Abstract
This brief review analyses the progress of noninvasive ventilation (NIV) over the last decade. NIV has gained the dignity of first line intervention for acute exacerbation of chronic obstructive pulmonary disease, assuring reduction of the intubation rate, rate of infection and mortality. Despite positive results, NIV still remains controversial as a treatment for acute hypoxemic respiratory failure, largely due to the different pathophysiology of hypoxemia. The infection rate reduction effect achieved by NIV application is crucial for immunocompromised patients for whom the endotracheal intubation represents a high risk. Improvements in skills acquired with experience over time progressively allowed successful treatment of more severe patients.
Keywords: COPD, helmet, hypoxemic respiratory failure, immunocompromised, noninvasive ventilation
Introduction
The term 'acute respiratory failure' (ARF) indicates a severe deterioration in gas exchange, often requiring mechanical ventilatory support with endotracheal intubation (ETI). Placement of an endotracheal tube is associated with increased risk for complications such as tracheal stenosis [1,2] and ventilation associated pneumonia [3]. Noninvasive ventilation (NIV; i.e. delivery of assisted breaths without an invasive artificial airway) is a safe and effective tool in correcting the pathophysiological mechanisms of ARF, and reduces the work of breathing while concomitant treatments correct the causes of the ARF. NIV has been used primarily in patients with acute hypercapnic ventilatory failure, and especially for acute exacerbation of chronic obstructive pulmonary disease (COPD; Table 1).
Table 1.
Selection criteria for noninvasive ventilation candidates
| Conscious and cooperative patient (the patient with chronic obstructive pulmonary disease may be an exception) |
| No need for urgent intubation to protect the airways or to remove copious secretions |
| No acute facial trauma (helmet interface may permit an exception in selected cases) |
| No recent gastroesophageal surgery |
| No active gastrointestinal bleeding |
| No impairment in swallowing |
| Hemodynamic and rhythm stability |
| Adequate fitting of the interface |
Hypercapnic respiratory failure
The efficacy of NIV in treating acute exacerbations of COPD was investigated by Brochard and colleagues [4] in a European, randomized, multicenter study conducted in 85 COPD patients assigned to receive conventional treatment (oxygen therapy plus drugs) or NIV. The group of patients treated with NIV had fewer intubations (26% versus 74%; P < 0.001), fewer complications (14% versus 45%; P < 0.01), shorter length of hospital stay (23 ± 17 days versus 35 ± 33 days; P < 0.02), and lower mortality (9% versus 29%; P < 0.02).
In a recent meta analysis, Keenan and coworkers [5] reported that the addition of noninvasive positive pressure ventilation (NPPV) to standard care in patients with acute exacerbations of COPD decreased the rate of ETI (28% risk reduction, 95% confidence interval [CI] 15–40%), length of hospital stay (4.57 days, 95% CI 2.30–6.83 days), and in-hospital mortality rate (10% risk reduction, 95% CI 5–15%). Subgroup analysis showed that these beneficial effects occurred in those patients who were more severely ill, and not in those with milder exacerbations. That meta-analysis also included studies conducted in patients who had milder exacerbations of COPD. In these investigations the mean arterial pH of the study populations was close to normal and the benefit from NIV was limited. In these studies the enrolment of patients with only slightly compromised respiratory function may account for the absence of relevant physiological effects of NIV. A point that the meta-analysis did not elucidate is whether patients with severe hypercapnia or acidemia at hospital admission are less likely to respond to NIV, as was reported in previous studies [6,7].
A recent Cochrane systematic review and meta-analysis [8] evaluated randomized controlled trials that compared NPPV with usual medical care in COPD patients with ARF. It found that NPPV is associated with lower mortality (relative risk 0.41, 95% CI 0.26–0.64), decreased rate of ETI (relative risk 0.42, 95% CI 0.31–0.59), and greater improvements in arterial carbon dioxide tension (PaCO2) and respiratory rate after 1 hour of treatment. Also, there were fewer complications with NPPV (relative risk 0.32, 95% CI 0.18–0.56).
Success rates with NIV improve with increasing physician experience. Carlucci and coworkers [9] reported an 8-year retrospective study in which they investigated the changes in clinical practice of NIV in COPD patients. In that survey the failure rate with NIV was constant over time, regardless of the increasing severity of illness over the years. Between 1992 and 1996 the risk for NIV failure in more severely ill patients (pH < 7.25 at admission) was threefold higher than during the period 1997–1999. The authors concluded that improvements in skills acquired with experience over time progressively allowed successful treatment of patients who are more severely ill.
NIV works not only in more experienced academic centers but also in the real, clinical world. In a multicenter trial of the use of NIV in general respiratory wards, Plant and colleagues [10] randomly assigned 236 patients suffering from COPD exacerbations to standard medical therapy alone or medical therapy combined with NIV. Intubation and mortality rates in the NIV group were lower than in the conventional therapy group (15% versus 27% [P = 0.02] and 10% versus 20% [P = 0.05], respectively). Patients in the NIV group had a more rapid improvement in arterial pH and respiratory rate. In patients who were more acidotic (pH < 7.30) the benefit from NIV was limited, suggesting that the appropriate location for treatment of this more severely ill subgroup of patients is the intensive care unit (ICU) and not the general ward.
The British Thoracic Society guidelines on the use of NIV in ARF stated that it can be considered the first-line intervention for hypercapnic COPD with ARF, and that expert staff and the facilities necessary to apply NIV should be available 24 hours a day in all hospitals that are likely to admit such patients [11]. A recent review defined NPPV as a standard of care for moderate-to-severe COPD exacerbation [12].
Hypoxemic respiratory failure
The use of NIV in the treatment of hypoxemic ARF is still controversial. Part of this controversy stems from the heterogeneity of patients classified as being hypoxemic, who respond differently to the application of NIV. Four prospective randomized studies have evaluated the usefulness of NIV in immunocompetent patients suffering from hypoxemic ARF of various origins [13-16].
Wysocki and coworkers [13] randomly assigned 41 patients with ARF to NIV via facemask or conventional medical therapy. NIV reduced the need for ETI (36% versus 100%; P = 0.02), the duration of ICU stay (13 ± 15 days versus 32 ± 30 days; P = 0.04), and mortality rate (9% versus 66%; P = 0.06) in patients with hypercapnia only (PaCO2> 45 mmHg); it conferred no significant advantages in the purely hypoxemic group.
Antonelli and colleagues [14] conducted a study in 64 consecutive patients with hypoxemic ARF who met well defined criteria for mechanical ventilation, in which they compared NIV via a face mask versus ETI with conventional mechanical ventilation. After 1 hour of mechanical ventilation both groups had a significant improvement in arterial oxygen tension (PaO2)/fractional inspired oxygen (FiO2) ratio. Ten (31%) patients randomized to NIV required ETI. Patients randomized to conventional ventilation developed more frequent and serious complications (38% versus 66%; P = 0.02) and infectious complications (pneumonia or sinusitis) related to the presence of the endotracheal tube (3% versus 31%; P = 0.004). Among the survivors patients randomly assigned to NIV had a lesser duration of mechanical ventilation (P = 0.006) and a shorter ICU stay (P = 0.002).
In another prospective, randomized trial Martin and coworkers [15] compared NIV using bilevel positive airway pressure with usual medical care for therapy of ARF. Patients were subgrouped according to the disease that led to ARF and were then randomly assigned to to NIV or medical treatment. A total of 32 patients with hypoxemic ARF were included in the study; 14 out of 32 were treated by NIV and were compared with the 18 patients treated with conventional medical therapy. The NIV group had a lower ETI rate than did those in the conventional therapy group (7.46 intubations/100 ICU days versus 22.64 intubations/100 ICU days; P = 0.026).
Recently, Ferrer and colleagues [16] reported a randomized controlled trial conducted in 105 consecutive patients with hypoxemic ARF. When compared with oxygen therapy, NIV decreased the need for intubation (25% versus 52%: P = 0.010), the incidence of septic shock (12% versus 31%; P = 0.028), and the ICU mortality (18% versus 39%; P = 0.028). Multivariate analysis showed that NIV was independently associated with decreased risk for intubation and decreased 90-day mortality.
Hypoxemic ARF can be the end-point of several pathologies, and the mechanisms responsible for PaO2 decrease may be quite different (shunt, ventilation/perfusion mismatch, impairment of alveolar capillary diffusion). Many reported clinical studies have been focused on specific pathologic conditions, such as cardiopulmonary edema (CPE), community acquired pneumonia (CAP), ARF occurring after thoracic surgery, or ARF occurring in immunosuppressed patients [17-25]. In these cases the efficacy of NIV, and therefore the patient's outcome, depends not only on gas exchange impairment as measured by PaO2/FiO2 ratio but also, and predominantly, on the underlying pathology.
Domenighetti and coworkers [26], in a prospective observational study, compared the acute effects of NIV in two groups of hypoxemic ARF patients with CAP and CPE. Oxygenation improved significantly in both groups, but the subsequent outcomes differed and were strictly dependent on the nature of the acute lung injury. The mean total time spent on NIV was 9 ± 6.3 hours in the CPE and 37 ± 36 hours in the CAP group (P = 0.01). ICU mortaliy rate was 6.6% in the CPE and 28% in the CAP group. In general, CPE with hypoxemic ARF is a condition that responds well to NIV.
The major advantages of NIV are related to the effects generated by the increase in intrathoracic pressure. These benefits include increases in functional residual capacity and oxygenation, reduction in the work of breathing, and reductions in preload and afterload.
Five randomized controlled studies were conducted in 336 patients with hypoxemic ARF due to CPE [17-21]. Patients were treated using continuous positive airway pressure (CPAP) in three studies [17-19], and bilevel positive ariway pressure [20] and pressure support ventilation [21] in the remaining two. ETI was required in 28 (16%) of the 167 patients assigned to the NIV group and in 54 (32%) of the 169 patients assigned to conventional treatment. The absolute risk reduction for ETI was 16%, and the number of patients needed to treat with NIV to avoid one ETI was six. The mortality rate was 13% (22/167) in the conventional therapy group and 8% (14/169) in the NIV group, with an absolute risk reduction of 5%, corresponding to 21 NIV treatments to save one life.
In a multicenter prospective cohort study conducted in 354 patients with a diagnosis of hypoxemic ARF [27], the intubation rate was lower for patients with CPE (10%), pulmonary contusion (18%), and atelectasis (32%). In contrast, a high failure rate with ETI was observed in patients with acute respiratory distress syndrome (51%) and CAP (50%). Multivariate analysis identified acute respiratory distress syndrome, CAP, and lack of improvement in PaO2/FiO2 ratio after 1 hour of treatment as independent risk factors for failure of NIV.
Reduction in infections
Randomized and observational studies including more than 300 immunocompetent and immunocompromised patients showed that NIV, by avoiding ETI, drastically reduces rates of infection and sepsis [4,14,16,24,25,28,29]. In a study conducted in COPD patients with acute exacerbation [4], the rate of pneumonia was 17% in the group with conventional medical treatment and 5% in the NIV group. In another randomized controlled study of the use of NIV in the treatment of hypoxemic patients [14], those randomly assigned to conventional ventilation developed more frequent infectious complications (pneumonia or sinusitis) related to the presence of the endotracheal tube (3% versus 31%; P = 0.004). This beneficial effect of NIV is important in immunocompromised or immunosuppressed patients.
A prospective randomized study conducted in 40 solid organ transplant recipients with acute hypoxemic respiratory failure [24] compared NIV with standard treatment with supplemental oxygen. The use of NIV was associated with a significant reduction in the rate of ETI (20% versus 70%; P = 0.002), rate of severe sepsis and septic shock (20% versus 50%; P = 0.05), length of ICU stay in survivors (5.5 ± 3 days versus 9 ± 4 days; P = 0.03), and ICU mortality (20% versus 50%; P = 0.05). Hospital mortality was not different.
Hilbert and coworkers [25], in a randomized controlled trial in 104 immunocompromised patients with severe hypoxemic ARF, compared intermittent NIV with standard treatment and supplemental oxygen. Twelve patients in the NIV group compared with 20 in the standard treatment group required ETI (P = 0.03); NIV was associated with less serious complications, namely pneumonia and sepsis (P = 0.02), and with lower mortality (P = 0.02).
New interfaces
Recently, Navalesi and coworkers [30] elucidated the importance of the ventilatory interfaces for the success of NIV. Intolerance is one of the leading causes of NIV failure (Table 2) [9]. In an attempt to improve tolerability, a helmet (Fig. 1) has been proposed as a new interface for clinical use with CPAP and NIV.
Table 2.
Criteria for discontinuation of noninvasive ventilation and endotracheal intubation
| Mask intolerance (discomfort or claustrophobia) |
| Inability to improve gas exchanges and dyspnea |
| Hemodynamic instability or evidence of cardiac ischemia or ventricular dysarrhythmia |
| Need for urgent endotracheal intubation (because of inadequate management of secretions and protection of the airways) |
| Failure to improve mental status, within 30 min after the application of noninvasive ventilation, in agitated hypoxemic patients |
Figure 1.
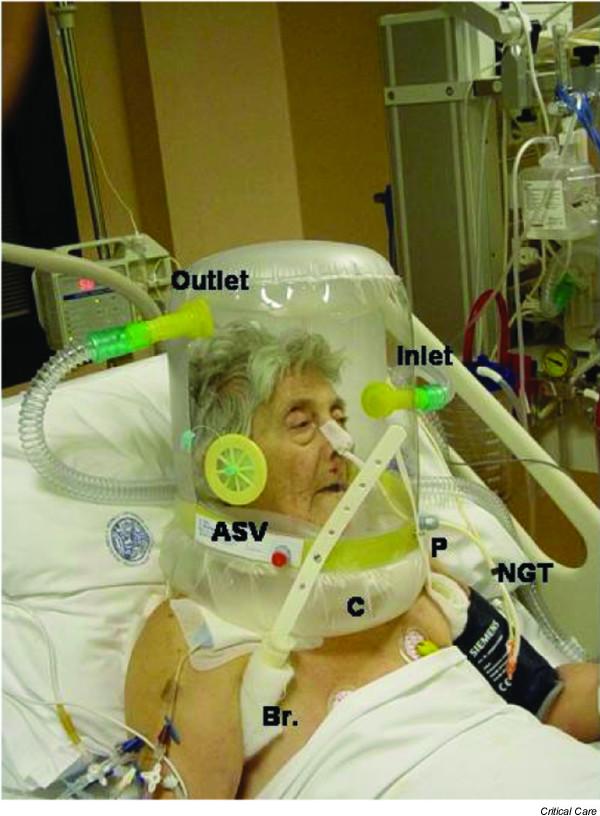
Patient undergoing pressure support ventilation with a helmet. The transparency of the device permits interaction of the patient with their surroundings. ASV, antisuffocation valve, which opens automatically if disconnection from the ventilator occurs; Br, armpit braces that keep the helmet attached to the patient; C, collar; Inlet, inlet of medical gases, connected to the inspiratory port of the ventilator by conventional tubing; Outlet, outlet of gases, connected to expiratory port of the ventilator; P, sealed passage for the nasogastric tube (NGT), through which the patient can receive enteral feeding or drink through a straw (picture taken with patient permission).
In a randomized physiologic study, Patroniti and collaborators [31] investigated the differences between facial mask and helmet in delivering CPAP. The authors concluded that both interfaces were effective and well tolerated, with similar reduction in the work of breathing, but the helmet needed flow rates greater than 30–40 l/min to prevent rebreathing.
In a prospective clinical pilot study [32], 33 patients with hypoxemic ARF, treated with noninvasive pressure support ventilation delivered by helmet, were compared with 66 matched control individuals treated with the same ventilatory technique by face mask. Oxygenation improved in both groups after NIV, and the number of patients requiring intubation was similar. No patients failed NIV because of intolerance to the technique in the helmet group, in comparison with eight patients (38%) in the mask group (P = 0.05). The duration of uninterrupted NIV was longer in the helmet group (36 ± 29 hours versus 26 ± 13 hours; P = 0.04).
A similar study was conducted in 33 COPD patients with acute exacerbation, who were treated with helmet NPPV and compared with 33 patients with similar characteristics treated by face mask [33]. The ratio of patients requiring intubation and outcomes were similar in the two groups, with a significant reduction in Pa CO2. However, PaCO2 decrease was more marked and faster in patients treated with the face mask (P = 0.01). The slower PaCO2 decrease was not related to rebreathing, which was similar with the two interfaces, but to the large volumes dissipated to distend the helmet.
Nonivasive ventilation and weaning
Three randomized trials conducted in Europe investigated the role of NIV in weaning failure [34-36]. In selected patients with COPD exacerbations and weaning failure, NIV facilitated extubation [34] and improved 3-month survival [35]. Ferrer and collaborators [36] investigated the role of NIV in patients who met criteria for a weaning attempt but failed a spontaneous breathing trial for 3 consecutive days. In that study NIV was effective in shortening the duration of invasive ventilation, and decreasing the incidence of nosocomial infections, mortality, and length of ICU and hospital stays. The International Consensus Conference [37] on the use of NIV in the treatment of ARF concluded that NIV may be effective in the setting of failed extubation.
Recently, Keenan and coworkers [38] reported a small, single-center, randomized trial that compared the use of NIV with standard medical therapy in patients who had respiratory failure within 48 hours after extubation. They found no difference either in the rate of reintubation or in mortality.
A multicenter randomized controlled trial recently reported by Esteban and colleagues [39] evaluated the impact that NIV had on extubation failure and mortality in a group of patients electively extubated after at least 48 hours of mechanical ventilation. There was no difference between the NIV and the standard therapy group in the need for reintubation (48% in both groups; P = 0.99). The rate of ICU death in the NIV group was greater than that in the standard therapy group (25% versus 14%; P = 0.048; relative risk 1.78, 95% CI 1.03–3.20) and the median time from respiratory failure to reintubation was longer in the NIV group (12 hours versus 2.5 hours; P = 0.02). The authors concluded that NIV failed to reduce mortality or the need for reintubation among patients who had respiratory failure after extubation, and may be harmful.
Conclusion
NIV is now a first-line intervention for acute exacerbation of COPD; also, a growing body of evidence supports the use of NIV in hypoxemic respiratory failure, but this application requires careful selection of patients and close monitoring, in a setting in which intubation devices are readily available. Although NIV can be useful in avoiding weaning failure in selected patients admitted to specialized centers, at present there is no strong evidence to support extensive use of NIV to prevent weaning failure.
Competing interests
None declared.
Abbreviations
ARF = acute respiratory failure; CAP = community acquired pneumonia; CI = confidence interval; COPD = chronic obstructive pulmonary disease; CPAP = continuous positive airway pressure; CPE = cardiopulmonary edema; ETI = endotracheal intubation; FiO2 = fractional inspired oxygen; ICU = intensive care unit; NIV = noninvasive ventilation; NPPV = noninvasive positive pressure ventilation; PaCO2 = arterial carbon dioxide tension; PaO2 = arterial oxygen tension.
References
- Torres A, Aznar R, Gatell JM, Jimenez P, Gonzalez J, Ferrer A, Celis R, Rodriguez-Roisin R. Incidence, risk and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990;142:523–528. doi: 10.1164/ajrccm/142.3.523. [DOI] [PubMed] [Google Scholar]
- Burns HP, Dayal VS, Scott A, Van Nostran ANP, Bryce DP. Laryngotracheal trauma: observations on its pathogenesis and its prevention following prolonged orotracheal intubation in the adult. Laryngoscope. 1979;89:1316–1325. doi: 10.1002/lary.1979.89.8.1316. [DOI] [PubMed] [Google Scholar]
- Nourdine K, Combes P, Carton MJ, Beuret P, Cannamela A, Ducreux JC. Does NIV reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Med. 1999;25:567–573. doi: 10.1007/s001340050904. [DOI] [PubMed] [Google Scholar]
- Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simonneau G, Benito S, Gasparetto A, Lemaire F, et al. NIV for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from non-invasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med. 2003;138:864–870. doi: 10.7326/0003-4819-138-11-200306030-00007. [DOI] [PubMed] [Google Scholar]
- Plant PK, Owen JL, Elliott MW. Non-invasive ventilation in acute exacerbations of chronic obstructive pulmonary disease: long term survival and predictors of in-hospital outcome. Thorax. 2001;56:708–712. doi: 10.1136/thorax.56.9.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo Hoo GW, Santiago S, Williams AJ. Nasal mechanical ventilation for hypercapnic respiratory failure in chronic obstructive pulmonary disease: determinants of success and failure. Crit Care Med. 1994;22:1253–1261. doi: 10.1097/00003246-199408000-00009. [DOI] [PubMed] [Google Scholar]
- Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ. 2003;326:185. doi: 10.1136/bmj.326.7382.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlucci A, Delmastro M, Rubini F, Fracchia C, Nava S. Changes in the practice of noninvasive ventilation in treating COPD patients over 8 years. Intensive Care Med. 2003;29:419–425. doi: 10.1007/s00134-002-1574-1. [DOI] [PubMed] [Google Scholar]
- Plant PK, Owen JL, Elliott MW. Early use of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000;355:1931–1935. doi: 10.1016/S0140-6736(00)02323-0. [DOI] [PubMed] [Google Scholar]
- British Thoracic Society Standards of Care Committee Non-invasive ventilation in acute respiratory failure. Thorax. 2002;57:192–211. doi: 10.1136/thorax.57.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill NS. Noninvasive ventilation for chronic obstructive pulmonary disease. Respir Care. 2004;49:72–89. [PubMed] [Google Scholar]
- Wysocki M, Tric L, Wolff MA, Millet H, Herman B. Noninvasive pressure support ventilation in patients with acute respiratory failure. A randomized comparison with conventional therapy. Chest. 1995;107:761–768. doi: 10.1378/chest.107.3.761. [DOI] [PubMed] [Google Scholar]
- Antonelli M, Conti G, Rocco M, Bufi M, De Blasi RA, Vivino G, Gasparetto A, Meduri GU. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998;339:429–435. doi: 10.1056/NEJM199808133390703. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Hovis JD, Costantino JP, Bierman MI, Donahoe MP, Rogers RM, Kreit JW, Sciurba FC, Stiller RA, Sanders MH. A randomized, prospective evaluation of noninvasive ventilation for acute respiratory failure. Am J Respir Crit Care Med. 2000;161:807–813. doi: 10.1164/ajrccm.161.3.9808143. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Esquinas A, Leon M, Gonzalez G, Alarcon A, Torres A. Noninvasive ventilation in severe hypoxemic respiratory failure: a randomized clinical trial. Am J Respir Crit Care Med. 2003;168:1438–1444. doi: 10.1164/rccm.200301-072OC. [DOI] [PubMed] [Google Scholar]
- Lin M, Yang Y, Chiany H, Chang M, Chiany BN, Chitlin MD. Reappraisal of continuous positive airway pressure therapy in acute cardiogenic pulmonary edema: short-term results and long-term follow-up. Chest. 1995;107:1379–1386. doi: 10.1378/chest.107.5.1379. [DOI] [PubMed] [Google Scholar]
- Bersten AD, Holt AW, Vedic AE, Skowronski GA, Baggoley CJ. Treatment of severe cardiogenic pulmonary edema with continuous positive airway pressure delivered by face mask. N Engl J Med. 1991;325:1825–1830. doi: 10.1056/NEJM199112263252601. [DOI] [PubMed] [Google Scholar]
- Rasanen J, Heikkila J, Downs J, Nikki P, Vaisanen I, Viitanen A. Continuous positive airway pressure by face mask in acute cardiogenic pulmonary edema. Am J Cardiol. 1985;55:296–300. doi: 10.1016/0002-9149(85)90364-9. [DOI] [PubMed] [Google Scholar]
- Mehta S, Jay GD, Woolard RH, Hipona RA, Connolly EM, Cimini DM, Drinkwine JH, Hill NS. Randomized prospective trial of bilevel versus continous positive airway pressure in acute pulmonary edema. Crit Care Med. 1997;25:620–628. doi: 10.1097/00003246-199704000-00011. [DOI] [PubMed] [Google Scholar]
- Nava S, Carbone G, DiBattista N, Bellone A, Baiardi P, Casentini R, Marengo M, Giostra F, Borasi G, Groff P. Noninvasive ventilation in cardiogenic pulmonary edema. A multicenter randomized trial. Am J Respir Crit Care Med. 2003;168:1432–1437. doi: 10.1164/rccm.200211-1270OC. [DOI] [PubMed] [Google Scholar]
- Auriant I, Jallot A, Herve P, Cerrina J, Le Roy Ladurie F, Fournier JL, Lescot B, Parquin F. Noninvasive ventilation reduces mortality in acute respiratory failure during lung resection. Am J Respir Crit Care Med. 2001;164:1231–1235. doi: 10.1164/ajrccm.164.7.2101089. [DOI] [PubMed] [Google Scholar]
- Confalonieri M, Potena A, Carbone G, Di Battista N, Bellone A, Baiardi P, Casentini R, Marenco M, Giostra F, Borasi G, et al. Noninvasive ventilation in cardiogenic pulmonary edema a multicenter randomized trial. Am J Respir Crit Care Med. 1999;160:1585–1591. doi: 10.1164/rccm.200211-1270OC. [DOI] [PubMed] [Google Scholar]
- Antonelli M, Conti G, Bufi M, Costa MG, Lappa A, Rocco M, Gasparetto A, Meduri GU. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation. JAMA. 2000;283:235–241. doi: 10.1001/jama.283.2.235. [DOI] [PubMed] [Google Scholar]
- Hilbert G, Gruson D, Vargas F, Valentino R. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever and acute respiratory failure. N Engl J Med. 2001;344:481–487. doi: 10.1056/NEJM200102153440703. [DOI] [PubMed] [Google Scholar]
- Domenighetti G, Gayer R, Gentilini R. Noninvasive pressure support ventilation in non COPD patients with acute cardiogenic pulmonary edema and severe community acquired penumonia: acute effect and outcome. Intensive Care Med. 2002;28:1226–1232. doi: 10.1007/s00134-002-1373-8. [DOI] [PubMed] [Google Scholar]
- Antonelli M, Conti G, Moro ML, Esquinas A, Gonzalez-Diaz G, Confalonieri M, Pelaia P, Principi T, Gregoretti C, Beltrame F, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi center study. Intensive Care Med. 2001;27:1718–1728. doi: 10.1007/s00134-001-1114-4. [DOI] [PubMed] [Google Scholar]
- Girou E, Schortgen F, Delclaux C, Brun-Buisson C, Blot F, Lefort Y, Lemaire F, Brochard L. Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients. JAMA. 2000;284:2361–2367. doi: 10.1001/jama.284.18.2361. [DOI] [PubMed] [Google Scholar]
- Girou E, Brun-Buisson C, Taille S, Lemaire F, Brochard L. Secular trends in nosocomial infections and mortality associated with noninvasive ventilation in patients with exacerbation of COPD and pulmonary edema. JAMA. 2003;290:2985–2991. doi: 10.1001/jama.290.22.2985. [DOI] [PubMed] [Google Scholar]
- Navalesi P, Fanfulla F, Frigerio P, Gregoretti C, Nava S. Physiologic evaluation of noninvasive mechanical ventilation delivered with three types of masks in patients with chronic hypercapnic respiratory failure. Crit Care Med. 2000;28:1785–1790. doi: 10.1097/00003246-200006000-00015. [DOI] [PubMed] [Google Scholar]
- Patroniti N, Foti G, Mangio A, Coppo A, Bellini G, Pesenti A. Head helmet versus face mask for non-invasive continuous positive airway pressure: physiological study. Intensive Care Med. 2003;29:1680–1687. doi: 10.1007/s00134-003-1931-8. [DOI] [PubMed] [Google Scholar]
- Antonelli M, Conti G, Pelosi P, Gregoretti C, Pennisi MA, Costa R, Severgnini P, Chiaranda M, Proietti R. New treatment of acute hypoxemic respiratory failure: Non invasive pressure support ventilation delivered by helmet: a pilot controlled trial. Crit Care Med. 2002;30:602–608. doi: 10.1097/00003246-200203000-00019. [DOI] [PubMed] [Google Scholar]
- Antonelli M, Pennisi MA, Pelosi P, Gregoretti C, Squadrone V, Rocco M, Cecchini L, Chiumello D, Severgnini P, Proietti R, et al. Noninvasive positive pressure ventilation using a Helmet in patients with acute exacerbation of COPD: a feasibility study. Anesthesiology. 2004;100:16–24. doi: 10.1097/00000542-200401000-00007. [DOI] [PubMed] [Google Scholar]
- Girault C, Daudenthun I, Chevron V, Tamion F, Leroy , Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute on chronic respiratory failure: a prospective randomized controlled study. Am J Respir Crit Care Med. 1999;160:86–92. doi: 10.1164/ajrccm.160.1.9802120. [DOI] [PubMed] [Google Scholar]
- Nava S, Ambrosino N, Clini E, Prato M, Orlando G, Vitacca M, Brigada P, Fracchia C, Rubini F. Noninvasive mechanical in the weaning of patients with respiratory failure due to COPD: a randomized controlled study. Ann Intern Med. 1998;128:721–728. doi: 10.7326/0003-4819-128-9-199805010-00004. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Esquinas A, Arancibia F, Thonmas Bauer T, Gonzalez G, Carrillo A, Rodriguez-Roisin R, Torres A. Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med. 2003;168:70–76. doi: 10.1164/rccm.200209-1074OC. [DOI] [PubMed] [Google Scholar]
- International Consensus Conferences in Intensive Care Medicine Noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 2001;163:283–291. doi: 10.1164/ajrccm.163.1.ats1000. [DOI] [PubMed] [Google Scholar]
- Keenan SP, Powers C, Mc Cormack DG, Block G. Noninvasive positive-pressure ventilation for poextubation respiratory distress: a randomized controlled trial. JAMA. 2002;287:3238–3244. doi: 10.1001/jama.287.24.3238. [DOI] [PubMed] [Google Scholar]
- Esteban A, Frutos-Vivar F, Ferguson ND, Arabi Y, Apzteguia C, Gonzalez M, Epstein SK, Hill NS, Nava S, Soares MA, et al. Non-invasive positive pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452–2460. doi: 10.1056/NEJMoa032736. [DOI] [PubMed] [Google Scholar]


