Abstract
ICU-acquired limb and respiratory muscle weakness is a common, serious ICU syndrome, increasing in frequency with prolonged ICU stay and sepsis. A systematic approach facilitates precise localization of the problem within central or peripheral nervous system. Most cases relate to critical illness polyneuropathy or myopathy or a combination of both (critical illness neuromyopathy). Within the latter entity, the relative contribution of neuropathy versus myopathy varies considerably among affected patients. Muscle enzyme testing, electromyography-nerve conduction and muscle biopsy are valuable investigative tests. Nerve biopsy is less commonly needed, but is useful when vascultis is suspected.
Keywords: ICU, myopathy, neuropathy, ventilator, weakness
The syndrome of severe, acute, intensive care unit (ICU)-acquired neuromuscular weakness poses a common and serious diagnostic, prognostic, and therefore management issue. It goes by various names, some of which presuppose a mechanism: acute necrotizing myopathy of intensive care, acute quadriplegic myopathy, critical care myopathy, critical illness myopathy (CIM), critical illness neuromuscular disease, critical illness neuromyopathy, critical illness polyneuromyopathy, critical illness polyneuropathy (CIP), ICU-acquired paresis, quadriplegic and areflexic ICU illness, rapidly evolving myopathy with myosin-deficient fibres and thick filament myopathy [1-6]. The problem affects at least 1.7% of children in paediatric ICUs, more than half of adult patients admitted to general ICUs for more than 1 week and more than 70% of those with sepsis and multiorgan failure [7-9]. Neuromuscular weakness typically becomes apparent when an attempt is made to wean the patient from the ventilator, although there are earlier clues, which include grimacing without movement with noxious stimuli before recovery of consciousness, relative lack of movement after regaining consciousness, and (not inevitably) loss of deep tendon reflexes that had been present earlier [10].
Precise diagnosis is vital for management. Although most cases will turn out to be critical illness polyneuromyopathy [11] – a term that embraces CIP, CIM, or a combination of the two – other potential causes should not be overlooked. A systematic approach is suggested in Fig. 1. The algorithm illustrates the early ruling in or out of spinal cord disease (e.g. in cases of trauma, coagulation disturbance, West Nile virus infection, acute disseminated encephalomyelitis, etc.), and then moving on to a clinical–biochemical–electromyographic assessment. A neuromuscular transmission defect (e.g. slow inactivation of neuromuscular blocking agents, unrecognized myasthenia gravis, or myasthenic [Lambert–Eaton] syndrome) is easily detected with repetitive nerve stimulation, revealing either a decremental or incremental response. Neuropathies other than CIP that may manifest after ICU admission include Guillain–Barré syndrome/acute immune-mediated demyelinating polyneuropathy (and its various subtypes), porphyria and recurrent chronic inflammatory demyelinating polyneuropathy. These are usually easily ruled in or out. Demyelinating inflammatory neuropathies usually cause slowing of conduction velocity and conduction block on electromyographic studies and produce increased protein in the cerebrospinal fluid. Biochemical screening for porphyria during acute attacks should be positive. Other conditions, such as inflammatory myopathies or unrecognized motor neurone disease, are not discussed here because they are usually diagnosed before admission to ICU, although they sometimes present with respiratory weakness that requires ICU admission.
Figure 1.
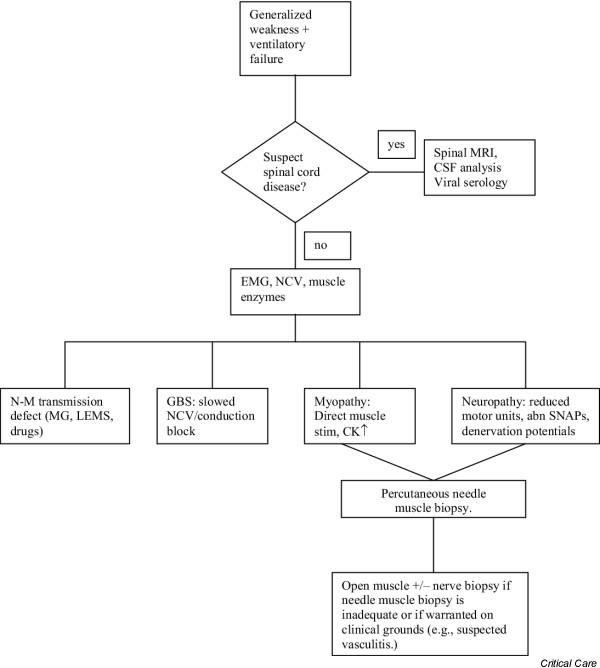
A flow chart giving an approach to generalized weakness and/or ventilatory failure in the intensive care unit. CK, creatine kinase; CSF, cerebrospinal fluid; GBS, Guillain–Barré syndrome; EMG, electromyography; MRI, magnetic resonance imaging; LEMS, Lambert–Eaton (myasthenic) syndrome; MG, myasthenia gravis; NCV, nerve conduction velocity studies; N-M, neuromuscular transmission; SNAPs, sensory nerve action potentials. Modified from Bolton and Young [16].
The last step, differentiating the most common causes of ICU-acquired generalized weakness (myopathy, neuropathy, or a combination of the two), is practical because their prognoses differ considerably. Some cases of myopathy appear to be merely 'membranopathies', with normal histology and rapid recovery. Presumably, the muscle membrane becomes dysfunctional, inexcitable and leaky, allowing myoglobin to leave the muscle. Some cases behave like disuse atrophy with selective type-2 fibre loss. Others, especially in those treated with corticosteroids and neuromuscular blocking agents, exhibit a relative loss of thick myosin filaments. Many cases show variable panfascicular necrosis, which can sometimes be widespread and severe. In general, the prognosis for recovery from myopathies is favourable, with most patients recovering fully within 1–3 months [6]. Patients with widespread muscle necrosis may recover incompletely, however. Severe CIP patients recover slowly because the axons regenerate at 1 mm/day. This takes many months and recovery is often incomplete, leaving patients with significant weakness, sensory loss, and absent reflexes distally in the lower limbs and variably more proximally. Patients with CIP who fail to show significant recovery by 4 weeks are often disabled with diminished quality of life [8]. Evidence of polyneuropathy is apparent after 5 years in over 90% of CIP patients [12]. The mildest residua may include reduced stamina for walking. Some older patients may fail to survive or wean from the ventilator because their recovery is so protracted and other complications ensue.
Electromyography is helpful in differentiating CIP from CIM but it has limitations [13]. Reduced or absent sensory nerve action potentials favours a neuropathy, but sensory potentials may be difficult to record if there is considerable oedema or a pre-existing polyneuropathy (e.g. from diabetes mellitus) may have clouded the issue. Direct muscle stimulation (not commonly done) may reveal no or absent response in CIM but normal responses in CIP [14]. Unfortunately, needle electrode studies of muscle can show similar features in CIP and CIM; both may exhibit spontaneous activity (fibrillations and positive sharp waves). Often CIP and CIM coexist and their relative contributions to the weakness may vary considerably when this occurs. Elevated serum creatine kinase may help to identify CIM, but the peak may be missed in the membranopathy/necrotizing varieties or creatine kinase may not be significantly elevated in cases with loss of myosin filaments. To determine the relative contributions of nerve versus muscle disease in explaining weakness, muscle or both nerve and muscle biopsies have been utilized and recommended [11], most recently in this issue by Kerbaul and colleagues [15]. In most cases muscle biopsy will address the relative contribution of myopathy to the picture because the neuropathy can be adequately assessed electrophysiologically. Percutaneous muscle biopsy, although providing limited tissue, has a number of advantages over operative biopsies: greater spatial sampling, minimal bleeding, negligible infection rate (we have had none in over 1000 biopsies), portability (done at the bedside), no general anaesthetic, speed of performance and ease of arrangement. Nerve and muscle biopsy is seldom necessary in the ICU, unless a vasculitis is suspected. An open muscle biopsy may also be necessary if the needle biopsies are inadequate.
Being aware of the incidence and signs of ICU-acquired weakness with ventilatory failure and having an approach to such disorders will prove valuable in management. Some conditions are treatable. Further insights into the mechanisms of CIP and CIM may provide some preventive strategies that will ameliorate their severity, shorten the duration of ICU stays and improve long-term outcomes.
Abbreviations
CIM = critical illness myopathy; CIP = critical illness polyneuropathy; ICU = intensive care unit.
Competing interests
The author(s) declare that they have no competing interests.
See related research article http://ccforum.com/content/8/6/R358
References
- De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- Zochodne DW, Bolton CF, Wells GA, Gilbert JJ, Hahn AF, Brown JD, Sibbald WA. Critical illness polyneuropathy: a complication of sepsis and multi-organ failure. Brain. 1987;110:819–842. doi: 10.1093/brain/110.4.819. [DOI] [PubMed] [Google Scholar]
- Latronico N, Fenzi F, Recupero D, Guarneri B, Tomelleri G, Tonin P, De Maria G, Antonini L, Rizzuto N, Candiani A. Critical illness myopathy and neuropathy. Lancet. 1996;347:1579–1582. doi: 10.1016/S0140-6736(96)91074-0. [DOI] [PubMed] [Google Scholar]
- Rich MM, Teener JW, Raps EC, Schotland DL, Bird SJ. Muscle is electrically inexcitable in acute quadriplegic myopathy. Neurology. 1996;46:731–736. doi: 10.1212/wnl.46.3.731. [DOI] [PubMed] [Google Scholar]
- Hund E. Myopathy in critically ill patients. Crit Care Med. 1999;27:2544–2547. doi: 10.1097/00003246-199911000-00036. [DOI] [PubMed] [Google Scholar]
- Lacomas D, Zochodne DW, Bird SJ. Critical illness myopathy: what's in a name? Muscle Nerve. 2000;23:1785–1788. doi: 10.1002/1097-4598(200012)23:12<1785::AID-MUS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Banwell BL, Mildner RJ, Hassall AC, Becker LE, Vajsar J, Shemie SD. Muscle weakness in critically ill children. Neurology. 2003;61:1779–1782. doi: 10.1212/01.wnl.0000098886.90030.67. [DOI] [PubMed] [Google Scholar]
- Leijten FS, Harinck-de Weerd JE, Poortvliet DC, de Weerd AW. The role of polyneuropathy in motor convalescence after prolonged mechanical ventilation. JAMA. 1995;274:1221–1225. doi: 10.1001/jama.274.15.1221. [DOI] [PubMed] [Google Scholar]
- Witt NJ, Zochodne DW, Bolton CF, Grand'Maison F, Wells G, Young GB, Sibbald WJ. Peripheral nerve function in sepsis and multiple organ failure. Chest. 1991;99:176–184. doi: 10.1378/chest.99.1.176. [DOI] [PubMed] [Google Scholar]
- Bolton CF, Gilbert JJ, Hahn AF, Sibbald WJ. Polyneuropathy in critically ill patients. J Neurol Neurosurg Psychiatry. 1984;47:1223–1231. doi: 10.1136/jnnp.47.11.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarik J, Lukas Z, Vondracek P. Critical illness polyneuropathy: the electrophysiological components of a complex entity. Intensive Care Med. 2003;29:1505–1514. doi: 10.1007/s00134-003-1858-0. [DOI] [PubMed] [Google Scholar]
- Fletcher SN, Kennedy DD, Ghosh IR, Misra VP, Kiff K, Coakley JH, Hinds CJ. Persistent neuromuscular and neurophysiologic abnormalities in long-term survivors of prolonged critical illness. Crit Care Med. 2003;31:1012–1016. doi: 10.1097/01.CCM.0000053651.38421.D9. [DOI] [PubMed] [Google Scholar]
- Bolton CF. Electrophysiologic studies in critically ill patients. Muscle Nerve. 1987;10:129–135. doi: 10.1002/mus.880100205. [DOI] [PubMed] [Google Scholar]
- Rich MM, Bird SJ, Raps EC, McCluskey LF, Teener JW. Direct muscle stimulation in acute quadriplegic myopathy. Muscle Nerve. 1997;20:665–673. doi: 10.1002/(SICI)1097-4598(199706)20:6<665::AID-MUS2>3.3.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kerbaul F, Brousse M, Collart F, Pellissier J-F, Planche D, Fernan-dez C, Gouin F, Guidon C. Combination of histopathological and electromyographic patterns can help to evaluate functional outcome of critical ill patients with neuromuscular weakness syndromes. Crit Care. 2004;8:R358–R366. doi: 10.1186/cc2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton CF, Young GB. The neurological consultation and neurological syndromes in the intensive care unit. Balliere's Clin Neurol. 1996;5(3):447–475. [PubMed] [Google Scholar]


