Abstract
We describe an approach for determining the genetic composition of Bacteroides and Prevotella populations in gut contents based on selective amplification of 16S rRNA gene sequences (rDNA) followed by cleavage of the amplified material with restriction enzymes. The relative contributions of different ribotypes to total Bacteroides and Prevotella 16S rDNA are estimated after end labelling of one of the PCR primers, and the contribution of Bacteroides and Prevotella sequences to total eubacterial 16S rDNA is estimated by measuring the binding of oligonucleotide probes to amplified DNA. Bacteroides and Prevotella 16S rDNA accounted for between 12 and 62% of total eubacterial 16S rDNA in samples of ruminal contents from six sheep and a cow. Ribotypes 4, 5, 6, and 7, which include most cultivated rumen Prevotella strains, together accounted for between 20 and 86% of the total amplified Bacteroides and Prevotella rDNA in these samples. The most abundant Bacteroides or Prevotella ribotype in four animals, however, was ribotype 8, for which there is only one known cultured isolate, while ribotypes 1 and 2, which include many colonic Bacteroides spp., were the most abundant in two animals. This indicates that some abundant Bacteroides and Prevotella groups in the rumen are underrepresented among cultured rumen Prevotella isolates. The approach described here provides a rapid, convenient, and widely applicable method for comparing the genotypic composition of bacterial populations in gut samples.
Methods for enumerating gut bacteria that are based on cultivation, isolation, and biochemical testing are generally laborious and do not guarantee recovery of the less easily cultivated species. This is a particular problem for obligately anaerobic bacteria, which make up the great majority of organisms present in densely populated gut habitats such as the rumen and hind gut (13, 31). For this reason, there has been increasing interest in the rapid enumeration of microbial groupings by analysis of nucleic acids extracted from gut samples. Probing of extracted RNA with radiolabelled or fluorescently labelled oligonucleotide probes has been used in several studies (6, 14, 20, 30) but relies on developing panels of probes for different groups from available sequence data. Sequencing of random PCR-amplified 16S rRNA gene (rDNA) clones has provided valuable information on total eubacterial diversity for human fecal microflora (37). However, more rapid approaches to the study of diversity that allow the examination of large numbers of samples are required, and a semiquantitative PCR detection approach based on serial dilution has been reported for some of the predominant gut anaerobes (35). The approach we take here is to perform selective PCR amplification of 16S rRNA genes from the gram-negative anaerobic genera Bacteroides and Prevotella by using DNA extracted from gut samples and then to estimate the genotypic composition of samples from restriction enzyme cleavage patterns (restriction fragment length polymorphism [RFLP]) of the amplified DNA (PCR-RFLP). 16S rDNA PCR-RFLP approaches have proved valuable for typing isolated bacterial strains (see, e.g., references 10 and 15) and assessing the diversity of cloned, amplified 16S rDNA sequences from bacteria at hydrothermal vents (23), but they do not appear to have been applied previously to sequences directly amplified from mixed gut communities.
Members of the Bacteroides-Cytophaga-Flexibacter phylum (25, 38) are often reported to be among the most numerous culturable microbes present in the rumen and hind gut, where they play important roles in the breakdown of protein and carbohydrate and, in some cases, act as opportunistic pathogens (28). Rumen Prevotella spp. form a diverse group that is distinct from the human hind-gut Bacteroides spp. based on 16S rRNA sequencing and other criteria (3, 18, 29). The single species recognized formerly, Prevotella ruminicola, contained considerable variation, and its recently proposed reclassification into four species, P. ruminicola, P. bryantii, P. brevis, and P. albensis (4), is followed here.
MATERIALS AND METHODS
Bacteria.
The origins of the Prevotella spp. have been described previously (3, 19). Bacteroides uniformis 1004 was obtained from A. Salyers, University of Illinois; B. vulgatus 10583, B. ovatus 11153, and B. levii 11028 were obtained from the National Collection of Type Cultures, Aberdeen, United Kingdom; B. vulgatus 1447 was from the DSM collection, Braunschweig, Germany. Bacteria were grown anaerobically (8) at 38°C in M2GSC medium (22) under O2-free CO2.
Animals and diets.
DNA was extracted from samples of rumen fluid removed from cannulated animals (one cow and six sheep). Unless otherwise stated, the samples were obtained 2 h after the morning feed and the microbial DNA was immediately extracted. Diet 1 consisted of 500 g of grass hay, 299.5 g of barley, 100 g of molasses, 91 g of white fishmeal, and 9.1 g of mineral-vitamin mixture per kg (cow, 4 to 5 kg, once daily; sheep 1, 0.7 kg, twice daily). Diet 2 consisted of 300 g of grass hay and 150 g of grass nut (sheep 2 and 3, fed twice daily). Sheep 4 was a defaunated animal that received diet 1 (1.4 kg, once daily). Diet 3 consisted of 400 g of bruised barley, 100 g of hay, twice daily, and diet 4 consisted of 200 g of bruised barley and 300 g of hay, twice daily (sheep 5 and 6).
DNA extraction.
DNA was extracted from isolated strains as described previously (2, 3). DNA was extracted from rumen and fecal samples by a modification of the method of Stahl et al. (30). A sterile 2-ml screw-cap Eppendorf tube was half filled with sterile zirconium beads, 0.1 mm in diameter, and 1 ml of sample was added so that the tube was filled completely. The sample was beaten with a mini bead beater (Biospec Products) for 30 s and then chilled on ice for at least 1 min. This procedure was carried out six times, and the sample was then added immediately to an equal volume of 1:1 (vol/vol) phenol-chloroform and vortexed. Further extractions were performed until the aqueous phase no longer appeared cloudy. Nucleic acids were recovered from the aqueous phase by ethanol precipitation and resuspended in a suitable volume of sterile distilled H2O (dH2O).
Humic material had to be removed from the DNA extracted from rumen fluid and feces prior to PCR amplification. This was achieved by passing the DNA through an Elutip-d column as specified by the manufacturer (Schleicher and Schuell, Dassel, Germany). The DNA was then precipitated in 2 volumes of ethanol and resuspended in sterile dH2O. This procedure had to be performed at least twice to obtain DNA of a quality suitable for amplification.
Oligonucleotide primers and probes.
The universal eubacterial primers fD1 (5′-AGAGTTTGATCCTGGCTCAG, positions 7 to 26 in the Escherichia coli 16S rRNA gene [7]) and rP2 (ACGGCTACCTTGTTACGACTT, positions 1513 to 1494) are those used in reference 36. The Uni16S primer (ACGGGCGGTGTGTACAAGGCC, positions 1383 to 1402) is that used in reference 30. The Bacteroides- and Prevotella-specific primer BacPre (GAGTACGCCGGCAACGGTGA, positions 887 to 907) its reverse complement rBacPre (TCACCGTTGCCGGCGTACTC), and the P. ruminicola 23-specific probe (ATCTTGAGTGAGTTCGATGTTGG, positions 650–673) are those used in reference 3. For end labelling of primers or probes, 100 ng of the oligonucleotide was diluted to a final volume of 16 μl with sterile dH2O, incubated at 70°C for 1 min, and immediately placed on ice. T4 polynucleotide kinase buffer, 50 μCi of [γ-32P]ATP, and 10 U of T4 polynucleotide kinase were added in a final volume of 25 μl, and the mixture was incubated for 30 min at 37°C. The reaction was stopped by heating to 70°C for 10 min. Unincorporated 32P was removed by passing the mixture through Chroma spin-10 columns (Clontech) as specified by the manufacturer.
PCR amplification of ruminal 16S rDNA and PCR-RFLP analysis.
Approximately 200 to 250 ng of chromosomal DNA was amplified with a Techne PHC-3 thermal cycler in a 100-μl reaction mix containing 0.04 mM each deoxynucleoside triphosphate, 20 pmol of each primer, and 1× reaction buffer, 0.5 U of Taq polymerase. Reaction conditions for the amplification with the forward fD1 primer and the reverse rBacPre primer involved an initial cycle of 94°C for 5 min, 60°C for 2 min, and 72°C for 2 min, followed by 29 cycles of 94°C for 2 min, 60°C for 30 s, and 72°C for 2 min, with a final cycle step at 72°C for 10 min. Amplification with the universal primers, fD1 and rP2, was performed under the same conditions, except that the annealing temperature was 57°C.
For PCR-RFLP analysis, PCR products were digested to completion with the appropriate enzyme and analyzed by electrophoresis in either 1.5% agarose or 3% MetaPhor agarose (Flowgen) gels. Radioactive bands resulting from 5′-end labelling of the rBacPre primer were analyzed with a Packard InstantImager after the gel was dried.
Some additional sequencing of 16S rDNA amplified from isolated Prevotella strains was undertaken with an ABI373 automated sequencer to extend the previous partial-sequence information.
Estimation of Bacteroides and Prevotella DNA by hybridization.
PCR products were transferred to positively charged nylon membranes (Boehringer Mannheim) by Southern blotting. After transfer, the DNA was fixed to the membrane by UV cross-linking at 120 mJ. The membranes were prehybridized for 3 to 4 h at 65°C in 0.2 volume of 20× Denhardt’s solution (0.2 mg of bovine serum albumin, 0.2 mg of Ficoll, and 0.2 mg of polyvinylpyrrolidone in 10 ml of sterile dH2O)–0.2 volume of 1% herring sperm DNA–0.2 volume of 25× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.06 volume of 5% sodium dodecyl sulfate (SDS)–0.34 volume of sterile dH2O. This solution was boiled for 2 to 3 min and then chilled on ice for 2 to 3 min before being added to the membrane. Labelled oligonucleotide (100 ng) was then added, and the membrane was incubated overnight at 54°C. Hybridized membranes were washed twice with 2× SSC–0.1% SDS and twice with 0.1× SSC–0.1% SDS, all for 15 min at 54°C. The membranes were then sealed in a bag and placed in a Packard InstantImager or exposed to X-ray film.
Nucleotide sequence accession number.
The sequence for P. bryantii B14 is available as accession no. AJ00647.
RESULTS
Restriction enzyme profiles of 16S rDNA sequences amplified with a PCR primer combination specific for Bacteroides and Prevotella spp.
The aim of this work was to derive information on the relative abundance in the community of different Bacteroides and Prevotella ribotypes from restriction enzyme cleavage of 16S rDNA sequences amplified from gut samples. A universal eubacterial primer, fD1 (36), and rBacPre, the reverse complement of a primer specific for Prevotella spp. and Bacteroides spp. (3), were used to amplify a 900-bp portion of the 16S rRNA gene. The recognition spectrum of the rBacPre oligonucleotide was established by using the Checkprobe program, which confirmed a 100% match for all 26 species of Prevotella and Bacteroides listed in the Ribosomal database (17), except for B. levii and B. splanchnicus, which showed a 90% match. Seven species not belonging to either of these genera (two Flectobacillus, Flexibacter, Runella, two Cytophaga, and Thermonema) were also recognized, but none of these have been found in rumen contents.
The rBacPre-plus-fD1 primer combination was used to amplify 16S rDNA sequences from isolated strains, and restriction enzyme cleavage patterns were analyzed for the enzymes HhaI, AatII, and StuI, which were predicted from computer analysis to discriminate between Prevotella species (Fig. 1). Combining the results obtained with the three enzymes, it was possible to define 11 ribotypes for the 26 rumen Prevotella and 6 Bacteroides strains studied (Table 1). It should be noted that certain species of human colonic or oral origin, not studied here, are predicted to belong to additional ribotypes that were not detected in this work (Table 1, footnote b).
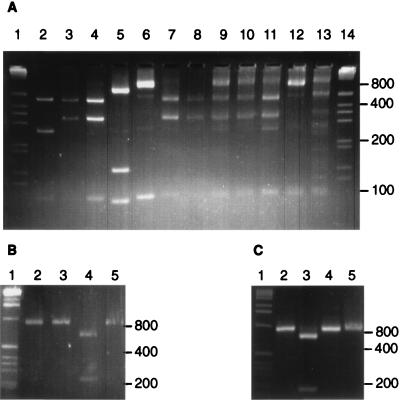
FIG. 1.
Restriction enzyme cleavage of PCR amplification products from 16S rDNA. Cleavage of amplified sequences from isolated strains P. bryantii B14 (lane 2), P. ruminicola 23 (lane 3), P. brevis GA33 (lane 4), P. albensis M384 (lane 5), and B. vulgatus 1447 (lane 6) by HhaI (A), AatII (B), and StuI (C) are shown. Lanes 7 to 13 show HhaI-cut amplification products from rumen samples derived from a cow (lane 7) and from sheep 1 to 4 (lanes 8 to 11) and human and porcine fecal samples (lanes 12 and 13, respectively). Size markers (1-kb ladder [Gibco BRL]) are shown in lanes 1 and 14.
TABLE 1.
Restriction fragment patterns obtained from rumen Prevotella isolates and from colonic Bacteroides spp. after cleavage of an 896- to 898-bp region of 16S rDNA amplified with the rBacPre-plus-fD1 primer set
| Bacterium | Ribotype | Enzyme | Observed band size or database prediction size (bp)a | Database predictionb |
|---|---|---|---|---|
| Bacteroides levii 11028 | 1 | HhaI | 897 | |
| StuI | 897 | |||
| AatII | 897 | |||
| Bacteroides vulgatus 1447 | 2 | HhaI | 794–799, 101–104 | B. forsythus, B. maccacae, B. distasonis,d Porphyromonas salivosa, Porphyromonas oulera |
| Bacteroides vulgatus 10583 | StuI | 892–900 | ||
| Bacteroides uniformis 1004 | AatII | 892–900 | ||
| Bacteroides ovatus 11153 | ||||
| Bacteroides thetaiotaomicron 5482 | 3 | HhaI | 519, 275, 102–104 | B. fragilis |
| StuI | 896–898 | |||
| AatII | 896–898 | |||
| Prevotella ruminicola 23 | 4 | HhaI | 472, 323, 102 | |
| Prevotella ruminicola 118Bc | StuI | 736, 161 | ||
| Prevotella ruminicola TC18c | AatII | 897 | ||
| Prevotella ruminicola TC35c | ||||
| Prevotella ruminicola TC44c | ||||
| Prevotella ruminicola TC27c | ||||
| Prevotella ruminicola TS1-2c | ||||
| Prevotella ruminicola TF1-2c | ||||
| Prevotella bryantii B14 | 5 | HhaI | 472, 266, 102, 57 | P. oralis,d P. disiensd |
| Prevotella bryantii TC1-1c | StuI | 897 | ||
| Prevotella bryantii 92/2c | AatII | 897 | ||
| Prevotella ruminicola TF1-5c | ||||
| Prevotella ruminicola TF1-10c | ||||
| Prevotella brevis GA33 | 6 | HhaI | 472, 323, 102 | |
| Prevotella brevis FC2c | StuI | 897 | ||
| Prevotella brevis FC4c | AatII | 661, 237 | ||
| Prevotella brevis FC6c | ||||
| Prevotella ruminicola-like TC2-24c | ||||
| Prevotella albensis M384c | 7 | HhaI | 529, 158, 108, 102 | P. veroralis, P. melaninogenica |
| Prevotella albensis 52/3c | StuI | 897 | ||
| Prevotella albensis 79/1c | AatII | 897 | ||
| Prevotella ruminicola TC2-3c | 8 | HhaI | 470, 325, 100 | |
| StuI | 900 | |||
| AatII | 900 | |||
| Prevotella brevis-like TS2-7c | 9 | HhaI | 520, 170, 100 | |
| StuI | 900 | |||
| AatII | 900 | |||
| Prevotella ruminicola-like TF2-5c | 10 | HhaI | 470, 325, 100 | |
| StuI | 740 160 | |||
| AatII | 660 240 | |||
| Prevotella ruminicola-like 223/M2/7c | 11 | HhaI | 510, 160, 110, 100, 20 | |
| StuI | 900 | |||
| AatI | 900 |
The rBacPre terminal fragment is indicated in boldface type.
Additional species predicted to give the same ribotype with respect to the rBacPre terminal fragment; other species predicted to belong to additional ribotypes (not shown) include B. eggerthii, B. heparinolytica, B. zoogleoformans, P. oris, P. buccae, P. bivia, P. buccalis, P. loeschii, P. intermedia, P. denticola, P. corporis, P. nigrescens, and Porphyromonas gingivalis.
Full 16S rDNA sequence not available; either approximate fragment sizes or the fragment sizes for sequenced representatives of the same ribotype are shown.
Predicted to show the same rBacPre terminal fragment, but differing in other HhaI digest products.
Analysis of 16S rDNA sequences amplified from rumen samples.
DNA suitable for PCR amplification with the rBacPre-plus-fD1 primer combination was extracted from rumen samples as described in Materials and Methods. When the amplified products were cleaved with HhaI, AatII, or StuI, most of the products of restriction enzyme cleavage correlated with bands obtained for the isolated strains (Fig. 1). The relatively simple banding patterns obtained and the ability to correlate these bands with ribotypes of isolated strains are consistent with highly specific amplification by the rBacPre-plus-fD1 primer pair. The 323-bp band obtained after HhaI cleavage, predicted for ribotypes 4 and 6, was shown to hybridize with a signature oligonucleotide probe designed to recognize strains related to P. ruminicola 23, which belongs to ribotype 4. No hybridization was obtained for the same probe when the amplified DNA was cut with TaqI, which is known to cut within the target site for the P. ruminicola 23 probe (results not shown).
As a test for bias in amplification, DNA was extracted from mixtures containing different proportions of P. ruminicola 23 and P. bryantii B14 cells and amplified with the rBacPre-plus-fD1 primer set. No evidence of bias was found, since the intensity of diagnostic bands for each strain reflected the relative contributions of the input cells (Fig. 2). The same result was obtained when purified DNA from the two strains was mixed in different proportions and subjected to amplification (results not shown).
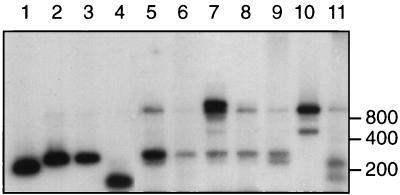
FIG. 2.
StuI digests of PCR amplification products obtained from mixtures of P. ruminicola 23 and P. bryantii B14 cells, using the rBacPre-plus-fD1 primer combination. Lanes: 1 B14 DNA only; 11, 23 DNA only; 2 to 10 contained B14 and 23 cells in the ratios 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, and 1:9, respectively.
Estimating the relative abundance of different Bacteroides and Prevotella rDNA ribotypes.
PCR amplifications in which the rBacPre primer was end labelled with [γ-32P]dATP were next performed. This simplifies the banding pattern, since only one terminal fragment is labelled, and also allowed the proportional contributions of particular labelled bands to the total radioactivity present in the amplified PCR product to be estimated by using a Packard β scanner (Fig. 3). Since only one 32P atom is present per fragment, detection is independent of fragment size. The sizes of the labelled restriction fragments were predicted by computer analysis for all of the Bacteroides and Prevotella spp. that gave an exact match with the rBacPre primer (Table 1). This confirmed that the ribotypes that include P. ruminicola 23 and P. brevis GA33 (ribotypes 4 and 6, respectively) do not include any other known organisms that give a PCR product with the rBacPre-plus-fD1 primer combination. Ribotypes 5 and 7 are predicted to include some other Prevotella spp. in addition to P. bryantii B14 and P. albensis M384 (Table 1). The end-labelling approach could not distinguish between ribotypes 7 and 11 or between ribotypes 3 and 9, and these pairs are treated together here, as are ribotypes 1 and 2.
FIG. 3.
Detection of 32P-labelled fragments derived from digestion of 16S rDNA sequences amplified with rBacPre and end-labelled fD1 primer. Lanes: 1 to 4 PCR-amplified fragments from P. bryantii B14, P. ruminicola 23, P. brevis GA33, and P. albensis M384, respectively, cut with HhaI; 5 to 9, HhaI-cut amplification products from rumen samples derived from a cow (lane 5) and from sheep 1 to 4 (lanes 6 to 9); 10 and 11, human and porcine fecal samples. Material in lane 7 was incompletely digested in this gel.
The relative abundance of the six most common ribotypes in rumen samples is shown in Table 2 for four sheep and one cow and in Table 3 for two further sheep. Ribotypes 4, 5, 6, and 7 plus 11, which include the best-defined rumen Prevotella species, together accounted for between 20 and 86% of the total amplified material from these animals. Up to 47% was due to ribotype 8, for which only one cultured rumen isolate (P. ruminicola TC2-3) is currently available. Ribotype 8 may represent a genetically divergent group that is underrepresented because its members are difficult to culture, and the functional properties of this group are largely unknown. Surprisingly, between 10 and 56% of ruminal material was due to representatives of ribotypes 1 plus 2, which include Bacteroides and Porphyromonas spp. Other recent studies have found evidence for Bacteroides-related organisms in rumen contents (5, 12).
TABLE 2.
Estimation of the relative abundance of different Bacteroides and Prevotella ribotypes in amplified 16S rDNA sequences from gut samples
| Sample and source | % of Prevotella and Bacteroides 16S rDNA present as ribotype:
|
% of Prevotella and Bacteroides in total eubacterial 16S rDNA | |||||
|---|---|---|---|---|---|---|---|
| 1 + 2 | 4 | 5 | 6 | 7 + 11 | 8 | ||
| Rumen, cow | 15.6 | 13.1 | 16.0 | 8.5 | 4.1 | 42.7 | 36.5 |
| Rumen, sheep | |||||||
| 1 | 15.8 | 5.1 | 24.1 | 12.5 | 7.9 | 34.5 | 12.4 |
| 2 | 56.2 | 0.9 | 9.9 | 7.6 | 1.9 | 24.4 | 22.6 |
| 3 | 52.3 | 2.7 | 14.5 | 7.7 | 1.9 | 20.9 | 18.8 |
| 4 | 25.1 | 3.7 | 27.0 | 3.0 | 3.0 | 38.2 | 19.2 |
| Feces, human | 89.8 | 0.0 | 3.5 | 1.3 | 0.0 | 5.3 | 5.8 |
| Feces, pig | 39.7 | 0.0 | 27.7 | 0.0 | 13.2 | 19.4 | 2.4 |
TABLE 3.
Changes in Bacteroides and Prevotella ribotypes with diet and sampling time in rumen liquor from two sheep
| Sample and source | Sampling timea | Dietb | % of Prevotella and Bacteroides 16S rDNA present as ribotype:
|
% of Prevotella and Bacteroides in total eubacterial 16S rDNA | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 + 2 | 4 | 5 | 6 | 7 + 11 | 8 | ||||
| Rumen, sheep 5 | Pre | 3 | 12.7 | 15.0 | 8.4 | 5.4 | 14.7 | 43.7 | 62.0 |
| Post | 3 | 14.1 | 11.4 | 9.0 | 3.3 | 15.4 | 46.7 | 45.0 | |
| Pre | 4 | 42.2 | 7.3 | 21.6 | 0.0 | 0.0 | 28.7 | 54.0 | |
| Post | 4 | 42.7 | 14.8 | 21.5 | 0.0 | 10.2 | 10.8 | 42.0 | |
| Rumen, sheep 6 | Pre | 3 | 10.8 | 4.5 | 49.5 | 29.3 | 6.5 | 0.0 | 45.0 |
| Post | 3 | 12.3 | 3.0 | 55.4 | 26.2 | 1.6 | 1.5 | 49.0 | |
| Pre | 4 | 30.3 | 7.9 | 12.2 | 1.7 | 22.8 | 20.1 | 57.0 | |
| Post | 4 | 44.1 | 3.2 | 7.7 | 2.6 | 21.3 | 21.4 | 60.0 | |
Pre, samples taken immediately before feeding; Post, samples taken 2 h after feeding.
The two animals were first fed diet 3 and then switched to diet 4 (see the text) and sampled again 28 days later.
To examine the stability of the rumen community with respect to Bacteroides and Prevotella ribotypes, rumen samples were taken from two sheep before and after a change in diet (Table 3). The results reveal a considerable difference initially in the strain profiles of the two animals. Apart from a consistent increase in ribotypes 1 plus 2, the effects of the dietary shift were quite different in the two animals. A likely explanation for this is that the two sheep harbored functionally distinct strains belonging to the same ribotypes.
A human fecal sample gave a low proportion (<10%) of bands characteristic of Prevotella ribotypes and a high proportion (90%) of bands of ribotypes 1 plus 2, corresponding to Bacteroides spp. A fecal sample from a pig gave significant proportions of ribotypes 1 plus 2, 5, and 7 plus 11, which include Bacteroides spp., P. bryantii B14, and P. albensis M384, but no detectable material closely related to ribotypes 4 and 6, which include P. ruminicola 23 and P. brevis GA33, respectively (Table 2). Prevotella strains apparently related to ruminal isolates have been isolated from the large intestinal contents of pigs (27).
Contribution of Bacteroides and Prevotella 16S rDNA sequences to total eubacterial 16S rDNA.
To estimate the amount of Bacteroides and Prevotella DNA relative to total eubacterial DNA, two universal eubacterial primers, fD1 and rP2 (36), were used to amplify most of the 16S rRNA gene. Amplified material was transferred to filters by Southern blotting and probed with a general eubacterial oligonucleotide, Uni16S (30), or with the Bacteroides- and Prevotella-specific oligonucleotide BacPre. The approximate proportion of Bacteroides and Prevotella 16S rDNA, shown in Tables 2 and 3, was calculated from the relative binding of these two probes to material amplified from gut samples and from pure cultures, correcting for any differences in probe-specific activity or hybridization kinetics (Fig. 4). Bacteroides and Prevotella sequences were estimated to account for between 12 and 62% of total eubacterial 16S rDNA in the rumen samples examined here (Tables 2 and 3).
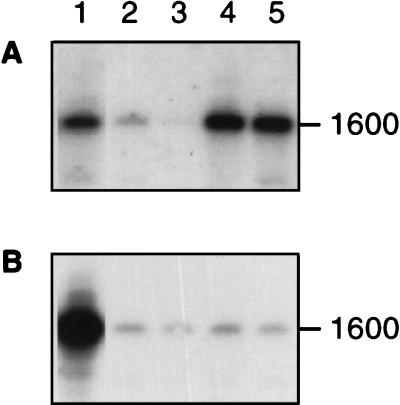
FIG. 4.
Estimation of the contribution of Bacteroides and Prevotella 16S rDNA to total eubacterial 16S rDNA sequences. Amplified sequences were transferred onto a filter by Southern blotting and probed with either the Uni16S eubacterial probe (A) or the BacPre probe (B). Lanes: 1, amplified DNA from P. ruminicola 23 control; 2 to 5, DNA from four different sheep rumen samples. To obtain the data shown in the final columns in Tables 2 and 3, radioactivity was estimated for each band by using a Packard beta scanner. The proportion of eubacterial 16S rDNA sequences due to Bacteroides and Prevotella was estimated as (ae/ab) × (bb/be) where ae and be are the counts obtained for the control and unknown cultures, respectively, with the universal eubacterial probe uni16S, and ab and bb are the corresponding counts obtained with the BacPre probe.
Combining the estimates of the relative abundance of Prevotella and Bacteroides ribotypes with the estimated contribution of Prevotella and Bacteroides sequences to total eubacterial rDNA allows calculation of the contributions of individual Prevotella ribotypes. For example, the greatest abundance for ribotypes 4, 5, 6, and 7 plus 11 was 9, 27, 13, and 13% respectively, as percentages of total eubacterial 16S rDNA in the rumen samples studied here.
DISCUSSION
The four ribotypes that include the major rumen Prevotella species identified previously by culture approaches were present as a significant proportion of Bacteroides and Prevotella 16S rDNA sequences in all seven ruminant animals examined here and accounted for 20 to 86% of Bacteroides and Prevotella rDNA or 4 to 43% of the total eubacterial rDNA. At present, the largest single group of cultured rumen Prevotella strains (9) is probably represented by ribotype 4. Among Prevotella isolates from silage-fed cattle studied by van Gylswyk (33), more than 50% were P. ruminicola belonging to ribotype 4 (3). On the other hand, isolations of strains showing dipeptidyl aminopeptidase I (DAPI) activity (thought to be characteristic of rumen Prevotella strains) from sheep fed similar diets and held at the same site as those studied here (19) yielded mainly P. bryantii, P. brevis, or P. albensis. The present observation that ribotypes 5 and 6 were more abundant than ribotype 4 in sheep rumen samples is therefore consistent with the results of previous isolation studies. On the other hand, the most abundant Bacteroides and Prevotella ribotypes in six of the seven animals (ribotypes 8 and 1 plus 2) are represented by very few cultured strains of rumen origin. Recent investigations through random sequencing of amplified 16S rDNA from the rumen have indicated a greater diversity of Bacteroides and Prevotella spp. than previously recognized (5, 12). It appears, therefore, that certain groupings may be underrepresented among cultured strains because of difficulties in their recovery through cultivation. Studies of other ecosystems have revealed large discrepancies between viable and direct microscopic microbial counts (1), although there are reasons to expect that discrepancies would be smaller for gut ecosystems in which a certain growth rate is required to prevent washout from the system. The viable count from the rumen was previously found to vary between 14 and 75% of the total direct count for cattle fed two different diets, depending on the diet and the time after feeding (16). These discrepancies may reflect a failure to recover the full range of rumen microbial diversity, as well as changes in the viability of known organisms (21).
It is possible that certain Bacteroides and Prevotella strains are overrepresented in amplified 16S rDNA due to PCR bias (32, 34), or differential extractability of nucleic acids, but there was little evidence of this in the control experiments reported here. PCR bias was detected by Wilson and Blitchington (37), who obtained slightly different estimates of relative sequence abundance after 35 cycles compared with 9 cycles of PCR in amplifications of rDNA sequences from human fecal material, although the amplified region was larger than in the present study. In addition, the number of rRNA operons can vary among different bacteria (11, 24, 26), and it is not known how much variation occurs between strains of Bacteroides and Prevotella. In general, such biases may prove less of a problem when comparisons are being made, as here, within a phylogenetic grouping than among dissimilar groupings.
The approach described here offers a simple, rapid, and convenient method for obtaining information on the population structure of bacteria present in gut ecosystems. In future, more convenient quantification should be possible, for example by using fluorescently labelled rather than radioactively labelled primers for PCR. Although it cannot be assumed that ribotype frequencies correspond precisely to the abundance of different genotypes in the sample, for reasons discussed above, they can nevertheless provide important indicators of population changes between samples. This simple profiling approach therefore appears ideally suited for testing hypotheses to explain in vivo population dynamics and interanimal variability of important components of gut microbial communities. For the Bacteroides and Prevotella group, it should prove directly applicable to other anaerobic systems such as the human and animal hind gut.
ACKNOWLEDGMENTS
This work was supported by the Scottish Office Agriculture, Environment and Fisheries Department (SOAEFD) and by a BBSRC studentship award to Jacqueline Wood.
We thank Freda McIntosh for her help with analysis of sheep rumen samples and Jennifer Martin for DNA sequence determination.
REFERENCES
- 1.Amman R I, Ludwig W, Schleifer K-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D M, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 3.Avguštin G, Wright F, Flint H J. Genetic diversity and phylogenetic relationships among strains of Prevotella (Bacteroides) ruminicola from the rumen. Int J Syst Bacteriol. 1994;44:246–255. doi: 10.1099/00207713-44-2-246. [DOI] [PubMed] [Google Scholar]
- 4.Avguštin G, Wallace R J, Flint H J. Phenotypic diversity among rumen isolates of Prevotella ruminicola: proposal for Prevotella brevis sp. nov., Prevotella bryantii sp. nov., Prevotella albensis sp. nov., and redefinition of Prevotella ruminicola. Int J Syst Bacteriol. 1997;47:284–288. doi: 10.1099/00207713-47-2-284. [DOI] [PubMed] [Google Scholar]
- 5.Avguštin G, Ramšak A, Peterka M, Nekrep F V, Flint H J. Evolutionary relationships of the rumen bacteria belonging to the Cytophaga-Flexibacter-Bacteroides phylum. In: Chesson A, Stewart C S, Flint H J, editors. Reproduction, nutrition and development. Supplement: Evolution of the rumen microbial ecosystem. Paris, France: Elsevier; 1997. pp. 27–28. [Google Scholar]
- 6.Briesacher S L, May T, Grigsby K N, Kerley M S, Anthony R V, Paterson J A. Use of DNA probes to monitor nutritional effects on the ruminal prokaryotes and Fibrobacter succinogenes S85. J Anim Sci. 1992;70:289–295. doi: 10.2527/1992.701289x. [DOI] [PubMed] [Google Scholar]
- 7.Brosius J, Dull T J, Sleeter D, Noller H F. Gene organisation and primary structure of a ribosomal DNA operon from Escherichia coli. J Mol Biol. 1981;148:107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- 8.Bryant M P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972;25:1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- 9.Bryant M P, Small N, Bouma C, Chu H. Bacteroides ruminicola n. sp. and Succinimonas amylolytica, the new species and genus. J Bacteriol. 1958;76:15–23. doi: 10.1128/jb.76.1.15-23.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardarelli-Leite P, Blom K, Patton C M, Nicholson M A, Steigerwalt A G, Hunter S B, Brenner D J, Barrett T J, Swaminthan B. Rapid identification of Campylobacter species by restriction fragment length polymorphism analysis of a PCR-amplified fragment of the gene coding for 16S rRNA. J Clin Microbiol. 1996;34:62–67. doi: 10.1128/jcm.34.1.62-67.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrelly V, Rainey F A, Stackebrandt E. Effect of genome size and rrn gene copy number on PCR amplification of 16S rRNA genes from a mixture of bacterial species. Appl Environ Microbiol. 1995;61:2798–2801. doi: 10.1128/aem.61.7.2798-2801.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forster R J, Whitford M F, Beard C E, Gong J. An investigation of microbial diversity in the rumen of dairy cattle using comparative sequence analysis of cloned 16S rRNA genes. In: Chesson A, Stewart C S, Flint H J, editors. Reproduction, nutrition and development. Supplement: evolution of the rumen microbial ecosystem. Paris, France: Elsevier; 1997. pp. 28–29. [Google Scholar]
- 13.Hespell R B, Akin D E, Dehority B A. Bacteria, fungi and protozoa of the rumen. In: Mackie R I, White B A, Isaacson R E, editors. Gastrointestinal microbiology. Vol. 2. New York, N.Y: Chapman and Hall; 1996. pp. 59–141. [Google Scholar]
- 14.Krause D O, Russell J B. An rRNA approach for assessing the role of obligate amino acid-fermenting bacteria in ruminal amino acid deamination. Appl Env Microbiol. 1996;62:815–821. doi: 10.1128/aem.62.3.815-821.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laguerre G, Allard M-R, Revoy F, Amrger N. Rapid identification of rhizobia by restriction fragment length polymorphism analysis of PCR-amplified 16S rRNA genes. Appl Environ Microbiol. 1994;60:56–63. doi: 10.1128/aem.60.1.56-63.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leedle J A Z, Bryant M P, Hespell R B. Diurnal variations in bacterial numbers and fluid parameters in ruminal contents of animals fed low- or high-forage diets. Appl Environ Microbiol. 1982;44:402–412. doi: 10.1128/aem.44.2.402-412.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maidak B L, Olsen G J, Larsen N, Overbak R, McCaughey M J, Woese C R. The ribosomal database project (RDP) Nucleic Acids Res. 1996;24:82–85. doi: 10.1093/nar/24.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannarelli B M, Ericsson L D, Stack R J. Taxonomic relationships among strains of the anaerobic bacterium Bacteroides ruminicola determined by DNA and extracellular polysaccharide analysis. Appl Environ Microbiol. 1991;57:2975–2980. doi: 10.1128/aem.57.10.2975-2980.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKain N, Wallace R J, Watt N D. Selective isolation of bacteria with dipeptidyl aminopeptidase I activity from the rumen. FEMS Microbiol Lett. 1992;95:169–174. doi: 10.1016/0378-1097(92)90424-m. [DOI] [PubMed] [Google Scholar]
- 20.McSweeney C S, Mackie R I, Odenyo A A, Stahl D A. Development of an oligonucleotide probe targeting 16S rRNA and its application for detection and quantification of the ruminal bacterium Synergistes jonesii in a mixed population chemostat. Appl Environ Microbiol. 1993;59:1607–1612. doi: 10.1128/aem.59.5.1607-1612.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mink R W, Hespell R B. Long-term nutrient starvation of continuously cultured (nutrient limited) Selenomonas ruminantium. J Bacteriol. 1981;148:541–546. doi: 10.1128/jb.148.2.541-550.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazaki K, Martin J C, Marinsek-Logar R, Flint H J. Degradation and utilization of xylans by the rumen anaerobe Prevotella bryantii (formerly P. ruminicola subsp. brevis) B14. Anaerobe. 1997;3:375–381. doi: 10.1006/anae.1997.0125. [DOI] [PubMed] [Google Scholar]
- 23.Moyer C L, Dobbs F C, Karl D M. Estimation of diversity and community structure through restriction fragment length polymorphism distribution analysis of bacterial 16S rRNA genes from a microbial mat at an active, hydrothermal vent system, Loihi Seamount, Hawaii. Appl Environ Microbiol. 1994;60:871–879. doi: 10.1128/aem.60.3.871-879.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nübel U, Engelen B, Felske A, Snaidar J, Weishuber A, Amann R I, Ludwig W, Backhaus H. Sequence heterogeneities of gene encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J Bacteriol. 1996;178:5636–5643. doi: 10.1128/jb.178.19.5636-5643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paster B J, Ludwig W, Weisburg W G, Stackbrandt E, Hespell R B, Hatan C M, Reichenbach K, Stetter O, Woese C R. A phylogenetic grouping of the bacteroides, cytophagas and certain flavobacteria. Syst Appl Microbiol. 1985;6:34–42. [Google Scholar]
- 26.Rainey F A, Ward-Rainey N L, Janssen P H, Hippe H, Stackebrandt E. Clostridium paradoxum DSM 7308T contains multiple 16S rRNA genes with heterogeneous intervening sequences. Microbiology. 1996;142:2087–2095. doi: 10.1099/13500872-142-8-2087. [DOI] [PubMed] [Google Scholar]
- 27.Robinson I M, Whipp S C, Bucklin J A, Allison M J. Characterization of predominant bacteria from the colons of normal and dysenteric pigs. Appl Environ Microbiol. 1984;48:964–969. doi: 10.1128/aem.48.5.964-969.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah H N. The genus Bacteroides and related taxa. In: Balows A, Trüper H G, Dworkin M, Harder W, Scheifer K H, editors. The prokaryotes: a handbook on the biology of bacteria, isolation, identification, application. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 3593–3607. [Google Scholar]
- 29.Shah H N, Collins M D. Prevotella, a new genus to include Bacteroides melaninogenicus and related species formerly classified in the genus Bacteroides. Int J Syst Bacteriol. 1990;40:205–208. doi: 10.1099/00207713-40-2-205. [DOI] [PubMed] [Google Scholar]
- 30.Stahl D A, Flesher B, Mansfield H R, Montgomery L. Use of phylogenetically based hybridization probes for studies in ruminal microbial ecology. Appl Environ Microbiol. 1988;54:1079–1084. doi: 10.1128/aem.54.5.1079-1084.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart C S, Flint H J, Bryant M P. The rumen bacteria. In: Hobson P N, Stewart C S, editors. The rumen microbial ecosystem. London, United Kingdom: Blackie; 1997. pp. 10–72. [Google Scholar]
- 32.Suzuki M T, Giovannoni S J. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol. 1996;62:625–630. doi: 10.1128/aem.62.2.625-630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Gylswyk N O. Enumeration and presumptive identification of some functional groups of bacteria in the rumen of dairy cows fed grass silage based diets. FEMS Microbiol Ecol. 1990;73:243–254. [Google Scholar]
- 34.von Wintingerode F, Göbel U B, Stackebrandt E. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev. 1997;21:213–229. doi: 10.1111/j.1574-6976.1997.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang R-F, Cao W-W, Cernaglia C E. PCR detection and quantitation of predominant anaerobic bacteria in human and animal fecal samples. Appl Environ Microbiol. 1996;62:1242–1247. doi: 10.1128/aem.62.4.1242-1247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson K H, Blitchington R B. Human colonic biota studied by ribosomal DNA sequence analysis. Appl Environ Microbiol. 1996;62:2273–2278. doi: 10.1128/aem.62.7.2273-2278.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]