Abstract
Background
Pet dogs and cats exert an unquestionable beneficial effect in the well‐being of their owners, but can also act as a source of zoonotic infections if improperly cared.
Objectives
We investigated the occurrence, risk factors, genetic variability and zoonotic potential of intestinal parasites in dogs and cats attended in a clinical veterinary setting in Spain.
Methods
Canine (n = 252) and feline (n = 35) faecal samples were collected during 2017–2019 and analysed by coproparasitological methods. A rapid lateral immunochromatographic test (ICT) was used for detecting Giardia duodenalis and Cryptosporidium sp. Samples positive at microscopy examination and/or ICT were reassessed by molecular methods.
Results
Overall, 48.8% (123/252) of dogs and 48.6% (17/35) of cats were infected by enteric parasites. In dogs, G. duodenalis was the most prevalent species (40.9%), followed by Cystoisospora sp. (7.1%), and Toxocara canis (5.2%). In cats, Joyeuxiella sp. and Toxocara cati were the dominant species (20.0% each), followed by G. duodenalis (14.3%), D. caninum (5.7%) and Cystoisospora felis and Toxascaris leonina (2.9% each). Pups and kittens were more likely to harbour intestinal parasites and develop clinical signs. Sequence analyses of dog isolates revealed the presence of assemblages A (n = 1), C (n = 4), D (n = 4) and C+D (n = 1) within G. duodenalis; C. parvum (n = 1) and C. canis (n = 4) within Cryptosporidium and PtEb IX (n = 1) in Enterocytozoon bieneusi. A novel C. canis subtype family, named XXi, is reported.
Conclusions
Our results highlight that (i) well‐cared dogs carry zoonotic enteric protozoan parasites of public health relevance, (ii) proper hygiene practices and routine veterinary treatment are essential to prevent zoonotic infections, (iii) vulnerable populations should avoid contact with pups/kittens with diarrhoea and (iv) infected dogs might be major contributors to the environmental contamination with soil‐transmitted helminths (STHs) eggs.
Keywords: genotyping, helminths, prevention, protists, small animal clinic, transmission, zoonosis
One in two pet dogs and cats is infected by intestinal parasites. Some of them have the potential to infect humans.
Pups and kittens were at higher risk of infection. Performing a coproparasitological analysis is essential to establish a correct deworming protocol in these animals.
We provide a comprehensive description of the presence, risk factors and molecular diversity (for G. duodenalis, Cryptosporidium sp. and E. bieneusi) of enteric parasites in well‐kept pet dogs and cats in an affluent area of central Spain.
1. INTRODUCTION
Pet ownership can have a beneficial impact on human health, providing emotional, social and physical well‐being benefits (Friedman & Krause‐Parello, 2018). Interacting with dogs and cats has been demonstrated beneficial for improve mental health by reducing stress, anxiety and depression, and by promoting feelings of happiness and companionship (Boldig & Butala, 2021). Owning a pet also encourages physical activity and contributes to improved physical fitness and cardiovascular health (Arhant‐Sudhir et al., 2011; Cutt et al., 2007). However, pet owning comes with the potential for zoonotic infections (Baneth et al., 2016). Domestic dogs and cats can carry a large variety of bacterial, viral and parasitic pathogens which can be transmitted to humans through bites, scratches, saliva, urine, faeces or contaminated surfaces (Overgaauw et al., 2020). Young children, pregnant women, elderly individuals and immunocompromised individuals might be more susceptible to zoonotic infections (Meers et al., 2022). In addition to adequate hygiene practices (e.g. regular handwashing, proper handling and disposal of dog waste), routine veterinary care is essential to prevent the transmission of zoonotic diseases and ensure the health and safety of dogs and their owners (ESCCAP, [Link], [Link]; Miró et al., 2020; Morelli et al., 2022).
Among parasites, enteric protists and helminths are significant causes of morbidity and mortality in dogs and cats, particularly pups and kittens, senior animals or those with weakened immune systems (Gorgani‐Firouzjaee et al., 2022; Raza et al., 2018; Traversa, 2012). These parasites include helminths (e.g. members of the families Ancylostomatidae, Dipylididae, Taeniidae, Toxocara canis, Toxocara cati, Toxascaris leonina, Strongyloides sp. and Trichuris vulpis) as well as protists (e.g. Giardia duodenalis, Cryptosporidium sp., Cystoisospora sp.), and are significant causes of diarrhoea with subsequent dehydration, weight loss, abdominal pain and occasionally, anaemia (Miller, 2020; Scorza & Tangtrongsup, 2010; Tangtrongsup & Scorza, 2010; Tysnes et al., 2014).
Regular anti‐parasitic treatment of dogs is the most effective measure to minimise the risk of infection and transmission of zoonotic diseases. However, the commonly used anti‐parasitic treatment used in the clinical practice consists in the administration of anthelmintic drugs, known to be ineffective against infections caused by protist parasites including G. duodenalis and Cryptosporidium sp. (ESCCAP [Link], [Link]). Furthermore, prescribed treatments are generic and do not take into consideration epidemiological risk factors such as the likelihood of reinfections or the genetic variants of the parasite involved in the infection (Bagster & Elsheikha, 2022; McNamara et al., 2018). Furthermore, the complex transmission cycles of parasitic helminths, which include multiple hosts and life stages, often pose significant challenges for treatment efforts, requiring specific dosages and active drugs for effective eradication (Mengarda et al., 2023). Considering all the stated above, regular coproparasitological examination of canine and feline faecal samples should be carried out to monitor and evaluate the efficacy of the prescribed treatments.
This study aims at investing the occurrence of enteric helminthic and protist parasites in dogs and cats attended in a small animal clinic setting in central Spain, highlighting the importance of conducting coproparasitological analyses for prompt treatment and reducing the likelihood of zoonotic transmission events. Additional molecular studies were conducted to investigate the frequency and diversity of genetic variants of G. duodenalis and Cryptosporidium sp. circulating in the surveyed dog population.
2. MATERIALS AND METHODS
2.1. Ethical statement
This study was carried out in accordance with Spanish legislation guidelines (RD 8/2003) and with the International Guiding Principles for Biomedical Research Involving Animals issued by the Council for International Organization of Medical Sciences and the International Council for Laboratory Animal Science (RD 53/2013).
2.2. Study design and setting
This is an observational, retrospective epidemiological study summarising the results of the coproparasitological analyses conducted in faecal samples from dogs (n = 252) and cats (n = 35) attended in a small animal veterinary clinic in the Majadahonda municipality (northwest Madrid) from January 2017 to December 2021. Majadahonda has 72,179 inhabitants and 3482 cats and 10,366 dogs censed in 2022 (Instituto Nacional de Estadística, 2022; RIAC). It is one of the Spain´s wealthiest municipalities ranking eight in average annual income per person and household (Instituto Nacional de Estadística, 2020). A total of 10 veterinary clinics and a large veterinary hospital provide health care services to the canine and feline populations in the local community.
2.3. Microscopy detection
Three consecutive faecal samples from each investigated dog or cat were collected by pet owners after spontaneous defecation of their animals. Faecal matter was transferred into sterile polystyrene plastic flasks and kept at 4°C until further processing at the veterinary clinic. The cohort included animals of all age groups and breeds with and without gastrointestinal or respiratory disease manifestations. Dogs and cats with a previous diagnosis for one or more enteric parasites undergoing treatment follow‐up were excluded from the study.
Intestinal parasites were diagnosed by the microscopic detection of their developmental stages (larvae, eggs, cysts, oocysts and trophozoites) in the faecal material using the modified Telemann concentration technique coupled with merthiolate‐iodine‐formaldehyde (MIF) staining (De Rivas, 1928; Telemann, 1908). No modified Ziehl–Neelsen staining for the detection of coccidian protozoa (e.g. Cryptosporidium sp.) in faecal smears was conducted. Faecal samples from dogs or cats with respiratory symptoms were analysed using the Baermann method for the specific detection of larvae of Strongyloides sp. and lungworms (ESCCAP, 2022).
2.4. Immunochromatographic rapid test
All canine and feline faecal samples were investigated by an immunochromatographic rapid test (immunochromatographic test (ICT), Stick Crypto‐Giardia, Operon, Zaragoza, Spain) for the simultaneous detection of the protozoa G. duodenalis and Cryptosporidium sp. following the manufacturer´s instructions.
2.5. DNA extraction and purification
Faecal concentrates that tested positive or dubious for enteric parasites by microscopy (G. duodenalis, Strongyloides sp.) or ICT (G. duodenalis, Cryptosporidium sp.) were stored for up to 3 months at −20°C and subsequently shipped to the Parasitology Reference and Research Laboratory, National Centre for Microbiology, Majadahonda (Madrid), for downstream molecular testing. Genomic DNA was isolated from about 200 mg of each concentrated faecal sample using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Samples mixed with InhibitEX buffer were incubated for 10 min at 95°C. Extracted and purified DNA samples were eluted in 200 μL of PCR‐grade water and kept at 4°C for up to 6 months until further PCR analysis.
2.6. Molecular detection and characterisation of Giardia duodenalis
Detection of G. duodenalis DNA was achieved using a real‐time PCR (qPCR) method targeting the gene codifying the small subunit ribosomal RNA (ssu rRNA) of the parasite (Verweij et al., 2003).
For assessing the molecular diversity of the parasite, we adopted a sequence‐based multilocus genotyping (MLST) scheme targeting the genes encoding for the ssu rRNA, the glutamate dehydrogenase (gdh), β‐giardin (bg) and triose phosphate isomerase (tpi) proteins of the parasite. For assessing the molecular diversity of G. duodenalis at the assemblage level, a nested PCR was used to amplify a fragment of the ssu rRNA gene (Appelbee et al., 2003; Hopkins et al., 1997). The molecular diversity of the parasite at the sub‐assemblage level was investigated only in Giardia isolates that tested positive by qPCR and yielded cycle threshold (CT) values ≤32. A semi‐nested PCR was used to amplify a fragment of the gdh gene (Read et al., 2004), and nested PCRs were used to amplify fragments of the bg and tpi genes, respectively (Cacciò et al., 2002; Lalle et al., 2005; Sulaiman et al., 2003).
2.7. Molecular detection and characterisation of Cryptosporidium sp
The presence of Cryptosporidium sp. was assessed using a nested‐PCR protocol to amplify a fragment of the ssu rRNA gene of the parasite (Tiangtip & Jongwutiwes, 2002). Subtyping tools based on the amplification of partial sequences of the 60‐kDa glycoprotein (gp60) gene were used to ascertain intra‐species genetic diversity in samples that tested positive for C. canis (Jiang et al., 2021) and C. parvum (Feltus et al., 2006).
2.8. Molecular detection of Strongyloides sp
Identification of Strongyloides sp. was carried out by a qualitative qPCR method using genus‐specific primers targeting the ssu rRNA gene of the parasite (Saugar et al., 2015; Verweij et al., 2009).
2.9. Molecular detection of Blastocystis sp
Identification of Blastocystis sp. was achieved by a direct PCR protocol targeting a fragment of the ssu rRNA gene of the parasite (Scicluna et al., 2006).
2.10. Molecular detection and characterisation of Enterocytozoon bieneusi
Detection of E. bieneusi was conducted by a nested PCR protocol to amplify a fragment of the internal transcribed spacer (ITS) region as well as portions of the flanking large and small subunit of the ribosomal RNA gene as previously described (Buckholt et al., 2002).
2.11. General PCR and electrophoretic procedures
Detailed information on the PCR cycling conditions and oligonucleotide sequences used for the molecular identification and/or characterisation of the protozoan parasites investigated in the present study is presented in Tables S1 and S2, respectively.
All qPCR protocols described above were carried out on a Corbett Rotor GeneTM 6000 real‐time PCR system (Qiagen). Reaction mixes included 2× TaqMan® Gene Expression Master Mix (Applied Biosytems, Foster City, CA, USA) or 1× Quantimix EasyMaster Mix (Biotools B&M Laboratories, Madrid, Spain) and 0.5 μL of 50× SybrGreen (Invitrogen, San Diego, CA, USA) for detection of G. duodenalis and Strongyloides sp., respectively. All the direct, semi‐nested and nested PCR protocols described above were conducted on a 2720 Thermal Cycler (Applied Biosystems). Reaction mixes always included 2.5 units of MyTAQTM DNA polymerase (Bioline GmbH, Luckenwalde, Germany), and 5–10 μL MyTAQTM Reaction Buffer containing 5 mM dNTPs and 15 mM MgCl2. Laboratory‐confirmed positive and negative DNA samples of human and animal origin for each parasitic species investigated were routinely used as controls and included in each round of PCR. PCR amplicons were visualised on 1.5% D5 agarose gels (Conda, Madrid, Spain) stained with Pronasafe (Conda) nucleic acid staining solutions. A 100 bp DNA ladder (Boehringer Mannheim GmbH, Baden‐Wurttemberg, Germany) was used for the sizing of obtained amplicons.
2.12. Sanger sequencing analyses
Positive‐PCR products of the expected size were directly sequenced in both directions using appropriate internal primer sets (Table S2). DNA sequencing was conducted by capillary electrophoresis using the BigDye® Terminator chemistry on an on ABI PRISM 3130 automated DNA sequencer (Applied Biosystems). Generated DNA consensus sequences were aligned to appropriate reference sequences obtained in GenBank using the Basic Local Alignment Search Tool (BLAST) and the MEGA X software (Kumar et al., 2018) for species confirmation and genotype identification. The sequences obtained in this study have been deposited in GenBank under accession numbers OQ722809–OQ722811 and OQ679953–OQ679968 (G. duodenalis), OQ722812–OQ722814 and OQ679969 (Cryptosporidium sp.), and OQ722933 (Enterocytozoon bieneusi).
2.13. Statistical analysis
The chi‐square test (χ 2) was used to determine potential statistically significant associations between the occurrence of individual enteric parasite species in dogs and cats and variables including age, sex, seasonality, anti‐parasitic treatment in the 3 months previous to sampling, presence of gastrointestinal or respiratory clinical manifestations and faecal consistency (1: hard, 2: formed, 3: soft, 4: liquid). A P‐value < 0.05 was considered statistically significant.
The Cohen's Kappa test was estimated to assess the agreement of the diagnostic results obtained with the Stick Crypto‐Giardia (Operon) ICT test and conventional microscopy examination. Cohen's Kappa ranges between 0 (no agreement between the two raters) and 1 (perfect agreement between the two raters). A Cohen's kappa value between 0.81 and 0.99 was considered as 'near perfect agreement'.
3. RESULTS
In this study, we tested faecal samples from 252 dogs and 35 cats that attended a small animal clinic during the period 2017−2021 in Madrid, central Spain (Table S3). The median age of the investigated dogs was 4.5 months [range: 1−156; standard deviation (SD): 42.3]. The median age of the investigated cats was 4.0 months (range: 1−228; SD: 49.1]. Near half of the dogs (49.6%, 125/252) and cats (54.3%, 19/35) were ≤4 months of age. The male/female ratios for dogs and cats were 1.3 in both cases. Collection of canine and feline faecal samples was similarly distributed across seasons (spring: n = 93; summer: n = 37; autumn: n = 78, winter; n = 79). Overall, 32.9% (83/252) of dogs and 11.4% (4/35) of cats received anthelmintic treatment in the 3 months previous to sample collection. Gastrointestinal and respiratory clinical manifestations were observed in 51.6% (130/252) and 5.2% (13/252) of dogs and in 28.6% (10/35) and 5.7% (2/35) of cats, respectively. Most of the faecal samples collected were formed both in dogs (55.2%, 139/252) and cats (77.1%, 27/35).
3.1. Microscopy
Overall, 48.8% (123/252) of dogs and 48.6% (17/35) of cats were infected by at least a single species of enteric parasites. Infections by protists were more frequent than infections by helminth parasites in dogs (43.7% vs. 9.5%), whereas the opposite pattern was observed in cats (14.3% vs. 45.7%).
In dogs, G. duodenalis (cysts) was the most common parasite species found (40.9%, 103/252; 95% CI: 34.7−47.2), followed by Cystoisospora sp. (7.1%, 18/252; 95% CI: 4.3−11.0), and T. canis (5.2%, 13/252; 95% CI: 2.8−8.7). Cryptosporidium sp. oocysts, T. leonine eggs, D. caninum proglottids, hookworms eggs, and Strongyloides sp. larvae were all detected at low (<2%) prevalence rates (Table 1).
TABLE 1.
Microscopy‐based frequencies of enteric parasites in the canine (n = 252) and feline (n = 35) populations investigated in the region of Madrid (Spain), and 95% confidence intervals (95% CI) are indicated.
| Dogs (n = 252) | Cats (n = 35) | All (n = 287) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Species | Pos. (n) | % | 95% CI | Pos. (n) | % | 95% CI | Pos. (n) | % | 95% CI |
| Protozoa | Giardia duodenalis | 103 | 40.9 | 34.7−47.2 | 5 | 14.3 | 4.8−30.3 | 108 | 37.6 | 32.0−43.5 |
| Cystosisospora sp. | 18 | 7.1 | 4.3−11.0 | 1 | 2.9 | 0.07−14.9 | 19 | 6.6 | 4.0−10.2 | |
| Nematoda | Toxocara canis/cati | 13 | 5.2 | 2.8−8.7 | 7 | 20.0 | 8.4−36.9 | 20 | 7.0 | 4.3−10.6 |
| Toxascaris leonina | 4 | 1.6 | 0.4−4.0 | 1 | 2.9 | 0.07−14.9 | 5 | 1.7 | 0.6−4.0 | |
| Fam. Ancylostomatidae | 3 | 1.2 | 0.3−3.4 | 0 | 0.0 | – | 3 | 1.1 | 0.2−3.0 | |
| Strongyloides sp. | 2 | 0.8 | 0.1−2.8 | 0 | 0.0 | – | 2 | 0.7 | 0.08−2.5 | |
| Cestoda | Dipylidium caninum | 4 | 1.6 | 0.4−4.0 | 2 | 5.7 | 0.7−19.2 | 6 | 2.1 | 0.8−4.5 |
| Joyeuxiella sp. | 0 | 0.0 | – | 7 | 20.0 | 8.4−36.9 | 7 | 2.4 | 1.0−5.0 | |
In cats, Joyeuxiella sp. and T. cati were the dominant enteric parasite species found (20.0%, 7/35; 95% CI: 8.4−36.9 each), followed by G. duodenalis (14.3%, 5/35; 95% CI: 4.8−30.3), D. caninum (5.7%, 2/35; 95% CI: 0.7−19.2) and Cystoisospora felis and T. leonina (2.9%, 1/35; 95% CI: 0.07−14.9 each) (Table 1).
Coinfections by two or more enteric parasite species were identified in 10.3% (26/252) of dogs and 14.3% (5/35) of cats, respectively. The combination G. duodenalis + Cystoisospora sp. was the coinfection most frequently detected in dogs (42.3%, 11/26) and G. duodenalis + T. cati in cats (40.0%, 2/5). The distribution of infections by single or multiple enteric parasites in the joint canine and feline populations is shown in Table 2.
TABLE 2.
Microscopy‐based frequencies of enteric parasites in monoinfection and coinfection in the canine (n = 252) and feline (n = 35) populations investigated in the region of Madrid (Spain).
| Parasite species | Positive (n) | Frequency (%) a |
|---|---|---|
| None | 147 | 51.2 |
| In monoinfection | ||
| G. duodenalis | 83 | 28.9 |
| Toxocara canis/cati | 8 | 2.8 |
| Joyeuxiella sp. | 7 | 2.4 |
| Cystoisospora sp. | 5 | 1.7 |
| Dipylidium caninum | 3 | 1.0 |
| Fam. Ancylostomatidae | 3 | 1.0 |
| Toxascaris leonina | 1 | 0.3 |
| In co‐infection | ||
| G. duodenalis + Cystoisospora sp. | 12 | 4.2 |
| G. duodenalis + Toxocara canis/cati | 8 | 2.8 |
| G. duodenalis + D. caninum | 2 | 0.7 |
| T. canis + Cystoisospora sp. | 2 | 0.7 |
| T. canis+ T. leonine | 2 | 0.7 |
| G. duodenalis + Strongyloides sp. | 1 | 0.3 |
| G. duodenalis + Toxascaris leonina | 1 | 0.3 |
| T. cati + D. caninum | 1 | 0.3 |
| G. duodenalis + Strongyloides sp. + T. leonina | 1 | 0.3 |
| Total | 287 | 100 |
Over the total of fecal samples (n = 287) examined.
3.2. Diagnostic performance of immunochromatographic rapid test using microscopy as gold standard
The ICT test yielded positive results for G. duodenalis in 113 faecal samples (39.4%, 113/287), 106 from dogs (42.1%; 106/252) and seven from cats (20.0%; 7/35). The frequency of agreement between microscopy and ICT was 98.3%. The ICT test identified five more Giardia‐positive samples (three in dogs and two in cats) than conventional microscopy. The Kappa coefficient showed an almost perfect agreement between both diagnostic methods (κ = 0.963).
In addition, five canine faecal samples (2.0%, 5/252) were tested positive for Cryptosporidium sp. by ICT. No feline faecal samples were tested positive for this pathogen by ICT.
3.3. Molecular characterisation of Giardia duodenalis isolates
Out of the 113 canine and feline faecal samples with a positive result either by microscopy or ICT, a total of 86 (82 from dogs and four from cats) were available for qPCR testing. Out of the 82 canine isolates tested, 86.6% (71/82) were confirmed by qPCR, yielding cycle threshold (CT) values ranging from 18.6 to 38.6 (median: 30.8; SD: 4.8). Out of the four feline isolates tested, 50% (2/4) were confirmed by qPCR, yielding CT values ranging of 34.8 and 41.8, respectively.
A total of 11 G. duodenalis isolates of canine origin were successfully amplified at one or more of the four (ssu rRNA, gdh, bg and tpi) genetic markers used for genotyping purposes (Table 3). Four isolates were amplified at a single locus or two loci (36.4% each), and the remaining three (27.2%) at three independent loci. None of the 11 isolates were simultaneously amplified at the four loci. Nucleotide sequence analyses revealed the presence of zoonotic assemblage A (18.2%, 2/11) and canine‐adapted assemblages C (36.3%, 4/11) and D (27.3%, 3/11). An additional isolate (18.2%, 2/11) was identified as a mixed C+D infection (Table 3).
TABLE 3.
Multilocus sequence typing results of the 11 G. duodenalis‐positive samples of canine origin successfully genotyped at any of the four loci investigated in the present survey.
| Sample ID | Host | Age (months) | CT value in qPCR | ssu rRNA | gdh | bg | tpi | Assigned genotype |
|---|---|---|---|---|---|---|---|---|
| 166 | Dog | 18 | 22.6 | – | A1 | A1 | – | A1 |
| 214 | Dog | 3 | 20.0 | – | C | C+D | C | C+D |
| 217 | Dog | 12 | 24.1 | – | C | – | – | C |
| 230 | Dog | 2 | 18.6 | – | D | D | C | C+D |
| 242 | Dog | 2 | 28.6 | C | – | – | – | C |
| 245 | Dog | 3 | 23.8 | – | D | D | – | D |
| 250 | Dog | 2 | 29.3 | – | D | D | – | C |
| 252 | Dog | 12 | 33.9 | A | – | – | – | A |
| 255 | Dog | 24 | 26.4 | – | C | – | – | C |
| 258 | Dog | 7 | 22.7 | D | D | D | – | D |
| 279 | Dog | 4 | 26.9 | – | D | D | – | D |
Table 4 summarises the molecular data generated at the ssu rRNA, gdh, bg and tpi loci. The three nucleotide sequences amplified at the ssu rRNA gene were assigned to assemblages A, C and D, respectively. These sequences were identical, or differed by a single nucleotide polymorphism (SNP), with their respective reference sequences.
TABLE 4.
Frequency and molecular diversity of the G. duodenalis, Cryptosporidium spp. and E. bieneusi sequences successfully genotyped in the canine population investigated in the region of Madrid (Spain). GenBank accession numbers are provided.
| Species | Genotype | Sub‐genotype | Locus | No. isolates | Reference sequence | Stretch | Single nucleotide polymorphisms | GenBank ID |
|---|---|---|---|---|---|---|---|---|
| Giardia duodenalis | A | – | ssu rRNA | 1 | M54878 | 51−289 | G113T | OQ722809 |
| C | – | ssu rRNA | 1 | AF199449 | 14−290 | A199R | OQ722810 | |
| D | – | ssu rRNA | 1 | AF199443 | 70−291 | None | OQ722811 | |
| A | AI | gdh | 1 | L40509 | 64−491 | C447Y | OQ679953 | |
| C | – | gdh | 2 | U60984 | 76−496 | None | OQ679954 | |
| C | – | gdh | 1 | U60984 | 76−491 | A212T | OQ679955 | |
| D | – | gdh | 1 | U60986 | 67−491 | None | OQ679956 | |
| D | – | gdh | 1 | U60986 | 73−491 | T240C, T429C, G441A | OQ679957 | |
| D | – | gdh | 1 | U60986 | 64−423 | T240C, T429C, G441A, T459A | OQ679958 | |
| D | – | gdh | 1 | U60986 | 125−417 | T312Y, C375Y | OQ679959 | |
| D | – | gdh | 1 | U60986 | 76−496 | C375Y, T429Y, G441R | OQ679960 | |
| A | AI | bg | 1 | AY655702 | 33−523 | None | OQ679961 | |
| D | – | bg | 1 | AY545647 | 98−596 | None | OQ679962 | |
| D | – | bg | 1 | AY545647 | 98−590 | A159G, A201G, T251Y | OQ679963 | |
| D | – | bg | 2 | AY545647 | 102−555 | A201G | OQ679964 | |
| D | – | bg | 1 | AY545647 | 102−590 | A201G, C207Y | OQ679965 | |
| C+D | – | bg | 1 | AY545647 | 96−594 | T123Y, T132Y, T150Y, A159W, T165K, T177Y, G183S, A194R, A201G, A202R, C207S, A231M, T243Y, T255Y, G273R, T276Y, A282M, A291R, C309Y, A312M, G327S, A387R, T390Y, T426Y, T441Y, G496R, T510Y, T513Y, T519Y, A552R, A570R, A573C, T579K | OQ679966 | |
| C | – | tpi | 1 | AY228641 | 21−532 | G37A, C63T, G136T, T316C, C369T, A379C | OQ679967 | |
| C | – | tpi | 1 | AY228641 | 19−531 | C49Y, G136K, T316Y, A368R, C369Y, A379M | OQ679968 | |
| Cryptosporidium parvum | – | – | ssu rRNA | 1 | AF112571 | 528−1030 | A646G, T649G, 686_689DelTAAT, T693A, T709C | OQ722812 |
| Cryptosporidium canis | – | – | ssu rRNA | 4 | AF112576 | 538−1021 | None | OQ722813 |
| – | XXi1a | gp60 | 1 | – | – | – | OQ679969 | |
| Enterocytozoon bieneusi | – | PtEb IX | ITS | 1 | AF059610 | 34−416 | None | OQ722933 |
bg, β‐giardin; Del, deletion; gdh, glutamate dehydrogenase; ITS, internal transcribed spacer; K, T/G; M, C/A; R, A/G; S, G/C; ssu rRNA, small subunit ribosomal RNA; tpi, triose phosphate isomerase; Y: C/T.
At the gdh locus, one isolate was confirmed as sub‐assemblage A1, differing by a single SNP from its reference sequence (GenBank accession number L4050917) (Table 4). Out of the three assemblage C sequences identified, two showed 100% identity with the reference U60984, with the remaining one differing from it by a single SNP. A higher level of genetic diversity was observed among the five assemblage D sequences found: only one was identical to reference sequence, whereas the remaining four differed from it by two to four SNPs (Table 5). Out of the six isolates amplified at the bg locus, one showed 100% identity with sub‐assemblage AI reference sequence AY655702, confirming the results previously obtained at the ssu rRNA and gdh loci. Four isolates were identified as assemblage D, one being identical to reference sequence AY545647, and the other three differing from it by one to three SNPs. The sixth bg isolate corresponded to a mixed C+D infection. Taking AY545647 as reference sequence, this isolate differed from it by 33 SNPs, 32 of them corresponding to ambiguous (double peak) positions (Table 4). The two isolates amplified at the tpi loci were assigned to assemblage C, differing by four to six SNPs between them and from reference sequence AY228641 (Table 4).
TABLE 5.
Frequencies of enteric parasites in the joint canine (n = 252) and feline (n = 35) populations investigated in the region of Madrid (Spain) according to age group, sex, seasonality, clinical manifestations and faecal consistency. Bolded values indicate statistical significance.
| Variable | Animals (n) | Any parasite (%) | Cystoisospora sp. (%) | Giardia duodenalis (%) | Toxocara canis (%) | Toxascaris leonina (%) | Fam. Ancylostomatidae (%) | Strongyloides sp. (%) | Dipylidum caninum (%) | Joyeuxiella spp. (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (months) | ||||||||||
| ≤ 4 | 146 | 98 (67.1) | 16 (11.0) | 76 (52.1) | 18 (12.3) | 4 (2.7) | 1 (0.7) | 2 (1.4) | 5 (3.4) | 1 (0.7) |
| >4 ≤ 8 | 22 | 11 (50.0) | 2 (9.1) | 9 (40.9) | 1 (4.5) | 0 (0.0) | 1 (4.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| >8 ≤ 12 | 18 | 10 (55.6) | 0 (0.0) | 9 (50.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| > 12 ≤ 24 | 24 | 10 (41.7) | 0 (0.0) | 7 (29.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (12.5) |
| > 24 | 77 | 11 (14.3) | 1 (1.3) | 7 (9.1) | 1 (1.3) | 1 (1.3) | 0 (0.0) | 0 (0.0) | 1 (1.3) | 3 (3.9) |
| Total | 287 | 140 (48.8) | 19 (6.6) | 108 (37.6) | 20 (7.0) | 5 (1.7) | 3 (1.0) | 2 (0.7) | 6 (2.1) | 7 (2.4) |
| P‐value | P < 0.001 | P = 0.025 | P < 0.001 | P = 0.009 | P = 0.723 | P = 0.116 | P = 0.746 | P = 0.579 | P = 0.008 | |
| Sex | ||||||||||
| Male | 164 | 84 (51.2) | 10 (6.1) | 65 (39.6) | 13 (7.9) | 5 (3.0) | 2 (1.2) | 1 (0.6) | 4 (2.4) | 4 (2.4) |
| Female | 123 | 56 (45.5) | 9 (7.3) | 43 (35.0) | 7 (5.7) | 0 (0.0) | 1 (0.8) | 1 (0.8) | 2 (1.6) | 3 (2.4) |
| Total | 287 | 140 (48.8) | 19 (6.6) | 108 (37.6) | 20 (7.0) | 5 (1.7) | 3 (1.0) | 2 (0.7) | 6 (2.1) | 7 (2.4) |
| P‐value | P = 0.340 | P = 0.681 | P = 0.419 | P = 0.462 | P = 0.051 | P = 0.738 | P = 0.838 | P = 0.634 | P = 1.000 | |
| Seasonality | ||||||||||
| Spring | 93 | 34 (36.6) | 6 (6.5) | 27 (29) | 6 (6.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.2) | 1 (1.1) |
| Summer | 37 | 14 (37.8) | 2 (5.4) | 11 (29.7) | 3 (8.1) | 1 (2.7) | 0 (0.0) | 1 (2.7) | 0 (0.0) | 0 (0.0) |
| Autumn | 78 | 40 (51.3) | 4 (5.1) | 29 (37.2) | 7 (9.0) | 3 (3.8) | 3 (3.8) | 1 (1.3) | 4 (5.1) | 0 (0.0) |
| Winter | 79 | 52 (65.8) | 7 (8.9) | 41 (51.9) | 4 (5.1) | 1 (1.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (7.6) |
| Total | 287 | 140 (48.8) | 19 (6.6) | 108 (37.6) | 20 (7.0) | 5 (1.7) | 3 (1.0) | 2 (0.7) | 6 (2.1) | 7 (2.4) |
| P‐value | P < 0.001 | P = 0.798 | P = 0.013 | P = 0.792 | P = 0.265 | P = 0.044 | P = 0.290 | P = 0.112 | P = 0.006 | |
| Deworming <3 months | ||||||||||
| Yes | 87 | 48 (55.2) | 9 (10.3) | 43 (49.4) | 1 (1.1) | 2 (2.3) | 0 (0.0) | – | 2 (2.3) | – |
| No | 31 | 18 (58.1) | 4 (12.9) | 10 (32.3) | 7 (22.6) | 0 (0.0) | 2 (6.5) | – | 2 (6.5) | – |
| Total | 118 | 66 (55.9) | 13 (11.0) | 53 (44.9) | 8 (6.8) | 2 (1.7) | 2 (1.7) | – | 4 (3.4) | – |
| P‐value | P = 0.781 | P = 0.696 | P = 0.099 | P < 0.001 | P = 0.395 | P = 0.017 | – | P = 0.273 | – | |
| Digestive signs | ||||||||||
| Yes | 141 | 82 (58.2) | 15 (10.6) | 70 (49.6) | 9 (6.4) | 2 (1.4) | 2 (1.4) | 2 (1.4) | 2 (1.4) | 1 (0.7) |
| No | 146 | 58 (39.7) | 4 (2.7) | 38 (26.0) | 11 (7.5) | 3 (2.1) | 1 (0.7) | 0 (0.0) | 4 (2.7) | 6 (4.1) |
| Total | 287 | 140 (48.8) | 19 (6.6) | 108 (37.6) | 20 (7.0) | 5 (1.7) | 3 (1.0) | 2 (0.7) | 6 (2.1) | 7 (2.4) |
| P‐value | P = 0.002 | P = 0.007 | P < 0.001 | P = 0.702 | P = 0.680 | P = 0.541 | P = 0.149 | P = 0.434 | P = 0.062 | |
| Respiratory signs | ||||||||||
| Yes | 15 | 10 (66.7) | 0 (0.0) | 9 (60.0) | 2 (13.3) | 1 (6.7) | 0 (0.0) | 2 (13.3) | 0 (0.0) | 0 (0.0) |
| No | 271 | 130 (48.3) | 19 (7.1) | 97 (36.1) | 18 (6.7) | 4 (1.5) | 3 (1.1) | 0 (0.0) | 6 (2.2) | 7 (2.6) |
| Total | 286 | 140 (49.0) | 19 (6.6) | 108 (37.8) | 20 (7.0) | 5 (1.7) | 3 (1.0) | 2 (0.7) | 6 (2.1) | 7 (2.4) |
| P‐value | P = 0.124 | P = 0.526 | P = 0.034 | P = 0.573 | P = 0.324 | P = 0.909 | P < 0.001 | P = 0.824 | P = 0.797 | |
| Faecal consistency | ||||||||||
| Hard | 1 | 1 (100) | 0 (0.0) | 1 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Formed | 166 | 67 (40.4) | 4 (2.4) | 45 (27.1) | 13 (7.8) | 4 (2.4) | 1 (0.6) | 0 (0.0) | 4 (2.4) | 6 (3.6) |
| Soft | 90 | 56 (62.2) | 10 (11.1) | 48 (53.3) | 5 (5.6) | 1 (1.1) | 1 (1.1) | 2 (2.2) | 2 (2.2) | 1 (1.1) |
| Liquid | 30 | 16 (53.3) | 5 (16.7) | 14 (46.7) | 2 (6.7) | 0 (0.0) | 1 (3.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 287 | 140 (48.8) | 19 (6.6) | 108 (37.6) | 20 (7.0) | 5 (1.7) | 3 (1.0) | 2 (0.7) | 6 (2.1) | 7 (2.4) |
| P‐value | P = 0.006 | P = 0.005 | P < 0.001 | P = 0.908 | P = 0.755 | P = 0.605 | P = 0.221 | P = 0.861 | P = 0.493 | |
None of the two feline isolates with a Giardia‐positive result at microscopy examination or ICT were genotyped at the ssu rRNA, gdh, bg or tpi loci.
3.4. Molecular characterisation of Cryptosporidium sp. isolates
All five canine samples that tested positive for Cryptosporidium sp. by ICT yielded amplicons of the expected size in ssu‐PCR. Sequence analyses of the obtained amplicons allowed the identification of zoonotic C. parvum (n = 1) and host‐specific C. canis (n = 4) (Table 4). The C. parvum sequence was identified as the bovine genotype of the parasite, differing from reference sequence AF112571 by six SNPs including the distinctive TAAT deletion in positions 686_689 (Table 4). This isolate was not be amplified at the gp60 locus.
The four C. canis sequences showed 100% identity with reference sequence AF112576. Only a single isolate could be molecularly characterised at the gp60 locus. Sequence analysis confirmed the presence of a novel C. canis subtype family that we named XXi1 in agreement with the established nomenclature for Cryptosporidium subtype families (Xiao & Feng, 2017).
Figure 1 shows the maximum‐likelihood tree generated with representative sequences of the eight C. canis subtype families (XXa, XXb, XXc, XXd, XXe, XXf, XXg and XXh) described to date. As expected, our XXi1 isolate formed an independent cluster in the topology of the generated tree.
FIGURE 1.
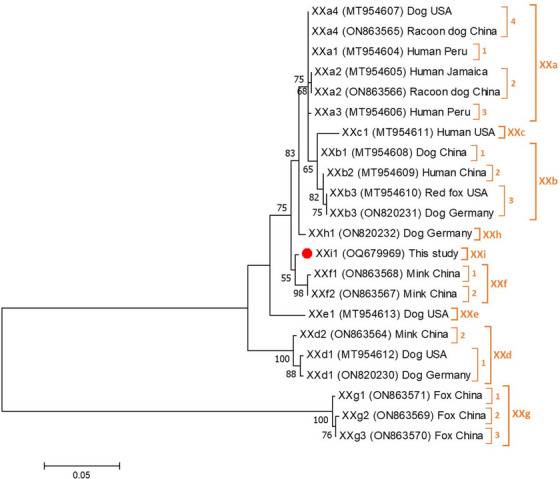
Phylogenetic relationship among nine Cryptosporidium canis subtype families (XXa–XXh) revealed by a maximum likelihood analysis of the partial gp60 gene. Substitution rates were calculated by using the general time reversible model. Numbers on branches are percent bootstrapping values over 50% using 1000 replicates. The filled red circle indicates the nucleotide sequence of the novel subtype XXi1 generated in the present study.
3.5. Molecular identification and characterisation of Strongyloides sp., Blastocystis sp. and E. bieneusi
The two canine isolates positive to Strongyloides sp. by conventional microscopy examination were confirmed by qPCR. Obtained qPCR CT values were 28 and 32.
To maximise the generated material, all faecal DNA samples available from canine (n = 82) and feline (n = 4) origin were re‐assessed for the presence of the Stramenopile Blastocystis sp. and the Microsporidia E. bieneusi. None of these two protist species were identified at microscopy examination. All analysed samples were tested negative for Blastocystis sp., but a dog isolate were tested positive for E. bieneusi. Nucleotide sequence analysis revealed the presence of canine‐adapted genotype PtEb IX. This sequence showed 100% identity with reference sequence AF059610 (Table 4).
3.6. Risk association analysis
Table 5 summarises the results of the statistical analyses conducted to demonstrate potential associations between individual enteric parasites and the epidemiological variables considered in the study. To increase statistical power canine and feline populations were analysed in combination.
Dogs and cats younger than 4 months of age were at higher risk of infection by any enteric parasite (P < 0.001) including Cystoisospora sp. (P = 0.025), G. duodenalis (P < 0.001) and Toxocara sp. (P = 0.009). Joyeuxiella sp. was more likely to infect cats in the age group of 12−24 months (P = 0.008). Sex was not identified as a risk factor for infections by enteric parasites.
Infections by enteric parasites were significantly higher in the winter months (P < 0.001), this being particularly true for G. duodenalis (P = 0.013). Hookworms infections were only detected during the autumn months (P = 0.044).
Dogs and cats receiving antiparasitic treatment the 3 months before sampling were significantly less infected by Toxocara sp. (P < 0.001) and hookworms (P = 0.017) than their untreated counterparts.
Dogs and cats infected by any enteric parasite were more likely to develop gastrointestinal manifestations (P = 0.002), being Cystoisospora sp. (P = 0.007) and G. duodenalis (P < 0.001) the major contributors to the occurrence of symptoms. Dogs and cats infected with G. duodenalis (P = 0.034) and Strongyloides sp. (P < 0.001) were significantly more prone to develop respiratory symptoms.
Soft faecal samples were significantly associated with infections by any enteric parasite (P = 0.006), particularly Cystoisospora sp. (P = 0.005) and G. duodenalis (P < 0.001).
4. DISCUSSION
Spain is the fifth largest pet market in Europe, behind the United Kingdom, France, Germany and Italy with 28 million registered pets, among them 6.7 million dogs and 3.8 million cats (International Trade Administration). Four out of 10 Spanish households have pets (ANFAAC, 2021). Given these large number of pet dogs and cats and the proximity and bond of these animals with their owners, understanding and preventing the zoonotic diseases that these companions bring with them are of paramount importance (Baneth et al., 2016; Esch & Petersen, 2013; Overgaauw et al., 2020). Under this approach, we present here novel data on the occurrence, risk factors and genetic variability of intestinal parasites in a large veterinary‐visiting pet population in Spain, because few studies have been conducted in animals attending clinical settings in this country (Causapé et al., 1996).
Using microscopy as screening method, we detected G. duodenalis cysts in 40.9% and 14.3% canine and feline faecal samples, respectively. Most of these infections occurred in pups and kittens with clinical manifestations, indicating that young animals are more vulnerable to this pathogen. Our results agree with previous studies showing that younger animals are prone to infections with intestinal parasites including G. duodenalis, Cystoisospora sp. and Toxocara sp. that mainly affect animals under 1 year of age (Barutzki & Schaper, 2013) and are more prevalent in breeding kennels than among household animals (Gothe & Reichler, 1990). Lower microscopy‐based prevalence rates of 1−16% have been previously reported in all‐age dogs from different populations and geographical areas in Spain (Causapé et al., 1996; Dado et al., 2012b; Marbella et al., 2022; Martínez‐Carrasco et al., 2007; Martínez‐Moreno et al., 2007; Miró et al., 2007; Regidor‐Cerrillo et al., 2020). Giardia duodenalis infection rates of 4.2% and 5% were found in sheltered and free‐roaming cats, respectively, in central Spain (Dado et al., 2012b; Montoya et al., 2018). Using more sensitive detection methods, a composite prevalence rate of 35.4% was obtained by direct immunofluorescence assay (DFA) in different dog populations in eastern Spain (Sanchez‐Thevenet et al., 2019), whereas prevalence rates of 29−33 and 6−9% have been reported by PCR in sheltered or owned dogs and cats in the northern part of the country (de Lucio et al., 2017; Gil et al., 2017).
In relation to clinical manifestations, respiratory signs are common in puppies and kittens infected with heavy burdens of migrating larvae of STHs including Strongyloides stercoralis and Toxocara sp. (Díez Baños et al., 2001; Schnyder et al., 2022). In the present study, respiratory signs have also been associated with G. duodenalis infections.
This study showed that ICT performed equally well than conventional microscopy for the detection of G. duodenalis cysts in faecal samples (Kappa coefficient, κ = 0.963). Because ICT is relatively cheap, easy to use and provides diagnostic results in minutes, this assay is increasingly used in veterinary clinical practice to minimise the high amount of labour work required in microscopy testing. Using ICT, G. duodenalis has been identified in 25% of faecal samples from dogs and 5−15% from cats (Epe et al., 2010; Montoya et al., 2018) in previous Spanish surveys. Of note, 13 faecal samples that tested positive for G. duodenalis by microscopy and/or ICT yielded a negative result by qPCR. Several reasons can explain this discrepancy, including inefficient removal of PCR inhibitors during the DNA extraction and purification process or suboptimal amount of quality template DNA.
Giardia duodenalis is the only Giardia species known to naturally infect dogs and cats. It comprises eight distinct genotypes or assemblages (A‐H) with marked differences in host specificity and range (Ryan et al., 2021). Assemblages A and B have the broadest host range and are therefore regarded as zoonotic (Cai et al., 2021). Assemblages C and D are mainly found in canine animals, and assemblage E in wild and domestic ungulates, whereas assemblages F, G and H have been mostly reported in felids, rodents and seals, respectively (Ryan et al., 2021). Sporadic reports of zoonotic assemblages A and B in dogs and cats have raised concerns about the actual role of these companion animals as sources of human giardiasis (Ballweber et al., 2010). Despite the fact that studies investigating simultaneously the presence and genetic variants on the parasite in human and animal populations sharing household have not demonstrated the occurrence of zoonotic transmission events (de Lucio et al., 2017; Lucio‐Forster et al., 2010; Rehbein et al., 2019), this possibility cannot be overlooked. Indeed, previous molecular‐based studies conducted in Spain have found zoonotic assemblages A and B at similar or higher proportions than host‐adapted assemblages C, D and F in unowned dogs and cats in northern (Gil et al., 2017) and eastern (Adell‐Aledón et al., 2018) Spain. Whether this unexpected high proportion of assemblages A and B in dogs and cats is the result of natural spreading or infections of anthropic nature (e.g. via contamination of water with human faecal material) is something that should be addressed and elucidated in further investigations. In the present study, only 15.5% (11/71) of the Giardia‐positive samples by qPCR were successfully genotyped at any of the four (ssu rRNA, gdh, bg and tpi) loci used for subtyping purposes. This is most likely due to the fact that the gdh, bg and tpi markers are single‐copy genes with limited diagnostic sensitivities, making them unsuitable for amplifying samples with little amount of parasitic DNA. This result was expected taking into consideration that most (62%, 44/71) of the Giardia‐positive samples tested yielded CT values ≥30 at qPCR.
We detected the presence of Cryptosporidium sp. antigens by ICT in 2.0% (5/252) of dogs, but none of the 35 cats investigated tested positive by this method. Since apicomplexan (including Cryptosporidium sp.) parasites were not specifically searched at microscopy examination, no diagnostic performance agreement between this method and ICT was conducted. Previous studies carried out in Spain have estimated the prevalence of the parasite in dogs at 7.4% by conventional microscopy (Causapé et al., 1996) and at 6.8% by direct immunofluorescence (Sanchez‐Thevenet et al., 2019). These surveys did not assess the occurrence of Cryptosporidium sp. in feline populations. Using PCR, canine and feline cryptosporidiosis have been estimated in the range of 4−9% (de Lucio et al., 2017; Gil et al., 2017).
The genus Cryptosporidium comprises at least 44 recognised species and more than 120 genotypes of uncertain taxonomic status. Nineteen species (mainly C. hominis, C. parvum and C. meleagridis, and, to a lesser extent, C. canis and C. felis) have been reported in humans (Ryan et al., 2021). Molecular data on the genetic diversity of Cryptosporidium infections in Spanish canine and feline populations are scarce. Host‐adapted C. canis and C. felis were the species primarily found in dogs and cats, respectively (de Lucio et al., 2017; Gil et al., 2017). However, a sheltered dog has been identified carrying C. hominis (Gil et al., 2017), a species formerly thought to be human‐specific (Widmer et al., 2020). None of the Cryptosporidium isolates of canine or feline origin identified in those studies could be subtyped at the gp60 locus. This trend was also observed in our study, where C. canis was predominantly found. Using a specific gp60 genotyping tool (Jiang et al., 2021), we managed to amplify one of the four C. canis isolates and identify it as XXi1, the first member of novel subtype family XXi. To date, nine (XXa to XXh) subtype families of C. canis have been recognised in a variety of animal hosts including dogs, foxes, minks and racoon dogs, in addition to humans (Elmahallawy et al., 2023; Jiang et al., 2021; Murnik et al., 2022; Wang et al., 2022). The fifth canine isolate was identified as the bovine genotype of C. parvum (unknown subtype family), a zoonotic genetic variant whose primary host species are cattle and humans (Guo et al., 2022). Taken together, these findings suggest that dogs can carry Cryptosporidium species (C. parvum and C. canis) that might represent a public health concern for vulnerable populations such as children and immunocompromised individuals.
The Stramenopile Blastocystis sp. was not identified by PCR in any of the 106 canine and seven feline DNA samples available for molecular testing, corroborating previous data about the rare occurrence of Blastocystis sp. in various carnivore species (Calero‐Bernal et al., 2020; Paulos et al., 2018). However, the Microsporidia E. bieneusi was found in a single canine isolate. Nucleotide sequence analysis confirmed the presence of canine‐adapted genotype PtEb IX, a genetic variant that has not been described in humans and therefore considered of limited or no zoonotic potential (Li et al., 2019). It should be highlighted that the potential role of pet dogs and cats as sources of human microsporidiosis by E. bieneusi should not be overlooked, as both host species have been shown to carry zoonotic genotypes (e.g. BEB6, D and Peru11) of the protist in Spain (Dashti et al., 2019).
The apicomplexan Cystoisospora sp. (formerly known as Isospora sp.) was identified by conventional microscopy in 7.1% of dogs. This figure is well in the range of those (1−10%) typically found in other Spanish canine populations (Causapé et al., 1996; Gracenea et al., 2009; Martínez‐Carrasco et al., 2007; Martínez‐Moreno et al., 2007; Miró et al., 2007). Of the four Cystoisospora species known to infect dogs (C. canis, C. ohioensis, C. burrowsi and C. neorivolta), C. canis is morphologically distinctive because of its large‐sized oocysts, whereas the latter three are grouped as C. ohioensis‐like because their oocysts overlap in size (Dubey & Lindsay, 2019). We found oocysts of Cystoisospora sp. in 2.9% of feline faecal samples, similar to previous studies in free‐rooming cats (2.5%) in central Spain (Montoya et al., 2018). Of note, cats are infected by two Cystoisospora species, namely C. felis and C. rivolta (Dubey, 2018). Cystoisospora felis (9.3%) and Cystoisospora rivolta (1.7%) have been previously identified in feral cats in Canary Island, Spain (Marbella et al., 2022). Because dogs and cats harbour canine‐ and feline‐adapted Cystoisospora species, their role as potential source of human infections (primarily infected by C. belli) is considered negligible.
Dogs and cats are known to be suitable hosts for a range of zoonotic STHs of public veterinary health relevance, including members of the genera Ancylostoma, Strongyloides and Toxocara (Gorgani‐Firouzjaee et al., 2022; Ketzis & Lucio‐Forster, 2020; Traub et al., 2021). Among them, Toxocara infections were predominant both in our canine (5.2%) and feline (20.0%) populations. These figures were in agreement with those (dogs: 6−33%; cats: 11−35%) documented in previous epidemiological studies conducted in Spain (Conde Garcia et al., 1989; Marbella et al., 2022; Martínez‐Carrasco et al., 2007; Martínez‐Moreno et al., 2007; Millán and Casanova, 2009; Miró et al., 2007, 2004; Montoya et al., 2018; Regidor‐Cerrillo et al., 2020; Rodríguez‐Ponce et al., 2016; Sánchez‐Thevenet et al., 2019). On the other hand, infections by T. leonina were less common (<3%), and, in the case of hookworms, rare in dogs and absent in cats. Importantly, Toxocara‐infected puppies or kittens shed large number of eggs with their faeces that contaminate the environment where they undergo development for 2−4 weeks to reach the infective stage (embryonated eggs). Human infections are predominantly acquired from ingestion of embryonated eggs by geophagia in sandpits, parks or playgrounds where cats and dogs have defecated (Dado et al., 2012a; Köchle et al., 2022). Taken together, these findings highlight that (i) our canine and feline populations harboured STH infections (mainly by T. canis and T. cati) that represent a public health concern, (ii) hygiene practices and regular deworming are crucial to reducing the burden of STH infections and their zoonotic impact and (iii) pet owners must be educated raising the awareness on the relevance of proper waste disposal to prevent environmental contamination. In this regard, it should be noted that STH eggs can rapidly develop into infective forms (e.g. larva 3 in hookworms) under adequate humidity and temperature levels. Precipitations contribute to improve soil moisture conditions, allowing higher survival rates of these infective forms, which, in the case of hookworms, are highly sensitive to desiccation. Rainfall can also extract Giardia cysts and Cryptosporidium oocysts from soil and grass. Taken together, frequent precipitation events during autumn and winter could potentially explain the superior infection rates by G. duodenalis observed during these months in our surveyed pet population (Short et al., 2017; Weaver et al., 2010).
Strongyloides sp. (presumably S. stercoralis) was identified in two dogs (0.8%) by microscopy. These infections were confirmed by qPCR, but lack of sequencing data precluded us to unambiguously confirm the species involved. Similar low prevalence rates have been reported in other Spanish canine populations (Sánchez‐Thevenet et al., 2019). Strongyloides sp. was not identified in any of the faeces of feline origin. However, cats are primarily infected by feline‐adapted Strongyloides species including S. felis, S. tumefaciens and S. planiceps (Thamsborg et al., 2017), suggesting that they play a negligible role as a source of human strongyloidiasis.
Regarding cestode infections, D. caninum was identified in 1.6% of dogs and 5.7% of cats. These rates were similar to those reported in other Spanish epidemiological studies in canine (1−13%) and feline (1−65%) populations (Calvete et al., 1998; Martínez‐Carrasco et al., 2007; Martínez‐Moreno et al., 2007; Miró et al., 2007, 2004). No members of the family Taeniidae (including Echinococcus granulosus) were identified. Finally, Joyeuxiella sp. was the most prevalent enteric parasite (20%, together with T. cati) found in cats. Of note, J. pasqualei has been identified 55% of stray cats in north‐east Spain (Calvete et al., 1998) and in 76% of feral cats in Majorca Island, Spain (Millán and Casanova, 2009).
This study has some limitations that must be taken into consideration when interpreting the results obtained and the conclusions reached. Firstly, conventional microscopy was used as a primary screening method. Because this technique has limited diagnostic sensitivity, it is likely that some of our prevalence data are underestimated. We attempted to minimise this drawback by analysing three consecutive samples from each investigated dog/cat. Secondly, and due to practical reasons, we did not conduct specific staining methods for apicomplexan parasites. This fact precluded us to comparatively assess the diagnostic performance of microscopy versus ICT for the detection of Cryptosporidium sp. Thirdly, we only managed to generate genotyping data for a relatively modest number of G. duodenalis and Cryptosporidium sp. isolates. It is likely that rare or underrepresented genetic variants of these pathogens have been missed. And fourth, obtained results were restricted to relatively small canine and feline population from northern Madrid and might not be representative of the whole epidemiological scenario in this and other regions of the country.
5. CONCLUSIONS
We provided a thorough account of the occurrence, risk factors and molecular diversity (for G. duodenalis, Cryptosporidium sp. and E. bieneusi) of enteric parasites in well‐cared pet dogs and cats in a wealthy area in central Spain. Our results show that one in two dogs and cats was infected by at least one species of protozoan, nematode or cestode parasites. Pups and kittens were at higher risk of infection. Using molecular (PCR and Sanger sequencing) tools, we found that dogs and cats were primarily infected by host‐adapted genetic variants of diarrhoea‐causing enteric protozoa, but they were also capable of carrying zoonotic strains including G. duodenalis assemblage A and C. parvum. We described a novel C. canis subtype family named XXi and provided diagnostic evidence demonstrating that ICT performed equally well than conventional microscopy for the detection of G. duodenalis, representing a cost‐effective option for the rapid detection of this pathogen. Dogs and cats were also frequently infected by STH of public health relevance including T. canis, T. cati, hookworms and, to a much lesser extent, Strongyloides sp. Taken together these results indicate that (i) adequate hygiene practices and routine veterinary care are essential to prevent enteric parasite (particularly helminthic) infections and minimise the risk of zoonotic transmission, (ii) vulnerable population (children, the elderly, immunocompromised individuals) should avoid contact with pups/kittens with diarrhoea, (iii) untreated, infected dogs might be major contributors to the environmental contamination with STH eggs and (iv) veterinarian have the duty to notify pet owners about parasitological findings and educate them on their zoonotic potential and public veterinary health implications. Adequate canine and feline waste disposal is important to reduce the spreading of these infections.
AUTHOR CONTRIBUTIONS
Marta Mateo: Conceptualisation; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualisation; Writing—original draft; Writing—review & editing. Ana Montoya: Data curation; Formal analysis; Writing—original draft; Writing—review & editing. Begoña Bailo: Methodology. Alejandro Dashti: Data curation; Investigation; Methodology; Writing—review & editing. Carolina Hernández‐Castro: Investigation; Methodology; Writing—review & editing. Pablo Matas: Investigation; Methodology; Writing—review & editing. Lihua Xiao: Data curation; Writing—review & editing. David Carmena: Formal analysis; Investigation; Methodology; Supervision; Validation; Writing—original draft; Writing—review & editing
CONFLICT OF INTEREST STATEMENT
The authors have declared no conflict of interest.
FUNDING INFORMATION
This study was partially funded by the Health Institute Carlos III (ISCIII), Spanish Ministry of Economy and Competitiveness under project PI19CIII/00029, and by Alfonso X el Sabio Foundation under proyect 1.011.019 (Grant / Award Number:).
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page have been adhered to. No ethical approval was required as this study did not cause any pain or discomfort to the animals because they were not directly or indirectly handled.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1002/vms3.1270.
Supporting information
TABLE S1. PCR cycling conditions used for the molecular identification and/or characterisation of the protist and helminthic pathogens of canine and feline origin investigated in this study.
TABLE S2. Oligonucleotides used for the molecular identification and/or characterisation of the protist and helminthic pathogens of canine and feline origin investigated in this study.
TABLE S3. Full dataset summarising the main epidemiological, clinical, diagnostic and genotyping results generated in the canine (n = 252) and feline (n = 35) populations investigated in the region of Madrid (Spain).
ACKNOWLEDGEMENTS
AD is the recipient of a predoctoral PFIS contract (FI20CIII/00002) funded by the Spanish Ministry of Science and Innovation and Universities. CHC is the recipient of a predoctoral fellowship funded by the Fundación Carolina (Spain) and the University of Antioquia, Medellín (Colombia). The authors are grateful to all the veterinarians of the Virgen of Iciar clinic for their collaboration and technical assistance throughout the whole study period.
Mateo, M. , Montoya, A. , Bailo, B. , Köster, P. C. , Dashti, A. , Hernández‐Castro, C. , Saugar, J. M. , Matas, P. , Xiao, L. , & Carmena, D. (2023). Prevalence and public health relevance of enteric parasites in domestic dogs and cats in the region of Madrid (Spain) with an emphasis on Giardia duodenalis and Cryptosporidium sp.. Veterinary Medicine and Science, 9, 2542–2558. 10.1002/vms3.1270
Contributor Information
Marta Mateo, Email: mmateo14@ucm.es.
David Carmena, Email: dacarmena@isciii.es.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available within the main body of the manuscript.
REFERENCES
- Adell‐Aledón, M. , Köster, P. C. , de Lucio, A. , Puente, P. , Hernández‐de‐Mingo, M. , Sánchez‐Thevenet, P. , Dea‐Ayuela, M. A. , & Carmena, D. (2018). Occurrence and molecular epidemiology of Giardia duodenalis infection in dog populations in eastern Spain. BMC Veterinary Research, 14, 26. https://doi.org10.1186/s12917‐018‐1353‐z [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANFAAC, Asociación Nacional de Fabricantes de Alimentos para Animales de Compañía . (2021). https://www.anfaac.org/anfaac/documentos/material‐educativo_18_1_ap.html
- Appelbee, A. J. , Frederick, L. M. , Heitman, T. L. , & Olson, M. E. (2003). Prevalence and genotyping of Giardia duodenalis from beef calves in Alberta, Canada. Veterinary Parasitology, 112, 289–294. 10.1016/s0304-4017(02)00422-3 [DOI] [PubMed] [Google Scholar]
- Arhant‐Sudhir, K. , Arhant‐Sudhir, R. , & Sudhir, K. (2011). Pet ownership and cardiovascular risk reduction: Supporting evidence, conflicting data and underlying mechanisms. Clinical and Experimental Pharmacology and Physiology, 38, 734–738. 10.1111/j.1440-1681.2011.05583.x [DOI] [PubMed] [Google Scholar]
- Bagster, A. , & Elsheikha, H. (2022). Perception of UK companion animal veterinarians on risk assessment based parasite control. Veterinary Parasitology: Regional Studies and Reports, 34, 100774. 10.1016/j.vprsr.2022.100774 [DOI] [PubMed] [Google Scholar]
- Baneth, G. , Thamsborg, S. M. , Otranto, D. , Guillot, J. , Blaga, R. , Deplazes, P. , & Solano‐Gallego, L. (2016). Major parasitic zoonoses associated with dogs and cats in Europe. Journal of Comparative Pathology, 155, S54–S74. 10.1016/j.jcpa.2015.10.179 [DOI] [PubMed] [Google Scholar]
- Ballweber, L. R. , Xiao, L. , Bowman, D. D. , Kahn, G. , & Cama, V. A. (2010). Giardiasis in dogs and cats: Update on epidemiology and public health significance. Trends in Parasitology, 26, 180–189. 10.1016/j.pt.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Barutzki, D. , & Schaper, R. (2013). Age‐dependant prevalence of endoparasites in young dogs and cats up to one year of age. Parasitology Research, 112, 119–131. 10.1007/s00436-013-3286-6 [DOI] [PubMed] [Google Scholar]
- Boldig, C. M. , & Butala, N. (2021). Pet therapy as a nonpharmacological treatment option for neurological disorders: A review of the literature. Cureus, 13, e16167. 10.7759/cureus.16167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholt, M. A. , Lee, J. H. , & Tzipori, S. (2002). Prevalence of Enterocytozoon bieneusi in swine: An 18‐month survey at a slaughterhouse in Massachusetts. Applied and Environmental Microbiology, 68, 2595–2599. 10.1128/AEM.68.5.2595-2599.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciò, S. M. , De Giacomo, M. , & Pozio, E. (2002). Sequence analysis of the beta‐giardin gene and development of a polymerase chain reaction‐restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. International Journal for Parasitology, 32, 1023–1030. 10.1016/s0020-7519(02)00068-1 [DOI] [PubMed] [Google Scholar]
- Cai, W. , Ryan, U. , Xiao, L. , & Feng, Y. (2021). Zoonotic giardiasis: An update. Parasitology Research, 120, 4199–4218. 10.1007/s00436-021-07325-2 [DOI] [PubMed] [Google Scholar]
- Calero‐Bernal, R. , Santín, M. , Maloney, J. G. , Martín‐Pérez, M. , Habela, M. A. , Fernández‐García, J. L. , Figueiredo, A. , Nájera, F. , Palacios, M. J. , Mateo, M. , Balseiro, A. , Barral, M. , Lima‐Barbero, J. F. , Köster, P. C. , & Carmena, D. (2020). Blastocystis sp. subtype diversity in wild carnivore species from Spain. Journal of Eukaryotic Microbiology, 67, 273–278. 10.1111/jeu.12772 [DOI] [PubMed] [Google Scholar]
- Calvete, C. , Lucientes, J. , Castillo, J. A. , Estrada, R. , Gracia, M. J. , Peribáñez, M. A. , & Ferrer, M. (1998). Gastrointestinal helminth parasites in stray cats from the mid‐Ebro Valley, Spain. Veterinary Parasitology, 75, 235–240. 10.1016/s0304-4017(97)00182-9 [DOI] [PubMed] [Google Scholar]
- Causapé, A. C. , Quílez, J. , Sánchez‐Acedo, C. , & del Cacho, E. (1996). Prevalence of intestinal parasites, including Cryptosporidium parvum, in dogs in Zaragoza city, Spain. Veterinary Parasitology, 67, 161–167. 10.1016/s0304-4017(96)01033-3 [DOI] [PubMed] [Google Scholar]
- Conde Garcia, L. , Muro Alvarez, A. , & Simon Martin, F. (1989). Epidemiological studies on toxocariasis and visceral larva migrans in a zone of western Spain. Annals of Tropical Medicine and Parasitology, 83, 615–620. 10.1080/00034983.1989.11812395 [DOI] [PubMed] [Google Scholar]
- Cutt, H. , Giles‐Corti, B. , Knuiman, M. , & Burke, V. (2007). Dog ownership, health and physical activity: A critical review of the literature. Health Place, 13, 261–272. 10.1016/j.healthplace.2006.01.003 [DOI] [PubMed] [Google Scholar]
- Dado, D. , Izquierdo, F. , Vera, O. , Montoya, A. , Mateo, M. , Fenoy, S. , Galván, A. L. , García, S. , García, A. , Aránguez, E. , López, L. , del Águila, C. , & Miró, G. (2012a). Detection of zoonotic intestinal parasites in public parks of Spain. Potential epidemiological role of microsporidia. Zoonoses Public Health, 59, 23–28. 10.1111/j.1863-2378.2011.01411.x [DOI] [PubMed] [Google Scholar]
- Dado, D. , Montoya, A. , Blanco, M. A. , Miró, G. , Saugar, J. M. , Bailo, B. , & Fuentes, I. (2012b). Prevalence and genotypes of Giardia duodenalis from dogs in Spain: possible zoonotic transmission and public health importance. Parasitology Research, 111, 2419–2422. 10.1007/s00436-012-3100-x [DOI] [PubMed] [Google Scholar]
- Dashti, A. , Santín, M. , Cano, L. , de Lucio, A. , Bailo, B. , de Mingo, M. H. , Köster, P. C. , Fernández‐Basterra, J. A. , Aramburu‐Aguirre, J. , López‐Molina, N. , Fernández‐Crespo, J. C. , Calero‐Bernal, R. , & Carmena, D. (2019). Occurrence and genetic diversity of Enterocytozoon bieneusi (Microsporidia) in owned and sheltered dogs and cats in Northern Spain. Parasitology Research, 118, 2979–2987. 10.1007/s00436-019-06428-1 [DOI] [PubMed] [Google Scholar]
- de Lucio, A. , Bailo, B. , Aguilera, M. , Cardona, G. A. , Fernández‐Crespo, J. C. , & Carmena, D. (2017). No molecular epidemiological evidence supporting household transmission of zoonotic Giardia duodenalis and Cryptosporidium spp. from pet dogs and cats in the province of Álava, Northern Spain. Acta Tropica, 170, 48–56. 10.1016/j.actatropica.2017.02.024 [DOI] [PubMed] [Google Scholar]
- De Rivas, D. (1928). An efficient and rapid method of concentration for the detection of ova and cysts of intestinal parasites. American Journal of Tropical Medicine, 8, 63–72. 10.4269/ajtmh.1928.s1-8.63 [DOI] [Google Scholar]
- Díez Baños, P. , Díez Baños, N. , & Morrondo Pelayo, M. P. (2001). Estrongiloidosis. In del Campillo M. C. (Ed.), Parasitología Veterinaria (p. 648). McGraw‐Hill. [Google Scholar]
- Dubey, J. P. (2018). A review of Cystoisospora felis and C. rivolta‐induced coccidiosis in cats. Veterinary Parasitology, 263, 34–48. 10.1016/j.vetpar.2018.09.016 [DOI] [PubMed] [Google Scholar]
- Dubey, J. P. , & Lindsay, D. S. (2019). Coccidiosis in dogs‐100 years of progress. Veterinary Parasitology, 266, 34–55. 10.1016/j.vetpar.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Elmahallawy, E. K. , Gareh, A. , Abu‐Okail, A. , Köster, P. C. , Dashti, A. , Asseri, J. , Gouda, A. A. , Mubaraki, M. A. , Mohamed, S. A.‐A. , Mohamed, Y. M. , Hassan, E. A. , Elgendy, M. , Hernández‐Castro, C. , Bailo, B. , González‐Barrio, D. , Xiao, L. , & Carmena, D. (2023). Molecular characteristics and zoonotic potential of enteric protists in domestic dogs and cats in Egypt. Frontiers in Veterinary Science, 10, 1229151. 10.3389/fvets.2023.1229151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epe, C. , Rehkter, G. , Schnieder, T. , Lorentzen, L. , & Kreienbrock, L. (2010). Giardia in symptomatic dogs and cats in Europe—Results of a European study. Veterinary Parasitology, 173, 32–38. 10.1016/j.vetpar.2010.06.015 [DOI] [PubMed] [Google Scholar]
- ESCCAP (European Specialist Counsel Companion Animal Parasites) . (2018). Control of intestinal protozoa in dogs and cats. ESCCAP Guideline 06 Second Edition. https://www.esccap.org/guidelines/gl6/
- ESCCAP (European Specialist Counsel Companion Animal Parasites) . (2021). Worm control in dogs and cats. ESCCAP Guideline 01 Sixth Edition. https://www.esccap.org/guidelines/gl1/
- ESCCAP (European Specialist Counsel Companion Animal Parasites) . (2022). Parasitological Diagnosis in Cats, Dogs and Equines. ESCCAP Guideline 04 First Edition. https://www.esccap.org/guidelines/gl4/
- Esch, K. J. , & Petersen, C. A. (2013). Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clinical Microbiology Reviews, 26, 58–85. 10.1128/CMR.00067-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltus, D. C. , Giddings, C. W. , Schneck, B. L. , Monson, T. , Warshauer, D. , & McEvoy, J. M. (2006). Evidence supporting zoonotic transmission of Cryptosporidium spp. in Wisconsin. Journal Clinical Microbiology, 44, 4303–4308. 10.1128/JCM.01067-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, E. , & Krause‐Parello, C. A. (2018). Companion animals and human health: Benefits, challenges, and the road ahead for human‐animal interaction. Revue Scientifique et Technique, 37, 71–82. 10.20506/rst.37.1.2741 [DOI] [PubMed] [Google Scholar]
- Gil, H. , Cano, L. , de Lucio, A. , Bailo, B. , de Mingo, M. H. , Cardona, G. A. , Fernández‐Basterra, J. A. , Aramburu‐Aguirre, J. , López‐Molina, N. , & Carmena, D. (2017). Detection and molecular diversity of Giardia duodenalis and Cryptosporidium spp. in sheltered dogs and cats in Northern Spain. Infection, Genetics and Evolution, 50, 62–69. 10.1016/j.meegid.2017.02.013 [DOI] [PubMed] [Google Scholar]
- Gorgani‐Firouzjaee, T. , Kalantari, N. , Chehrazi, M. , Ghaffari, S. , & Shahdin, S. (2022). Global prevalence of Strongyloides stercoralis in dogs: A systematic review and meta‐analysis. Journal of Helminthology, 96, e11. 10.1017/S0022149x21000808 [DOI] [PubMed] [Google Scholar]
- Gothe, R. , & Reichler, I. (1990). Zur befallshüfigkeit von kokzidien bei hundefamilien unterschiedlicher haltung und rassen in Süddeutschland [The frequency of coccidial infection in dog families of different husbandry and breeds in south Germany]. Tierärztliche Praxis, 18, 407–413. German. [PubMed] [Google Scholar]
- Gracenea, M. , Gómez, M. S. , & Torres, J. (2009). Prevalence of intestinal parasites in shelter dogs and cats in the metropolitan area of Barcelona (Spain). Acta Parasitologica, 54, 73–77. 10.2478/s11686-009-0005-7 [DOI] [Google Scholar]
- Guo, Y. , Ryan, U. , Feng, Y. , & Xiao, L. (2022). Emergence of zoonotic Cryptosporidium parvum in China. Trends in Parasitology, 38, 335–343. 10.1016/j.pt.2021.12.002 [DOI] [PubMed] [Google Scholar]
- Hopkins, R. M. , Meloni, B. P. , Groth, D. M. , Wetherall, J. D. , Reynoldson, J. A. , & Thompson, R. C. (1997). Ribosomal RNA sequencing reveals differences between the genotypes of Giardia isolates recovered from humans and dogs living in the same locality. Journal of Parasitology, 83, 44–51. [PubMed] [Google Scholar]
- Instituto Nacional de Estadística . (2020). https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736177088&menu=ultiDatos&idp=1254735976608
- Instituto Nacional de Estadística . (2022). https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176951&menu=ultiDatos&idp=1254735572981
- International Trade Administration . https://www.trade.gov/market‐intelligence/spain‐pet‐sector
- Jiang, W. , Roellig, D. M. , Guo, Y. , Li, N. , Feng, Y. , & Xiao, L. (2021). Development of a subtyping tool for zoonotic pathogen Cryptosporidium canis . Journal of Clinical Microbiology, 59, e02474–e024720. 10.1128/JCM.02474-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketzis, J. K. , & Lucio‐Forster, A. (2020). Toxocara canis and Toxocara cati in domestic dogs and cats in the United States, Mexico, Central America and the Caribbean: A review. Advances in Parasitology, 109, 655–714. 10.1016/bs.apar.2020.01.027 [DOI] [PubMed] [Google Scholar]
- Köchle, B. R. , Garijo‐Toledo, M. M. , Llobat, L. , & Sansano‐Maestre, J. (2022). Prevalence of Toxocara eggs in public parks in the City of Valencia (Eastern Spain). Veterinary Science, 9, 232. 10.3390/vetsci9050232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. , Li, M. , Knyaz, C. , & Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549. 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalle, M. , Pozio, E. , Capelli, G. , Bruschi, F. , Crotti, D. , & Cacciò, S. M. (2005). Genetic heterogeneity at the beta‐giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. International Journal of Parasitology, 35, 207–213. 10.1016/j.ijpara.2004.10.022 [DOI] [PubMed] [Google Scholar]
- Li, W. , Feng, Y. , & Santin, M. (2019). Host Specificity of Enterocytozoon bieneusi and public health implications. Trends in Parasitology, 35, 436–451. 10.1016/j.pt.2019.04.004 [DOI] [PubMed] [Google Scholar]
- Lucio‐Forster, A. , Griffiths, J. K. , Cama, V. A. , Xiao, L. , & Bowman, D. D. (2010). Minimal zoonotic risk of cryptosporidiosis from pet dogs and cats. Trends in Parasitology, 26, 174–179. 10.1016/j.pt.2010.01.004 [DOI] [PubMed] [Google Scholar]
- McNamara, J. , Drake, J. , Wiseman, S. , & Wright, I. (2018). Survey of European pet owners quantifying endoparasitic infection risk and implications for deworming recommendations. Parasite & Vectors, 11, 571. 10.1186/s13071-018-3149-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbella, D. , Santana‐Hernández, K. M. , & Rodríguez‐Ponce, E. (2022). Small islands as potential model ecosystems for parasitology: Climatic influence on parasites of feral cats. Journal of Helminthology, 96, e51. 10.1017/S0022149x22000451 [DOI] [PubMed] [Google Scholar]
- Martínez‐Carrasco, C. , Berriatua, E. , Garijo, M. , Martínez, J. , Alonso, F. D. , & de Ybáñez, R. R. (2007). Epidemiological study of non‐systemic parasitism in dogs in southeast Mediterranean Spain assessed by coprological and post‐mortem examination. Zoonoses and Public Health, 54, 195–203. 10.1111/j.1863-2378.2007.01047.x [DOI] [PubMed] [Google Scholar]
- Martínez‐Moreno, F. J. , Hernández, S. , López‐Cobos, E. , Becerra, C. , Acosta, I. , & Martínez‐Moreno, A. (2007). Estimation of canine intestinal parasites in Córdoba (Spain) and their risk to public health. Veterinary Parasitology, 143, 7–13. 10.1016/j.vetpar.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Meers, L. L. , Contalbrigo, L. , Samuels, W. E. , Duarte‐Gan, C. , Berckmans, D. , Laufer, S. J. , Stevens, V. A. , Walsh, E. A. , & Normando, S. (2022). Canine‐assisted interventions and the relevance of welfare assessments for human health, and transmission of zoonosis: A literature review. Frontiers in Veterinary Science, 9, 899889. 10.3389/fvets.2022.899889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengarda, A. C. , Silva, T. C. , Silva, A. S. , Roquini, D. B. , Fernandes, J. P. S. , & de Moraes, J. (2023). Toward anthelmintic drug candidates for toxocariasis: Challenges and recent developments. European Journal of Medicinal Chemistry, 251, 115268. 10.1016/j.ejmech.2023.115268 [DOI] [PubMed] [Google Scholar]
- Millán, J. , & Casanova, J. C. (2009). High prevalence of helminth parasites in feral cats in Majorca Island (Spain). Parasitology Research, 106, 183–188. 10.1007/s00436-009-1647-y [DOI] [PubMed] [Google Scholar]
- Miller, A. D. (2020). Pathology of larvae and adults in dogs and cats. Advances in Parasitology, 109, 537–544. 10.1016/bs.apar.2020.01.024 [DOI] [PubMed] [Google Scholar]
- Miró, G. , Gálvez, R. , Montoya, A. , Delgado, B. , & Drake, J. (2020). Survey of Spanish pet owners about endoparasite infection risk and deworming frequencies. Parasite & Vectors, 13, 101. 10.1186/s13071-020-3976-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miró, G. , Mateo, M. , Montoya, A. , Vela, E. , & Calonge, R. (2007). Survey of intestinal parasites in stray dogs in the Madrid area and comparison of the efficacy of three anthelmintics in naturally infected dogs. Parasitology Research, 100, 317–320. 10.1007/s00436-006-0258-0 [DOI] [PubMed] [Google Scholar]
- Miró, G. , Montoya, A. , Jiménez, S. , Frisuelos, C. , Mateo, M. , & Fuentes, I. (2004). Prevalence of antibodies to Toxoplasma gondii and intestinal parasites in stray, farm and household cats in Spain. Veterinary Parasitology, 126, 249–255. 10.1016/j.vetpar.2004.08.015 [DOI] [PubMed] [Google Scholar]
- Montoya, A. , García, M. , Gálvez, R. , Checa, R. , Marino, V. , Sarquis, J. , Barrera, J. P. , Rupérez, C. , Caballero, L. , Chicharro, C. , Cruz, I. , & Miró, G. (2018). Implications of zoonotic and vector‐borne parasites to free‐roaming cats in central Spain. Veterinary Parasitology, 251, 125–130. 10.1016/j.vetpar.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Morelli, S. , Colombo, M. , Traversa, D. , Iorio, R. , Paoletti, B. , Bartolini, R. , Barlaam, A. , & Di Cesare, A. (2022). Zoonotic intestinal helminthes diagnosed in a 6‐year period (2015‐2020) in privately owned dogs of sub‐urban and urban areas of Italy. Veterinary Parasitology: Regional Studies and Reports, 29, 100689. 10.1016/j.vprsr.2022.100689 [DOI] [PubMed] [Google Scholar]
- Murnik, L. C. , Daugschies, A. , & Delling, C. (2022). Cryptosporidium infection in young dogs from Germany. Parasitology Research, 121, 2985–2993. 10.1007/s00436-022-07632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaauw, P. A. M. , Vinke, C. M. , Hagen, M. A. E. V. , & Lipman, L. J. A. (2020). A One Health perspective on the human‐companion animal relationship with emphasis on zoonotic aspects. International Journal of Environmental Research and Public Health, 17, 3789. 10.3390/ijerph17113789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulos, S. , Köster, P. C. , de Lucio, A. , Hernández‐de‐Mingo, M. , Cardona, G. A. , Fernández‐Crespo, J. C. , Stensvold, C. R. , & Carmena, D. (2018). Occurrence and subtype distribution of Blastocystis sp. in humans, dogs and cats sharing household in northern Spain and assessment of zoonotic transmission risk. Zoonoses and Public Health, 65, 993–1002. 10.1111/zph.12522 [DOI] [PubMed] [Google Scholar]
- Raza, A. , Rand, J. , Qamar, A. G. , Jabbar, A. , & Kopp, S. (2018). Gastrointestinal parasites in shelter dogs: Occurrence, pathology, treatment and risk to shelter workers. Animals (Basel), 8, 108. 10.3390/ani8070108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, C. M. , Monis, P. T. , & Thompson, R. C. (2004). Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR‐RFLP. Infection, Genetics and Evolution, 4, 125–130. 10.1016/j.meegid.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Rehbein, S. , Klotz, C. , Ignatius, R. , Müller, E. , Aebischer, A. , & Kohn, B. (2019). Giardia duodenalis in small animals and their owners in Germany: A pilot study. Zoonoses and Public Health, 66, 117–124. 10.1111/zph.12541 [DOI] [PubMed] [Google Scholar]
- Regidor‐Cerrillo, J. , Arranz‐Solís, D. , Moreno‐Gonzalo, J. , Pedraza‐Díaz, S. , Gomez‐Bautista, M. , Ortega‐Mora, L. M. , & Collantes‐Fernandez, E. (2020). Prevalence of intestinal parasite infections in stray and farm dogs from Spain. Revista Brasileira de Parasitologia Veterinária, 29, e014920. 10.1590/S1984-29612020063 [DOI] [PubMed] [Google Scholar]
- RIAC (Registro de identificación de animales de compañía) . https://www.colvema.org/riac.asp
- Rodríguez‐Ponce, E. , González, J. F. , Conde de Felipe, M. , Hernández, J. N. , & Raduan Jaber, J. (2016). Epidemiological survey of zoonotic helminths in feral cats in Gran Canaria island (Macaronesian archipelago‐Spain). Acta Parasitologica, 61, 443–450. 10.1515/ap-2016-0059 [DOI] [PubMed] [Google Scholar]
- Ryan, U. M. , Feng, Y. , Fayer, R. , & Xiao, L. (2021). Taxonomy and molecular epidemiology of Cryptosporidium and Giardia—A 50 year perspective (1971‐2021). International Journal of Parasitology, 51, 1099–1119. 10.1016/.ijpara.2021.08.007 [DOI] [PubMed] [Google Scholar]
- Schnyder, M. , Reichler, I. M. , Eichenberger, R. M. , Hofer‐Inteeworn, N. , Kümmerle‐Fraune, C. , & Grimm, F. (2022). Strongyloides stercoralis in Swiss dogs—A retrospective study suggests an increasing occurrence of this potentially zoonotic parasite as a consequence of dog imports. Schweizer Archiv für Tierheilkunde, 164, 89–104. 10.17236/sat00340 [DOI] [PubMed] [Google Scholar]
- Sanchez‐Thevenet, P. , Carmena, D. , Adell‐Aledón, M. , Dacal, E. , Arias, E. , Saugar, J. M. , Rodríguez, E. , & Dea‐Ayuela, M. A. (2019). High prevalence and diversity of zoonotic and other intestinal parasites in dogs from eastern Spain. Vector Borne and Zoonotic Diseases, 19, 915–922. 10.1089/vbz.2019.2468 [DOI] [PubMed] [Google Scholar]
- Saugar, J. M. , Merino, F. J. , Martín‐Rabadán, P. , Fernández‐Soto, P. , Ortega, S. , Gárate, T. , & Rodríguez, E. (2015). Application of real‐time PCR for the detection of Strongyloides spp. in clinical samples in a reference center in Spain. Acta Tropica, 142, 20–25. 10.1016/j.actatropica.2014.10.020 [DOI] [PubMed] [Google Scholar]
- Scicluna, S. M. , Tawari, B. , & Clark, C. G. (2006). DNA barcoding of Blastocystis . Protist, 157, 77–85. 10.1016/j.protis.2005.12.001 [DOI] [PubMed] [Google Scholar]
- Scorza, V. , & Tangtrongsup, S. (2010). Update on the diagnosis and management of Cryptosporidium spp. infections in dogs and cats. Topics in Companion Animal Medicine, 25, 163–169. 10.1053/j.tcam.2010.07.007 [DOI] [PubMed] [Google Scholar]
- Short, E. E. , Caminade, C. , & Thomas, B. N. (2017). Climate change contribution to the emergence or re‐emergence of parasitic diseases. Infectious Diseases, 10, 1178633617732296. 10.1177/1178633617732296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman, I. M. , Fayer, R. , Bern, C. , Gilman, R. H. , Trout, J. M. , Schantz, P. M. , Das, P. , Lal, A. A. , & Xiao, L. (2003). Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis . Emerging and Infectious Diseases, 9, 1444–1452. 10.3201/eid0911.030084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangtrongsup, S. , & Scorza, V. (2010). Update on the diagnosis and management of Giardia spp. infections in dogs and cats. Topics in Companion Animal Medicine, 25, 155–162. 10.1053/j.tcam.2010.07.003 [DOI] [PubMed] [Google Scholar]
- Thamsborg, S. M. , Ketzis, J. , Horii, Y. , & Matthews, J. B. (2017). Strongyloides spp. infections of veterinary importance. Parasitology, 144, 274–284. 10.1017/S0031182016001116 [DOI] [PubMed] [Google Scholar]
- Telemann, W. (1908). Eine methode zur erleichterung der auffindung von parasiteneiern in den faeces. Deutsche Medizinische Wochenschrift.
- Tiangtip, R. , & Jongwutiwes, S. (2002). Molecular analysis of Cryptosporidium species isolated from HIV‐infected patients in Thailand. Tropical Medicine and International Health, 7, 357–364. 10.1046/j.1365-3156.2002.00855.x [DOI] [PubMed] [Google Scholar]
- Traub, R. J. , Zendejas‐Heredia, P. A. , Massetti, L. , & Colella, V. (2021). Zoonotic hookworms of dogs and cats—Lessons from the past to inform current knowledge and future directions of research. International Journal for Parasitology, 51, 1233–1241. 10.1016/j.ijpara.2021.10.005 [DOI] [PubMed] [Google Scholar]
- Traversa, D. (2012). Pet roundworms and hookworms: A continuing need for global warming. Parasite & Vectors, 5, 91. 10.1186/1756-3305-5-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysnes, K. R. , Skancke, E. , & Robertson, L. J. (2014). Subclinical Giardia in dogs: A veterinary conundrum relevant to human infection. Trends in Parasitology, 30, 520–527. 10.1016/j.pt.2014.08.007 [DOI] [PubMed] [Google Scholar]
- Verweij, J. J. , Canales, M. , Polman, K. , Ziem, J. , Brienen, E. A. , Polderman, A. M. , & van Lieshout, L. (2009). Molecular diagnosis of Strongyloides stercoralis in faecal samples using real‐time PCR. Transactions of the Royal Society of Tropical Medicine and Hygiene, 103, 342–346. 10.1016/j.trstmh.2008.12.001 [DOI] [PubMed] [Google Scholar]
- Verweij, J. J. , Schinkel, J. , Laeijendecker, D. , van Rooyen, M. A. , van Lieshout, L. , & Polderman, A. M. (2003). Real‐time PCR for the detection of Giardia lamblia . Molecular and Cellular Probes, 17, 223–225. 10.1016/s0890-8508(03)00057-4 [DOI] [PubMed] [Google Scholar]
- Wang, W. , Wei, Y. , Cao, S. , Wu, W. , Zhao, W. , Guo, Y. , Xiao, L. , Feng, Y. , & Li, N. (2022). Divergent Cryptosporidium species and host‐adapted Cryptosporidium canis subtypes in farmed minks, raccoon dogs and foxes in Shandong, China. Frontiers in Cellular and Infection Microbiology, 12, 980917. 10.3389/fcimb.2022.980917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver, H. J. , Hawdon, J. M. , & Hoberg, E. P. (2010). Soil‐transmitted helminthiases: Implications of climate change and human behavior. Trends in Parasitology, 26, 574–581. 10.1016/j.pt.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Xiao, L. , & Feng, Y. (2017). Molecular epidemiologic tools for waterborne pathogens Cryptosporidium spp. and Giardia duodenalis . Food and Waterborne Parasitology, 8–9, 14–32. 10.1016/j.fawpar.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer, G. , Köster, P, C. , & Carmena, D. (2020). Cryptosporidium hominis infections in non‐human animal species: revisiting the concept of host specificity. International Journal for Parasitology, 50, 253–262. 10.1016/j.ijpara.2020.01.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. PCR cycling conditions used for the molecular identification and/or characterisation of the protist and helminthic pathogens of canine and feline origin investigated in this study.
TABLE S2. Oligonucleotides used for the molecular identification and/or characterisation of the protist and helminthic pathogens of canine and feline origin investigated in this study.
TABLE S3. Full dataset summarising the main epidemiological, clinical, diagnostic and genotyping results generated in the canine (n = 252) and feline (n = 35) populations investigated in the region of Madrid (Spain).
Data Availability Statement
The data that support the findings of this study are available within the main body of the manuscript.


