomes Genom. 2011;5:24–39. [Google Scholar]
- 9.Amil-Ruiz F., Garrido-Gala J., Gadea J., Blanco-Portales R., Muñoz-Mérida A., Trelles O., de Los Santos B., Arroyo F.T., Aguado-Puig A., Romero F. Partial activation of SA-and JA-defensive pathways in strawberry upon Colletotrichum acutatum interaction. Front. Plant Sci. 2016;7:1036. doi: 10.3389/fpls.2016.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forcelini B.B., Peres N.A. Widespread resistance to QoI fungicides of Colletotrichum acutatum from strawberry nurseries and production fields. Plant Health Prog. 2018;19:338–341. doi: 10.1094/PHP-08-18-0050-RS. [DOI] [Google Scholar]
- 11.Dale A., Hughes B.R., Donnelly D. The role of micropropagation in producing specific pathogen-tested plants. HortScience. 2008;43:74–77. doi: 10.21273/HORTSCI.43.1.74. [DOI] [Google Scholar]
- 12.Forcelini B.B., Gonçalves F.P., Peres N.A. Effect of inoculum concentration and interrupted wetness duration on the development of anthracnose fruit rot of strawberry. Plant Dis. 2017;101:372–377. doi: 10.1094/PDIS-08-16-1175-RE. [DOI] [PubMed] [Google Scholar]
- 13.Poling E.B. Anthracnose on strawberry: Its etiology, epidemiology, and pathology, together with management strategies for strawberry nurseries: Introduction to the workshop. HortScience. 2008;43:59–65. doi: 10.21273/HORTSCI.43.1.59. [DOI] [Google Scholar]
- 14.Miller-Butler M.A., Smith B.J., Curry K.J., Blythe E.K. Evaluation of detached strawberry leaves for anthracnose disease severity using image analysis and visual ratings. HortScience. 2019;54:2111–2117. doi: 10.21273/HORTSCI14321-19. [DOI] [Google Scholar]
- 15.Damm U., Cannon P., Woudenberg J., Crous P. The Colletotrichum acutatum species complex. Stud. Mycol. 2012;73:37–113. doi: 10.3114/sim0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weir B., Johnston P., Damm U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012;73:115–180. doi: 10.3114/sim0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard C., Albregts E. Anthracnose of strawberry fruit caused by Glomerella cingulata in Florida. Plant Dis. 1984;68:824–825. doi: 10.1094/PD-69-824. [DOI] [Google Scholar]
- 18.Liu F., Ma Z., Hou L., Diao Y., Wu W., Damm U., Song S., Cai L. Updating species diversity of Colletotrichum, with a phylogenomic overview. Stud. Mycol. 2022;101:1–56. doi: 10.3114/sim.2022.101.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agusti L., Bonaterra A., Moragrega C., Camps J., Montesinos E. Biocontrol of root rot of strawberry caused by Phytophthora cactorum with a combination of two Pseudomonas fluorescens strains. J. Plant Pathol. 2011;93:363–372. [Google Scholar]
- 20.Denoyes-Rothan B., Guérin G., Délye C., Smith B., Minz D., Maymon M., Freeman S. Genetic diversity, and pathogenic variability among isolates of Colletotrichum species from strawberry. Phytopathology. 2003;93:219–228. doi: 10.1094/PHYTO.2003.93.2.219. [DOI] [PubMed] [Google Scholar]
- 21.Mertely J., Peres N. Root Necrosis of Strawberries Caused by Colletotrichum acutatum. UF/IFAS Extension; Gainesville, FL, USA: 2008. [Google Scholar]
- 22.Seijo T.E., Chandler C.K., Mertely J.C., Moyer C., Peres N.A. Resistance of strawberry cultivars and advanced selections to anthracnose and Botrytis fruit rots. Proc. Fla. State Hortic. Soc. 2008;121:246–248. [Google Scholar]
- 23.Simmonds J. A study of the species of Colletotrichum causing ripe fruit rots in Queensland. Qld. J. Agric. Anim. Sci. 1966;22:437–459. [Google Scholar]
- 24.Von Arx J.A. A Revision of the Fungi Classified as Gloeosporium. Wiley; Hoboken, NJ, USA: 1970. A revision of the fungi classified as Gloeosporium. [Google Scholar]
- 25.Jayawardena R., Bhunjun C., Hyde K., Gentekaki E., Itthayakorn P. Colletotrichum: Lifestyles, biology, morpho-species, species complexes and accepted species. Mycosphere. 2021;12:519–669. doi: 10.5943/mycosphere/12/1/7. [DOI] [Google Scholar]
- 26.Cho B.-J., Choi H.-W., Kim D.-H., Kyu L.J. Colletotrichum spp. Agents of Anthracnose on Blueberry Leaves in Gangwon Province, Korea. J. For. Environ. Sci. 2021;37:154–162. [Google Scholar]
- 27.Talhinhas P., Baroncelli R. Colletotrichum species and complexes: Geographic distribution, host range and conservation status. Fungal Divers. 2021;110:109–198. doi: 10.1007/s13225-021-00491-9. [DOI] [Google Scholar]
- 28.Bhunjun C.S., Phukhamsakda C., Jayawardena R.S., Jeewon R., Promputtha I., Hyde K.D. Investigating species boundaries in Colletotrichum. Fungal Divers. 2021;107:107–127. doi: 10.1007/s13225-021-00471-z. [DOI] [Google Scholar]
- 29.Strand L.L. Integrated Pest Management for Strawberries. Volume 3351 UCANR Publications; Davis, CA, USA: 2008. [Google Scholar]
- 30.Yang X., Madden L., Wilson L., Ellis M. Effects of surface topography and rain intensity on splash dispersal of Colletotrichum acutatum. Phytopathology. 1990;80:1115–1120. doi: 10.1094/Phyto-80-1115. [DOI] [Google Scholar]
- 31.Smith B.J. Epidemiology and pathology of strawberry anthracnose: A North American perspective. HortScience. 2008;43:69–73. doi: 10.21273/HORTSCI.43.1.69. [DOI] [Google Scholar]
- 32.Leandro L., Gleason M., Nutter F., Jr., Wegulo S., Dixon P. Germination and sporulation of Colletotrichum acutatum on symptomless strawberry leaves. Phytopathology. 2001;91:659–664. doi: 10.1094/PHYTO.2001.91.7.659. [DOI] [PubMed] [Google Scholar]
- 33.Prusky D. Pathogen quiescence in postharvest diseases. Annu. Rev. Phytopathol. 1996;34:413–434. doi: 10.1146/annurev.phyto.34.1.413. [DOI] [PubMed] [Google Scholar]
- 34.King W., Madden L., Ellis M., Wilson L. Effects of temperature on sporulation and latent period of Colletotrichum spp. infecting strawberry fruit. Plant Dis. 1997;81:77–84. doi: 10.1094/PDIS.1997.81.1.77. [DOI] [PubMed] [Google Scholar]
- 35.Howard C.M. Anthracnose of strawberry caused by the Colleiotrichum complex in Florida. Plant Dis. 1992;76:976–981. doi: 10.1094/PD-76-0976. [DOI] [Google Scholar]
- 36.Zhang X. Doctoral Dissertation. Iowa State University; Ames, IA, USA: 2015. Detection and Management of Colletotrichum Acutatum Sensu Lato on Strawberry. [Google Scholar]
- 37.Madden L., Wilson L., Ellis M. Field spread of anthracnose fruit rot of strawberry in relation to ground cover and ambient weather conditions. Plant Dis. 1993;77:861–866. doi: 10.1094/PD-77-0861. [DOI] [Google Scholar]
- 38.Madden L., Yang X., Wilson L. Effects of rain intensity on splash dispersal of Colletotrichum acutatum. Phytopathology. 1996;86:864–874. doi: 10.1094/Phyto-86-864. [DOI] [Google Scholar]
- 39.Ntahimpera N., Wilson L., Ellis M., Madden L. Comparison of rain effects on splash dispersal of three Colletotrichum species infecting strawberry. Phytopathology. 1999;89:555–563. doi: 10.1094/PHYTO.1999.89.7.555. [DOI] [PubMed] [Google Scholar]
- 40.Agostini J., Timmer L. Population dynamics and survival of strains of Colletotrichum gloeosporioides on citrus in Florida. Phytopathology. 1994;84:420–425. doi: 10.1094/Phyto-84-420. [DOI] [Google Scholar]
- 41.Lilja A.T., Parikka P.K., Pääskynkivi E.A., Hantula J.I., Vainio E.J., Vartiamäki H.A., Lemmetty A.H., Vestberg M.V. Phytophthora cactorum and Colletotrichum acutatum: Survival and Detection. Agric. Conspec. Sci. 2006;71:121–128. [Google Scholar]
- 42.MacKenzie S., Peres N.A., Barquero M., Arauz L., Timmer L. Host range and genetic relatedness of Colletotrichum acutatum isolates from fruit crops and leatherleaf fern in Florida. Phytopathology. 2009;99:620–631. doi: 10.1094/PHYTO-99-5-0620. [DOI] [PubMed] [Google Scholar]
- 43.Han Y., Zeng X., Xiang F., Ren L., Chen F., Gu Y. Distribution and characteristics of Colletotrichum spp. associated with anthracnose of strawberry in Hubei, China. Plant Dis. 2016;100:996–1006. doi: 10.1094/PDIS-09-15-1016-RE. [DOI] [PubMed] [Google Scholar]
- 44.Malarczyk D., Panek J., Frąc M. Alternative molecular-based diagnostic methods of plant pathogenic fungi affecting berry crops—A Review. Molecules. 2019;24:1200. doi: 10.3390/molecules24071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aljawasim B., Vincelli P. Evaluation of Polymerase Chain Reaction (PCR)-Based Methods for Rapid, Accurate Detection and Monitoring of Verticillium dahliae in Woody Hosts by Real-Time PCR. Plant Dis. 2015;99:866–873. doi: 10.1094/PDIS-05-14-0528-RE. [DOI] [PubMed] [Google Scholar]
- 46.Freeman S., Rodriguez R. Differentiation of Colletotrichum species responsible for anthracnose of strawberry by arbitrarily primed PCR. Mycol. Res. 1995;99:501–504. doi: 10.1016/S0953-7562(09)80653-9. [DOI] [Google Scholar]
- 47.Fazari A., Pellicer-Valero O.J., Gómez-Sanchıs J., Bernardi B., Cubero S., Benalia S., Zimbalatti G., Blasco J. Application of deep convolutional neural networks for the detection of anthracnose in olives using VIS/NIR hyperspectral images. Comput. Electron. Agric. 2021;187:106252. doi: 10.1016/j.compag.2021.106252. [DOI] [Google Scholar]
- 48.Lu J., Ehsani R., Shi Y., Abdulridha J., de Castro A.I., Xu Y. Field detection of anthracnose crown rot in strawberry using spectroscopy technology. Comput. Electron. Agric. 2017;135:289–299. doi: 10.1016/j.compag.2017.01.017. [DOI] [Google Scholar]
- 49.Yeh Y.-H., Chung W.-C., Liao J.-Y., Chung C.-L., Kuo Y.-F., Lin T.-T. Strawberry foliar anthracnose assessment by hyperspectral imaging. Comput. Electron. Agric. 2016;122:1–9. doi: 10.1016/j.compag.2016.01.012. [DOI] [Google Scholar]
- 50.Alijani Z., Amini J., Ashengroph M., Bahramnejad B., Mozafari A.A. Biocontrol of strawberry anthracnose disease caused by Colletotrichum nymphaeae using Bacillus atrophaeus strain DM6120 with multiple mechanisms. Trop. Plant Pathol. 2022;47:245–259. doi: 10.1007/s40858-021-00477-7. [DOI] [Google Scholar]
- 51.Veys C., Chatziavgerinos F., AlSuwaidi A., Hibbert J., Hansen M., Bernotas G., Smith M., Yin H., Rolfe S., Grieve B. Multispectral imaging for presymptomatic analysis of light leaf spot in oilseed rape. Plant Methods. 2019;15:4. doi: 10.1186/s13007-019-0389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khaliq A., Comba L., Biglia A., Ricauda Aimonino D., Chiaberge M., Gay P. Comparison of satellite and UAV-based multispectral imagery for vineyard variability assessment. Remote Sens. 2019;11:436. doi: 10.3390/rs11040436. [DOI] [Google Scholar]
- 53.Yuan L., Yan P., Han W., Huang Y., Wang B., Zhang J., Zhang H., Bao Z. Detection of anthracnose in tea plants based on hyperspectral imaging. Comput. Electron. Agric. 2019;167:105039. doi: 10.1016/j.compag.2019.105039. [DOI] [Google Scholar]
- 54.Canteri M.H., Renard C.M., Le Bourvellec C., Bureau S. ATR-FTIR spectroscopy to determine cell wall composition: Application on a large diversity of fruits and vegetables. Carbohydr. Polym. 2019;212:186–196. doi: 10.1016/j.carbpol.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 55.Zhang D., Zhou X., Zhang J., Lan Y., Xu C., Liang D. Detection of rice sheath blight using an unmanned aerial system with high-resolution color and multispectral imaging. PLoS ONE. 2018;13:e0187470. doi: 10.1371/journal.pone.0187470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu C., Liu W., Lu X., Ma F., Chen W., Yang J., Zheng L. Application of multispectral imaging to determine quality attributes and ripeness stage in strawberry fruit. PLoS ONE. 2014;9:e87818. doi: 10.1371/journal.pone.0087818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang C., Chung W., Liao J., Chung C., Kuo Y., Lin T. Strawberry anthracnose disease assessment using hyperspectral imaging; Proceedings of the 6th International Symposium on Machinery and Mechatronics for Agriculture and Biosystems Engineering (ISMAB); Jeonju, Korea. 18–20 June 2012. [Google Scholar]
- 58.Pérez-Roncal C., López-Maestresalas A., Lopez-Molina C., Jarén C., Urrestarazu J., Santesteban L.G., Arazuri S. Hyperspectral imaging to assess the presence of powdery mildew (Erysiphe necator) in cv. Carignan noir grapevine bunches. Agronomy. 2020;10:88. doi: 10.3390/agronomy10010088. [DOI] [Google Scholar]
- 59.Jiang Q., Wu G., Tian C., Li N., Yang H., Bai Y., Zhang B. Hyperspectral imaging for early identification of strawberry leaves diseases with machine learning and spectral fingerprint features. Infrared Phys. Technol. 2021;118:103898. doi: 10.1016/j.infrared.2021.103898. [DOI] [Google Scholar]
- 60.Wu G., Fang Y., Jiang Q., Cui M., Li N., Ou Y., Diao Z., Zhang B. Early identification of strawberry leaves disease utilizing hyperspectral imaging combing with spectral features, multiple vegetation indices and textural features. Comput. Electron. Agric. 2023;204:107553. doi: 10.1016/j.compag.2022.107553. [DOI] [Google Scholar]
- 61.Liu C., Cao Y., Wu E., Yang R., Xu H., Qiao Y. A Discriminative Model for Early Detection of Anthracnose in Strawberry Plants Based on Hyperspectral Imaging Technology. Remote Sens. 2023;15:4640. doi: 10.3390/rs15184640. [DOI] [Google Scholar]
- 62.Pham H., Lim Y., Gardi A., Sabatini R., Pang E. A novel bistatic lidar system for early-detection of plant diseases from unmanned aircraft; Proceedings of the 31th Congress of the International Council of the Aeronautical Sciences (ICAS 2018); Belo Horizonte, Brazil. 9–14 September 2018. [Google Scholar]
- 63.Zhang C., Kovacs J.M. The application of small unmanned aerial systems for precision agriculture: A review. Precis. Agric. 2012;13:693–712. doi: 10.1007/s11119-012-9274-5. [DOI] [Google Scholar]
- 64.Messina G., Peña J.M., Vizzari M., Modica G. A comparison of UAV and satellites multispectral imagery in monitoring onion crop. An application in the ‘Cipolla Rossa di Tropea’(Italy) Remote Sens. 2020;12:3424. doi: 10.3390/rs12203424. [DOI] [Google Scholar]
- 65.Chandel A.K., Khot L.R., Sallato B. Apple powdery mildew infestation detection and mapping using high-resolution visible and multispectral aerial imaging technique. Sci. Hortic. 2021;287:110228. doi: 10.1016/j.scienta.2021.110228. [DOI] [Google Scholar]
- 66.Miller-Butler M.A. Ph.D. Thesis. The University of Southern Mississippi; Hattiesburg, MS, USA: 2016. Screening Strawberry Clones for Anthracnose Disease Resistance Using Traditional Techniques and Molecular Markers. [Google Scholar]
- 67.Simpson D., Berrie A., Johnson A. Hot Water Treatment to Eliminate Colletotrichum acutatum from Strawberry Runner Cuttings; Proceedings of the V International Strawberry Symposium; Coolum Beach, Australia. 5–10 September 2004; pp. 255–258. [Google Scholar]
- 68.Mertely J.C., Peres N.A. Anthracnose Fruit Rot of Strawberry: PP-207/PP130, rev. 9/2012. EDIS. 2012;2012:1–4. doi: 10.32473/edis-pp130-2012. [DOI] [Google Scholar]
- 69.Chechi A., Stahlecker J., Dowling M., Schnabel G. Diversity in species composition and fungicide resistance profiles in Colletotrichum isolates from apples. Pestic. Biochem. Physiol. 2019;158:18–24. doi: 10.1016/j.pestbp.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 70.Sengupta P., Sen S., Mukherjee K., Acharya K. Postharvest diseases of Indian gooseberry and their management: A review. Int. J. Fruit Sci. 2020;20:178–190. doi: 10.1080/15538362.2019.1608889. [DOI] [Google Scholar]
- 71.Melanson R.A., Johnson C., Schnabel G., Ferguson M., Desaeger J., Schmidt-Jeffris R., Burrack H.J., Pfeiffer D.G., Hale F., Jennings K. 2020 Southeast Regional Strawberry Integrated Pest Management Guide For Plasticulture Production. University of Georgia Cooperative Extension; Athens, GA, USA: 2020. [Google Scholar]
- 72.Rahman M., Ballington J., Louws F. Role of foliar hemibiotrophic and fruit resistance in anthracnose-resistant strawberry genotypes for annual hill plasticulture systems. Ann. Appl. Biol. 2013;163:102–113. doi: 10.1111/aab.12038. [DOI] [Google Scholar]
- 73.Ballington J., Shuman J., Hokanson S., Smith B., Giménez G. Breeding strawberries (Fragaria × ananassa) for resistance to anthracnose caused by Colletotrichum acutatum; Proceedings of the IV International Strawberry Symposium; Tampere, Finland. 9–14 July 2000; pp. 89–92. [Google Scholar]
- 74.Whitaker V., Lee S., Osorio L., Verma S., Roach J., Mangandi J., Noh Y.-H., Gezan S., Peres N. Advances in strawberry breeding at the University of Florida; Proceedings of the VIII International Strawberry Symposium; Québec, QC, Canada. 13–17 August 2016; pp. 1–6. [Google Scholar]
- 75.Salinas N.R., Zurn J.D., Mathey M., Mookerjee S., Denoyes B., Perrotte J., Potier A., Finn C.E., Hancock J.F., Stewart P. Validation of molecular markers associated with perpetual flowering in octoploid Fragaria germplasm. Mol. Breed. 2017;37:70. doi: 10.1007/s11032-017-0672-2. [DOI] [Google Scholar]
- 76.Adhikari T.B., Aryal R., Redpath L.E., Van den Broeck L., Ashrafi H., Philbrick A.N., Jacobs R.L., Sozzani R., Louws F.J. RNA-Seq and Gene Regulatory Network Analyses Uncover Candidate Genes in the Early Defense to Two Hemibiotrophic Colletorichum spp. in Strawberry. Front. Genet. 2022;12:805771. doi: 10.3389/fgene.2021.805771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma L., Haile Z.M., Sabbadini S., Mezzetti B., Negrini F., Baraldi E. Functional characterization of MANNOSE-BINDING LECTIN 1, a G-type lectin gene family member, in response to fungal pathogens of strawberry. J. Exp. Bot. 2023;74:149–161. doi: 10.1093/jxb/erac396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He T., Fan J., Jiao G., Liu Y., Zhang Q., Luo N., Ahmad B., Chen Q., Wen Z. Bioinformatics and Expression Analysis of the Chitinase Genes in Strawberry (Fragaria vesca) and Functional Study of FvChi-14. Plants. 2023;12:1543. doi: 10.3390/plants12071543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shuman J.L. Ph.D. Thesis. North Carolina State University; Raleigh, NC, USA: 2001. Anthracnose Fruit Rot Resistance in Strawberry. [Google Scholar]
- 80.Shi X.-C., Wang S.-Y., Duan X.-C., Wang Y.-Z., Liu F.-Q., Laborda P. Biocontrol strategies for the management of Colletotrichum species in postharvest fruits. Crop Prot. 2021;141:105454. doi: 10.1016/j.cropro.2020.105454. [DOI] [Google Scholar]
- 81.Kim Y.S., Balaraju K., Jeon Y. Biological control of apple anthracnose by Paenibacillus polymyxa APEC128, an antagonistic rhizobacterium. Plant Pathol. J. 2016;32:251. doi: 10.5423/PPJ.OA.01.2016.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moreira R.R., Nesi C.N., De Mio L.L.M. Bacillus spp. and Pseudomonas putida as inhibitors of the Colletotrichum acutatum group and potential to control Glomerella leaf spot. Biol. Control. 2014;72:30–37. doi: 10.1016/j.biocontrol.2014.02.001. [DOI] [Google Scholar]
- 83.Verma N., MacDonald L., Punja Z. Inoculum prevalence, host infection and biological control of Colletotrichum acutatum: Causal agent of blueberry anthracnose in British Columbia. Plant Pathol. 2006;55:442–450. doi: 10.1111/j.1365-3059.2006.01401.x. [DOI] [Google Scholar]
- 84.Lopes M.R., Klein M.N., Ferraz L.P., da Silva A.C., Kupper K.C. Saccharomyces cerevisiae: A novel and efficient biological control agent for Colletotrichum acutatum during pre-harvest. Microbiol. Res. 2015;175:93–99. doi: 10.1016/j.micres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 85.McInnes T., Black L., Gatti J., Jr. Disease-free plants for management of strawberry anthracnose crown rot. Plant Dis. 1992;76:260–264. doi: 10.1094/PD-76-0260. [DOI] [Google Scholar]
- 86.Dowling M., Peres N., Villani S., Schnabel G. Managing Colletotrichum on fruit crops: A “complex” challenge. Plant Dis. 2020;104:2301–2316. doi: 10.1094/PDIS-11-19-2378-FE. [DOI] [PubMed] [Google Scholar]
- 87.Oldfield T.L., Achmon Y., Perano K.M., Dahlquist-Willard R.M., VanderGheynst J.S., Stapleton J.J., Simmons C.W., Holden N.M. A life cycle assessment of biosolarization as a valorization pathway for tomato pomace utilization in California. J. Clean. Prod. 2017;141:146–156. doi: 10.1016/j.jclepro.2016.09.051. [DOI] [Google Scholar]
- 88.Matthiessen J.N., Kirkegaard J.A. Biofumigation and enhanced biodegradation: Opportunity and challenge in soilborne pest and disease management. Crit. Rev. Plant Sci. 2006;25:235–265. doi: 10.1080/07352680600611543. [DOI] [Google Scholar]
- 89.Hansen Z., Keinath A. Increased pepper yields following incorporation of biofumigation cover crops and the effects on soilborne pathogen populations and pepper diseases. Appl. Soil Ecol. 2013;63:67–77. doi: 10.1016/j.apsoil.2012.09.007. [DOI] [Google Scholar]
- 90.Morra M., Kirkegaard J. Isothiocyanate release from soil-incorporated Brassica tissues. Soil Biol. Biochem. 2002;34:1683–1690. doi: 10.1016/S0038-0717(02)00153-0. [DOI] [Google Scholar]
- 91.Butler D.M., Kokalis-Burelle N., Muramoto J., Shennan C., McCollum T.G., Rosskopf E.N. Impact of anaerobic soil disinfestation combined with soil solarization on plant–parasitic nematodes and introduced inoculum of soilborne plant pathogens in raised-bed vegetable production. Crop Prot. 2012;39:33–40. doi: 10.1016/j.cropro.2012.03.019. [DOI] [Google Scholar]
- 92.Shrestha U., Augé R.M., Butler D.M. A meta-analysis of the impact of anaerobic soil disinfestation on pest suppression and yield of horticultural crops. Front. Plant Sci. 2016;7:1254. doi: 10.3389/fpls.2016.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molendijk L., Bleeker P., Lamers J., Runia W. Perspectives of anaerobic soil disinfestation; Proceedings of the VII International Symposium on Chemical and Non-Chemical Soil and Substrate Disinfestation; Leuven, Belgium. 13–18 September 2009; pp. 277–283. [Google Scholar]
- 94.Shrestha U., Dee M.E., Piya S., Ownley B.H., Butler D.M. Soil inoculation with Trichoderma asperellum, T. harzianum or Streptomyces griseoviridis prior to anaerobic soil disinfestation (ASD) does not increase ASD efficacy against Sclerotium rolfsii germination. Appl. Soil Ecol. 2020;147:103383. doi: 10.1016/j.apsoil.2019.103383. [DOI] [Google Scholar]
- 95.Butler D.M., Rosskopf E.N., Kokalis-Burelle N., Albano J.P., Muramoto J., Shennan C. Exploring warm-season cover crops as carbon sources for anaerobic soil disinfestation (ASD) Plant Soil. 2012;355:149–165. doi: 10.1007/s11104-011-1088-0. [DOI] [Google Scholar]
- 96.Shennan C., Muramoto J., Koike S., Baird G., Fennimore S., Samtani J., Bolda M., Dara S., Daugovish O., Lazarovits G. Anaerobic soil disinfestation is an alternative to soil fumigation for control of some soilborne pathogens in strawberry production. Plant Pathol. 2018;67:51–66. doi: 10.1111/ppa.12721. [DOI] [Google Scholar]
- 97.Testen A.L., Bosques Martinez M., Jimenez Madrid A., Deblais L., Taylor C., Paul P.A., Miller S.A. On-farm evaluations of anaerobic soil disinfestation and grafting for management of a widespread soilborne disease complex in protected culture tomato production. Phytopathology. 2020;111:954–965. doi: 10.1094/PHYTO-07-20-0288-R. [DOI] [PubMed] [Google Scholar]
- 98.Hernández-Muñiz P., Borrero C., Ordóñez-Martín J., Pastrana A.M., Avilés M. Optimization of the Use of Industrial Wastes in Anaerobic Soil Disinfestation for the Control of Fusarium Wilt in Strawberry. Plants. 2023;12:3185. doi: 10.3390/plants12183185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu D., Samtani J., Johnson C., Zhang X., Butler D.M., Derr J. Brewer’s Spent Grain with Yeast Amendment Shows Potential for Anaerobic Soil Disinfestation of Weeds and Pythium irregulare. Agronomy. 2023;13:2081. doi: 10.3390/agronomy13082081. [DOI] [Google Scholar]
- 100.Song Z., Yan D., Fang W., Zhang D., Jin X., Li Y., Wang Q., Wang G., Li Q., Cao A. Response of Strawberry Fruit Yield, Soil Chemical and Microbial Properties to Anaerobic Soil Disinfestation with Biochar and Rice Bran. Agriculture. 2023;13:1466. doi: 10.3390/agriculture13071466. [DOI] [Google Scholar]
- 101.Lamers J., Mazzola M., Rosskopf E., Kokalis-Burelle N., Momma N., Butler D., Shennan C., Muramoto J., Kobara Y. Anaerobic soil disinfestation for soil borne disease control in strawberry and vegetable systems: Current knowledge and future directions; Proceedings of the VIII International Symposium on Chemical and Non-Chemical Soil and Substrate Disinfestation; Turin, Italy. 13–17 July 2014; pp. 165–175. [Google Scholar]
- 102.Liu D. Ph.D. Thesis. Virginia Polytechnic Institute and State University; Blacksburg, NA, USA: 2021. Evaluation of Anaerobic Soil Disinfestation Using brewers Spent Grain and Yeast Inoculation on Weed Control in Annual Hill Plasticulture Strawberry Production. [Google Scholar]
- 103.Momma N., Kobara Y., Uematsu S., Kita N., Shinmura A. Development of biological soil disinfestations in Japan. Appl. Microbiol. Biotechnol. 2013;97:3801–3809. doi: 10.1007/s00253-013-4826-9. [DOI] [PubMed] [Google Scholar]
- 104.Kundan R., Pant G., Jadon N., Agrawal P. Plant growth promoting rhizobacteria: Mechanism and current prospective. J. Fertil Pestic. 2015;6:9. doi: 10.4172/2471-2728.1000155. [DOI] [Google Scholar]
- 105.Jayaprakashvel M., Chitra C., Mathivanan N. Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms. Springer; Berlin/Heidelberg, Germany: 2019. Metabolites of plant growth-promoting rhizobacteria for the management of soilborne pathogenic fungi in crops; pp. 293–315. [Google Scholar]
- 106.Aljawasim B.D., Khaeim H.M., Manshood M.A. Assessment of arbuscular mycorrhizal fungi (Glomus spp.) as potential biocontrol agents against damping-off disease Rhizoctonia solani on cucumber. J. Crop Prot. 2020;9:141–147. [Google Scholar]
- 107.Rahman M., Sabir A.A., Mukta J.A., Khan M., Alam M., Mohi-Ud-Din M., Miah M., Rahman M., Islam M.T. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018;8:2504. doi: 10.1038/s41598-018-20235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mei C., Amaradasa B.S., Chretien R.L., Liu D., Snead G., Samtani J.B., Lowman S. A Potential Application of Endophytic Bacteria in Strawberry Production. Horticulturae. 2021;7:504. doi: 10.3390/horticulturae7110504. [DOI] [Google Scholar]
- 109.de Melo Pereira G.V., Magalhães K.T., Lorenzetii E.R., Souza T.P., Schwan R.F. A multiphasic approach for the identification of endophytic bacterial in strawberry fruit and their potential for plant growth promotion. Microb. Ecol. 2012;63:405–417. doi: 10.1007/s00248-011-9919-3. [DOI] [PubMed] [Google Scholar]
- 110.Baysal F. Comparative performance of fungicides and biocontrol products in suppression of Rhizoctonia root rot in viburnum. J. Plant Pathol. Microbiol. 2018;9:1000451 [Google Scholar]
- 111.Rahman M., Islam T., Jett L., Kotcon J. Probiotic Bacteria, Anaerobic Soil Disinfestation and Mustard Cover Crop Biofumigation Suppress Soilborne Disease and Increase Yield of Strawberry in a Perennial Organic Production System. Plant Dis. 2023;107:2490–2499. doi: 10.1094/PDIS-10-22-2402-RE. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.