Summary
CRISPR/Cas‐based genome editing is now extensively used in plant breeding and continues to evolve. Most CRISPR/Cas current applications in plants focus on gene knock‐outs; however, there is a pressing need for new methods to achieve more efficient delivery of CRISPR components and gene knock‐ins to improve agronomic traits of crop cultivars. We report here a genome editing system that combines the advantages of protoplast technologies with recent CRISPR/Cas advances to achieve seamless large fragment insertions in the model Solanaceae plant Nicotiana tabacum. With this system, two resistance‐related regions of the N′ gene were replaced with homologous fragments from the N′alata gene to confer TMV‐U1 resistance in the T0 generation of GMO‐free plants. Our study establishes a reliable genome‐editing tool for efficient gene modifications and provides a detailed description of the optimization process to assist other researchers adapt this system for their needs.
Keywords: protoplast regeneration, gene knock‐in, gene replacement, CRISPR/Cas9, long fragment insertion, TMV resistance
Introduction
CRISPR/Cas‐based genome editing is now commonly used in plant breeding and has resulted in tremendous progress in genome engineering (Gao, 2021; Jinek et al., 2012; Zhu et al., 2020). Although there are still challenges due to the lack of efficient gene insertions in polyploid crops, the delivery of CRISPR/Cas component procedure is rapidly improving.
Although CRISPR/Cas‐based system improvements, such as base editing, prime editing, and multiplex editing, now result in more efficient and versatile genome editing in plants (Armario Najera et al., 2019; Cox et al., 2017; Jin et al., 2021; Li et al., 2020, 2022; Lin et al., 2021; Liu et al., 2019; Ma et al., 2021; Miki et al., 2018; Pan et al., 2022; Reider Apel et al., 2017; Rothan et al., 2019; Shen et al., 2017; Stuttmann et al., 2021; Zhang et al., 2019; Zhong et al., 2018), most current CRISPR/Cas applications have only focused on knock‐outs or knock‐downs of specific genes by introducing indels at targeted sites or conversion of individual nucleotides. Therefore, there is a pressing need for new gene knock‐ins to improve desired traits for crop cultivars.
Gene knock‐ins can be achieved during DNA repair by either homologous recombination or nonhomologous end joining (NHEJ) pathways after the creation of double‐strand breaks (DSBs). Several research groups have obtained accurate gene modifications by homologous recombination, but these advances have serious limitations (Baltes et al., 2014; Bibikova et al., 2003; Čermák et al., 2015; Puchta et al., 1996; Shimatani et al., 2015; Shukla et al., 2009; Sun et al., 2016; Svitashev et al., 2015; Townsend et al., 2009). A robust method for gene insertion and replacements has been established in rice based on NHEJ (Li et al., 2016) and more recently, the efficiency of this technique has been improved by using donor DNA with chemical modifications at both ends to insert short and long fragment insertions into rice (Lu et al., 2020).
CRISPR/Cas‐based gene editing has also been used to confer resistance against plant pathogens (Karmakar et al., 2022). The latest report using the PrimeRoot system to insert robust long fragment inserts has delivered a 4.5 kb pigmR cassette into an untranslated region of the rice genome to confer resistance against rice blast disease (Sun et al., 2023). Our current application focuses on CRISPR/Cas‐mediated resistance against plant viruses by using CRISPR/Cas defences to target virus genomes directly or to knock out susceptibility factors (S‐gene) that limit virus infections (Khan et al., 2022). However, conferring virus resistance in plants by knocking in resistance genes (R‐gene) has not been described.
Improving genomics of Solanaceae crops has enormous potential for alleviating worldwide food security challenges. Genome editing has already been used for Solanaceae crop improvement (Yamamoto et al., 2018), and CRISPR/Cas9 systems have been used to create gene insertions and replacements in Solanaceae plants (Hsu et al., 2021; Yu et al., 2017). However, the insertions and replacements only included small fragments of a few dozen bases, and the efficiencies were relatively low. Thus, these advances are not sufficient to fulfil the increasing demands for large‐scale targeted genome modifications in Solanaceae plants.
Applying protoplast technologies to genome editing has resulted in the creation of crop plants without transgenic components, and this strategy circumvents chimeric problems and eliminates genetically modified organism (GMO) concerns (Lin et al., 2018). More recently, protoplast‐associated CRISPR/Cas9 methods have been applied to generate genome editing of wild tomatoes (Lin et al., 2022). The combined applications of modified gene insertions in rice, accompanied by the use of protoplasts in editing the tomato genome thus provide new tools for crop plant applications.
Using protoplasts for genome editing has two major advantages: First, transfection of protoplasts does not depend on methods such as agrobacterium transformation or particle bombardment. This eliminates transgenic components and permits the direct introduction of gene‐edited plant materials. A second advantage of protoplasts is that multiple plasmids and donor DNA can be introduced simultaneously to facilitate multiplex editing (Lin et al., 2022).
However, the biggest problem with the protoplast methods is that plant regeneration is relatively difficult. To overcome this obstacle, we have optimized a series of protocols, including protoplast isolation, protoplast transfection, and plant regeneration to develop a stable and efficient regeneration procedure for the model solanaceous plant, N. tabacum.
The tobacco mosaic virus (TMV) N′ resistance gene from N. tabacum lacks introns and is 4143 bp in length. Unfortunately, the N′ gene only mediates resistance against the TMV crucifer‐infecting strain (TMV‐Cg) but is not effective against the common U1 strain (TMV‐U1). The N′ protein has a typical structure of a coiled‐coil domain (CC), a nucleotide‐binding site (NBS) and leucine‐rich repeat (LRR) domains, and its corresponding avirulence determinant is the TMV‐U1 coat protein (Sekine et al., 2012; Taraporewala and Culver, 1996; Yuan et al., 2015). The related orthologous gene, N′alata (accession PI42334), has the same length and structure as N′ and exhibits resistance to both the TMV‐Cg and TMV‐U1 strains. By conducting phylogenetic analyses of N′ homologous genes in different wild tobacco species, our group has found that N′ homologous genes with resistance functions have a common ancestor distributed in a well‐supported clade of the phylogenetic tree and that their resistance variation likely results from random mutations during evolution (Yuan et al., 2015).
The N′ and N′alata genes are similar in sequence (>97%) but differ in TMV‐U1 resistance, so they provide an ideal model for the addition of agronomic traits through genome editing. Therefore, the N′alata and N′ genes were divided into eight regions to represent different domains. Then, we identified the TMV‐U1 resistance‐determining regions of N′alata by a series of recombinations between the eight regions of N′alata and the N′ genes. The results revealed that amino acids 265 to 798 and 1130 to 1233 together determine N′alata resistance. Hence, this provided the potential to modify the N′ resistance‐affecting regions in N. tabacum to expand the resistance spectrum.
Therefore, we first developed a CRISPR/Cas9‐mediated gene insertion protocol for N. tabacum by combining recent advances in gene insertion methods with the advantages of protoplast delivery methods to permit the generation of foreign DNA‐free genome‐edited T0 plants. Then we optimized several critical protocol steps to improve gene insertion and plant regeneration efficiencies. Finally, we used our optimized gene insertion system to swap the two regions of the N′ gene that affect TMV resistance in N. tabacum with the corresponding fragments from N′alata to engineer GMO‐free plants with TMV‐U1 resistance.
Our procedure permits the seamless replacement of an 1819 bp fragment in the exon of a functional gene to generate GMO‐free plants that possess the desired phenotype. This provides an effective gene knock‐in tool for genome editing that can be adapted by other researchers to achieve their goals.
Results
Gene knock‐ins in N. tabacum protoplasts
The CRISPR/Cas9 system has recently been employed to create gene insertions and replacements in tomato (Yu et al., 2017), but these modifications involved only small fragments. To establish a more robust and versatile gene knock‐in system for the Solanaceae, we used N. tabacum, as a model. The goal of our research was to establish a transgene‐free CRISPR/Cas9 system, so we used transfection of N. tabacum protoplasts to facilitate CRISPR/Cas9 plasmids and donor DNA deliveries into regenerated plants. This strategy relies on transient expression of the Cas9 protein and avoids stable nuclear transformation of DNA encoding the Cas9 protein and single guide RNA (sgRNA).
To initiate the experiments, we first inserted the eGFP sequence into the N′ gene of N. tabacum protoplasts. This eGFP reporter gene was constructed by using 5′‐phosphorylated primers, containing two phosphorothioate linkages at the 5′ end as described by Lu et al. (2020). Chemically modified eGFP‐1 and eGFP‐2 primers were designed to generate eGFP donors with start codons harbouring phosphorothioate linkages and phosphorylation at both ends (Figure 1a). Two sgRNAs targeting the N′ coding sequence were designed and used as a basis for the construction of the corresponding CRISPR/Cas9 derivatives (Figure 1b).
Figure 1.

Targeted insertion of GFP into N′ gene. (a) Schematic diagram of the eGFP donor. The eGFP, 5′‐phosphorylated (indicated with “P‐”) donor harbouring two phosphorothioate‐linkages (indicated with “*”) at both 5′‐ends, was amplified by using chemically modified primers GFP‐1 and GFP‐2. (b) Schematic diagram of N′ gene and eGFP inserted chimeric N′ gene with the targets (underlined), PAM sequences (orange boxed and emboldened letters), cleavage sites (red arrowhead), and primers (arrows). The N′ gene is illustrated in dark green and GFP donor is in light green. (c) GFP fluorescence signals in eGFP donor and Cas9 plasmid transfected protoplasts, and protoplast transfected with only eGFP donor as control. GFP channel, DIC channel and merged channel are single microscope sections. Bar = 100 μm. (d) PCR verification of eGFP insertions. The N′ specific primer N′F1 and the eGFP‐specific GFP‐R1 primer were used for verification of the targeted insertions. The protoplasts were transfected with the donor eGFP and Cas9 plasmids or with only the donor eGFP as a control. Three experiments were performed. Protoplast DNA samples with targeted insertions resulted in a PCR product of ~546 bp, while samples from protoplast transfected with only donor eGFP did not result in target bands.
Nicotiana tabacum protoplasts were prepared and transfected with the eGFP and CRISPR/Cas9 plasmids by use of a PEG‐Ca2+ transfection method (Yoo et al., 2007). Then transfected protoplasts were cultured for 7 days in the dark at 25 °C in protoplast culture media without antibiotics and analysed by fluorescence microscopy and a cell counter to identify GFP‐expressing cells (Figure 1c).
Protoplasts transfected with the eGFP donor and the Cas9 plasmid had 0.6% GFP expression, but those transfected with only eGFP failed to develop GFP fluorescence (Figure S1a). As a positive control, protoplasts transfected with a 35S promoter fused to eGFP as a positive control had 1.6% GFP expression.
To extend the fluorescence results, the N′F1 and GFP‐R1 primers (Figure 1a, and Table S7) were used to verify gene insertions. DNAs from the co‐transfected protoplasts or those transfected with only the eGFP donor were extracted and amplified by PCR. As expected, the PCRs resulted in an ~523 bp amplicon (Figure 1a). As shown in Figure 1d, GFP amplicons were only detected in protoplasts co‐transfected with the eGFP donor and the Cas9 plasmid. The amplicons were also sequenced and the eGFP sequence was confirmed (Figure S1b). In contrast, eGFP sequences were not detected in protoplasts transfected with only the donor eGFP.
Optimization of the key steps of protoplast isolation, transfection and whole plant regeneration
After verification of the protoplast gene insertion, the next bottleneck to the success of the genome editing system was to develop efficient whole plant regeneration of the protoplasts. Therefore, we first optimized protoplast procedures described in a recently reported protoplast regeneration protocol (Jeong et al., 2021). These experiments emphasized isolation, transfection, and regeneration to improve the recovery of genome‐edited plants from the protoplasts.
The quantity and quality of protoplasts recovered in the isolation step proved to be critical for optimal transfection and whole‐plant regeneration. For this purpose, 4‐ to 6‐week‐old N. tabacum plants were grown under sterile conditions and selected for protoplast preparation. We found that protoplasts of excellent quality could be obtained by cutting fully expanded leaves into 0.3 × 0.3 cm square sections and incubating the sections in an enzyme solution (Figure 2a,b, Table S1). The optimal enzyme digestion period was 12–14 h in the dark at 25 °C with gentle shaking, and longer times increased the protoplast fragility.
Figure 2.

Optimization of N. tabacum protoplast isolation, transfection and regeneration. (a) Fully expanded 4‐ to 6‐week‐old N. tabacum leaves were cut into small sections and immersed in the enzyme solution. (b) The enzyme solution containing the leaf sections was incubated in the dark overnight at room temperature with gentle shaking to release the protoplasts. (c) W58 salt solution was overlaid on released protoplasts. (d) Accumulation of protoplasts at the bottom of the W58 solution after centrifugation at 100 g for 5 min. The arrow points to the dark green protoplast band at the junction of the enzyme and W58 salt solutions. (e) Protoplast pellet produced after low‐speed centrifuge washing in a 50 mL conical centrifuge tube. (f) Washed protoplasts after transfer into MMG solution before transfection. (g) Transfected protoplasts overlaid with low‐melting agarose gel containing protoplast culture media. (h) Transformation of protoplasts into microcalli after incubation for 4–6 weeks. (i) Small microcallus pieces before incubation for 3 weeks in liquid shoot regeneration media. (j) Microcallus pieces on solid shoot regeneration media. (k) Regenerated microcalli forming shoots and roots. White scale bar = 1 cm, black scale bar = 100 μm.
After digestion, the released protoplasts were sieved, suspended, centrifuged, washed, and resuspended using our optimal conditions and improved agent concentrations (Figure 2c–f, Tables S1 and S2).
Finally, we evaluated several protoplast concentrations before transfection and found that the best transfection and regeneration were achieved between 0.8 and 1.5 × 106 cells/mL.
For protoplast transfections, we used endotoxin‐free plasmids and chemically modified donor DNAs. The efficiency of different transfection incubation times and plasmid concentrations were next evaluated by testing variations of the CRISPR/Cas9 plasmid and GFP donor DNA. The final proportion of cells expressing GFP at 7 days after completion of the isolation procedure revealed that the optimal transfection time was 5–10 min. Longer PEG‐CaCl2 transfection times provided a higher proportion of protoplasts expressing GFP, but the regeneration rates of the protoplasts were reduced drastically.
It is vital to select optimal culture conditions for isolated protoplasts to obtain the most efficient plant regeneration. Several variables, including liquid culture compositions and hydrogel embedding procedures, were evaluated to determine the best cell division and regeneration conditions for the transfected protoplasts (Davey et al., 2005). The hydrogel methods provided the highest cell proliferation and regeneration rates. Among these methods (Miao et al., 2018), agarose‐ and alginate‐based gels were similar; therefore, we optimized a protoplast embedding method using Kao and Michayluk‐agarose media (see Tables S1–S5 for details).
Protoplasts in agarose culture media were mixed with an equal volume of Kao and Michayluk‐agarose media at <37 °C. Multiple small drops of the agarose mixture were pipetted onto sterile petri dishes (Figure 2g). After the agarose solidified, the beads were overlaid with 15 mL of protoplast culture media and incubated in a growth chamber at 20–25 °C in the dark. The protoplast density in the agarose gels was a key factor for cell proliferation. After a series of dilution assessments, we found that 0.5 × 106 protoplasts/mL in the gel facilitated cell division greatly and that lower or higher cell densities negatively impacted cell growth and division.
To assist regeneration, antibiotics were used to select protoplasts expressing the CRISPR/Cas9 plasmid. Because the plasmid was transiently expressed in the protoplasts rather than integrated into the genome, it was important to determine the details of the antibiotic administration needed to permit growth of cells harbouring the CRISPR/Cas9 while suppressing protoplasts lacking the plasmid. Therefore, we conducted a series of antibiotic treatments differing in timing, concentration and duration. These experiments showed that the best antibiotic addition time was 3 days after protoplast transfection when the antibiotic resistance genes were being expressed efficiently. We used a hygromycin concentration of 10 mg/L and also added the same antibiotic concentration to the protoplast culture media that initially overlayed the agarose‐embedded protoplasts. This initial solution was replaced 3 days later with culture media without antibiotics. Higher hygromycin concentrations or longer incubation times were found to significantly reduce the protoplast regeneration rates.
According to our observations, a key requirement for efficient protoplast regeneration was to conduct protoplast proliferation steps in darkness and to conduct the callus proliferation steps under light under conditions similar to those used by Lin et al. (2022).
According to Lin et al. (2022), the most critical steps in protoplast regeneration steps are conducted in liquid culture. Therefore, we evaluated different modifications to the culture media. The best results were obtained by hormonal treatments with 1‐naphthaleneacetic acid (NAA) and 6‐benzylaminopurine (BAP) at a ratio of 1 : 20 for callus induction (Table S3). After approximately four weekly changes of regeneration media containing NAA and BAP, microcalli were visible in the agarose bed (Figure 2h). Then agarose containing the microcalli was carefully divided into small pieces to ensure access to nutrients needed for optimal callus growth (Figure 2i). The microcallus pieces were then overlayed with liquid shoot regeneration media containing optimal NAA:BAP at 1 : 10 ratios (Table S4). Microcallus growth, shoot regeneration and rooting were performed according to canonical protocols (Figure 2j,k). The optimized protocols and the reagents used for the protocols above are described in more detail in Tables S1–S5.
N′ gene fragment replacements conferring resistance to TMV‐U1
The N′ gene from N. tabacum elicits resistance to the TMV‐Cg strain, but not to the TMV‐U1 strain. In contrast, the N′ homologue N′alata provides both TMV‐Cg and TMV‐U1 resistance by recognizing the coat protein (CP) of both TMV strains (Sekine et al., 2012; Taraporewala and Culver, 1996; Yuan et al., 2015).
We amplified the N′ and N′alata genes from N. tabacum and N. alata (accession PI42334) and inserted them between 35S promoter and the octopine synthase terminator. The N′ and N′alata genes were then divided into eight regions representing different domains and a series of recombinant genes were constructed (Figure 3a,b). These recombinant genes were then transiently co‐expressed with TMV‐U1 CP in N. benthamiana leaves by agroinfiltration, and the resistance of the chimera genes was evaluated by observations of the hypersensitive responses (HR) of infiltrated leaves. When the TMV‐U1 CP and the N′ constructs were co‐expressed, the infiltrated leaves failed to develop HR responses, but the N′alata genes induced robust HR activities (Figure 3c).
Figure 3.

Verification of the N′alata gene resistance‐determining regions. (a) Schematic diagram of the N′ gene domain structure. CC represents the coiled‐coil domain. NB‐ARC represents the nucleotide‐binding adaptor shared by the APAF‐1, R proteins, and the CED‐4 domain. LRR represents the leucine‐rich repeat domain. The amino acid distributions are labelled below each domain. (b) Schematic diagram of N′alata, N′ and recombinant N′alata with fragments swapped from the N′ gene. The N′alata and N′ genes were divided into eight regions to facilitate construction of different domains. The amino acid ranges are labelled for each domain. N′alata, N′ and recombinant N′alata genes were inserted between the 35S promoter and octopine synthase terminator. (c) Transient co‐expression of the N′alata, N′ and recombinant N′alata genes along with the TMV‐U1 CP gene in N. benthamiana leaves. Two days after agroinfiltration, the N. benthamiana leaves exhibited hypersensitive response (HR) symptoms (left) or no HR symptoms (right). The HR implies that the recombinant gene is resistant to TMV‐U1, whereas the absence of HR indicates susceptibility to TMV‐U1. The proportions of infiltration sites showing the HR for each construct are shown based on counting 60 infiltration sites per construct in three independent experiments. Under most circumstances, the N′alata::b, N′alata::c and N′alata::d genes failed to induce HR symptoms, but they occasionally induced weak HR. Other constructs induced only one dominant symptom after infiltration. (d) Schematic diagram of the N′ gene with fragments swapped from the N′alata gene. The BCD and G regions were truncated into the B'CD and G' regions respectively to more precisely locate the resistance‐determining regions. (e) Transient co‐expression of recombinant N′ genes with TMV‐U1 CP compared with only the recombinant N′ genes in N. benthamiana leaves. The Infiltrated areas of each structure are indicated with dotted red circles. Transient expression of recombinant N′ genes without the TMV‐U1 CP was used as controls to eliminate spontaneous HR responses to infiltration. The N′ gene is shown in green and the N′alata gene is in cyan. All infiltration assays were repeated three times and a total of 60 infiltrations were conducted for each structure.
By replacing each of the eight regions of N′alata individually with corresponding fragments from the N′ gene, four N′ regions representing amino acids 171 to 337, 338 to 485, 486 to 798, and 1121 to 1233 were shown to compromise the N′alata HR activity (Figure 3b,c). Interestingly, when N′alata amino acids 171 to 337, 338 to 485 and 486 to 798 were swapped individually, the resulting chimeras occasionally induced a weak HR, whereas swaps of amino acids 1121 to 1233 totally eliminated the resistance response (Figure 3b,c).
We then conducted reverse swaps by replacing the four resistance‐related regions of N′ with the corresponding fragments from N′alata (Figure 3d). These chimeric genes elicited resistance against TMV‐U1 in a transient expression assay (Figure 3e). Further truncation of the confirmed resistance‐related regions demonstrated that amino acid regions 265–798 and 1130–1233 represented the minimum forms needed to ensure robust resistance (Figure 3d,e).
We optimized in vivo gene replacement system in protoplasts in order to insert critical N′ regions encoding resistance to TMV‐U1. The first step of the procedure was conducted with N′ amino acids 265 to 798. Two sgRNAs (sgRNA1 and sgRNA2) targeting N′ amino acids 250 and 857 were designed and introduced into the pCambia‐AtUbi‐Cas9 vector under control of the U3 and U6 promoters, respectively. Donor DNA was amplified by PCR with the chemically modified primers DonorF1 and DonorR1 (Figure 4a). Synonymous mutant termini were also inserted into the donor DNAs to eliminate the PAM site and avoid secondary cleavage by Cas9 (Figure 4a). Then, the resulting CRISPR/Cas9 plasmid and the donor DNAs were transfected into N. tabacum protoplasts with the optimized procedure.
Figure 4.

Two‐step targeted substitution of N′ with N′alata fragments. (a) Schematic diagram of donor fragment, N′ and the first step gene substitution. Donor DNA was amplified with chemically modified primers. Verified sgRNA targets (underlined) were used for gene replacements. Genotyping primers are indicated by arrows showing synthesis directions. A portion of the N′ gene was replaced by the corresponding region from the N′alata gene to provide the chimericN′1 structure in planta. (b) Reference sequences and Sanger sequencing chromatograms for gene replacement in line No. 376. (c) Genotyping of T0 seedlings to illustrate the first step of the targeted forward gene replacement. The bands marked by black horizontal arrows indicate the forward targeted gene replacement, marked by green arrows indicate the non‐edited allele of the N′ gene, marked by orange arrow indicate that at least one allele of N′ was either not edited or completely replaced, marked by red arrow indicate the fragment deletion between the two targets in one of the N′ alleles. WT, wild type. (d) Schematic diagram of the donor fragment and the second step of gene substitution. A portion of the ChimericN′1 gene was partially replaced by the corresponding region from the N′alata gene to produce the chimericN′2 structure in planta. (e) Reference sequences and Sanger sequencing chromatograms to illustrate the gene replacement in line No. 25. (f) Genotyping of T0 seedlings with targeted forward gene replacements after the second replacement step. The bands marked by black horizontal arrows indicate the forward targeted gene replacement, marked by green arrows indicate the non‐edited allele of the ChimericN′1 gene, marked by orange arrows indicate that at least one allele of ChimericN′1 was either not edited or was completely replaced, marked by red arrows indicate that fragment deletion between the two targets in one of the ChimericN′1 alleles. (g) Resistance verification of edited N. tabacum seedlings. Coat proteins of TMV‐Cg (CgCP) and TMV‐U1 (U1CP) were transiently expressed in wild‐type (WT), seedlings with the ChimericN′1 gene or the ChimericN′2 gene. The CgCP can induce an HR in all plants while U1CP can only induce HR in ChimericN′2 seedlings. This result shows that the gene substitution conferred resistance to TMV‐U1 in N. tabacum. 5′‐phosphorylation is indicated by “P‐”, while phosphorothioate‐linkages are indicated by “*”. The cyan‐coloured nucleotides show the polymorphism in N′alata and also help to identify the gene substitution region. Single‐guide RNAs are underlined. Synonymous mutations in the donor are shown in red. The donor DNA terminus was deliberately designed to eliminate the PAM site by synonymous mutations to avoid secondary cleavage by Cas9 after ligation. The bold nucleotides in the orange box indicate the PAM sites and the red arrowheads indicate the cleavage sites. N′ gene is shown in green and N′alata gene in cyan. Ref means the expected sequences as reference. Donor DNA is indicated in the black box. No. equals the number of sequenced plantlets.
Hundreds of plantlets were obtained by protoplast regeneration and subjected to DNA extraction and genotyping. DNA fragments were amplified with the primer pairs (N′F1/N′R1, N′F1/N′alataR1 and N′alataF1/N′R2) flanking the sgRNA1 and sgRNA2 target sites (Figure 4a: Note the primer sequences in Table S7). N′F1, N′R1 and N′R2 contained common sequences shared by the N′ and N′alata genes, whereas N′alataR1 and N′alataF1 were designed to correspond to N′alata nucleotide polymorphisms and only annealed to the N′alata sequence (see the genotyping primer positions in Figure S2).
The targeted forward gene replacement was expected to produce an amplicon of 644 bp when the N′F1 and N′alataR1 primers were used for PCR and to yield an amplicon of 482 bp with the N′alataF1 and N′R2 primers (Figure 4a). We used a PCR verification assay to identify plantlets with targeted gene replacements and found ten lines with forward replacements out of a total of 450 lines tested. The confirmed replacement lines were sequenced to verify the 5′ and 3′ junctions at the sgRNA1 and sgRNA2 sites. Nine of the ten lines contained indels, whereas only one line (No. 376) had a seamless fragment replacement that produced the ChimericN′1 gene structure (Figure 4a). The sequencing results showed that the donor was prone to elicit indels at junctions during insertions into the PAM cleavage sites (Figure S3). Thus, the proportion of indels at the donor ends was similar to a previous report on rice in which particle bombardment was used for donor delivery (Lu et al., 2020).
To verify whether lines with sequence replacements were homozygous, the primer pairs N′F1/N′R1 and N′F1/N′R2 (Figure 4a,c) were used for PCR amplification of genomic DNAs. The DNA from the N′ allele before replacement harboured a 387 bp sequence that could be amplified with the N′F1 and N′R1 primers, whereas N′ did not provide amplified fragments when the same primers were used for genotyping of regenerated plants. N′ alleles with fragment deletions between the sgRNA1 and sgRNA2 sites had smaller 217 bp amplicons, whereas PCRs of contact alleles resulted in a 2036 bp fragment when the N′F1 and N′R2 primers were used. According to PCR genotyping assessments (Figure 4c) all ten lines were heterozygous, and the desired gene replacement only occurred once in the 10 lines harbouring the N′ alleles. In addition, deletions between sgRNA1 and sgRNA2 were verified in one of the N′ alleles.
We self‐pollinated T0 plant 376, which contained the ChimericN′1 gene and then used the resulting homozygous T1 seedlings with seamless sequence replacements for editing with the second region of the N′ gene. Then the T1 insertions were genotyped by PCR analyses to verify the insertions. In summary, these results showed that the targeted gene replacement could be transmitted to the T1 generation.
The gene replacement results encouraged us to replace the other resistance‐related region of the N′ gene. For this purpose, the homozygous T1 seedling harbouring the ChimericN′1 gene was used to initiate replacement with the second resistance‐related region. A similar procedure was conducted by using sgRNA3 and sgRNA4 to target N′ amino acids 1126 to 1240. Donor DNAs were amplified by PCR with the chemically modified DonorF2 and DonorR2 primers (Figure 4d). The donor DNA termini were also designed by synonymous mutations to eliminate the PAM site (Figure 4d).
After protoplast transfections and regenerations, DNA was extracted from the resulting lines and genotyped. DNA fragments were amplified with the primer pairs N′F2/N′R3, N′F2/N′alataR2, and N′alataF2/N′R4 that flank the target sites of sgRNA3 and sgRNA4 as shown in Figure 4d (Note the primer sequences in Table S7). N′F2, N′R2, and N′R3 were designed according to a common sequence shared by N′ and N′alata, whereas N′alataR2 and N′alataF2 were designed to anneal specifically to the N′alata sequence (Figure S2).
The targeted replacements were expected to produce an amplicon of 236 bp with the N′F2 and N′alataR2 primers, and an amplicon of 495 bp was anticipated with the N′alataF2 and N′R4 primers. PCR verification assays confirmed that 10 of 10 T0 lines had replacements. One line (No. 25) had a seamless replacement, but the other nine lines had indels at the 5′ or 3′ junctions of lines with ChimericN′2 gene structures (Figure 4b,e).
To verify whether the lines with sequence replacements were homozygous, the primer pairs N′F2/N′R3 and N′F2/N′R4 (Figure 4f) were used for genotyping. N′ alleles without replacements had a 286 bp fragment amplification with the N′F2 and N′R3 primers. N′ alleles with deletions between the sgRNA3 and sgRNA4 sites contained a 527 bp fragment, whereas contact alleles had an 871 bp amplicon when the N′F2 and N′R4 primers were used for PCR.
Twenty tested lines were heterozygous according to the PCR results (Figure 4f), and 14 of the 20 lines were shown to be resistant by phenotypic analyses. In addition, a gene replacement only occurred in one of the N′ alleles. In six lines, deletions between sgRNA3 and sgRNA4 were verified in one of the N′ alleles. Subsequently, homozygous T1 seedlings were obtained by self‐pollinating T0 plant number 25, which harboured the ChimericN′2 structure (Figure 4d).
The resulting T1 homozygous lines were subjected to resistance testing by transiently expressing the CPs from TMV‐Cg (CgCP) and TMV‐U1 (U1CP) in wild‐type N. tabacum and homozygous T1 seedlings harbouring ChimericN′1 and ChimericN′2. The ChimericN′2 lines exhibited an HR in response to both CgCP and U1CP, but the wild‐type and ChimericN′1 lines only developed an HR in response to CgCP (Figure 4g). Then, wild‐type T1 N. tabacum, seedlings at the six‐leaf stage that harboured ChimericN′1 and ChimericN′2 were inoculated with purified TMV‐U1 virus for resistance evaluation (Figure 5a). At 3 days post inoculation (dpi), only the ChimericN′2 line exhibited an HR on inoculated leaves, whereas the wild‐type and the ChimericN′1 lines failed to develop lesions (Figure 5b). At 13 dpi, wild‐type and ChimericN′1 lines developed typical mosaic symptoms on the upper uninoculated leaves, but symptoms were not observed in the ChimericN′2 line (Figure 5c,d). Western blotting was performed to assess the accumulation of TMV CP in the inoculated seedlings. These results showed that the TMV CP was only present in the wild‐type and ChimericN′1 seedlings (Figure 5e). Thus, the two‐step replacement conferred resistance to TMV‐U1 strain in N. tabacum.
Figure 5.
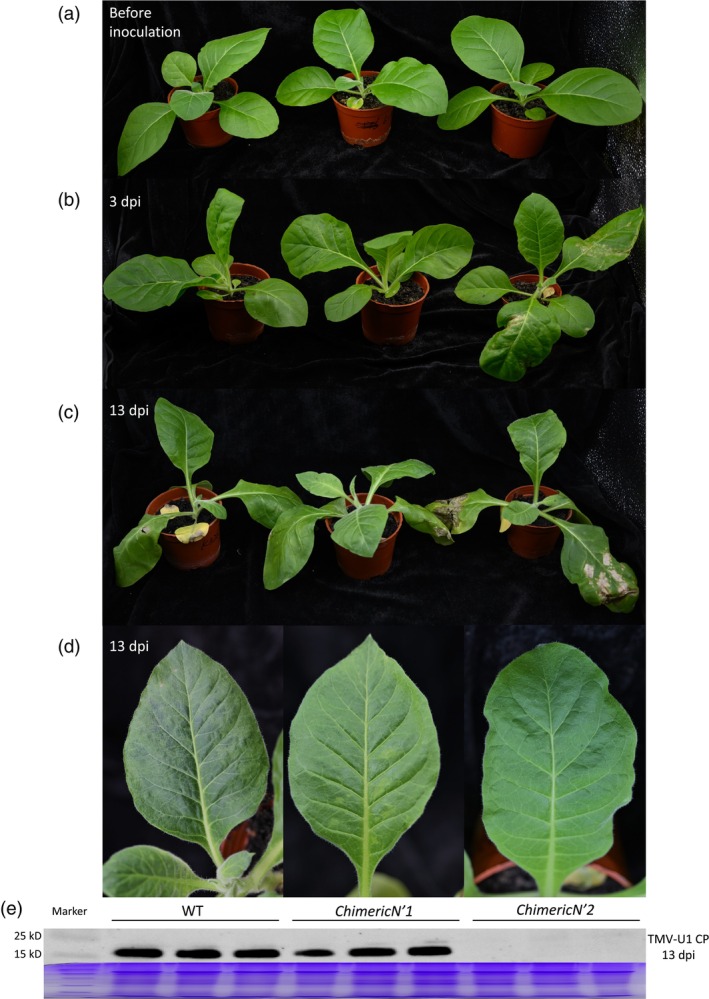
Resistance verification of edited N. tabacum seedlings. Virus inoculation assay was used for evaluating the resistance of genome‐edited N. tabacum. (a) Healthy seedlings of six‐leaf stage were used for virus inoculation assay. Wild‐type (WT) seedlings and seedlings with either the ChimericN′1 or ChimericN′2 gene were inoculated with the TMV‐U1 strain. (b) Three days post‐inoculation (dpi), seedlings with the ChimericN′2 gene exhibited a hypersensitive response (HR) on the inoculated leaves, while no symptoms were observed in WT or seedlings with ChimericN′1. (c, d) Thirteen dpi, upper leaves of WT and ChimericN′1 seedlings exhibited typical mosaic symptoms, but no symptoms were observed in seedlings with the ChimericN′2 gene. (e) Upper leaves of all three plant types were collected and analysed via western blotting to detect TMV CP. Results showed that TMV CP accumulated in upper leaves of WT and ChimericN′1 seedlings, while no TMV CP was detected in the upper leaves of seedlings with the ChimericN′2 gene.
To determine whether the Cas9 structure was integrated into the N. tabacum genome, all 20 T0 lines with targeted replacements were subjected to PCR verification with the Cas9‐F and Cas9‐R primers, but none of these lines harboured the Cas9 sequence (Table S6). We also tested all 450 lines in the first step replacement, and only 3 of these contained Cas9. This result suggests that there is a low probability of Cas9 integration during protoplast‐mediated genome editing.
The chimeric status of the edited T0 lines was also assessed. All 20 lines with ChimericN′1 or ChimericN′2 genes were self‐pollinated, and the progeny were genotyped. In most incidences, targeted insertions in the T0 plants were transmitted to the T1 progenies in a Mendelian fashion, suggesting that the T0 plants were not chimeras.
We also used qPCR to determine whether off‐target insertions occurred in the 20 T0 lines. After the first step of editing, four lines contained 2 to 4 copies of the 1819 bp fragment and six lines contained one copy. After the second editing step, all lines contained 2 to 8 copies of the 344 bp fragment (Table S6).
Our results show that our protoplast‐mediated genome editing system can create seamless replacements of targeted gene fragments to generate transgene‐free plants with desired traits and that the targeted insertions can be transmitted to progeny plants.
Discussion
The CRISPR/Cas system in plants usually uses Agrobacterium tumefaciens or biolistic methods for Cas and sgRNA‐related genomic DNA deliveries, both of which can create GMO concerns. Some efforts have been made to avoid GMO problems by using modified particle bombardment methods to achieve DNA‐free genome editing (Banakar et al., 2020; Liang et al., 2018; Svitashev et al., 2016), but application of these methods has been quite limited due to execution complexities. Compared with conventional methods, protoplast transfection enables delivery of preassembled RNP complexes and sgRNAs for direct DNA‐free genome editing, and also has a high delivery efficiency and capacity for transient RNP expression. These properties eliminate GMO concerns and greatly facilitate genome editing applications (Andersson et al., 2018; Hsu et al., 2021; Lin et al., 2018; Woo et al., 2015). Another advantage of applying protoplast methods for genome editing is that multiple plasmids and donor DNA structures can be introduced simultaneously, which permits multiple‐site editing (Lin et al., 2022).
Although the first protoplast regeneration of tobacco was reported 50 years ago (Takebe et al., 1971), this process is still a major bottleneck for genome editing because protoplast regeneration rates are reduced dramatically after PEG‐mediated transfection. To address these limitations, we have optimized a series of steps such as protoplast isolation, PEG‐mediated transfection, antibiotic selection, and plant regeneration. These advances have enabled us to develop a stable protoplast editing system for seamless gene insertions that proved to be suitable for the replacement of an 1819 bp fragment in the coding sequence of a functional gene.
In this study, we used CRISPR/Cas9‐related plasmids and donor DNA to transfect protoplasts. With this procedure, the Cas9 protein was transiently expressed rather than stably integrated into the plant genome. Therefore, screening regenerated plants, lines without transgenic components could be obtained directly, which circumvents many policy and regulatory restrictions and expands gene editing applications. However, previous reports have suggested that there is still a small possibility of stable genomic transformation during protoplast transfection (Lin et al., 2018). Therefore, after genome editing, we probed 470 transfected N. tabacum lines for the Cas9 sequence and found only 3 that harboured Cas9 DNA. Thus, the probability is relatively low (3 of 470 T0 seedlings) that edited plants regenerated from protoplasts will contain transgenic components, and this scenario is easy to detect by PCR screening. Our protoplast gene editing system could also use CRISPR ribonucleoproteins to avoid CRISPR‐related plasmids; hence our gene editing process can be completed without introducing any foreign DNA, as previously reported for another system (Lin et al., 2022).
Although some researchers have obtained gene insertions in N. benthamiana, via protoplast regenerations with DNA donors, only short fragments of ~60 nt were inserted into the protoplasts, and additional agronomic traits were not generated (Hsu et al., 2021). Therefore, we initially attempted to use ~700 bp GFP plasmids as donor DNAs for protoplast transfections, but GFP was not detected after whole plant regeneration. The presumed reason for this is that the longer fragments are prone to degradation during transfection. Therefore, we subsequently used a chemically modified donor to obtain end protection and promote ligation efficiency. In order to reduce the length limitations for DNA fragments, we incorporated chemical modifications at each end by using PCR to amplify fragments with 5′‐phosphorylated primers that contained two phosphorothioate linkages at the 5′ end.
The previously targeted editing of rice achieved fragment insertions as long as 2049 bp, but the fragments had indels at both ends (Lu et al., 2020). Therefore, the PAM sites for large fragment insertions in rice can only be selected in introns or untranslated regions to avoid disrupting the exon regions of targeted loci. The latest PrimeRoot report established an efficient long fragment insertion system in which a 11.1 kb insertion cassette was introduced into an untranslated region of the rice genome (Sun et al., 2023), but these insertions were not seamless and did not replace a gene of interest. In contrast, our gene editing system achieved seamless 1819 bp gene replacements, although most of our targeted insertions had indels at the junctions. However, plantlets with seamless replacements could be easily identified by PCR screening.
We used PEG‐mediated transfection for the CRISPR/Cas9 vector and donor DNA deliveries to recover 10 lines with 1819 bp fragment insertions out of 450 T0 plants. In contrast, targeted insertions in rice used bombardment for plasmid and donor deliveries, which resulted in 3 lines with targeted insertion out of 76 T0 plants (Lu et al., 2020). Considering that we only screened for forward insertions, we achieved comparable insertion efficiencies for long fragments compared with previous reports (Li et al., 2016; Lu et al., 2020; Sun et al., 2023). Furthermore, we were able to achieve seamless editing of exons within functional genes, so this achievement broadens genome editing applications in plants.
Protoplast transfections effectively provide high‐concentration donor deliveries, improve on‐target genome editing efficiencies and increase off‐target editing. According to our off‐target assessment results, longer fragment insertions had low copy numbers and a possibility of off‐target insertions, but high copy numbers of off‐target insertions also occurred when short fragments were inserted (Table S6). Although off‐target insertions can potentially limit breeding applications, the off‐target rates can usually be alleviated by selecting sgRNAs with higher specificity. However, this was not feasible in our study because the targeted insertions had to be conducted within specific N′ coding sequence regions where sgRNA selections were quite limited. However, this limitation can be addressed by screening larger numbers of T0 lines to select those with fewer copies and by crossing to reduce insertion frequencies.
Other genome editing methods commonly result in chimeric T0 plants, but our system had a very low frequency of chimeric lines. A possible reason is that the genome‐edited plants in our system were derived from single protoplasts and that the low levels of individual transfections effectively avoided chimerism in plants regenerated from the protoplasts.
This study constructed chimeric genes between the TMV‐U1 and TMV‐Cg resistant gene N′alata and the TMV‐Cg resistant gene N′. This helped us understand which regions of the N′ orthologs determine the recognition specificity of TMV‐U1 and TMV‐Cg. Transient expression results showed that only chimeric genes containing both the B′CD region (amino acids 265–798) and the G′ region (amino acids 1130–1233) from the N′alata gene could induce HR reactions when co‐expressed with the TMV‐U1 CP, whereas other chimeric genes did not have TMV‐U1 recognition specificity (Figure 3). Although most studies show that the LRR region determines the recognition specificity of disease resistance genes (Caplan et al., 2008; Ng and Xavier, 2011), our study indicates that the resistance‐determining regions that broaden the resistance spectrum of N′alata gene occur in both the NB‐ARC and the LRR domains (Figure 3), which may be caused by point mutation accumulations in regions B′CD and G′. Subsequent studies may be needed to further locate the resistance‐determining motifs and amino acids, which could help reveal the resistance mechanisms of N′ orthologs in Solanaceae plants.
Collectively, by combining the latest advances in gene insertion methods and the advantages of protoplast technologies for gene editing, we have developed an elegant system for gene insertions and replacements in N. tabacum. Although our method was more efficient for inserting short fragments, an 1819 bp fragment was also inserted. Overall, the approaches developed in our study provide a versatile tool for precise genome editing in the Solanaceae and enable efficient insertions of long DNA fragments into targeted sites. The simplicity and robustness of our methods will help advance precise genome editing during plant research and breeding, and the optimization steps can easily be applied to other research projects.
Experimental procedures
Plasmid constructions
First, we designed AtU6 and AtU3b promoter‐driven RNA scaffold expression modules, with multiple digestion sites at the 5′ and 3′ ends. These designs were sent to BGI (Beijing Gene Technology Corporation) for generation of the AtU6‐sgRNA‐CRR and AtU3b‐sgRNA‐CRR scaffold structure vectors with BsaI promoters.
BGI also synthesized a ptAtUBQCas9 module containing a 680 bp AtUBQ1 promoter, a code‐optimized Cas9 gene and a 208 bp AtUBQ1 terminator as described for Arabidopsis (Mao et al., 2013; Zhang et al., 2016). BGI next inserted the modules into the XmaI‐KpnI sites of the pCAMBIA1300 binary vector to generate the plant binary gene editing vector.
CRISPR software (http://crispr.hzau.edu.cn/cgi‐bin/CRISPR2/SCORE) was also used to identify sgRNA1‐4 spacer sequences in the N′ gene coding region. The sgRNA1 and sgRNA2 primer pairs (Nsg1‐F/Nsg1‐R and Nsg2‐F/Nsg2‐R) were annealed and ligated into BsaI‐digested AtU6‐sgRNA‐CRR and the AtU3b‐sgRNA‐CRR plasmids to generate the AtU6‐sgRNA1‐CRR and At3b‐sgRNA2‐CRR structures. Then the two structures were fused to form the AtU6‐sgRNA1‐AtU3b‐sgRNA2 module. This module was next inserted into the 5′‐KpnI and 3′‐EcoRI sites of pCambia‐AtUbi‐Cas9 to provide the pCambia‐sgRNA1‐sgRNA2‐Cas9 dual editing construct used to edit the N′ gene in N. tabacum.
By use of the strategy described above, the AtU6‐sgRNA3‐AtU3b‐sgRNA4 module was constructed with the Nsg3‐F/Nsg3‐R and Nsg4‐F/Nsg4‐R primers and cloned into pCambia‐AtUbi‐Cas9 to generate pCambia‐sgRNA3‐sgRNA4‐Cas9 for targeting of the sgRNA3 and sgRNA4 sites. The PCR primers for these and other manipulations described below can be found in Table S7.
For gene swapping assays, N′ and N′alata were amplified with the N′F and N′R primers, and then inserted between the XhoI and XbaI sites of pHellsgate8 by homologous recombination. Chimeric structures were chemically synthesized and inserted into pHellsgate8 using the same strategy.
Plant materials
For protoplast isolation, N. tabacum seeds were sterilized with 70% ethanol for 1 min and 20% bleach solution for 15 min, and the resulting seeds were washed with sterile water and placed on agar plates containing plant growth media (see Tables S1–S5). The germinated seedlings were then grown in a sterile environment under controlled growth conditions at 20–25 °C under 16 h of light at ~75 μmol photons/m2/s and 8 h of darkness.
Agrobacterium infiltration
For transient expression assays, plasmids encoding recombinant N′ or N′alata genes, the TMV‐Cg CP and the TMV‐U1 CP proteins were introduced into Agrobacterium strain EHA105. The bacteria were grown overnight at 25 °C and diluted to 0.5 A600 density and infiltrated into N. benthamiana leaves immediately above the cotyledon with a 1‐mL needleless syringe (Yuan et al., 2016). Infiltrated plants were grown in soil in an environmentally controlled chamber as described above.
For reproducibility and optimal resistance verification, tested plants were less than 4 weeks old and at the 4‐ to 6‐leaf stage. At least 3 plants were used for each experiment, and all experiments were repeated three times.
Plant genotyping
Genomic DNA was extracted from the leaves by use of the FastPure Plant DNA Isolation Mini Kit (Vazyme Cat. DC104‐1) and used to genotype the T0 edited lines and progenies from the first‐step gene replacements. To identify targeted replacement events, two primer pairs (N′F1/N′alataR1 and N′alataF1/N′R2) that contained target‐specific and donor‐specific primers were used to determine the donor insertion directions. Mutant plants containing both PCR amplicons were counted as targeted insertion plants. The PCR products containing junctions between the targeted sequence and the expected donor sequences were selectively cloned into the pMD19‐T TA cloning vector (Takara) and sequenced.
To assess homozygotes, the (N′F1/N′R1 and N′F1/N′R2) primer pairs flanking the target sites were used to detect fragment deletions or unedited N′ alleles. PCR amplicons with N′F1/N′R1 primers indicated that at least one N′ allele was unedited and that the failure of PCR amplification suggested a deletion between the two targeted sites. Multiple PCR bands from the N′F1/N′R2 amplifications indicated that one allele had a fragment deletion, whereas the other allele was intact. The same strategy was used for the second gene replacement steps with the N′F2, N′R3, N′alataF2, N′alataR2, and N′R4 primers (Figures 4d and 3f).
To verify Cas9 sequence integrations, the Cas9‐F and Cas9‐R primers were used for PCR amplifications. Plants with a Cas9 sequence contained a 265 bp fragment that could be amplified with the Cas9‐F and Cas9‐R primers.
Protoplast isolation, transfection, and whole plant regeneration
Details of the protoplast procedures and reagents are described in Tables S1–S5.
Fluorescence signal counting and microscopy observations
Seven days after transfection, protoplast samples transfected with both the eGFP donor and the Cas9 plasmid, or transfected with only the eGFP donor as a control were collected and counted with a Countstar Rigel S3 cell analyser. Intact cells expressing the highest GFP levels were determined by the use of parameters set to suppress chloroplast autofluorescence and to emphasize the brightest eGFP signals. Dead cells and fake eGFP signals were separated from the intact fluorescing cells by a default recognition method.
For fluorescence observations, images were collected with a Zeiss Axio Imager Z2 fluorescence microscope. A 475 nm LED module was used to excite the GFP protein, and a 550 nm filter was used to block chloroplast autofluorescence. All settings for image acquisition were used for different experiments. On average, 20–30 fields of view were examined for each experiment.
TMV inoculation and Western blotting
For virus inoculation, purified TMV‐U1 was diluted to 0.1 mg/mL in PBS buffer, and celite was added to a final concentration of 1%. The virus solution was then gently rubbed onto N. tabacum leaves at a volume of 5 μL per leaf. Only the two largest leaves of six‐leaf‐stage seedlings were inoculated.
Western blotting was performed to detect TMV coat protein accumulation in the inoculated seedlings. Total protein was extracted from leaves of Nicotiana tabacum seedlings at 13 dpi using RIPA buffer (Thermo Fisher Scientific) supplemented with protease inhibitors (Roche). The protein concentration was determined using the Pierce BCA protein assay kit (Thermo Fisher Scientific).
Equal amounts of protein (20 μg) were separated by 12% SDS–PAGE and transferred onto PVDF membranes (Millipore). The membranes were blocked with EveryBlot blotting buffer (Bio‐Rad) for 1 h at room temperature and incubated for 2 h at 37 °C with TMV CP primary antibody prepared from rabbits at a dilution of 1 : 1000. After washing three times with PBS‐T buffer, the membranes were incubated with a secondary immunofluorescence anti‐rabbit antibody (Alexa Fluor™ Plus 800, Invitrogen) at a dilution of 1 : 10 000 for 1 h at 37 °C. After washing the membranes three times with PBS‐T buffer, protein bands were identified with the Bio‐Rad Chemidoc MP imaging system.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
C.Y. conceived the research project. C.Y. and S.Z. designed the experiments and supervised the research. Y.L., C.H. and Y.L. performed most of the protoplast transfection and plant regeneration experiments. Y.L., X.Y., X.S. and J.Z. developed the constructs. X.Y., X.S., H.Y. and Z.T. cloned target genes and identified resistance‐related regions. D.F. and B.X. performed resistance verification of genome‐edited lines and phenotyping of the mutants. C.Y. wrote the paper, and all authors revised it.
Supporting information
Figure S1 eGFP insertion verification.
Figure S2 Partial comparisons between N′ and N′alata sequences, and location of genotyping primers.
Figure S3 Sequencing results of T0 seedlings with targeted forward gene replacement.
Table S1 Protocol for isolation of N. tabacum protoplasts.
Table S2 Protocol for protoplast PEG transformation of N. tabacum.
Table S3 Protocol for N. tabacum protoplast regeneration.
Table S4 Protocol for N. tabacum shoot and root regeneration.
Table S5 Reagents list.
Table S6 Genotyping of plantlets for copy numbers of inserted fragments and Cas9 sequence.
Table S7 Primer list.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 31860499) and the key projects of YNTC (No. 2022530000241014, No. 2019530000241002). We acknowledge Professor Ralph Dewey from NC state university for kindly providing detailed protocol of protoplast regeneration. Professor Andrew Jackson from UC Berkeley is also acknowledged for editing the manuscript and long‐term support to our research.
References
- Andersson, M. , Turesson, H. , Olsson, N. , Fält, A.S. , Ohlsson, P. , Gonzalez, M.N. , Samuelsson, M. et al. (2018) Genome editing in potato via CRISPR‐Cas9 ribonucleoprotein delivery. Physiol. Plant. 164, 378–384. [DOI] [PubMed] [Google Scholar]
- Armario Najera, V. , Twyman, R.M. , Christou, P. and Zhu, C. (2019) Applications of multiplex genome editing in higher plants. Currr. Opin. Biotechnol. 59, 93–102. [DOI] [PubMed] [Google Scholar]
- Baltes, N.J. , Gil‐Humanes, J. , Cermak, T. , Atkins, P.A. and Voytas, D.F. (2014) DNA replicons for plant genome engineering. Plant Cell 26, 151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banakar, R. , Schubert, M. , Collingwood, M. , Vakulskas, C. , Eggenberger, A.L. and Wang, K. (2020) Comparison of CRISPR‐Cas9/Cas12a ribonucleoprotein complexes for genome editing efficiency in the rice phytoene desaturase (OsPDS) gene. Rice 13, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikova, M. , Beumer, K. , Trautman, J.K. and Carroll, D. (2003) Enhancing gene targeting with designed zinc finger nucleases. Science 300, 764. [DOI] [PubMed] [Google Scholar]
- Caplan, J. , Padmanabhan, M. and Dinesh‐Kumar, S.P. (2008) Plant NB‐LRR immune receptors: from recognition to transcriptional reprogramming. Cell Host Microbe 3, 126–135. [DOI] [PubMed] [Google Scholar]
- Čermák, T. , Baltes, N.J. , Čegan, R. , Zhang, Y. and Voytas, D.F. (2015) High‐frequency, precise modification of the tomato genome. Genome Biol. 16, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, D.B.T. , Gootenberg, J.S. , Abudayyeh, O.O. , Franklin, B. , Kellner, M.J. , Joung, J. and Zhang, F. (2017) RNA editing with CRISPR‐Cas13. Science 358, 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey, M.R. , Anthony, P. , Power, J.B. and Lowe, K.C. (2005) Plant protoplast technology: current status. Acta Physiologiae Plantarum 27, 117–130. [Google Scholar]
- Gao, C. (2021) Genome engineering for crop improvement and future agriculture. Cell 184, 1621–1635. [DOI] [PubMed] [Google Scholar]
- Hsu, C.‐T. , Yuan, Y.‐H. , Lin, Y.‐C. , Lin, S. , Cheng, Q.‐W. , Wu, F.‐H. , Sheen, J. et al. (2021) Efficient and economical targeted insertion in plant genomes via protoplast regeneration. CRISPR J. 4, 752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, Y.Y. , Lee, H.‐Y. , Kim, S.W. , Noh, Y.‐S. and Seo, P.J. (2021) Optimization of protoplast regeneration in the model plant Arabidopsis thaliana . Plant Methods 17, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, S. , Lin, Q. , Luo, Y. , Zhu, Z. , Liu, G. , Li, Y. , Chen, K. et al. (2021) Genome‐wide specificity of prime editors in plants. Nat. Biotechnol. 39, 1292–1299. [DOI] [PubMed] [Google Scholar]
- Jinek, M. , Chylinski, K. , Fonfara, I. , Hauer, M. , Doudna, J.A. and Charpentier, E. (2012) A programmable dual‐RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar, S. , Das, P. , Panda, D. , Xie, K. , Baig, M.J. and Molla, K.A. (2022) A detailed landscape of CRISPR‐Cas‐mediated plant disease and pest management. Plant Sci. 323, 111376. [DOI] [PubMed] [Google Scholar]
- Khan, Z.A. , Kumar, R. and Dasgupta, I. (2022) CRISPR/Cas‐mediated resistance against viruses in plants. Int. J. Mol. Sci. 23, 2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Meng, X. , Zong, Y. , Chen, K. , Zhang, H. , Liu, J. , Li, J. et al. (2016) Gene replacements and insertions in rice by intron targeting using CRISPR–Cas9. Nat. Plants 2, 16139. [DOI] [PubMed] [Google Scholar]
- Li, C. , Zhang, R. , Meng, X. , Chen, S. , Zong, Y. , Lu, C. , Qiu, J.‐L. et al. (2020) Targeted, random mutagenesis of plant genes with dual cytosine and adenine base editors. Nat. Biotechnol. 38, 875–882. [DOI] [PubMed] [Google Scholar]
- Li, S. , Lin, D. , Zhang, Y. , Deng, M. , Chen, Y. , Lv, B. , Li, B. et al. (2022) Genome‐edited powdery mildew resistance in wheat without growth penalties. Nature 602, 455–460. [DOI] [PubMed] [Google Scholar]
- Liang, Z. , Chen, K. , Zhang, Y. , Liu, J. , Yin, K. , Qiu, J.‐L. and Gao, C. (2018) Genome editing of bread wheat using biolistic delivery of CRISPR/Cas9 in vitro transcripts or ribonucleoproteins. Nat. Protocol 13, 413–430. [DOI] [PubMed] [Google Scholar]
- Lin, C.‐S. , Hsu, C.‐T. , Yang, L.‐H. , Lee, L.‐Y. , Fu, J.‐Y. , Cheng, Q.‐W. , Wu, F.‐H. et al. (2018) Application of protoplast technology to CRISPR/Cas9 mutagenesis: from single‐cell mutation detection to mutant plant regeneration. Plant Biotechnol. J. 16, 1295–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Q. , Jin, S. , Zong, Y. , Yu, H. , Zhu, Z. , Liu, G. , Kou, L. et al. (2021) High‐efficiency prime editing with optimized, paired pegRNAs in plants. Nat. Biotechnol. 39, 923–927. [DOI] [PubMed] [Google Scholar]
- Lin, C.‐S. , Hsu, C.‐T. , Yuan, Y.‐H. , Zheng, P.‐X. , Wu, F.‐H. , Cheng, Q.‐W. , Wu, Y.‐L. et al. (2022) DNA‐free CRISPR‐Cas9 gene editing of wild tetraploid tomato Solanum peruvianum using protoplast regeneration. Plant Physiol. 188, 1917–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Zeng, J. , Yuan, C. , Guo, Y. , Yu, H. , Li, Y. and Huang, C. (2019) Cas9‐PF, an early flowering and visual selection marker system, enhances the frequency of editing event occurrence and expedites the isolation of genome‐edited and transgene‐free plants. Plant Biotechnol. J. 17, 1191–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. , Tian, Y. , Shen, R. , Yao, Q. , Wang, M. , Chen, M. , Dong, J. et al. (2020) Targeted, efficient sequence insertion and replacement in rice. Nat. Biotechnol. 38, 1402–1407. [DOI] [PubMed] [Google Scholar]
- Ma, W. , Xu, Y.‐S. , Sun, X.‐M. and Huang, H. (2021) Transposon‐associated CRISPR‐Cas system: a powerful DNA insertion tool. Trends Microbiol. 29, 565–568. [DOI] [PubMed] [Google Scholar]
- Mao, Y. , Zhang, H. , Xu, N. , Zhang, B. , Gou, F. and Zhu, J.K. (2013) Application of the CRISPR‐Cas system for efficient genome engineering in plants. Mol. Plant 6, 2008–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao, Z. , Lu, Z. , Wu, H. , Liu, H. , Li, M. , Lei, D. , Zheng, L. et al. (2018) Collagen, agarose, alginate, and Matrigel hydrogels as cell substrates for culture of chondrocytes in vitro: a comparative study. J. Cell. Biochem. 119, 7924–7933. [DOI] [PubMed] [Google Scholar]
- Miki, D. , Zhang, W. , Zeng, W. , Feng, Z. and Zhu, J.‐K. (2018) CRISPR/Cas9‐mediated gene targeting in Arabidopsis using sequential transformation. Nat. Commun. 9, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, A. and Xavier, R.J. (2011) Leucine‐rich repeat (LRR) proteins: integrators of pattern recognition and signaling in immunity. Autophagy 7, 1082–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, C. , Li, G. , Malzahn, A.A. , Cheng, Y. , Leyson, B. , Sretenovic, S. , Gurel, F. et al. (2022) Boosting plant genome editing with a versatile CRISPR‐Combo system. Nat. Plants 8, 513–525. [DOI] [PubMed] [Google Scholar]
- Puchta, H. , Dujon, B. and Hohn, B. (1996) Two different but related mechanisms are used in plants for the repair of genomic double‐strand breaks by homologous recombination. Proc. Natl. Acad. Sci. 93, 5055–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reider Apel, A. , d'Espaux, L. , Wehrs, M. , Sachs, D. , Li, R.A. , Tong, G.J. , Garber, M. et al. (2017) A Cas9‐based toolkit to program gene expression in Saccharomyces cerevisiae. Nucleic Acids Res. 45, 496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan, C. , Diouf, I. and Causse, M. (2019) Trait discovery and editing in tomato. Plant J. 97, 73–90. [DOI] [PubMed] [Google Scholar]
- Sekine, K.‐T. , Tomita, R. , Takeuchi, S. , Atsumi, G. , Saitoh, H. , Mizumoto, H. , Kiba, A. et al. (2012) Functional differentiation in the leucine‐rich repeat domains of closely related plant virus‐resistance proteins that recognize common avr proteins. Mol. Plant Microbe Interact. 25, 1219–1229. [DOI] [PubMed] [Google Scholar]
- Shen, J.P. , Zhao, D. , Sasik, R. , Luebeck, J. , Birmingham, A. , Bojorquez‐Gomez, A. , Licon, K. et al. (2017) Combinatorial CRISPR‐Cas9 screens for de novo mapping of genetic interactions. Nat. Methods 14, 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimatani, Z. , Nishizawa‐Yokoi, A. , Endo, M. , Toki, S. and Terada, R. (2015) Positive–negative‐selection‐mediated gene targeting in rice. Front. Plant Sci. 5, 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, V.K. , Doyon, Y. , Miller, J.C. , DeKelver, R.C. , Moehle, E.A. , Worden, S.E. , Mitchell, J.C. et al. (2009) Precise genome modification in the crop species Zea mays using zinc‐finger nucleases. Nature 459, 437–441. [DOI] [PubMed] [Google Scholar]
- Stuttmann, J. , Barthel, K. , Martin, P. , Ordon, J. , Erickson, J.L. , Herr, R. , Ferik, F. et al. (2021) Highly efficient multiplex editing: one‐shot generation of 8× Nicotiana benthamiana and 12× Arabidopsis mutants. Plant J. 106, 8–22. [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Zhang, X. , Wu, C. , He, Y. , Ma, Y. , Hou, H. , Guo, X. et al. (2016) Engineering herbicide‐resistant rice plants through CRISPR/Cas9‐mediated homologous recombination of acetolactate synthase. Mol. Plant 9, 628–631. [DOI] [PubMed] [Google Scholar]
- Sun, C. , Lei, Y. , Li, B. , Gao, Q. , Li, Y. , Cao, W. , Yang, C. et al. (2023) Precise integration of large DNA sequences in plant genomes using PrimeRoot editors. Nat. Biotechnol. 10.1038/s41587-023-01769-w [DOI] [PubMed] [Google Scholar]
- Svitashev, S. , Young, J.K. , Schwartz, C. , Gao, H. , Falco, S.C. and Cigan, A.M. (2015) Targeted mutagenesis, precise gene editing, and site‐specific gene insertion in maize using Cas9 and guide RNA. Plant Physiol. 169, 931–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitashev, S. , Schwartz, C. , Lenderts, B. , Young, J.K. and Mark Cigan, A. (2016) Genome editing in maize directed by CRISPR–Cas9 ribonucleoprotein complexes. Nat. Commun. 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe, I. , Labib, G. and Melchers, G. (1971) Regeneration of whole plants from isolated mesophyll protoplasts of tobacco. Naturwissenschaften 58, 318–320. [Google Scholar]
- Taraporewala, Z.F. and Culver, J.N. (1996) Identification of an elicitor active site within the three‐dimensional structure of the tobacco mosaic tobamovirus coat protein. Plant Cell 8, 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend, J.A. , Wright, D.A. , Winfrey, R.J. , Fu, F. , Maeder, M.L. , Joung, J.K. and Voytas, D.F. (2009) High‐frequency modification of plant genes using engineered zinc‐finger nucleases. Nature 459, 442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, J.W. , Kim, J. , Kwon, S.I. , Corvalán, C. , Cho, S.W. , Kim, H. , Kim, S.‐G. et al. (2015) DNA‐free genome editing in plants with preassembled CRISPR‐Cas9 ribonucleoproteins. Nat. Biotechnol. 33, 1162–1164. [DOI] [PubMed] [Google Scholar]
- Yamamoto, T. , Kashojiya, S. , Kamimura, S. , Kameyama, T. , Ariizumi, T. , Ezura, H. and Miura, K. (2018) Application and development of genome editing technologies to the Solanaceae plants. Plant Physiol. Biochem. 131, 37–46. [DOI] [PubMed] [Google Scholar]
- Yoo, S.D. , Cho, Y.H. and Sheen, J. (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572. [DOI] [PubMed] [Google Scholar]
- Yu, Q.‐H. , Wang, B. , Li, N. , Tang, Y. , Yang, S. , Yang, T. , Xu, J. et al. (2017) CRISPR/Cas9‐induced targeted mutagenesis and gene replacement to generate long‐shelf life tomato lines. Sci. Rep. 7, 11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, X. , Yan, C. , Wu, Z. , Ren, F. , Zhang, H. , Baker, B. , Chen, J. et al. (2015) Frequent gain and loss of resistance against tobacco mosaic virus in nicotiana species. Mol. Plant 8, 1813–1815. [DOI] [PubMed] [Google Scholar]
- Yuan, C. , Lazarowitz, S.G. and Citovsky, V. (2016) Identification of a functional plasmodesmal localization signal in a plant viral cell‐to‐cell‐movement protein. mBio 7, e02052‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Mao, Y. , Ha, S. , Liu, W. , Botella, J.R. and Zhu, J.K. (2016) A multiplex CRISPR/Cas9 platform for fast and efficient editing of multiple genes in Arabidopsis. Plant Cell Rep. 35, 1519–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Wang, J. , Wang, Z. , Zhang, Y. , Shi, S. , Nielsen, J. and Liu, Z. (2019) A gRNA‐tRNA array for CRISPR‐Cas9 based rapid multiplexed genome editing in Saccharomyces cerevisiae . Nat. Commun. 10, 1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, Z. , Zhang, Y. , You, Q. , Tang, X. , Ren, Q. , Liu, S. , Yang, L. et al. (2018) Plant genome editing using FnCpf1 and LbCpf1 nucleases at redefined and altered PAM sites. Mol. Plant 11, 999–1002. [DOI] [PubMed] [Google Scholar]
- Zhu, H. , Li, C. and Gao, C. (2020) Applications of CRISPR–Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 21, 661–677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 eGFP insertion verification.
Figure S2 Partial comparisons between N′ and N′alata sequences, and location of genotyping primers.
Figure S3 Sequencing results of T0 seedlings with targeted forward gene replacement.
Table S1 Protocol for isolation of N. tabacum protoplasts.
Table S2 Protocol for protoplast PEG transformation of N. tabacum.
Table S3 Protocol for N. tabacum protoplast regeneration.
Table S4 Protocol for N. tabacum shoot and root regeneration.
Table S5 Reagents list.
Table S6 Genotyping of plantlets for copy numbers of inserted fragments and Cas9 sequence.
Table S7 Primer list.


