Abstract
Introduction
Chronic alcoholic patients have a threefold to fourfold increased risk for developing a severe infection or septic shock after surgery, which might be due to altered immune response. The aim of this outcome matched study was to investigate proinflammatory and anti-inflammatory immune parameters during the course of infection and subsequent septic shock in chronic alcoholic patients, and to compare these parameters with those in nonalcoholic patients.
Methods
Twenty-eight patients from a cohort of fifty-six with either pneumonia or peritonitis and subsequent septic shock were selected. Fourteen patients were chronic alcoholics whereas fourteen were nonalcoholic patients. Chronic alcoholic patients met criteria (Diagnostic and Statistical Manual of Mental Disorders IV, of the American Psychiatric Association) for alcohol abuse or dependence. Measurements were performed during the onset of infection (within 24 hours after the onset of infection), in early septic shock (within 12 hours after onset of septic shock) and in late septic shock (72 hours after the onset). Blood measurements included proinflammatory and anti-inflammatory cytokines.
Results
Chronic alcoholic patients exhibited significantly lower plasma levels of IL-8 (P < 0.010) during the onset of infection than did matched nonalcoholic patients. In early septic shock, chronic alcoholic patients had significantly decreased levels of IL-1β (P < 0.015), IL-6 (P < 0.016) and IL-8 (P < 0.010). The anti-inflammatory parameters IL-10 and tumour necrosis factor receptors I and II did not differ between alcoholic and nonalcoholic patients.
Conclusion
At the onset of infection and during early septic shock, chronic alcoholic patients had lower levels of proinflammatory immune parameters than did nonalcoholic patients. Therefore, immunomodulatory therapy administered early may be considered in chronic alcoholic patients at the onset of an infection because of their altered proinflammatory immune response.
Keywords: alcohol, altered immune response, cytokines, severe infection
Introduction
Chronic alcoholic patients have a twofold to fivefold increased risk for postoperative morbidity after surgery as compared with nonalcoholic patients [1,2]. As a result of this increased postoperative morbidity, intensive care treatment and overall hospital stay are prolonged [1,2]. Among all complications, infections are the most serious and are associated with a worse outcome [1-3].
Prolonged and excessive consumption of alcohol has been shown to predispose to a variety of infectious complications, which may be due, in part, to an inability to produce important cytokines [4]. In experimental settings, T-cell mediated immunity was found to be suppressed by ethanol [4,5], which was associated with altered cytokine production [6,7]. A significant suppression of tumour necrosis factor (TNF)-α, as well as of IL-6 and IL-10, in a model of chronic alcoholism was reported [8-10]. TNF-α and IL-1β plasma cytokine levels are induced early as the 'first hit' of infection, whereas IL-10 is the subsequent response to this first hit, stimulated by macrophages and monocytes [10]. In clinical studies the delayed-type hypersensitivity skin response was decreased in chronic alcoholic patients before surgery and was further impaired after surgery [2,11,12]. Immediately after surgery the IL-6/IL-10 ratio was found to be depressed in chronic alcoholic patients [13], and the subsequent rate of infection was elevated. Several controversal clinical studies evaluated the impact of inflammatory and mediator release on the development of severe infection and subsequent septic shock [14,15]. Elevated levels of IL-6 and IL-8 were associated with higher mortality rates [14,16].
To the best of our knowledge, no other studies have been reported that investigated the progression from infection to septic shock in terms of immune modulating cytokines in chronic alcoholic patients. The aim of the present study was to investigate whether chronic alcoholic patients differed from nonalcoholic patients with respect to plasma cytokine levels at the onset of infection and in early septic shock.
Methods
The patients or relatives gave written, informed consent to participate in this institutionally approved, case–control study. A total of 14 chronic alcoholic patients with septic shock were included, along with 14 control individuals (nonalcoholic patients with septic shock). These 28 patients were selected from a cohort of 56 with either pneumonia or peritonitis and who subsequently developed septic shock. Patients with nosocomial pneumonia met criteria given by the US Centers for Diseases Control and Prevention [17], and nosocomial peritonitis was diagnosed according to the Mannheimer Peritonitis Index [18]. Subsequent septic shock criteria were defined as outlined in the consensus conference of 1992 [19].
Patients were excluded if they were younger than 18 years, if they had a diagnosis of liver cirrhosis, if consent could not be ontained, or if they were considered 'social drinkers', with an ethanol intake of about 20–60 g/day [1]. Basic patient characteristics such as age, height, weight and Acute Physiology and Chronic Health Evaluation III score [20] were documented.
Diagnosis of chronic alcohol abuse and alcohol dependence
The history was recorded, and an alcoholism related questionnaire – the CAGE Questionnaire [21] – was administered to all patients. (The acronym CAGE stands for 'Cutting down, Annoyance by criticism, Guilty feeling, and Eye-openers'.) All chronic alcoholic patients met criteria for alcohol abuse or dependence (Diagnostic and Statistical Manual of Mental Disorders IV, from the American Psychiatric Association [22]), had a daily ethanol intake in excess of 60 g, and had a CAGE score of 3 or more. Patients with a daily ethanol intake below 25 g and a CAGE score of 1 or less were considered to be nonalcoholic. Conventional laboratory markers such as γ-glutamyl transferase and mean corpuscular volume were determined, in accordance with routine clinical practice. In addition, a marker of higher sensitivity and specificity, namely carbohydrate deficient transferrin, was measured.
Monitoring and management
A radial artery catheter and a central venous line were inserted for routine cardiovascular monitoring. A fibreoptic, pulmonary artery flotation catheter (Swan-Ganz Oximetry/TD-Catheter model 93A-741h-7.5F; Baxter Edwards Laboratories, Irvine, CA, USA) was inserted in all patients with sepsis. Fluids were administered to achieve optimal left ventricular filling pressure, reaching the plateau value for left ventricular stroke work. If the cardiac index was less than 2.5 l/min per m2, then dobutamine was administered up to 20 μg/kg per min to maintain cardiac index between 3.0 and 3.5 l/min per m2. Noradrenaline (norepinephrine) was administered to patients in whom mean arterial pressure was below 70 mmHg. All patients were mechanically ventilated and received continuous analgesic sedation.
Haemodynamic measurements included heart rate and cardiovascular pressures, as measured from the mid-axillary line. Also, cardiac output measurements (as measured using the thermodilution method with 10 ml iced physiological saline solution as injectate, employing a cardiac computer [SAT-2 Oximeter/Cardiac Output Computer; Baxter Edwards Laboratories]) were taken in triplicate, with results expressed as the mean value. Blood samples were drawn simultaneously, slowly and continuously over 30 s.
Arterial and mixed venous blood samples were analyzed for oxygen and carbon dioxide tensions (ABL 300; Radiometer Inc., Copenhagen, Denmark) and for their haemoglobin content and oxygen saturation (Hemoximeter OSM 3; Radiometer Inc.). Oxygen content, delivery and consumption were calculated according to the standard formulae.
Measurements
Measurements were performed within 24 hours after the onset of infection (peritonitis/pneumonia), within 12 hours after the onset of septic shock, and 72–96 hours after the onset of septic shock. With each measurement, haemodynamic and oxygen transport related measures were recored and blood samples were drawn.
Laboratory parameters
Blood samples were collected in sterile tubes and centrifuged at 1800 g for 10 min, and serum was stored at -80°C. All mediators were analyzed at room temperature (i.e. 23°C). Measurements were done using commercially available enzyme-linked immunosorbent assay kits (Quantikine™ Immunoassay Kit; R&D Systems, Minneapolis, MN, USA) for the cytokines (TNF-α, TNF-receptors [TNF-Rs], IL-1β, IL-6 and IL-8). IL-10 was analyzed using a commercially available enzyme immunoassay (TiterZyme IL-10 enzyme immunoassay kit; Perseptive Diagnostic, Cambridge, MA, USA). Detection limits and variation coefficients were as follows: TNF-α, 4.4 pg/ml (5.1%); TNF-RI, 30.0 pg/ml (5.9%); TNF-RII, 10.0 pg/ml (3.2%); IL-1β, 1.0 pg/ml (5.2%); IL-6, 0.7 pg/ml (3.6%); IL-8, 10.0 pg/ml (6.7%); and IL-10, 1.0 pg/ml (5.8%). Routine laboratory markers, including leucocytes, lactate and C-reactive protein (CRP), were also determined.
Statistical analysis
Data are presented as median (range). The Wilcoxon matched pairs signed rank sum test was used to compare intergroup variables. The adjusted significance (Bonferroni method) for twice measured haemodynamic and oxygen transport parameters was P/2 = 0.025; the adjusted significance for the thrice measured parameters was P/3 = 0.017.
The Friedman test was used to identify significant intragroup differences from infection to early or late septic shock. If the global test revealed a significant difference, then the Wilcoxon test was used to define at which time point a significant change occurred. P < 0.05 was considered statistically significant. Correlation coefficients were calculated according to the Spearman rank correlation. The receiver operating characteristic curve was used to provide a presentation of the relationship between sensitivity and sensitivity of mediators that were found to be significantly different between groups, and possibly to provide diagnostic cutoff levels. The area under the receiver operating curve represents the probability of discrimination between chronic alcoholic patients and nonalcoholic patients [23].
Results
Basic patient characteristics did not differ significantly between groups (Table 1). There were significant differences with respect to alcoholism-related data and laboratory markers (Table 1).
Table 1.
Basic patient characteristics
| Characteristic | Chronic alcoholic patients | Nonalcoholic patients | P |
| Age (years) | 57 (24–72) | 60 (23–74) | 0.872 |
| Body surface area (m2) | 1.87 (1.57–2.40) | 2.08 (1.68–2.14) | 0.174 |
| Weight (kg) | 74 (50–120) | 85 (55–93) | 0.210 |
| Culture positive/culture negative (n) | 4/14 | 3/14 | >0.999 |
| Peritonitis/pneumonia (n) | 8/6 | 8/6 | >0.999 |
| Survivors/nonsurvivors (n) | 6/8 | 6/8 | >0.999 |
| Nicotine abuse (n [%]) | 9/14 (64%) | 6/14 (43%) | 0.153 |
| APACHE III score (admission) | 40 (26–76) | 27 (9–74) | 0.107 |
| Length of ICU stay (days) | 16 (3–50) | 19 (9–41) | 0.397 |
| CAGE score | 4 (3–4) | 0 (0–1) | <0.001 |
| Ethanol consumption (g/day) | 170 (60–380) | 0 (0–20) | <0.001 |
| CDT (mg/l) | 17.2 (5.1–70.1) | 3.2 (2.0–8.2) | <0.001 |
| MCV (fl) | 96.2 (77.2–106.0) | 90.2 (78.3–102.3) | 0.031 |
| GGT (U/l) | 31 (20–178) | 20 (10–97) | 0.074 |
Values are expressed as median (range), unless otherwise stated. APACHE, Acute Physiology and Chronic Health Evaluation; CAGE, alcohol-related questionnaire; CDT, carbohydrate-deficient transferrin; GGT, γ-glutamyl transferase; ICU, intensive care unit; MCV, mean corpuscular volume.
Immune parameters
At the onset of infection IL-8 plasma levels were significantly lower in chronic alcoholic patients than in nonalcoholic patients (Fig. 1), whereas no differences between groups occurred with respect to plasma IL-1β and IL-6 levels (Figs 2 and 3). During early septic shock, IL-1β, IL-6 and IL-8 were significantly lower in chronic alcoholic patients (Figs 1, 2, 3). IL-1β significantly increased in nonalcoholic patients from the onset of infection to early septic shock (Fig. 2). Also, IL-1β and IL-6 significantly decreased in the nonalcoholic patients from early to late septic shock (Figs 2 and 3).
Figure 1.
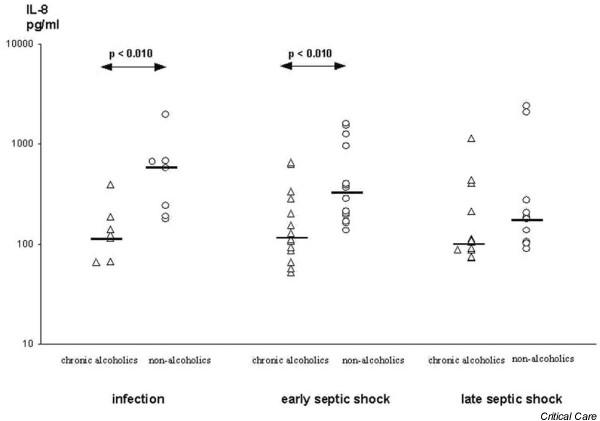
Interleukin (IL)-8 in the course of septic shock in chronic alcoholic patients and nonalcoholic patients.
Figure 2.
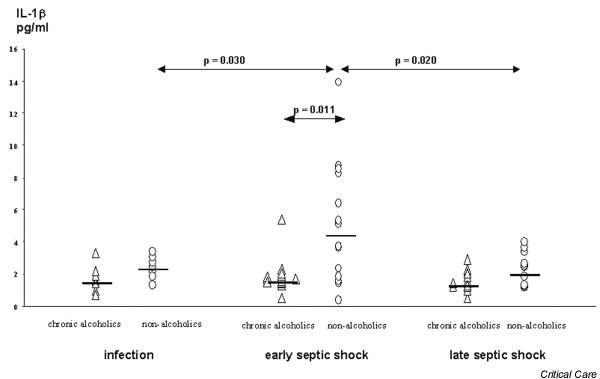
Interleukin (IL)-1β in the course of septic shock in chronic alcoholic patients and nonalcoholic patients.
Figure 3.
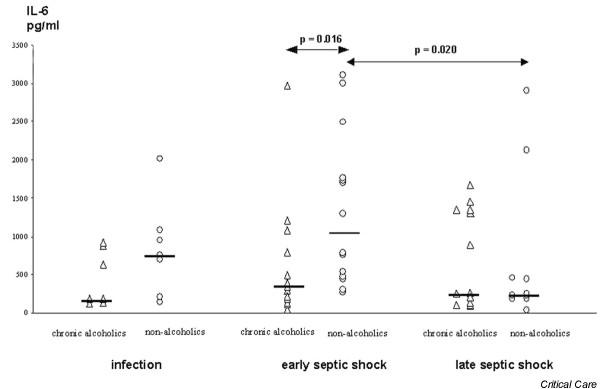
Interleukin (IL)-6 in the course of septic shock in chronic alcoholic patients and nonalcoholic patients.
In the chronic alcoholic patients significant increases occurred for soluble TNF-RI from infection to early septic shock (Table 2). However, no significant intergroup differences for soluble TNF-RI (Table 2) were found. In addition, IL-10 plasma levels did not differ between groups during infection to septic shock.
Table 2.
Immune parameters at onset of infection, early and late septic shock
| Parameter | Chronic alcoholic patients | Nonalcoholic patients | P |
| IL-10 (pg/ml) | |||
| Infection | 35 (2–37) | 41 (12–392) | 0.110 |
| Early septic shock | 37 (4–371) | 66 (2–255) | 0.590 |
| Late septic shock | 30 (2–118) | 35 (0–81) | 0.576 |
| Soluble TNF-RI (pg/ml) | |||
| Infection | 3440 (1280–6680)* | 4750 (3540–7880) | 0.153 |
| Early septic shock | 10,860 (2260–16,670)* | 7170 (3280–14,950) | 0.622 |
| Late septic shock | 9410 (1240–15,940) | 7920 (4130–15,000) | 0.775 |
| Soluble TNF-RII (pg/ml) | |||
| Infection | 7280 (3990–8500) | 7180 (5930–8890) | 0.668 |
| Early septic shock | 8070 (3500–8500) | 7900 (5100–10,000) | 0.895 |
| Late septic shock | 7980 (3050–8500) | 8120 (5480–10,660) | 0.567 |
| TNF-α (pg/ml) | |||
| Infection | 10 (4–18) | 19 (12–93) | 0.048 |
| Early septic shock | 14 (1–56) | 14 (3–29) | 0.385 |
| Late septic shock | 10 (1–29) | 10 (7–16) | 0.723 |
Values are expressed as median (range). IL, interleukin; TNF, tumour necrosis factor; TNF-R, tumour necrosis factor receptor. *Significant changes from infection to early septic shock, and from early septic shock to late septic shock.
For the significant intergroup differences, receiver operating curves were calculated for the progression from infection to septic shock. The corresponding area under the curve values were 0.60 for IL-1β, 0.62 for IL-6, and 0.66 for IL-8.
Conventional laboratory markers
CRP was significantly increased in chronic alcoholic patients at the onset of infection as compared with non-alcoholic patients (Table 3). During early septic shock, leucocyte count was significantly increased in the chronic alcoholic patients (Table 3).
Table 3.
Clinical and laboratory signs of infections
| Sign | Chronic alcoholic patients | Nonalcoholic patients | P |
| Temperature (°C) | |||
| Infection | 37.2 (36.8–38.1) | 37.5 (37.1–38.8) | 0.851 |
| Early septic shock | 37.8 (36.2–39.8) | 38.7 (37.6–39.9) | 0.037 |
| Late septic shock | 38.1 (36.6–38.8) | 37.2 (35.8–39.2) | 0.413 |
| Leucocytes (×109/l) | |||
| Infection | 8.9 (4.4–13.2) | 10.2 (3.4–10.2) | 0.663 |
| Early septic shock | 18.9 (6.5–45.8) | 9.5 (3.5–13.9) | <0.010 |
| Late septic shock | 15.0 (9.8–39.2) | 17.8 (12.3–33.2) | 0.717 |
| CRP (mg/l) | |||
| Infection | 110 (80–223) | 54 (8–72) | <0.010 |
| Early septic shock | 203 (75–266) | 144 (42–323) | 0.162 |
| Late septic shock | 124 (44–210) | 120 (90–258) | 0.690 |
| Lactate (mmol/l) | |||
| Infection | 1.6 (0.8–2.0) | 1.9 (1.0–5.1) | 0.174 |
| Early septic shock | 2.3 (1.4–3.2) | 1.9 (1.0–5.1) | 0.520 |
| Late septic shock | 2.0 (0.8–4.5) | 2.0 (0.7–2.8) | 0.702 |
Value are expressed as median (range). CRP, C-reactive protein.
Chronic alcoholic patients had a significantly lower oxygenation index during early and late septic shock than did nonalcoholic patients (Table 4). The oxygenation index in chronic alcoholic patients with pneumonia was not statistically different from that in chronic alcoholic patients with peritonitis.
Table 4.
Haemodynamic and oxygen transport related data and catecholamines
| Variables | Septic shock | Chronic alcoholic patients | Nonalcoholic patients | P |
| Heart rate (beats/min) | Early | 104 (69–143) | 122 (99–140) | 0.048 |
| Late | 111 (52–153) | 106 (91–146) | 0.696 | |
| MAP (mmHg) | Early | 84 (69–112) | 76 (59–94) | 0.060 |
| Late | 78 (68–113) | 78 (61–101) | 0.643 | |
| PCWP (mmHg) | Early | 16 (9–19) | 11 (8–15) | 0.021 |
| Late | 15 (8–20) | 10 (8–15) | 0.312 | |
| Cardiac index (l/min per m2) | Early | 5.1 (3.5–6.2) | 5.0 (3.0–10.4) | 0.940 |
| Late | 4.6 (3.4–7.00) | 4.0 (3.3–4.8) | 0.196 | |
| DO2 (ml/min per m2) | Early | 721 (489–888) | 718 (408–1366) | 0.870 |
| Late | 693 (485–922) | 580 (453–817) | 0.366 | |
| VO2 (ml/min per m2) | Early | 140 (114–237) | 181 (49–330) | 0.178 |
| Late | 162 (95–263) | 143 (33–253) | 0.437 | |
| PaO2/FiO2 (mmHg) | Early | 198 (110–256) | 282 (188–320) | <0.010 |
| Late | 193 (151–298) | 291 (161–511) | 0.020 | |
| Noradrenaline (μg/kg per min) | Early | 0.3 (0.1–0.8) | 0.5 (0.2–1.0) | 0.112 |
| Late | 1.3 (0.1–1.7) | 1.1(0.1–2.4) | 0.522 |
Values are expressed as median (range). DO2, oxygen delivery; MAP, mean arterial pressure; PaO2/FiO2, arterial oxygen tension/fractional inspired oxygen ratio (oxygenation index); PCWP, pulmonary capillary wedge pressure; VO2, oxygen consumption.
Outcome
In the present study the period between establishing infection and development of septic shock did not differ between chronic alcoholic patients (5 [2–10] days) and nonalcoholic patients (4 [1–129] days; P < 0.171). Numbers of culture-positive versus culture-negative samples did not differ between groups (Table 1). The identities of the positive cultures isolated from blood culture or peritoneal swab/histology were as follows: Enterococcus faecium (n = 3), Streptococcus pyogenes (n = 2), Staphylococcus aureus (n = 1) and Enterococcus faecalis (n = 1). All patients with peritonitis were surgical patients. All infections were hospital acquired.
The survival rate for nonalcoholic patients with septic shock was 53% (10/19), whereas only 43% (9/19) of the chronic alcoholic patients survived. With regard to mortality rates, 47% (9/19) of the nonalcoholic patients and 57% (12/21) of the chronic alcoholic patients died (Table 1).
Discussion
The most important finding was that chronic alcoholic patients had lower plasma levels of cytokines at the onset of infection and in early septic shock, in particular IL-1β, IL-6 and IL-8.
Proinflammatory cytokines
This is the first clinical study to demonstrate an altered plasma cytokine response in chronic alcoholic patients at the onset of infection and early septic shock.
Experimental studies have shown that ethanol modulates cytokine secretion and synthesis in vivo and in vitro [24]. In vitro, alcohol blunted the stimulation of cytokine production by lipopolysaccharide, and the alcohol induced decrease in cytokine synthesis was proportional to the level of alcohol consumption [6]. Also, levels of production of TNF-α and IL-8 in mast cells as well as in blood monocytes were downregulated by clinically relevant ethanol concentrations [25,26].
In a clinical study [27], alveolar macrophages and their ability to produce cytokines locally were studied because of the high risk for pneumonia associated with chronic alcoholism. In vitro stimulation of alveolar macrophages from chronic alcoholic persons resulted in significant suppression of TNF-α as compared with those from healthy control individuals. The local pulmonary suppressive effects of acute or chronic alcoholism have been described [8,10]. In chronic alcoholic patients, circulating IL-1β, IL-6 and TNF-α levels were significantly elevated as compared with nonusers of alcohol, and this elevation was correlated with liver disease [28]. In the present study, patients with active liver cirrhosis were excluded because of the different cytokine kinetics that occur in the presence of liver disease.
That levels of systemic proinflammatory cytokines were significantly lower in chronic alcoholic patients than in nonalcoholic patients in early sepsis is an important new finding. The proinflammatory response to invading micro-organisms might be fundamentally impaired in chronic alcoholism, and this may contribute to the clinically evident immune alteration in such patients. This is in contrast to a previous report that found that TNF-α is elevated in early infection in patients developing septic shock [29]. The latter may be the effect of a severe infection whereas the former may be the cause of progression of the disease. However, this remains speculative and requires further investigation.
Anti-inflammatory cytokines
In the present study no differences with respect to IL-10 levels were found between groups either during the initial development of infection or in early septic shock.
In previous studies IL-10 was produced in large amounts during septicaemia and septic shock [30]. Increased IL-10 production by human blood monocytes was observed after acute ethanol treatment [31]. Considering the ability of IL-10 to inhibit monocyte function, it is likely that elevated IL-10 levels contribute to the disturbed cellular immune response observed after acute alcohol treatment. However, we did not find a difference in response of chronic alcoholic patients with respect to IL-10. The reasons for this might be, first, that elevated IL-10 levels occur earlier in the development of infection and may not be seen later in the course of the disease [13]. Second, the reduced proinflammatory response might have contributed to lower IL-10 levels [14], and therefore no difference was found between groups. In addition, no significant intergroup differences were found with respect to soluble TNF-RI and TNF-RII. A significant rise in soluble TNF-RI was noted from the onset of infection to early septic shock in the chronic alcoholic patients. High values of soluble TNF-Rs were reported during severe sepsis [32], which was confirmed by previous data. One study evaluated the in vitro expression of TNF-Rs on macrophages in response to ethanol exposure [33]; ethanol significantly reduced the expression of TNF-Rs on interferon-γ stimulated pulmonary macrophages. In the present study we found that levels of TNF-Rs were not suppressed, and so hypothetically chronic alcoholic patients have a preferentially anti-inflammatory immune response.
In particular, plasma levels of IL-6 were significantly lower in chronic alcoholic patients in our study. Although IL-6 was initially found to be proinflammatory, recent findings suggest that IL-6 has anti-inflammatory effects [34]. Therefore, IL-6 might contribute to the resolution of acute and chronic inflammatory processes by direct suppression of IL-1β and TNF-α [35].
Patient characteristics
In the present study CRP was significantly increased in chronic alcoholic patients during infection. This is in accordance with a previous study conducted in patients with a daily alcohol intake of more than 80 g [36]. Chronic alcohol consumption is associated with higher CRP concentrations. CRP is mainly regulated by proinflammatory cytokines [37]. Proinflammatory cytokines were significantly decreased in early septic shock and did not differ in late septic shock. Additionally CRP might not be stimulated adequately in chronic alcoholic patients in early or late septic shock, which might be explained as an immune breakdown. There was a significant increase in leucocyte count in early septic shock. The leucocyte count has low predictive ability as a marker of infection [38]. A marked activation of the hypothalamic–pituitary–adrenal axis occurs during ethanol withdrawal, and this could result in an hypercortisolism mediated leucocytosis in early septic shock [39].
Chronic alcoholic patients had a significantly lower oxygenation index, which might be due to the higher number of smokers [40]. However, in the present study the percentage of smokers was not significantly different between the groups, and none of the patients were hypoxic because of a higher inspired oxygen fraction, indicating that these mechanisms were not relevant.
There were only a few significant differences in haemodynamic parameters. Chronic ethanol abuse can be associated with a variety of cardiovascular disorders, ranging from asymptomatic left ventricular dysfunction to hypertension, stroke, heart failure and sudden death from arrhythmias [2,41]. The higher pulmonary capillary wedge pressure could be interpreted as a result of a higher end-diastolic pressure leading to heart failure if there were normal ventricular compliance [41].
Because the number of patients with dual addiction (i.e. to both alcohol and nicotine) is more frequently seen than isolated or no addictions, we cannot totally exclude that the effects of ethanol on cytokine interactions were also influenced by nicotine. However, in the present study no significant difference between groups was seen. Spies and coworkers [42] examined patients with comorbid chronic alcoholism and nicotine abuse, and found that only TNF-α plasma levels were significantly higher in chronic alcoholic smokers than in chronic alcoholic nonsmokers.
Conclusion
In conclusion, chronic alcoholic patients exhibited lower levels of proinflammatory immune parameters and of IL-6 during infection and early sepsis, which might be due to an inhibitory effect on proinflammatory cytokine production induced by alcohol abuse and which might contribute to the clinically evident immune alteration. Therefore, chronic alcoholic patients may benefit from immune monitoring and early immune modulatory treatment at the onset of severe infections. However, this requires further investigation.
Key messages
• At the onset of infection and during early septic shock, chronic alcoholic patients had lower levels of proinflammatory immune parameters than did nonalcoholic patients
• Immunomodulatory therapy administered early may be considered in chronic alcoholic patients at the onset of an infection because of their altered proinflammatory immune response
Competing interests
None declared.
Abbreviations
CAGE = Cutting down, Annoyance by criticism, Guilty feeling, and Eye-openers; CRP = C-reactive protein; IL = interleukin; TNF = tumour necrosis factor; TNF-R = tumour necrosis factor receptor.
Acknowledgments
Acknowledgements
The authors should like to thank Jordan Rettig, a native English speaker, and Prof. Dr. K-D Wernecke for his statistical help (Head of the Institute for Medical Biometrics, Humboldt-University of Berlin). This study was supported in part by the German Research Society (DFG SP 432/1-1 and /1-2).
Contributor Information
Vera von Dossow, Email: vera.vdossow@charite.de.
Corinna Schilling, Email: corinna.schilling@charite.de.
Stefan Beller, Email: stefan.beller@charite.de.
Ortrud Vargas Hein, Email: ortrud.vargas@charite.de.
Christian von Heymann, Email: christian.von.heymann@rz.hu-berlin.de.
Wolfgang J Kox, Email: wkox@charite.de.
Claudia D Spies, Email: claudia.spies@charite.de.
References
- Spies CD, Nordmann A, Brummer G, Marks C, Conrad C, Berger G, Runkel N, Neumann T, Muller C, Rommelspacher H, et al. Intensive care unit stay is prolonged in chronic alcoholic men following tumor resection of the upper digestive tract. Acta Anaesthesiol Scand. 1996;40:649–656. doi: 10.1111/j.1399-6576.1996.tb04505.x. [DOI] [PubMed] [Google Scholar]
- Tønnesen H, Petersen KR, Højgaard L, Stokholm KH, Nielsen HJ, Knigge U, Kehlet H. Postoperative morbidity among symptom-free alcohol misusers. Lancet. 1992;340:334–337. doi: 10.1016/0140-6736(92)91405-W. [DOI] [PubMed] [Google Scholar]
- Fernández-Solá J, Junqué A, Estruch R, Monforte R, Torres A, Urbano-Marquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995;155:1649–1654. doi: 10.1001/archinte.155.15.1649. [DOI] [PubMed] [Google Scholar]
- Spies C, Tønnesen H, Andreasson S, Helander A, Conigrave K. Perioperative morbidity and mortality in chronic alcoholic patients. Alcohol Clin Exp Res. 2001. pp. 164S–170S. [DOI] [PubMed]
- Zisman DA, Strieter RM, Kunkel SL, Tsai WC, Wilkowski JM, Bucknell KA, Standiford TJ. Ethanol feeding impairs innate immunity and alters expression of Th1- and Th2-phenotype cytokines in murine Klebsiella pneumonia. Alcohol Clin Exp Res. 1998;22:621–627. doi: 10.1111/j.1530-0277.1998.tb04303.x. [DOI] [PubMed] [Google Scholar]
- Jerrels TR. Alcohol effects on the immune system. Third Annual Meeting of the Alcohol and Drug Abuse Immunology Symposium, Vail, Colorado, March 25–29. Alcohol. 1993;10:335–342. doi: 10.1016/0741-8329(93)90017-I. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Girouard L, Catalano D. Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-α, interleukin1β and elevated interleukin-10, and transforming growth factor-β production. Alcohol Clin Exp Res. 1996;20:900–907. doi: 10.1111/j.1530-0277.1996.tb05269.x. [DOI] [PubMed] [Google Scholar]
- D'Souza NB, Nelson S, Summer WR, Deaciuc IV. Alcohol modulates alveolar macrophage tumor necrosis factor-α, superoxide anion, and nitric oxide secretion in the rat. Alcohol Clin Exp Res. 1996;20:156–163. doi: 10.1111/j.1530-0277.1996.tb01059.x. [DOI] [PubMed] [Google Scholar]
- Xie J, Kolls J, Bagby G, Greenberg SS. Independent suppression of nitric oxide and TNF alpha in the lung of conscious rats by ethanol. FASEB J. 1995;9:253–261. doi: 10.1096/fasebj.9.2.7540157. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang DS, Giger PT, Watson RR. Influence of chronic dietary ethanol cytokine production by murine splenocytes and thymocytes. Alcohol Clin Exp Res. 1994;18:64–70. doi: 10.1111/j.1530-0277.1994.tb00882.x. [DOI] [PubMed] [Google Scholar]
- Woiciechowsky C, Asadullah K, Nestler D, Eberhardt B, Platzer C, Schoning B, Glockner F, Lanksch WR, Volk HD, Docke WD. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat Med. 1998;7:808–813. doi: 10.1038/nm0798-808. [DOI] [PubMed] [Google Scholar]
- Tonnesen H, Kaiser AH, Nielsen BB, Pederson AE. Reversibility of alcohol-induced immune depression. Br J Addict. 1992;87:1025–1028. doi: 10.1111/j.1360-0443.1992.tb03119.x. [DOI] [PubMed] [Google Scholar]
- Sander M, Irwin M, Sinha P, Naumann E, Kox WJ, Spies CD. Suppression of interleukin-6 to interleukin-10 ratio in chronic alcoholics: association with postoperative infections. Intensive Care Med. 2002;28:285–292. doi: 10.1007/s00134-001-1199-9. [DOI] [PubMed] [Google Scholar]
- Bonten MJ, Froon A, Gaillard C, Greve JW, Dentener MA, de Leeuw PW, Drent M, Stobberingh EE, Buurman WA. The systemic inflammatory response in the development of ventilator-associated pneumonia. Am J Respir Crit Care Med. 1997;156:1105–1113. doi: 10.1164/ajrccm.156.4.9610002. [DOI] [PubMed] [Google Scholar]
- Froon AH, Bonten MJ, Gaillard CA, Greve JW, Dentener MA, de Leeuw PW, Drent M, Stobberingh EE, Buurman WA. Prediction of clinical severity and outcome of ventilator-associated pneumonia. Comparision of simplified acute physiology score with systemic inflammatory mediators. Am J Respir Crit Care Med. 1998;158:1026–1031. doi: 10.1164/ajrccm.158.4.9801013. [DOI] [PubMed] [Google Scholar]
- Casey LC, Balk A, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with sepsis syndrome. Ann Intern Med. 1993;119:771–777. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- Linder MM, Wacha H, Feldmann U, Wesch G, Streifensand RA, Gundlach E. The Mannheim peritonitis index. An instrument for the intraoperative prognosis of peritonitis [in German] Chirurg. 1987;58:84–92. [PubMed] [Google Scholar]
- Members of the American College of Chest Physisians/ Society of Critical Care Medicine Consensus Conference Committee American College of Chest Physisians/Society of Critical Care Medicine Consensus Conference (1992) definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A, et al. The APACHE III Prognostic System. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- Ewing JA. Detecting alcoholism. The CAGE Questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . DSM-IV Options Book. Washington, DC: American Psychiatric Association; 1991. [Google Scholar]
- Beck JR, Schultz EK. The use of the relative operating characteristics (ROC) curves in test performance evaluation. Arch Pathol Lab Med. 1986;110:13–20. [PubMed] [Google Scholar]
- Ahluwalia B, Wesley B, Adeyiga O. Alcohol modulates cytokine secretion and synthesis in human fetus: an in vivo and in vitro study. Alcohol. 2000;21:207–213. doi: 10.1016/S0741-8329(00)00076-8. [DOI] [PubMed] [Google Scholar]
- Toivari M, Maki T, Suutarla S, Eklund KK. Ethanol inhibits IGE-induced degranulation and cytokine production in cultured mouse and human mast cells. Life Sci. 2000;67:2795–2806. doi: 10.1016/S0024-3205(00)00863-8. [DOI] [PubMed] [Google Scholar]
- Arbabi S, Garcia I, Bauer JG, Maier RV. Alcohol inhibits IL-8 and TNF: role of the p38 pathway. J Immunol. 1999;162:7441–7445. [PubMed] [Google Scholar]
- Omidvari K, Casey R, Nelson S, Olariu R, Shellito JE. Alveolar macrophage release of tumor-necrosis-factor-α in chronic alcoholic patients without liver disease. Alcohol Clin Exp Res. 1998;22:567–572. doi: 10.1111/j.1530-0277.1998.tb04294.x. [DOI] [PubMed] [Google Scholar]
- Khoruts A, Stahnke L, McClain C, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267–275. doi: 10.1016/0270-9139(91)92439-F. [DOI] [PubMed] [Google Scholar]
- Brede K, Kern H, Morciniec P, Katja B, Harmut K, Pawel M, Stefan B, Kox WJ, Spies CD. The value of immune modulating parameters in predicting the progression from peritonitis to septic shock. Shock. 2001;15:95–100. doi: 10.1097/00024382-200115020-00003. [DOI] [PubMed] [Google Scholar]
- Marchant A, Alegre ML, Hakim A, Pierard G, Marecaux G, Friedman G, De Groote D, Kahn RJ, Vincent JL, Goldman M. Clinical and biological significance of interleukin-10 plasma levels in patients with septic shock. J Clin Immunol. 1995;15:266–273. doi: 10.1007/BF01540884. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Girouard L, Szabo G. Human monocyte IL-10 production is increased by acute ethanol treatment. Cytokine. 1996;8:567–577. doi: 10.1006/cyto.1996.0076. [DOI] [PubMed] [Google Scholar]
- Ertel W, Scholl FA, Gallati H, Bonaccio M, Schildberg FW, Trentz O. Increased release of soluble tumor necrosis factor receptors into blood during clinical sepsis. Arch Surg. 1994;129:1330–1337. doi: 10.1001/archsurg.1994.01420360120017. [DOI] [PubMed] [Google Scholar]
- Bermudez LE, Wu M, Martinelli J, Young LS. Ethanol affects release of TNF and GM-CSF and membrane expression of TNF receptors by human macrophages. Lymphokine Cytokine Res. 1991;10:413–419. [PubMed] [Google Scholar]
- Tilg H, Dinarello CA, Mier J. IL-6 and APP: anti-inflammtory and immunosuppressive mediators. Immunol Today. 1997;18:428–432. doi: 10.1016/S0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- Mizuhara H, O'Neill E, Seki N, Ogawa T, Kusunoki C, Otsuka K, Satoh S, Niwa M, Senoh H, Fujiwara H. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med. 1994;179:1529–1537. doi: 10.1084/jem.179.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357:763–767. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- Oberhoffer M, Karzai W, Meier-Hellmann A, Bogel D, Fassbinder J, Reinhart K. Sensitivity and specifity of various markers of inflammation for the prediction of tumor necrosis factor-alpha and interleukin-6 in patients with sepsis. Crit Care Med. 1999;27:1814–1818. doi: 10.1097/00003246-199909000-00018. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Risher-Flowers D, De Jong J, Ravitz B, Bone GH, Nutt DJ, Roehrich L, Martin PR, Linnoila M. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am J Psychiatry. 1991;148:1023–1025. doi: 10.1176/ajp.148.8.1023. [DOI] [PubMed] [Google Scholar]
- Bobo JK. Nicotine dependence and alcoholism epidemiology and treatment. J Psychoactive Drugs. 1992;24:123–129. doi: 10.1080/02791072.1992.10471633. [DOI] [PubMed] [Google Scholar]
- Spies CD, Sander M, Stangl K, Fernandez-Sola J, Preedy VR, Rubin E, Andreasson S, Hanna EZ, Kox WJ. Effects of alcohol on the heart. Curr Opin Crit Care. 2001;7:337–343. doi: 10.1097/00075198-200110000-00004. [DOI] [PubMed] [Google Scholar]
- Spies CD, von Dossow V, Eggers V, Jetschmann G, El-Hilali R, Egert J, Fischer M, Schroder T, Hoflich C, Sinha P, et al. Altered cell-mediated immunity and increased postoperative infection rate in chronic alcoholic patients. Anesthesiology. 2004;100:1088–1100. doi: 10.1097/00000542-200405000-00010. [DOI] [PubMed] [Google Scholar]


