Abstract
Background
Nonrandomized studies of unilateral nerve-sparing (UNS) radical prostatectomy (RP) have reported improved recovery of erectile function if the sacrificed cavernous nerve is reconstructed with a sural nerve graft (SNG).
Objective
To determine whether UNS RP plus SNG results in a 50% relative increase in potency at 2 yr compared to UNS RP alone.
Design, setting, and participants
The study enrolled patients from October 2001[en]May 2006 from a single academic center and was randomized, open label. Participants were men with localized prostate cancer recommended for UNS RP, less than 66 yr old, normal baseline erectile function, and willing to participate in early erectile dysfunction (ED) therapy. Patients were followed up to 2 yr.
Intervention
Patients underwent UNS RP and ED therapy starting at 6 wk: oral prostaglandin type-5 (PDE5) inhibitor, vacuum erection device (VED), and intracavernosal injection therapy. In the SNG group, a plastic surgeon performed the procedure at the time of RP.
Measurements
The ability to have an erection suitable for intercourse with or without a PDE5 inhibitor at 2 yr. The hypothesis was that SNG would result in a 60% potency rate compared to 40% for controls (80% power, 5% two-way significance).
Results and Limitations
The trial planned to enroll 200 patients, but an interim analysis at 107 patients met criteria for futility and the trial was closed. For patients completing the protocol to 2 yr, potency was recovered in 32 of 45 (71%) of SNG and 14 of 21 (67%) of controls (p = 0.777). By intent-to-treat analysis, potency recovered in 32 of 66 (48.5%) of SNG and 14 of 41 (34%) of controls (p = 0.271). No differences were seen in time to potency or quality of life scores for ED and urinary function. Limitations included slower-than-expected accrual and poor compliance with ED therapy: <65% for VED and <40% for injections.
Conclusions
The addition of SNG to a UNS RP did not improve potency at 2 yr following surgery.
Trial Registration
ClinicalTrials.gov, Identifier: NCT00080808, http://www.clinicaltrials.gov/ct2/show/NCT00080808?term=NCT00080808&rank=1
Keywords: sural nerve, prostatectomy, erectile dysfunction, prostate cancer
1. Introduction
In 1999, Kim et al [1] reported a small series of patients with locally advanced prostate cancer treated by bilateral non[en]nerve-sparing radical prostatectomy (RP) with bilateral cavernous nerve reconstruction with sural nerve grafting (SNG). Further follow-up [2], as well as a series from our institution [3], confirmed erectile activity rates of 40[en]60% compared to historically observed rates of <5% without SNG. Spontaneous erections generally occurred 18[en]24 mo postoperatively. With prostate-specific antigen (PSA) screening, many newly diagnosed men with prostate cancer have early-stage disease [4] and can undergo a bilateral nerve-sparing prostatectomy. However, some can be predicted to have elevated risk for extraprostatic extension [5] and are better treated with wide resection of one or more neurovascular bundles. The expected outcomes of potency after radical prostatectomy are reviewed by Dubbelman et al [6] and range from 31[en]86% for bilateral nerve-sparing technique; outcomes will also vary by age and pretreatment function. The SNG technique represented a possible solution in that selected patients could undergo a radical prostatectomy with wider surgical margins while being reconstructed to improve the chance of recovery of spontaneous erections.
The more common scenario for a man with elevated risk of extraprostatic disease is for a dominant focus of cancer on one side. The patient may then be recommended for a unilateral nerve sparing (UNS) on the side with less risk and a non[en]nerve-sparing technique on the side of the dominant focus. With the technique of bilateral SNG described, the application of a unilateral SNG became the next topic of study. However, measuring the efficacy of the combination of UNS plus SNG is more challenging, as the UNS alone has the potential to spare potency, and the populations of patients may have different baseline erectile function and utilization of treatments for erectile dysfunction (ED). Therefore, we sought to study the efficacy of unilateral SNG in the context of a randomized trial, with all patients instructed to undergo early ED therapy as “penile rehabilitation.”
2. Methods
This single-institution study was approved by the University of Texas M.D. Anderson Cancer Center Institutional Review Board. Informed consent was obtained from each patient. The primary objective was to determine if unilateral SNG would result in an increased potency rate 2 yr after unilateral nerve-sparing (UNS) RP to 60% compared to 40% for UNS RP alone; both groups would undergo the same early ED therapy. The definition of potency after surgery used to asses the primary objective was a patient history of a spontaneous erection suitable for intercourse with or without phosphodiesterase type 5 (PDE5) inhibitors. Secondary objectives included time to first spontaneous erection, quality of erections, urinary function, and the incidence of extraprostatic disease on the side of the nerve bundle removed. The rationale to assess urinary function outcomes derived from reports of an association between neurovascular bundle preservation and urinary control [7]. A subsequent retrospective study found improved recovery of urinary control after UNS RP with SNG compared to UNS alone [8].
Patients were recruited from M.D. Anderson Cancer Center urology clinics from faculty surgeons experienced in SNG technique. Key inclusion criteria included: (1) selection for a UNS procedure, (2) age under 66 yr, and (3) normal baseline erectile function as assessed by the patient’s self-described ability to have successful penetration on at least 75% of attempts. Guidelines for sparing one nerve included: (1) <3 positive cores on the side to be spared with no cancer in the apical core(s), and (2) Gleason score ≤6 in the cores on the side to be spared. There were no criteria for selecting a nerve for resection[em]this was left to faculty discretion. Patients were educated on the 12- to 18-mo potential time frame needed for graft nerve regeneration and plans for immediate postoperative penile rehabilitation. Exclusion criteria included significant psychiatric illness, history of pelvic irradiation, and use of androgen deprivation.
Baseline assessment of quality of life utilized the International Index of Erectile Function (IIEF-6; 6-question version; maximum 30 points) [9] and the University of California, Los Angeles Prostate Cancer Index (UCLA-PCI) Urinary Function and Bother scale [10]. The randomization method utilized an institutional electronic randomization process during which patient inclusion and exclusion criteria are entered and the system assigns them to a treatment arm. The randomization ratio was 3:2 (SNG to control) to an open-labeled treatment arm[em]there was no blinding regarding a patient’s treatment arm.
The treatment plan for all patients included open radical retropubic prostatectomy with preservation of one neurovascular bundle using athermal technique and wide excision of the contralateral bundle with the goal of removing all posterior-lateral vascular and neural tissue from the apex to the seminal vesicle. The Cavermap (Blue Torch Medical Technologies Inc, Ashland, MA) was used in many patients to locate the ideal sites of anastomosis of SNG grafts [11]. The surgeon then marked both proximal and distal graft sites with a small clip or suture ligature to assist the plastic surgeon.
Select plastic surgeons with prior SNG experience performed SNG harvesting using a minimal incision technique and reconstruction (D. W. Chang, oral communication). SNG anastomoses were performed using the epineural technique with 7-0 polypropylene sutures. The urology team then performed the vesico-urethral anastomosis to complete the procedure.
Postoperative week 6, patients were referred to a specialty ED clinic where treatment was described to them as “penile rehabilitation.” The plan included all of the following: sildenafil 50 mg every other day; a vacuum erection device (VED) for 10 min per d, 5 d per wk; and intracavernosal injection (ICI) therapy with Trimix (12.5 μg/mL prostaglandin E1, 4.5 mg/mL papaverine, 0.125 mg/mL phentolamine in saline) 2 times per wk, 3 d apart. Compliance with VED therapy was rated good, poor, or none based upon usage of 2[en]3/wk, 1/wk, or none, respectively. Compliance with injection therapy was rated as good, poor, or none based upon usage of 1[en]2/wk, 0[en]1/wk, or none, respectively.
Follow-up evaluations occurred every 4 mo for 2 yr or until return of spontaneous erectile activity. Evaluations included monitoring for disease recurrence, an interview by a research nurse to assess the primary study objective, patient-completed written quality-of-life questionnaires, and assessment of penile rehabilitation compliance and side effects.
Statistical considerations assumed a 2-yr 40% potency rate without SNG and a 60% potency rate with SNG. A sample size of 200 evaluable patients (120 SNG, 80 control) would provide a power of 80% to detect such a difference using a two-sided test at a 5% significance level. Two interim analyses were planned, using the O’Brien-Fleming stopping rule [12]. The nominal significance levels of sequential tests for proportions were set at 0.0002, 0.0120, and 0.0450, respectively. Interim analysis for futility was performed using Bayesian posterior probability. Descriptive analyses including proportion, mean, and standard deviation (SD) were used to summarize the data. Fisher’s exact test was used to assess the dependence between two categorical variables. The Wilcoxon signed-rank test was applied to examine the differences between IIEF-6 scores. Kaplan-Meier curves were plotted for time to recovery of potency for both treatment groups, and the log-rank test was used to assess the difference in time to recovery of potency between the two treatment groups. A p-value of 0.05 was used as a cut-off for statistical significance. All p-values are two-sided. We performed the statistical analyses using SAS 9.1.3 and S-PLUS 2000.
3. Results
After 107 patients were randomized in a 3:2 ratio (66 SNG, 41 controls) between October 2001 and May 2006, a protocol-planned interim analysis was performed. Analysis of evaluable patients (potent at any time or nonpotent with 2-yr follow-up) showed potency rates of 18 of 41 (44%) in the SNG group and 10 of 23 (43%) in the control group. Based upon slower-than-estimated accrual (8/mo planned vs 2/mo actual) and a <5% posterior probability that the groups would show a difference, early termination of the trial was recommended by the Data Monitoring Committee. From May 2006[en]May 2007, we obtained additional potency follow-up, including IIEF-6 surveys by clinic visit and/or telephone interviews with a research nurse, and updated the patient database for the 107 patients.
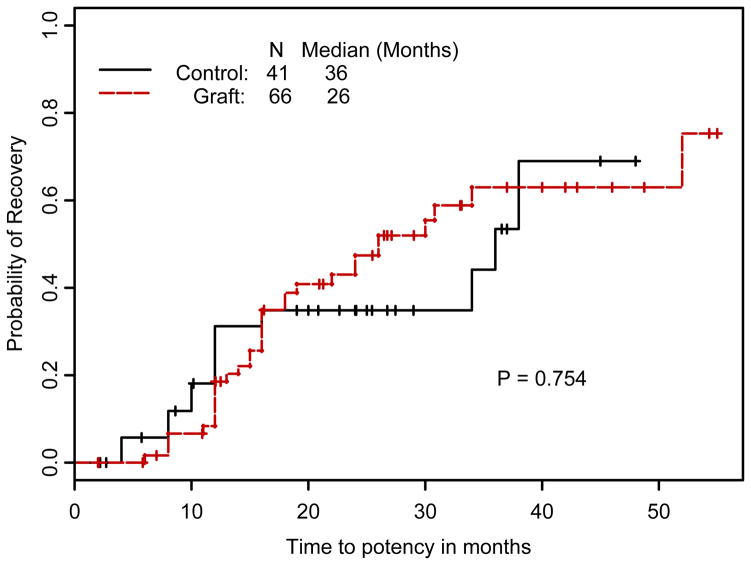
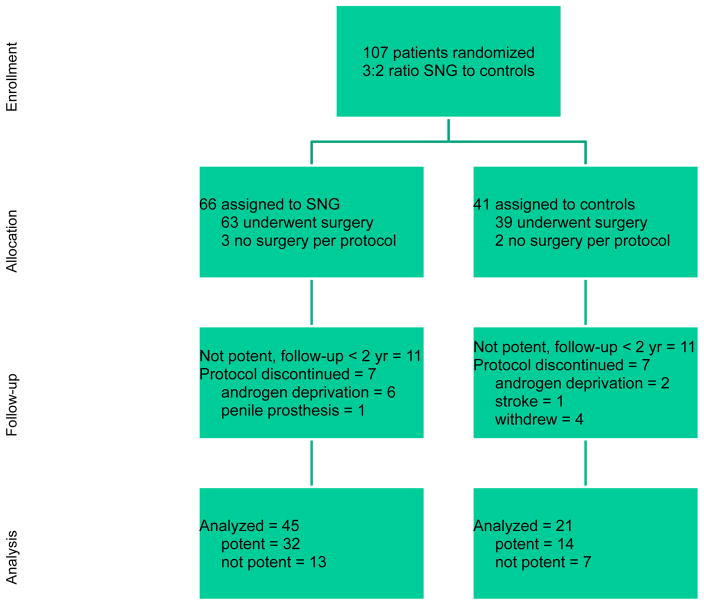
Table 1 shows baseline characteristics of the 107 randomized patients. Figure 1 shows the cumulative probability of potency recovery by treatment group. As shown at the bottom of Figure 2, the endpoints of potency or nonpotency with 2 yr of follow-up were reached by 45 SNG and 21 control patients. Eleven SNG and 11 control patients were still nonpotent with less than 2 yr follow-up but compliant. Finally, there were 10 SNG and 9 control patients who left the trial for reasons listed in Figure 2. Therefore, the outcome of the study can be reported in three ways:
Table 1.
[en] Patient characteristics of 107 patients randomized 3:2 to sural nerve grafting or control
| Characteristic | Sural nerve graft | Control | p-value |
|---|---|---|---|
|
| |||
| Total treated | 66 (62%) | 41 (38%) | |
|
| |||
| Mean age, yr (SD) | 57.3 (4.8) | 55.3 (6.1) | 0.165 |
|
| |||
| Race | 0.366 | ||
| Caucasian | 59 (89%) | 33 (80%) | |
| African American | 6 (9%) | 6 (15%) | |
| Other | 1 (2%) | 2 (5%) | |
|
| |||
| Clinical Stage | 0.297 | ||
| T1 | 37 (56%) | 17 (44%) | |
| T2 | 28 (42%) | 22 (56%) | |
| T3 | 1 (2%) | 0 | |
| Unknown | 0 | 2 | |
|
| |||
| Biopsy (Gleason score) | 0.394 | ||
| 3+3 | 17 (26%) | 8 (20%) | |
| 3+4 | 24 (36%) | 21 (51%) | |
| 4+3 | 17 (27%) | 10 (24%) | |
| 4+4 | 8 (12%) | 2 (5%) | |
|
| |||
| Number biopsy cores positive | Median (range) | Median (range) | |
| Side of NNS | 3 (1[en]9) | 3 (1[en]11) | 0.64 |
| Side of NS | 0 (0[en]4) | 0 (0[en]5) | 0.971 |
| Largest tumor focus (mm) | |||
| Side of NNS | 6 (1[en]14) | 8 (1[en]17) | 0.03 |
| Side of NS | 2 (1[en]8) | 1 (1[en]10) | 0.61 |
Abbreviations: NNS, non nerve sparing; NS, nerve sparing.
Figure 1.
Cumulative probability of potency recovery (intent to treat) by treatment group: 32 of 66 in SNG, median recovery 26 mo (95% CI of 18, 50+), and 14 of 41 controls, median recovery 36 mo (95% CI of 34, 50+).
Figure 2.
CONSORT flow diagram of enrollment, allocation, follow-up, and analyses of 107 patients randomized.
Endpoint analysis: Achieving potency at any time or nonpotency with 2-yr follow-up on protocol. By this definition, potency was achieved in 32 of 45 (71%) of SNG patients and 14 of 21 (67%) of control patients (p = 0.777).
Patients on protocol: Achieving potency at any time or nonpotency at any time and still on protocol. By this definition, potency was achieved in 32 of 56 (57%) of SNG patients and 14 of 32 (44%) of control patients (p = 0.164).
Intent to treat: The percentage of patients achieving potency out of all patients randomized to that group. By this definition, potency was achieved in 32 of 66 (48.5%) of SNG patients and 14 of 41 (34%) of control patients (p = 0.271).
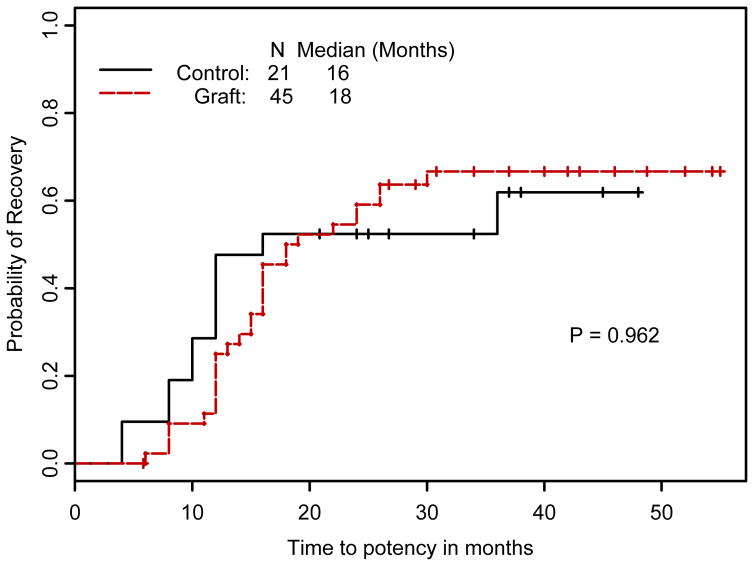
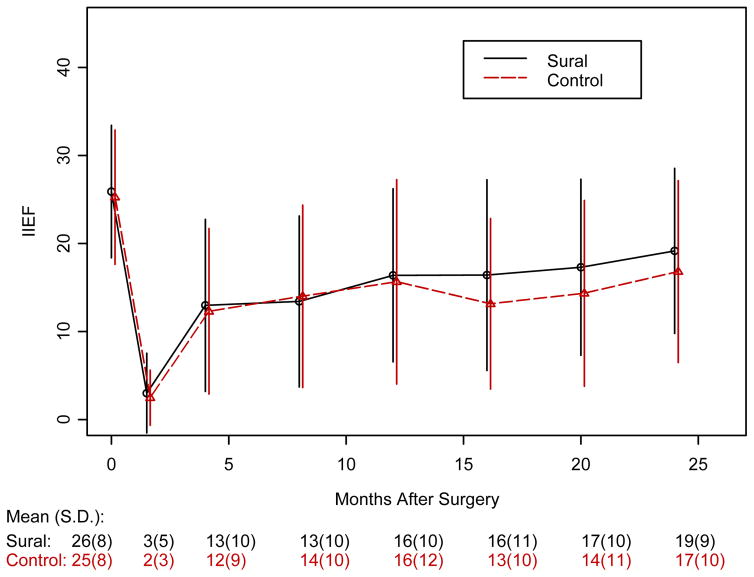
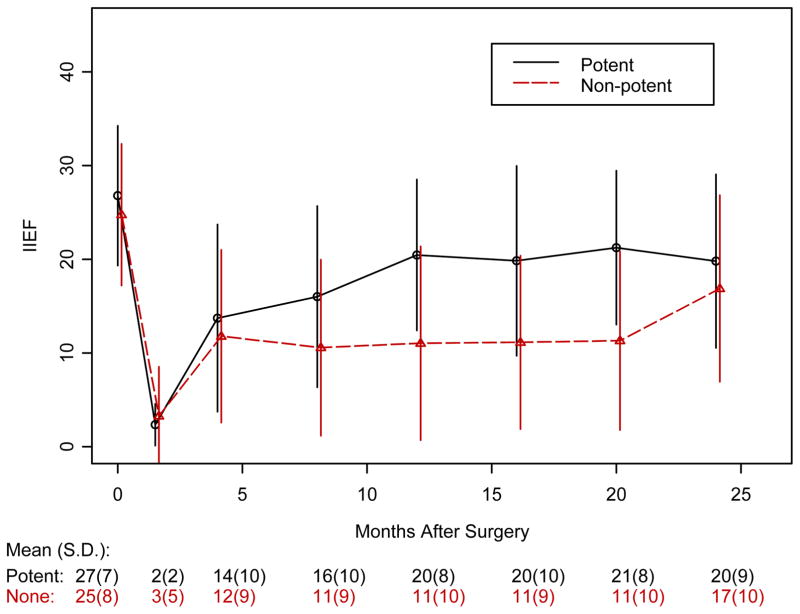
Figure 3 shows the Kaplan-Meier estimates of probability of recovery of potency for patients with 2 yr of follow-up. Erections returned at a median of 18 mo in the SNG arm and 16 mo in the control arm (p = 0.962). Figure 4 shows the results of the IIEF-6 surveys. In both groups, baseline scores between 25 and 30 dropped to <10 immediately after surgery and improved to the 15[en]20 range after 1 yr. There were no statistically significant differences between the two arms at these assessment times (p-values at different time points ranged from 0.643 to 0.999). In Figure 5, IIEF-6 scores are plotted for all patients who were classified as either potent or nonpotent. After 6 wk of follow-up, potent patients had significantly higher IIEF-6 scores than nonpotent patients, with differences remaining up to 20 mo (p-values are 0.001 at 12 mo and 0.02 at 16 and 20 mo). Among patients meeting the definition of potency after surgery, the IIEF-6 score at last follow-up was not statistically different between SNG and control patients: 23.6 (n = 27, SD = 6.5) and 24.8 (n = 12, SD = 4.47); p = 0.806.
Figure 3.
Cumulative probability of potency recovery by treatment group among patients with 2 yr of follow-up.
Figure 4.
IIEF-6 scores for SNG versus controls with standard of error bars.
Figure 5.
IIEF-6 scores for patients in either group reaching the endpoint of potency versus nonpotency.
Table 2a shows the compliance with VED and injection therapy. Good compliance with VED at 8 mo in both arms started at <65% and declined with follow-up. Good compliance with injection therapy at 8 mo started at <40% and declined with follow-up. Good compliance with VED was higher for SNG compared to controls at 8 mo (61% vs 34%, p = 0.016) and 12 mo (48% vs 20%, p = 0.02). Good compliance with injections was higher for SNG compared to controls at 8 mo (39% vs 26%, p = 0.047). Looking at IIEF-6 scores among patients with good compliance versus poor compliance, there were no significant differences at any assessment time. The same analysis for injection therapy showed an 8-mo follow-up mean IIEF-6 of 16.6 for good compliance versus 10.23 for poor compliance (p = 0.014). After 8 mo, the IIEF-6 scores were not significantly different (data not shown).
Table 2a.
[en] Compliance with vacuum erection device therapy (good = 2[en]3/wk, poor = 1/wk)
| Group | 8 mo | 12 mo | 16 mo | 20 mo |
|---|---|---|---|---|
| SNG (n = 66) | ||||
| Poor | 10 (16%) | 8 (13%) | 5 (9%) | 6 (13%) |
| Good | 37 (61%) | 29 (48%) | 21 (39%) | 8 (17%) |
| None | 14 (23%) | 23 (38%) | 28 (52%) | 32 (70%) |
| Not evaluated | 5 | 6 | 12 | 20 |
| Control (n = 41) | ||||
| Poor | 6 (16%) | 6 (17%) | 2 (6%) | 3 (11%) |
| Good | 13 (34%) | 7 (20%) | 6 (19%) | 2 (7%) |
| None | 19 (50%) | 22 (63%) | 23 (74%) | 22 (81%) |
| Not evaluated | 3 | 6 | 10 | 14 |
| p-value (Fisher’s test) | 0.016 | 0.02 | 0.141 | 0.532 |
The mean baseline UCLA-PCI urinary function scores were 93.7 and 96.2 for SNG and controls, respectively. The 12-mo follow-up scores for function decreased by 22.3 (SD = 29.7) for SNG and 26.4 (SD = 25.4) for controls (p = 0.478). The mean baseline UCLA-PCI Urinary Function and Bother scores were 91.7 and 94.8 for SNG and controls, respectively. The 12-mo follow-up scores for bother decreased by 13.1 (SD = 38.3) for SNG and 13.6 (SD = 27.5) for controls (p = 0.706).
Table 3 shows the pathologic results of 105 of 107 patients completing surgery. The majority of cases were organ-confined (69.5%). In 14 (13.3%) cases, the disease was non[en]organ-confined with a negative surgical margin, and in 13 of these cases, the extraprostatic disease included the nerve bundle resected.
Table 3.
[en] Pathologic staging results among 104 of 107 randomized patients completing radical prostatectomy (RP)
| Pathologic stage | Number (% of 104 RP) | Number with extra prostatic disease matching NNS (% of category of non[en]organ-confined) |
|---|---|---|
|
| ||
| Organ-confined N0 | 72 (69.2) | [en] |
| pT2 MOR negative N0 | 67 (64.4) | [en] |
| pT2 MOR positive N0 | 5 (4.8) | [en] |
|
| ||
| Non[en]organ-confined | 32 (30.7) | 23 (72) |
| pT3a MOR negative N0 | 14 (13.4) | 13 (93) |
| pT3a MOR negative N1 | 1 (0.96) | 1 (100) |
| pT3a MOR positive N0 | 3 (2.8) | 2 (66) |
| pT3b MOR negative N0 | 9 (8.6) | 6 (66) |
| pT3b MOR negative N1 | 2 (1.9) | 1 (50) |
| pT3b MOR positive N0 | 3 (2.8) | 0 (0) |
Abbreviations: pT2, pathologic organ-confined; pT3a, extraprostatic extension; pT3b, seminal vesicle invasion; MOR, margins of resection; N0, lymph node negative; N1, lymph node positive; NNS, non[en]nerve-sparing.
4. Discussion
Based upon our findings, we conclude that the addition of a unilateral SNG to a UNS RP does not produce a 50% relative improvement in potency at 2 yr. The difference between potency rates was not statistically significant in three different analyses of the data where the denominator changes by the definition: (1) all patients had 2-yr follow-up (endpoint analysis and the primary objective of the trial), (2) on protocol with any follow-up, and (3) all patients in each arm (intent to treat). Furthermore, we did not detect differences in time to potency, IIEF-6 scores, or UCLA PCI Urinary Function and Bother scores at 1 yr.
The strengths of this trial include its randomized design and the performance of the surgical procedure by a small group of experienced urologic oncologists and plastic surgeons. The surgical teams had experience with more than 100 cases, demonstrating 30% usable erections in patients who had undergone bilateral non[en]nerve-sparing/bilateral sural nerve grafts [3]. The inclusion criteria were designed to enroll potent younger men who were sexually active. The postoperative care included early penile rehabilitation in both arms. Together, these trial design features were aimed at creating balanced groups such that the presence or absence of the sural graft would be the main difference.
The limitations of this trial include slower-than-expected accrual and poorer-than-expected compliance with ED therapy in both arms. The planned accrual of 200 patients at 8 per month seemed reasonable in a department that performed 35[en]40 procedures per month. However, the indications for unilateral non[en]nerve-sparing RP declined as the trial progressed due to further PSA stage migration and increasing surgeon comfort with sparing nerve bundles in the presence of cancer with a Gleason score of 7. Another potential shortcoming of the study was the choice of the binary (yes/no) assessment of erectile function rather than the IIEF-6 score.
This study does not test a particular nerve-sparing selection algorithm, as this was left to faculty discretion. The pathology results showed 70% organ-confined, and in 17%, the cancer was in a nonconfined/locally advanced stage (pT3b, N+, etc.) and likely to receive hormone and/or radiation therapy in the future. Thus, the non[en]nerve-sparing approach benefited at most 13% of patients in the pT3a negative surgical margin category.
We observed poor compliance with ED therapy in both arms of the trial, and compliance declined further with follow-up. All components of penile rehabilitation[em]oral phosphodiesterase-5 inhibitors, VED, and ICI[em]have associated costs and side effects. It was hoped that our selected cohort of potentially motivated patients would overcome these drawbacks and comply with penile rehabilitation, but this did not occur.
Another limitation was patient attrition. Even though we had sufficient enrollment for the interim analysis, several patients were not evaluable due to noncompliance with surgery, medical risk for ED therapy, failure to return for follow-up, and biochemical relapse, leading to initiation of hormonal therapy. These various reasons for study dropout led to significant differences in defining potency rates, as shown in the results. Future studies should probably plan for a 20[en]30% attrition rate.
The published literature on SNG includes nonrandomized studies of bilateral and unilateral results[em]some of the latter conflicting with this report. In the Baylor College of Medicine [2], Memorial Sloan-Kettering [13], and M.D. Anderson Cancer Center [3] series of bilateral SNG studies, the efficacy was approximately 30%, with most recovery of erectile potency occurring around 18 mo. Sim et al [14] reported a large nonrandomized series of unilateral nerve-sparing SNG. At 24 mo, potency was seen in 24 of 38 (63.2%) of SNG versus 13 of 49 (26.5%) of controls. The potency rate was similar to our endpoint analysis for SNG (71%); our control patients fared much better at 67%, but they were an average of 5 yr younger (55 vs 60 yr old). Another study utilized similar definitions and IIEF scores but used a laparoscopic technique [15]. At 18 mo, there was no improvement in IIEF scores between the SNG and control arms. A large study from Japan with 3-yr follow-up used the UCLA-PCI measurement [16]. Bilateral nerve sparing, unilateral nerve sparing/SNG, and unilateral nerve sparing alone were all studied. At 24 mo, the results of bilateral nerve sparing and unilateral/SNG were equivalent. However, age and baseline function scores were unbalanced, and both the authors and the attached editorial emphasized the need for future studies to utilize a randomized design.
A multi-institutional study may allow for greater enrollment and greater power to detect smaller, clinically relevant differences than this study. The surgical techniques studied in this report were state of the art at the time of inception: sural nerve anastomosed with fine monofilament suture and using the Cavermap to assist with placement. Other nerve reconstruction techniques have since been described and may merit further study; for example, collagen tube grafting and autologous vein sheath [17]. Other centers have combined fibrin glue with reduced or no sutures [14]. A robot-assisted technique may be useful because it could result in reduced blood loss, magnified vision, and ability to use fine-scaled motions for suturing.
5. Conclusions
In a single-institution randomized study, unilateral SNG did not result in an increased potency rate at 2 yr compared to UNS RP alone based upon a threshold significance level of at least a 20% (absolute) improvement. Secondary endpoints also did not show an improvement in time to potency or urinary function at 1 yr. Based upon the power of this study, we cannot exclude a smaller benefit. Future study designs should anticipate inconsistent compliance with penile rehabilitation and 20[en]30% patient attrition.
Table 2b.
[en] Compliance with injection therapy (good = 1[en]2/wk, poor = 0[en]1/wk)
| Group | 8 mo | 12 mo | 16 mo | 20 mo |
|---|---|---|---|---|
| SNG (n = 66) | ||||
| Poor | 23 (38%) | 14 (23%) | 9 (17%) | 5 (11%) |
| Good | 24 (39%) | 23 (38%) | 18 (33%) | 10 (22%) |
| None | 14 (23%) | 23 (38%) | 27 (50%) | 31 (67%) |
| Not evaluated | 5 | 6 | 12 | 19 |
| Control (n = 41) | ||||
| Poor | 10 (26%) | 8 (23%) | 3 (10%) | 4 (15%) |
| Good | 10 (26%) | 7 (20%) | 5 (16%) | 1 (4%) |
| None | 18 (47%) | 20 (57%) | 23 (74%) | 22 (81%) |
| Not evaluated | 3 | 6 | 10 | 14 |
| p-value (Fisher’s test) | 0.047 | 0.141 | 0.105 | 0.107 |
Acknowledgments
Funding/Support and role of the sponsor: Timm Medical Technologies, Inc.
Footnotes
Author contributions: John Davis had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chang, Chevray, Pettaway, Pisters, Swanson, Babaian
Acquisition of data: Davis, Wang, Madsen, Huber, Troncoso
Analysis and interpretation of data: Davis, Chang
Drafting of the manuscript: Davis, Chang, Shen, Wen, Madsen, Babaian, Wood
Critical revision of the manuscript for important intellectual content: Davis, Shen, Wen, Pettaway, Pisters, Swanson, Troncoso, Wood
Statistical analysis: Shen, Wen
Obtaining funding: Wood
Administrative, technical, or material support: Chevray, Wang, Madsen, Huber, Wood
Supervision: Huber, Madsen, Wood
Other (specify): none
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: none
References
- 1.Kim ED, Scardino PT, Hampel O, et al. Interposition of sural nerve restores function of cavernous nerves resected during radical prostatectomy. J Urol. 1999;161:188. [en]92. [PubMed] [Google Scholar]
- 2.Kim ED, Nath R, Slawin KM, et al. Bilateral nerve grafting during radical retropubic prostatectomy: extended follow-up. Urology. 2001;58:983. doi: 10.1016/s0090-4295(01)01403-0. [en]7. [DOI] [PubMed] [Google Scholar]
- 3.Chang DW, Wood CG, Kroll SS, et al. Cavernous nerve reconstruction to preserve erectile function following non-nerve-sparing radical retropubic prostatectomy: a prospective study. Plast Reconstr Surg. 2003;111:1174. doi: 10.1097/01.PRS.0000047606.84539.F1. [en]81. [DOI] [PubMed] [Google Scholar]
- 4.Galper SL, Chen MH, Catalona WJ, et al. Evidence to support a continued stage migration and decrease in prostate cancer specific mortality. J Urol. 2006;175:907. doi: 10.1016/S0022-5347(05)00419-2. [en]12. [DOI] [PubMed] [Google Scholar]
- 5.Sokoloff MH, Brendler CB. Indications and contraindications for nerve-sparing radical prostatectomy. Urol Clin North Am. 2001;28:535. doi: 10.1016/s0094-0143(05)70161-0. [en]43. [DOI] [PubMed] [Google Scholar]
- 6.Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50:711. doi: 10.1016/j.eururo.2006.06.009. [en]20. [DOI] [PubMed] [Google Scholar]
- 7.Eastham JA, Kattan MW, Rogers E, et al. Risk factors for urinary incontinence after radical prostatectomy. J Urol. 1996;156:1707. [en]13. [PubMed] [Google Scholar]
- 8.Singh H, Karakiewicz P, Shariat SF, et al. Impact of unilateral interposition sural nerve grafting on recovery of urinary function after radical prostatectomy. J Urol. 2004;63:1122. doi: 10.1016/j.urology.2004.01.016. [en]7. [DOI] [PubMed] [Google Scholar]
- 9.Rosen RC, Riley A, Wagner G, et al. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822. doi: 10.1016/s0090-4295(97)00238-0. [en]30. [DOI] [PubMed] [Google Scholar]
- 10.Litwin MS, Hays RD, Fink A, et al. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002. doi: 10.1097/00005650-199807000-00007. [en]12. [DOI] [PubMed] [Google Scholar]
- 11.Walsh PC, Marschke P, Catalona WJ, et al. Efficacy of first-generation Cavermap to verify location and function of cavernous nerves during radical prostatectomy: a multi-institutional evaluation by experienced surgeons. Urology. 2001;57:491. doi: 10.1016/s0090-4295(00)01067-0. [en]4. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549. [en]56. [PubMed] [Google Scholar]
- 13.Secin FP, Koppie TM, Scardino PT, et al. Bilateral cavernous nerve interposition grafting during radical retropubic prostatectomy: Memorial Sloan-Kettering Cancer Center experience. J Urol. 2007;177:664. doi: 10.1016/j.juro.2006.09.035. [en]8. [DOI] [PubMed] [Google Scholar]
- 14.Sim HG, Kliot M, Lange PH, et al. Two-year outcome of unilateral sural nerve interposition graft after radical prostatectomy. Urology. 2006;68:1290. doi: 10.1016/j.urology.2006.08.1064. [en]4. [DOI] [PubMed] [Google Scholar]
- 15.Porpiglia F, Ragni F, Terrone C, et al. Is laparoscopic unilateral sural nerve grafting during radical prostatectomy effective in retaining sexual potency? BJU Int. 2005;95:1267. doi: 10.1111/j.1464-410X.2005.05501.x. [en]71. [DOI] [PubMed] [Google Scholar]
- 16.Namiki S, Saito S, Nakagawa H, et al. Impact of unilateral sural nerve graft on recovery of potency and continence following radical prostatectomy: 3-year longitudinal study. J Urol. 2007;178:212. doi: 10.1016/j.juro.2007.03.043. [en]6. [DOI] [PubMed] [Google Scholar]
- 17.Fujioka M, Tasaki I, Kitamura R, et al. Cavernous nerve graft reconstruction using an autologous nerve guide to restore potency. BJU Int. 2007;100:1107. doi: 10.1111/j.1464-410X.2007.07068.x. [en]9. [DOI] [PubMed] [Google Scholar]