Abstract
Background:
Staging laparoscopy for gastric cancer is recommended to assess the tumor’s locoregional extension and exclude peritoneal disease. As there is no consensus on optimizing the procedure’s diagnostic accuracy, we aimed to systematically review the literature on operative techniques, followed by peritoneal lavage fluid assessment in gastric cancer patients. Specifically, we sought to indicate the most common characteristics of the procedure and cytological evaluation.
Methods:
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The protocol for this systematic review was registered on PROSPERO database (CRD: 42022306746). On September 2022, a search was carried out using Embase, Medline ALL, Cochrane Central Register of Controlled Trials, and Web of Science Core Collection.
Results:
The search identified 1632 studies on staging laparoscopy and 2190 studies on peritoneal fluid assessment. Some 212 studies were included. Open Hasson was the method of choice in accessing the peritoneal cavity in 65% of the studies, followed by establishing a pneumoperitoneum at 10–12 mmHg in 52% of reports. Most frequently, the patient was positioned supine (70%), while a 30° scope and three ports were used to assess the peritoneal cavity clockwise (72%, 77%, and 85%, respectively). Right and left upper abdomen quadrants were the predominant area of laparoscopic exploration (both 65%), followed by the primary tumor region (54%), liver and pelvis (both 30%), and small bowel and spleen (19% and 17%, respectively). Regions of peritoneal lavage and aspiration were limited to the pelvis (50%), followed by right and left upper abdomen quadrants (37.5% and 50%, respectively). No studies compared different methods of operative techniques or analysis of ascites/fluid.
Conclusions:
This study indicates a high heterogeneity in the technique of staging laparoscopy and peritoneal fluid assessment in gastric cancer patients. Further research and initiatives to reach a consensus on the standardization of the procedure are warranted.
Keywords: gastric cancer, peritoneal fluid assessment, staging laparoscopy
Introduction
Highlights
This systematic review is first to summarize the literature on techniques of staging laparoscopy and peritoneal fluid assessment for gastric cancer.
The search identified 1632 studies on staging laparoscopy and 2190 studies on peritoneal fluid assessment. In total, 212 studies were included.
This study indicates a high heterogeneity in the technique of staging laparoscopy and peritoneal fluid assessment in gastric cancer patients. Further research and initiatives to reach a consensus on the standardization of the procedure are warranted.
Gastric cancer is the fifth most common malignancy worldwide, with an annual incidence of 1 000 000 cases1. Approximately 50% of patients present distant metastases at diagnosis, with the peritoneum being the most common site of dissemination2,3. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy may improve the prognosis of patients with peritoneal metastases, but the evidence is merely based on non-randomized cohort studies4.
European Society of Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) gastric cancer guidelines recommend computed tomography (CT) for clinical staging and risk assessment5,6. Nonetheless, CT sensitivity to detect peritoneal carcinomatosis is highly variable, ranging from 23% to 76%7. Positron emission tomography (PET)–CT imaging may improve sensitivity to nearly 80%; however, the negative predictive value (NPV) remains as low as 60%8. Of note, the accuracy of magnetic resonance imaging (MRI) in diagnosing peritoneal metastases may reach up to 83%9, but mainly among patients with severe carcinomatosis. Regardless of imaging choice, the precise assessment of dissemination is often underestimated due to decreased sensitivity for subcentimeter lesions10.
In order to improve the evaluation of radiologically and macroscopically occult peritoneal metastatic disease, staging laparoscopy is recommended for every gastric cancer patient with stage cT1b and higher3,11–13. Apart from the omission of unnecessary laparotomy in up to 25% of cases, staging laparoscopy enables the assessment of intraperitoneal lavage washings3. Positive cytology without macroscopic dissemination (P0Cyt+) is classified as stage IV gastric cancer with poor survival outcomes14–16. Although gastrectomy is ineffective in improving survival among this specific group of patients17, positive-to-negative cytology conversion after neoadjuvant chemotherapy is a significant prognostic factor, justifying surgical treatment in well-selected cases18. However, the cytological analysis lacks standardization, resulting in free cancer cell detection with sensitivity varying from 26 to 70.8%16,19.
Given the clinical importance of diagnosing irresectable and incurable disease prior to curative-intent treatment, the current study aimed to systematically review surgical techniques of staging laparoscopy and cytological assessment of peritoneal lavage fluid in gastric cancer patients. Specifically, we sought to evaluate the access, usage of instruments, areas of peritoneal cavity exploration, and potential complications after the procedure, together with assessing clinical considerations of intraperitoneal lavage washings.
Methods
The following study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, Supplemental Digital Content 1, http://links.lww.com/JS9/A854, Supplemental Digital Content 2, http://links.lww.com/JS9/A855, Supplemental Digital Content 3, http://links.lww.com/JS9/A856)20. The protocol for this systematic review was registered on PROSPERO database (CRD: 42022306746)21.
Search strategy
On September 2022, a search was carried out using Embase (from 1971), Medline ALL (from 1946), and Cochrane Central Register of Controlled Trials (from 1992) for studies on staging laparoscopy for gastric cancer. At the same time, a second search was conducted using the same database with the addition of the Web of Science Core Collection (from 1975) to assess peritoneal fluid in patients with gastric cancer. The search terms included multiple combinations and synonyms of the keywords “gastric cancer”, “gastroesophageal cancer”, “cancer staging”, “laparoscopy”, “peritoneal lavage fluid”, “ascites”, “assessment”, and “cytology”. The complete search strategy is available in the supplementary material (Supplementary Material Tables 1 and 2, Supplemental Digital Content 4, http://links.lww.com/JS9/A857). All duplicate records were removed before the screening.
Study questions
Two main clinical questions were addressed:
Surgical considerations: patient positioning, type of instruments used, peritoneal cavity access, number of ports/trocars, technical details of locoregional tumor assessment, and peritoneal metastases evaluation.
Peritoneal lavage considerations: type and volume of fluid used in assessment, the time interval between washings and cytological evaluation, area of fluid aspiration, and comparison of techniques and biomarkers used in the cytological workup.
Screening and study selection
Two independent authors (K.R.-P. and M.E.) screened the titles and abstracts to identify citations for inclusion. Any reviewer conflicts were resolved by discussion with the senior author (B.P.L.W.). The studies of interest included patients with gastric or esophagogastric cancer who underwent staging laparoscopy or/and peritoneal washings. Systematic reviews, meta-analyses, reviews, editorials/letters, case reports, posters, conferences, animal models, and studies published in languages other than English were excluded. The study selection procedure for the technique of staging laparoscopy and the peritoneal fluid assessment is presented according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, Supplemental Digital Content 1, http://links.lww.com/JS9/A854, Supplemental Digital Content 2, http://links.lww.com/JS9/A855, Supplemental Digital Content 3, http://links.lww.com/JS9/A856)20 flowcharts in Supplementary Figure 1 (Supplemental Digital Content 5, http://links.lww.com/JS9/A858) and Supplementary Figure 2 (Supplemental Digital Content 5, http://links.lww.com/JS9/A858), respectively.
Data extraction and data synthesis
Two authors (K.R.-P. and M.E.) extracted data from the included studies, focusing initially on the first author’s name, year of publication, country, cancer type, number of patients, and study period. Next, technical details on the surgical procedure were assessed, including patient positioning(s), peritoneum access, pressure of pneumoperitoneum, number of trocars used, type of laparoscope, regions of exploration, and classification of peritoneal dissemination. Furthermore, aspiration of ascites/peritoneal lavage fluid was evaluated, including timing, the volume of fluid aspirate and volume for cytology, possible type fluid containers used, followed by assessment methods. Finally, additional screening for procedure complications was conducted. The methodological quality of the studies was assessed using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement22,23 and AMSTAR guidelines, Supplemental Digital Content 6, http://links.lww.com/JS9/A859 (methodological quality – high, score 10)24. Due to substantial heterogeneity between the studies, a meta-analysis was waived upon the senior author’s discretion and approval. However, a descriptive analysis of all studies on the technical aspects of staging laparoscopy and peritoneal fluid assessment was performed.
Results
Characteristics of included studies
The initial literature search identified 1632 studies describing staging laparoscopy technique and 2190 studies reporting peritoneal fluid assessment. After duplicates were removed, 1132 and 1771 studies remained for screening. Based on the titles and abstracts, another 1855 were excluded. The full text was available for 100 studies on staging laparoscopy technique and 130 studies on peritoneal fluid assessment. In total, 212 publications were included in this systematic review. Out of 212 included studies, 175 (83%) were reported, according to the STROBE statement. Eighty-four studies reporting on staging laparoscopy technique were published between 1984 and 2021, with 5316 patients in total2,3,11–14,19,25–101. One hundred twenty-eight studies reporting peritoneal fluid assessment were published between 1993 and 2022, including 20 115 patients11,13,14,18,25,33,34,36,37,40–44,46,49,54,57,59,61,67,70,73,79,85,93,94,98,102–200. Peritoneal lavage fluid assessment was most commonly assessed with reverse transcriptase-polymerase chain reaction (RT-PCR) (31%), Papanicolau-alone (30.5%), or in combination with Giemsa staining (10%). Molecular biological techniques for detecting cancer were performed in 3% of included studies, whereas 25.5% of the studies did not report information on cytological examination. Nearly half (47.5%) of the studies lacked data on the volume of fluid used for analysis. Specific characteristics of staging laparoscopy technique and peritoneal fluid assessment are shown in Tables 1, 2, respectively.
Table 1.
Studies included in the technique of staging laparoscopy assessment.
| Number of included studies (references) | Year of publication | Median number of patients (IQR) | Continent/country of the study | Access to peritoneal cavity | Number of trocars | Type of laparoscope | Reported regions assessed during laparoscopy | Complications |
|---|---|---|---|---|---|---|---|---|
| 84 2,3,11–14,19,23–35,37–72,77–89,93–99,101,102,136–140 | 1984–2021 | 5316 (127; 40–167) | Europe (43.5%) | Open Hasson (55%) | 3 (82%) | 30° (90%) | Stomach (49%) | Intestinal perforation (5%) |
| Japan (20%) | Verres (45%) | 2 (13%) | 0° (5%) | Local tumor extension (63%) | Diaphragmatic perforation (3.5%) | |||
| China (11%) | 4-5 (5%) | 45° (5%) | Peritoneal surface (76%) | Pulmonary infection (2.5%) | ||||
| USA (10%) | Greater omentum (58%) | Blood loss (2.5%) | ||||||
| Other (15.5%) | Liver (69%) | |||||||
| Diaphragm (44%) | ||||||||
| Small bowel with mesenterium (25%) | ||||||||
| Pelvis (35%) | ||||||||
| Other (30%) |
Other: esophageal hiatus, pancreas, Winslow foramen, celiac trunk, and large bowel.
Table 2.
Studies included in peritoneal lavage fluid assessment.
| Number of included studies (references) | Year of publication | Median number of patients (IQR) | Country | Type of procedure | Volume of sample (ml) | Technique of cytological examination | Biomarker | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|---|---|---|---|---|---|
| 128 11,13,14,18,25,33,36,37,40,41,44,46,49,57,59,61,62,70,73–77,81,86,88,90–92,100,118,120–130,141–222 | 1993–2021 | 20 115 (114.5; 50.5–165) | Japan (41%) | Laparoscopy (55%) | 100 (20%) | Papanicolau (30.5%) | CEA (23%) | 31–88.5% | 79–97% | 64–82.5% |
| China (14%) | Laparotomy (33%) | 50 (8%) | RT-PCR (31%) | FCC (18%) | ||||||
| Europe (21%) | Paracentesis (12%) | 200 (8.5%) | Papanicolau+Giemsa (10%) | Other (15%) | ||||||
| U.S. (10.5%) | 250-300 (5.5%) | Other (3%) | No data (44%) | |||||||
| Korea (5.5%) | 500 (4%) | No data (25.5%) | ||||||||
| Other (8%) | 1000 (6.5%) | |||||||||
| Unknown (47.5%) |
CEA, carcinoembryonic antigen; FCC, free cancer cells; RT-PCR, reverse transcription polymerase chain reaction.
Staging laparoscopy technique
Access and pneumoperitoneum
The most preferred approach to staging laparoscopy technique among the included studies is depicted in Figure 1. Most studies (17/26, 65.3%) reported using the open Hasson technique for access to the peritoneal cavity13,32,39,47,48,51,61,63,65,68,70,72,79,84,87,88. In contrast, in seven (26.9%) studies, the Verres needle was used39,51,52,64,71,91,94, while in two studies (7.6%), both methods were proposed39,51. The pressure of pneumoperitoneum with CO2 was set at 8–15 mmHg, with 12 mmHg being the most commonly reported pressure (4 out of 17 studies, 23%)47,51,71,94.
Figure 1.
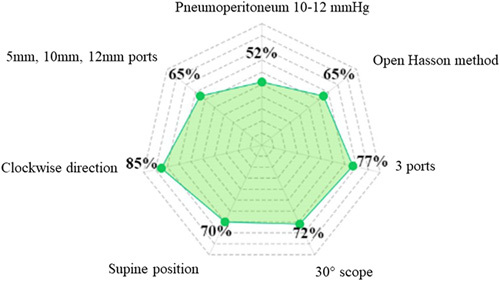
Radar chart indicating the most preferred approach to staging laparoscopy technique among included studies.
Ports/trocars
The number of ports used varied from 1 to 5, as was reported in 57 studies11–14,19,25,30,32–42,44,45,47–51,53–55,58,59,62,64–68,70–73,75,79–83,85–87,94,96,99,106,142,153. The optical port was most often placed periumbilical. The maneuver trocar diameter ranged from 5 to 12 mm. The right and left upper quadrants were the most common location for the two remaining trocars. One study reported on a single port Endocam with a 5 mm side channel, which was used for inserting laparoscopic instruments such as biopsy forceps and aspiration of ascites using a urethral stent72.
Type of laparoscope
A 30-degree laparoscope was predominantly used (27/37 studies, 73%)11,12,19,30,36–38,47,49,59,64,67,70,81,83,87,94,106,153.
Patient positioning
Most studies reported supine patient positioning (17/23 studies 74%)12,32–34,40,41,47,49,51,53,55,59,61,67,71,84,94. In other studies, the patient was in a French or lithotomy position (6/23 studies, 26%)19,25,30,33,37,94. Trendelenburg position was reported explicitly in half of the studies (13/26) to visualize the pouch of Douglas, the root of the mesentery, pelvis, and ovaries47,48,52,61,64,65,68,70,79,84,88,91,94. Five reports mentioned the anti-Trendelenburg position for better visualization of the upper abdomen by elevating the left liver lobe to approach the anterior surface of the stomach61,63,70,71,84. Such maneuvers allowed a more precise assessment of the extent of tumor infiltration on the gastric wall, the perigastric nodes along the greater and lesser curvature, and the gastrohepatic and hepatoduodenal ligaments.
Orientation
Seven studies reported on a clockwise exploration of the abdominal cavity to detect peritoneal disease, ascites, liver metastases, or suspected lymph nodes11,12,25,30,47,51,57. The inspection started at the right upper quadrant, followed by a visual exploration of the bilateral diaphragmatic dome, the left side of the anterior abdominal wall, the hypogastrium, and the right side of the anterior abdominal wall. A retrospective observational study from China presented a ‘Four-Step Procedure’ in which the examination of the surface of abdominal viscera was according to a so-called ‘S’ route11. The exploratory sequence began at the diaphragmatic surface of the left liver lobe, followed by the diaphragmatic surface of the right liver lobe, the surface of transverse colon, the great omentum from left to right, the left side of the abdominal wall, the left paracolic sulcus and the surface of descending colon, the inferior abdominal wall, the surface of the small intestine, the right side of abdominal wall, the right paracolic sulcus, and the surface of ascending colon.
Regions of intraoperative assessment
The most common areas of abdomen exploration during staging laparoscopy among included studies are depicted in Figure 2. Assessment of the primary tumor area and peritoneal cavity was described in detail in 71 studies11–14,19,25,27,30,32–45,47–51,53–59,61,62,64–75,79–83,85–87,92–96,98–100,106,110,114,142,153,159. The stomach and assessment of local tumor extension were reported in 35 (49.2%) of the studies, followed by the peritoneal surface (47/71 studies, 65%), pelvis, and Douglas pouch (29/71 studies 30%). Some studies reported specific details of surgical maneuvers to assess local ingrowth of the tumor or sites of peritoneal metastases19,33,37,57,64,75,85,93,96,110. These included lifting the left liver lobe to evaluate the complete anterior wall of the stomach, the lesser curvature, the lesser omentum, the undersurface of the left lobe, and hepatoduodenal ligament. In patients with a tumor located at the posterior wall, some studies reported inspection of the lesser sac through a small incision in the gastrocolic ligament or via the opening of the gastrohepatic ligament12,13,39,41,57,61,64. To this extent, the relationship of the tumor to retroperitoneal structures such as pancreas, celiac axis, and the peritoneal surface of the lesser sac or tumor fixation could be inspected.
Figure 2.
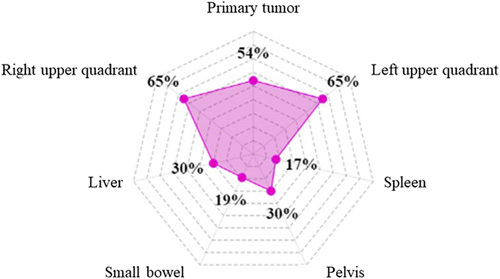
Radar chart evaluating the most common areas of abdomen exploration during staging laparoscopy among included studies.
Greater omentum and bowel
Half of the studies specifically reported assessing greater omentum and small bowel. Retraction of the greater omentum toward the left upper quadrant and elevation of the transverse colon allows inspection of the root of mesenteries, duodenum, the proximal part of jejunum, and the ligament of Treitz. The surface of the small and large intestines was assessed in 15 studies12,14,30,36,40,45,47,55,61,64,70,71,81,85,87 (21%), while the inspection of the spleen’s surface and the hepatorenal recess was reported in 3 studies (4%)14,40,88.
Pelvic cavity
Examination of the pelvic cavity was reported in 27 (38%) studies by elevating the foot end of the table (Trendelenburg)12,13,25,27,33,34,36,37,40,41,45,47,53,54,57,62,65,69,74,79,85,92,95,96,99,142. Nine studies (12%) reported the evaluation of the ovaries and the fallopian tubes to rule out ovarium metastases14,30,36,38,54,87,93,98,106. Furthermore, inspection for peritoneal deposits of tumor cells in the Douglas pouch was reported explicitly in three studies (4%)51,88,114.
Esophageal hiatus
The evaluation of the esophageal hiatus was discussed in nine studies34,38,39,44,61,81,83,86,89,95. Primarily, for tumors of the gastroesophageal junction, exploration of the diaphragmatic hiatus was achieved through an incision and a blunt dissection to the phrenoesophageal peritoneal fold.
Classification of peritoneal dissemination
Three studies reported a classification system to assess the extent of peritoneal carcinomatosis12,31,65. The peritoneal cavity was evaluated either using the Peritoneal Cancer Index (PCI)12,31 or according to the Japanese Research Society for Gastric Cancer (P0,1,2,3)65.
Complications
Perioperative complications after staging laparoscopy in gastric cancer were reported in 13 studies11,14,32,37,40,49,59,61,64,65,86,95,101. The most common reported complication was intestinal perforation (30%), followed by myocardial infarction and blood loss (23%). Vascular injury, diaphragmatic perforation, and pulmonary infection were documented in two studies (15%), while ileum perforation, urine infection, and subcutaneous emphysema were reported least frequently (7%).
Intraoperative peritoneal lavage
Out of 29 studies assessing the timing of peritoneal washings during staging laparoscopy, 25 (86%) reported lavage at the beginning of the procedure and before manipulation of the primary tumor12–14,25,26,28,30,32,34,36,44,47–51,59,61,63,65,78,83,84,93,95. In contrast, four studies (14%) reported peritoneal lavage performed after the abdominal cavity inspection and/or peritoneal biopsy3,70,79,80. No comparative studies were identified about intraoperative peritoneal lavage timing during staging laparoscopy. In two retrospective studies from the East, cytology of the fluid was performed only in tumors with serosal invasion as a supplementary investigation51,80. Moreover, according to 21 studies, a sample for cytological examination was obtained in the presence of ascites14,28,30,34,37–40,48,50,51,58,61,63,78,79,84,85,98.
Intraperitoneal lavage aspiration
No studies reported the timing of intra-abdominal fluid injection and aspiration. Two studies reported a 3–5-min interval between peritoneal lavage and fluid aspiration14,70. Before the collection of the fluid, a gentle peritoneal agitation was performed, as reported in eight studies (27%).
The volume of fluid and aspiration
Detailed information on the volume of peritoneal lavage fluid was reported in 38 studies11,12,14,19,25,26,28–34,36,37,41,42,44,46,47,49–51,54,57,59,61,63–65,70,73,78–80,83,84,95. With a range of 20–1000 ml, the most common volume of intraperitoneal lavage in the included reports was 200 ml (8/38 studies, 19%). All peritoneal washings were conducted with 0.9% saline solution. The following regions were described as areas of fluid aspiration: right and left upper abdominal quadrants lesser sac, right paracolic sulcus or quadrant, left paracolic sulcus or quadrant, pouch of Douglas, pelvic floor, subhepatic space, hepatorenal recess, splenic recess, and over the primary tumor area. Reported regions of intraperitoneal lavage aspiration are depicted in Figure 3. Although no studies compared the fluid volume aspiration, the amount of fluid sent for cytological evaluation ranged from 50 to 100 ml, according to 12 reports11,12,14,19,25,26,34,41,46,47,51,54,65,70,79,80,93. Most authors did not routinely describe the storage conditions of the aspirated intraperitoneal fluid. However, two studies reported transportation in universal containers with no additives79 or in conical tubes36. A summary of surgical techniques of staging laparoscopy in gastric cancer followed by peritoneal lavage assessment is shown in Supplementary Material Table 3, Supplemental Digital Content 4, http://links.lww.com/JS9/A857.
Figure 3.
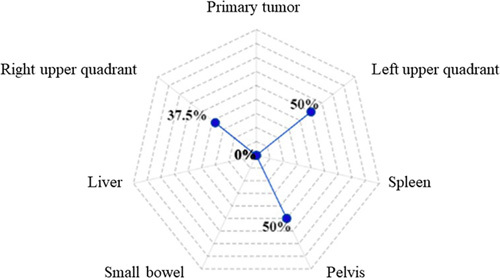
Radar chart evaluating the areas of peritoneal fluid aspiration during staging laparoscopy among included studies.
Discussion
Nearly four decades ago, Gross et al.201 reported in British Medical Journal that staging laparoscopy in gastric cancer patients was a clinically useful and safe procedure. Since that time, outcomes of over 5000 patients were reported in studies conducted in countries with the highest gastric cancer incidence and treatment experience. Advancements in minimally-invasive surgery have occurred since the time that the first documented peritoneal nodule biopsy was reported. Staging laparoscopy can now be enhanced with intraoperative ultrasonography and fluorescence imaging, which allow for staging optimization and treatment tailoring in cancer patients202. Our own group is currently evaluating the role of indocyanine-green (ICG) dye in nodal staging during diagnostic laparoscopy in a prospective and multi-institutional setting203. The current systematic review summarized the available data on staging laparoscopy technique and peritoneal fluid assessment among gastric cancer patients. Despite significant heterogeneity among the 212 included studies, several common characteristics of the procedure and cytological evaluation for gastric cancer were identified. For example, open Hasson was the method of choice to access the peritoneal cavity in 65% of studies, followed by establishing a pneumoperitoneum at 10–12 mmHg. Most frequently, the patient was positioned supine (70%), while a 30° scope and three ports were used to assess the peritoneal cavity clockwise (72%, 77%, and 85%, respectively) (Fig. 1). Right and left upper abdomen quadrants were the predominant area of laparoscopic exploration (both 65%), followed by primary tumor region (54%), liver and pelvis (both 30%), and small bowel and spleen (19% and 17%, respectively) (Fig. 2). Regions of peritoneal lavage and aspiration were limited to pelvis (50%), followed by right and left upper abdomen quadrants (37.5% and 50%, respectively) (Fig. 3). The technique of staging laparoscopy likely differs according to the surgeon’s preference, patient habitus, as well as primary tumor location. Although intraoperative findings regarding peritoneal carcinomatosis significantly differ among patients with gastric- and gastroesophageal junction cancers39, the proximal location of the tumor may require additional locoregional assessment, including dissection of phrenoesophageal ligament and hiatus. Meanwhile, cephalad extension of the disease should be additionally evaluated with endosonography, with possible fine-needle aspiration of suspected lymph nodes204. For tumors located in the posterior gastric wall, dissection of the omental bursa should be considered, despite an increased risk of postoperative complications3,54. Special attention should be paid to possible duodenum and hepatoduodenal ligament infiltration in distal gastric cancers. Additional evaluation of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) may be considered in such clinical settings205. Despite significant improvements in imaging over the last two decades206, radiological assessment of small bowel and small bowel mesentery remains challenging207. The abdominal area is crucial to access for peritoneal carcinomatosis and may be a reason for increased false-negative rates at staging laparoscopy41. To examine the small bowel mesentery and transverse mesocolon, intraoperative port placement and patient positioning modifications may be required. In a non-standardized setting, such adjustments may be time-consuming, raising a question of cost-effectiveness. Staging laparoscopy provides financial benefits in certain case-based scenarios, including signet ring histology, poor tumor differentiation, and lymphadenopathy208. From a surgical perspective, nodal involvement is one of the critical prognostic factors in gastric cancer209. However, estimating the resectability of bulky nodal disease around the celiac axis and its tributaries (N2 trier) is limited. Concomitant with the increasing implementation of minimally-invasive surgery in gastric cancer, a higher median lymph node harvest during gastrectomy has been observed210. Since laparoscopic and robotic techniques allow maintenance of oncological radicality and low mortality rate211, it has been suggested that extensive nodal assessment during staging laparoscopy may be used only when distant nodal disease is suspected. Staging laparoscopy combined with peritoneal cytology status may impact therapeutic decision-making212. When peritoneal disease is detected, palliative systemic or a combination of systemic and intraperitoneal chemotherapy is often indicated4. Administration of chemotherapy may converse with the positive cytology status, resulting in improved disease-specific survival (DSS)147. However, recommendations for routine laparoscopic workups vary between guidelines. Initially, the procedure was indicated for cT3-T4 tumors only213. NCCN guidelines recommend performing staging laparoscopy for cT1b or higher214, while the Japanese Gastric Cancer Association (JGCA) restricts staging laparoscopy to advanced tumors with neoadjuvant chemotherapy indications215. Generally, staging laparoscopy is suggested in all potentially resectable gastric cancer patients since considered a safe and minimally-invasive procedure216,217. Although the complication rate is likely low, serious adverse events can occur, particularly during intraoperative biopsies nearby vulnerable structures95. This systematic review did not aim to assess the diagnostic accuracy of laparoscopy as such. Leake et al.218 analyzed indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer over a decade ago. The accuracy of staging laparoscopy was independently evaluated according to T, N, and M stages. The accuracy assessment varied between 67–92.9% for the primary tumor, 64.3–66.7% for lymph node involvement, and 85–100% for distant metastases, including liver and peritoneal dissemination. Despite significant heterogeneity among studies evaluating cytological assessment of peritoneal lavage in gastric cancer patients, its contribution to staging laparoscopy for treatment decision-making has been underlined37. Conventional cytological evaluation (Papanicolaou or hematoxylin and eosin stains) presented low sensitivity and a poor negative predictive value, which led to the development of advanced techniques and improvement in detecting free cancer cells – immunoassays, immunohistochemistry (IHC), and reverse RT-PCR219. The latter is the most common alternative method for peritoneal lavage evaluation70,115,129,133,135,145,155,167,172,178–180,186,191,193,199. Although the sensitivity and specificity of the cytological assessment with several biomarkers (CEA, Ca19.9, Ca72-4, Ca15-3, AFP, cytokeratin 19, CYFRA 21.1) are ambiguous, the accuracy of the analysis increases with the clinical stage, reaching up to 87% in pT4 tumors. Moreover, patients with peritoneal recurrence will more likely be identified by a combination of RT-PCR and the cytological assay193. Conversely, RT-PCR detection rates may be unproportionally high due to CEA-mRNA expression in non-tumor cells105. Detecting peritoneal disease or positive cytology has significant consequences for gastric cancer patients’ treatment, particularly in the multimodal therapy setting5.
Although lacking standardization, the technique of staging laparoscopy has undoubtedly evolved over the last decades. Implementing narrow band imaging (NBI)220, near-infrared (NIR) fluorescent and ICG technologies may increase the accuracy of both staging laparoscopy and more complex minimally-invasive gastric cancer procedures221. However, objective measures are required to support the true impact of modern technologies on cancer surgery222. Compared with other surgical and staging procedures which established consensus on ‘how to do it’223,224, staging laparoscopy and peritoneal fluid assessment in gastric cancer patients remain highly variable. Although the most common approach to the procedure was pointed out in the current study, the best approach cannot be recommended due to the lack of studies directly comparing technical aspects intraoperatively. New research insights and initiatives to reach consensus globally are warranted. This systematic review has several limitations. Due to the use of various techniques for staging laparoscopy and peritoneal fluid assessment, the pooling of data was impossible. Moreover, selective outcome reporting in included studies might have led to clinical heterogeneity, and thus, our results should be interpreted cautiously. In conclusion, this systematic review demonstrated a high heterogeneity in the technique of staging laparoscopy and peritoneal fluid assessment in gastric cancer patients. Further research and initiatives to reach a consensus on the standardization of the procedure are warranted.
Ethical approval
Not applicable.
Consent
Not applicable.
Sources of funding
None, however the First author is a scholar of the Polish National Agency for Academic Exchange (NAWA) Franciszek Walczak program, which allowed conducting this study as a Research Fellow at the Department of Surgery, Ohio State University, Wexner Medical Center, Columbus, Ohio, USA.
Author contribution
K.R.-P.: conceptualization, search strategy, data retrieval, and writing – original manuscript, editing, and reviewing; M.E.: search strategy, data retrieval, and writing – original manuscript, editing, and reviewing; Z.P. and K.S.: data retrieval and writing – original manuscript, editing, and reviewing; W.P., T.M.P., and B.P.L.W.: correction and finalization of the manuscript and reviewing discussion.
Conflicts of interest disclosure
There are no conflicts of interest.
Research registration unique identifying number (UIN)
The protocol for this systematic review was registered on PROSPERO database (CRD: 42022306746).
Guarantor
Karol Rawicz-Pruszyński.
Data availability statement
Supplementary tables summarize the study search strategy and result in retrieval comprehensively. The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials, Supplemental Digital Content 4, http://links.lww.com/JS9/A857.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Footnotes
Karol Rawicz-Pruszyński and Maria Erodotou contributed equally to the manuscript.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 14 August 2023
Contributor Information
Karol Rawicz-Pruszyński, Email: krpruszynski@gmail.com.
Maria Erodotou, Email: mariaerodotou23@outlook.com.
Zuzanna Pelc, Email: zuzanna.torun@gmail.com.
Katarzyna Sędłak, Email: sedlak.katarz@gmail.com.
Wojciech Polkowski, Email: wojciechpolkowski@umlub.pl.
Timothy M. Pawlik, Email: tim.pawlik@osumc.edu.
Bas P.L. Wijnhoven, Email: b.wijnhoven@erasmusmc.nl.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.Fujita K, Omori T, Hara H, et al. Clinical importance of carcinoembryonic antigen messenger RNA level in peritoneal lavage fluids measured by transcription-reverse transcription concerted reaction for advanced gastric cancer in laparoscopic surgery 2022;36:2514–2523. [DOI] [PubMed] [Google Scholar]
- 3.Allen CJ, Blumenthaler AN, Das P, et al. Staging laparoscopy and peritoneal cytology in patients with early stage gastric adenocarcinoma. World J Surg Oncol 2020;18:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan H, Johnston FM. Current role for cytoreduction and HIPEC for gastric cancer with peritoneal disease. J Surg Oncol 2022;125:1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:1005–1020. [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:167–192. [DOI] [PubMed] [Google Scholar]
- 7.Saiz Martinez R, Dromain C, Vietti Violi N. Imaging of gastric carcinomatosis. J Clin Med 2021;10:5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darweesh AA, Barakat AF, Dawoud MF, et al. Diagnostic value of positron emission tomography/computed tomography (PET/CT) in detection of peritoneal carcinomatosis. Egypt J Radiol Nucl Med 2023;54:13. [Google Scholar]
- 9.Soussan M, Des Guetz G, Barrau V, et al. Comparison of FDG-PET/CT and MR with diffusion-weighted imaging for assessing peritoneal carcinomatosis from gastrointestinal malignancy. Eur Radiol 2012;22:1479–1487. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Yu J. The role of MRI in the diagnosis and treatment of gastric cancer. Diagn Interv Radiol 2020;26:176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu K, Chen XZ, Zhang WH, et al. “Four-Step Procedure” of laparoscopic exploration for gastric cancer in West China Hospital: a retrospective observational analysis from a high-volume institution in China. Surg Endosc 2019;33:1674–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Li Z, Zhang L, et al. Staging laparoscopy for locally advanced gastric cancer in Chinese patients: a multicenter prospective registry study. BMC Cancer 2018;18:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosogi H, Shinohara H, Tsunoda S, et al. Staging laparoscopy for advanced gastric cancer: significance of preoperative clinicopathological factors. Langenbecks Arch Surg 2017;402:33–39. [DOI] [PubMed] [Google Scholar]
- 14.Munasinghe A, Kazi W, Taniere P, et al. The incremental benefit of two quadrant lavage for peritoneal cytology at staging laparoscopy for oesophagogastric adenocarcinoma. Surg Endosc 2013;27:4049–4053. [DOI] [PubMed] [Google Scholar]
- 15.Yepuri N, Bahary N, Jain A, et al. Review and update on the role of peritoneal cytology in the treatment of gastric cancer. J Surg Res 2019;235:607–614. [DOI] [PubMed] [Google Scholar]
- 16.Virgilio E, Giarnieri E, Giovagnoli MR, et al. Gastric cancer cells in peritoneal lavage fluid: a systematic review comparing cytological with molecular detection for diagnosis of peritoneal metastases and prediction of peritoneal recurrences. Anticancer Res 2018;38:1255–1262. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi H, Honda M, Kawamura H, et al. Clinical impact of gastrectomy for gastric cancer patients with positive lavage cytology without gross peritoneal dissemination. J Surg Oncol 2022;125:615–620. [DOI] [PubMed] [Google Scholar]
- 18.Valletti M, Eshmuminov D, Gnecco N, et al. Gastric cancer with positive peritoneal cytology: survival benefit after induction chemotherapy and conversion to negative peritoneal cytology. World J Surg Oncol 2021;19:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlotto JRM, Carlotto FM, Vesco Neto MD, et al. Preoperative laparoscopy and peritoneal lavage in gastric adenocarcinoma: can the approach be modified? Rev Col Bras Cir 2020;46:e20192314. [DOI] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 21.Rawicz-Pruszyński K, Erodotou M, Pelc Z, et al. Techniques of staging laparoscopy and peritoneal fluid assessment in gastric cancer: a systematic review. International prospective register of systematic reviews (PROSPERO) registration, 2023. [DOI] [PMC free article] [PubMed]
- 22.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 23.da Costa BR, Cevallos M, Altman DG, et al. Uses and misuses of the STROBE statement: bibliographic study. BMJ Open 2011;1:e000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuksel C, Ersen O, Basceken SI, et al. The role of laparoscopic staging for the management of gastric cancer. Pol Przegl Chir 2021;93:1–8. [DOI] [PubMed] [Google Scholar]
- 26.Li ZY, Tang L, Li ZM, et al. Four-point computed tomography scores for evaluation of occult peritoneal metastasis in patients with gastric cancer: a region-to-region comparison with staging laparoscopy. Ann Surg Oncol 2020;27:1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rawicz-Pruszynski K, Mielko J, Pudlo K, et al. Yield of staging laparoscopy in gastric cancer is influenced by Lauren histologic subtype. J Surg Oncol 2019;120:1148–1153. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura N, Kinami S, Fujii Y, et al. The neutrophil/lymphocyte ratio as a predictor of peritoneal metastasis during staging laparoscopy for advanced gastric cancer: a retrospective cohort analysis. World J Surg Oncol 2019;17:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura M, Ojima T, Nakamori M, et al. Conversion surgery for gastric cancer with peritoneal metastasis based on the diagnosis of second-look staging laparoscopy. J Gastrointest Surg 2019;23:1758–1766. [DOI] [PubMed] [Google Scholar]
- 30.Bintintan VV, Cordos A, Chira R, et al. The value of staging laparoscopy for optimal multidisciplinary treatment in patients with gastric cancer. Chirurgia (Bucur) 2018;113:789–798. [DOI] [PubMed] [Google Scholar]
- 31.Brenkman HJF, Gertsen EC, Vegt E, et al. Evaluation of PET and laparoscopy in STagIng advanced gastric cancer: a multicenter prospective study (PLASTIC-study. BMC Cancer 2018;18:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irino T, Sano T, Hiki N, et al. Diagnostic staging laparoscopy in gastric cancer: a prospective cohort at a cancer institute in Japan. Surg Endosc 2018;32:268–275. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Luo H, Zhou C, et al. Yield of staging laparoscopy for incurable factors in Chinese patients with advanced gastric cancer. J Laparoendosc Adv Surg Tech A 2018;28:19–24. [DOI] [PubMed] [Google Scholar]
- 34.Leeman MF, Patel D, Anderson J, et al. Multidetector computed tomography versus staging laparoscopy for the detection of peritoneal metastases in esophagogastric junctional and gastric cancer. Surg Laparosc Endosc Percutan Tech 2017;27:369–374. [DOI] [PubMed] [Google Scholar]
- 35.Harris C, Ostwal V, Vallathol DH, et al. Calculation of a clinical predictive factors identifying peritoneal disease on a staging laparoscopy in gastric cancers. South Asian J Cancer 2019;8:166–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikoma N, Blum M, Chiang YJ, et al. Yield of staging laparoscopy and lavage cytology for radiologically occult peritoneal carcinomatosis of gastric cancer. Ann Surg Oncol 2016;23:4332–4337. [DOI] [PubMed] [Google Scholar]
- 37.Hu YF, Deng ZW, Liu H, et al. Staging laparoscopy improves treatment decision-making for advanced gastric cancer. World J Gastroenterol 2016;22:1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirza A, Galloway S. Laparoscopy, computerised tomography and fluorodeoxyglucose positron emission tomography in the management of gastric and gastro-oesophageal junction cancers. Surg Endosc 2016;30:2690–2696. [DOI] [PubMed] [Google Scholar]
- 39.Strandby RB, Svendsen LB, Fallentin E, et al. The multidisciplinary team conference’s decision on M-staging in patients with gastric- and gastroesophageal cancer is not accurate without staging laparoscopy. Scand J Surg 2016;105:104–108. [DOI] [PubMed] [Google Scholar]
- 40.Simon M, Mal F, Perniceni T, et al. Accuracy of staging laparoscopy in detecting peritoneal dissemination in patients with gastroesophageal adenocarcinoma. Dis Esophagus 2016;29:236–240. [DOI] [PubMed] [Google Scholar]
- 41.Miki Y, Tokunaga M, Tanizawa Y, et al. Staging laparoscopy for patients with cM0, type 4, and large type 3 gastric cancer. World J Surg 2015;39:2742–2747. [DOI] [PubMed] [Google Scholar]
- 42.Convie L, Thompson RJ, Kennedy R, et al. The current role of staging laparoscopy in oesophagogastric cancer. Ann R Coll Surg Engl 2015;97:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tourani SS, Cabalag C, Link E, et al. Laparoscopy and peritoneal cytology: important prognostic tools to guide treatment selection in gastric adenocarcinoma. ANZ J Surg 2015;85:69–73. [DOI] [PubMed] [Google Scholar]
- 44.Ishigami S, Uenosono Y, Arigami T, et al. Clinical utility of perioperative staging laparoscopy for advanced gastric cancer. World J Surg Oncol 2014;12:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang H, Jin JJ, Long ZW, et al. Three-port laparoscopic exploration is not sufficient for patients with T4 gastric cancer. Asian Pac J Cancer Prev 2014;15:8221–8224. [DOI] [PubMed] [Google Scholar]
- 46.Kishi K, Fujiwara Y, Yano M, et al. Diagnostic laparoscopy with 5-aminolevulinic-acid-mediated photodynamic diagnosis enhances the detection of peritoneal micrometastases in advanced gastric cancer. Oncology 2014;87:257–265. [DOI] [PubMed] [Google Scholar]
- 47.Santa-Maria AF, Valadao M, Iglesias AC. The role of staging laparoscopy in treatment of locally advanced gastric cancer. Surg Laparosc Endosc Percutan Tech 2014;24:434–439. [DOI] [PubMed] [Google Scholar]
- 48.Kikuchi H, Kamiya K, Hiramatsu Y, et al. Laparoscopic narrow-band imaging for the diagnosis of peritoneal metastasis in gastric cancer. Ann Surg Oncol 2014;21:3954–3962. [DOI] [PubMed] [Google Scholar]
- 49.Yamagata Y, Amikura K, Kawashima Y, et al. Staging laparoscopy in advanced gastric cancer: usefulness and issues requiring improvement. Hepatogastroenterology 2013;60:751–755. [DOI] [PubMed] [Google Scholar]
- 50.Cardona K, Zhou Q, Gonen M, et al. Role of repeat staging laparoscopy in locoregionally advanced gastric or gastroesophageal cancer after neoadjuvant therapy. Ann Surg Oncol 2013;20:548–554. [DOI] [PubMed] [Google Scholar]
- 51.Li Z, Ji J. Application of laparoscopy in the diagnosis and treatment of gastric cancer. Ann Transl Med 2015;3:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kakroo SM, Rashid A, Wani AA, et al. Staging laparoscopy in carcinoma of stomach: a comparison with CECT staging. Int J Surg Oncol 2013;2013:674965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murayama Y, Ichikawa D, Koizumi N, et al. Staging fluorescence laparoscopy for gastric cancer by using 5-aminolevulinic acid. Anticancer Res 2012;32:5421–5427. [PubMed] [Google Scholar]
- 54.Shelat VG, Thong JF, Seah M, et al. Role of staging laparoscopy in gastric malignancies – our institutional experience. World J Gastrointest Surg 2012;4:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahadevan D, Sudirman A, Kandasami P, et al. Laparoscopic staging in gastric cancer: an essential step in its management. J Minim Access Surg 2010;6:111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roviaro GC, Varoli F, Sonnino D, et al. Can routine laparoscopy help to reduce the rate of explorative laparotomies for gastric cancer? Laparoscopy in gastric cancer. Diagn Ther Endosc 2000;6:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsuchida K, Yoshikawa T, Tsuburaya A, et al. Indications for staging laparoscopy in clinical T4M0 gastric cancer. World J Surg 2011;35:2703–2709. [DOI] [PubMed] [Google Scholar]
- 58.Kapiev A, Rabin I, Lavy R, et al. The role of diagnostic laparoscopy in the management of patients with gastric cancer. Isr Med Assoc J 2010;12:726–728. [PubMed] [Google Scholar]
- 59.Shimizu H, Imamura H, Ohta K, et al. Usefulness of staging laparoscopy for advanced gastric cancer. Surg Today 2010;40:119–124. [DOI] [PubMed] [Google Scholar]
- 60.Hao YX, Zhong H, Yu PW, et al. Influence of laparoscopic gastrectomy on the detection rate of free gastric cancer cells in the peritoneal cavity. Ann Surg Oncol 2010;17:65–72. [DOI] [PubMed] [Google Scholar]
- 61.Muntean V, Mihailov A, Iancu C, et al. Staging laparoscopy in gastric cancer. Accuracy and impact on therapy. J Gastrointestinal Liver Diseases 2009;18:189–195. [PubMed] [Google Scholar]
- 62.Samee A, Moorthy K, Jaipersad T, et al. Evaluation of the role of laparoscopic ultrasonography in the staging of oesophagogastric cancers. Surg Endosc 2009;23:2061–2065. [DOI] [PubMed] [Google Scholar]
- 63.Badgwell B, Cormier JN, Krishnan S, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival. Ann Surg Oncol 2008;15:2684–2691. [DOI] [PubMed] [Google Scholar]
- 64.Osorio J, Rodriguez-Santiago J, Munoz E, et al. Outcome of unresected gastric cancer after laparoscopic diagnosis of peritoneal carcinomatosis. Clin Transl Oncol 2008;10:294–297. [DOI] [PubMed] [Google Scholar]
- 65.Song KY, Kim JJ, Kim SN, et al. Staging laparoscopy for advanced gastric cancer: is it also useful for the group which has an aggressive surgical strategy? World J Surg 2007;31:1228–3. [DOI] [PubMed] [Google Scholar]
- 66.de Graaf GW, Ayantunde AA, Parsons SL, et al. The role of staging laparoscopy in oesophagogastric cancers. Eur J Surg Oncol 2007;33:988–992. [DOI] [PubMed] [Google Scholar]
- 67.Nakagawa S, Nashimoto A, Yabusaki H. Role of staging laparoscopy with peritoneal lavage cytology in the treatment of locally advanced gastric cancer. Gastric Cancer 2007;10:29–34. [DOI] [PubMed] [Google Scholar]
- 68.Deogracias ML, Rodriguez-Sanjuan JC, de la Torre F, et al. Absence of port-site metastases following staging laparoscopy for gastric carcinoma. Rev Esp Enferm Dig 2006;98:755–759. [DOI] [PubMed] [Google Scholar]
- 69.Sarela AI, Lefkowitz R, Brennan MF, et al. Selection of patients with gastric adenocarcinoma for laparoscopic staging. Am J Surg 2006;191:134–138. [DOI] [PubMed] [Google Scholar]
- 70.Wilkiemeyer MB, Bieligk SC, Ashfaq R, et al. Laparoscopy alone is superior to peritoneal cytology in staging gastric and esophageal carcinoma. Surg Endosc 2004;18:852–856. [DOI] [PubMed] [Google Scholar]
- 71.Blackshaw GR, Barry JD, Edwards P, et al. Laparoscopy significantly improves the perceived preoperative stage of gastric cancer. Gastric Cancer 2003;6:225–229. [DOI] [PubMed] [Google Scholar]
- 72.Lee JH, Ryu KW, Kim YW, et al. Staging laparoscopy in gastric cancer: a single port method. J Surg Oncol 2003;84:50–52. [DOI] [PubMed] [Google Scholar]
- 73.Fujimura T, Kinami S, Ninomiya I, et al. Diagnostic laparoscopy, serum CA125, and peritoneal metastasis in gastric cancer. Endoscopy 2002;34:569–574. [DOI] [PubMed] [Google Scholar]
- 74.Lehnert T, Rudek B, Kienle P, et al. Impact of diagnostic laparoscopy on the management of gastric cancer: prospective study of 120 consecutive patients with primary gastric adenocarcinoma. Br J Surg 2002;89:471–475. [DOI] [PubMed] [Google Scholar]
- 75.Lavonius MI, Gullichsen R, Salo S, et al. Staging of gastric cancer: a study with spiral computed tomography, ultrasonography, laparoscopy, and laparoscopic ultrasonography. Surg Laparosc Endosc Percutan Tech 2002;12:77–81. [DOI] [PubMed] [Google Scholar]
- 76.Wakelin SJ, Deans C, Crofts TJ, et al. A comparison of computerised tomography, laparoscopic ultrasound and endoscopic ultrasound in the preoperative staging of oesophago-gastric carcinoma. Eur J Radiol 2002;41:161–167. [DOI] [PubMed] [Google Scholar]
- 77.Flett ME, Lim MN, Bruce D, et al. Prognostic value of laparoscopic ultrasound in patients with gastro-esophageal cancer. Dis Esophagus 2001;14:223–226. [DOI] [PubMed] [Google Scholar]
- 78.Onate-Ocana LF, Gallardo-Rincon D, Aiello-Crocifoglio V, et al. The role of pretherapeutic laparoscopy in the selection of treatment for patients with gastric carcinoma: a proposal for a laparoscopic staging system. Ann Surg Oncol 2001;8:624–631. [DOI] [PubMed] [Google Scholar]
- 79.Bryan RT, Cruickshank NR, Needham SJ, et al. Laparoscopic peritoneal lavage in staging gastric and oesophageal cancer. Eur J Surg Oncol 2001;27:291–297. [DOI] [PubMed] [Google Scholar]
- 80.Yano M, Tsujinaka T, Shiozaki H, et al. Appraisal of treatment strategy by staging laparoscopy for locally advanced gastric cancer. World J Surg Sep 2000;24:1130–1135; discussion 1135-6. [DOI] [PubMed] [Google Scholar]
- 81.Smith A, Finch MD, John TG, et al. Role of laparoscopic ultrasonography in the management of patients with oesophagogastric cancer. Br J Surg 1999;86:1083–1087. [DOI] [PubMed] [Google Scholar]
- 82.McCulloch P, Johnson M, Jairam R, et al. Laparoscopic staging of gastric cancer is safe and affects treatment strategy. Ann R Coll Surg Engl 1998;80:400–402. [PMC free article] [PubMed] [Google Scholar]
- 83.Stein HJ, Kraemer SJ, Feussner H, et al. Clinical value of diagnostic laparoscopy with laparoscopic ultrasound in patients with cancer of the esophagus or cardia. J Gastrointest Surg 1997;1:167–172; discussion 72-3. [DOI] [PubMed] [Google Scholar]
- 84.D’Ugo DM, Persiani R, Caracciolo F, et al. Selection of locally advanced gastric carcinoma by preoperative staging laparoscopy. Surg Endosc 1997;11:1159–1162. [DOI] [PubMed] [Google Scholar]
- 85.Asencio F, Aguilo J, Salvador JL, et al. Video-laparoscopic staging of gastric cancer. A prospective multicenter comparison with noninvasive techniques. Surg Endosc 1997;11:1153–1158. [DOI] [PubMed] [Google Scholar]
- 86.Finch MD, John TG, Garden OJ, et al. Laparoscopic ultrasonography for staging gastroesophageal cancer. Surgery 1997;121:10–17. [DOI] [PubMed] [Google Scholar]
- 87.D’Ugo DM, Coppola R, Persiani R, et al. Immediately preoperative laparoscopic staging for gastric cancer. Surg Endosc 1996;10:996–999. [DOI] [PubMed] [Google Scholar]
- 88.Shiraishi N, Morimoto A, Sato K, et al. Laparoscopy in the management of scirrhous gastric cancer. Gastric Cancer 1999;2:109–114. [DOI] [PubMed] [Google Scholar]
- 89.Molloy RG, McCourtney JS, Anderson JR. Laparoscopy in the management of patients with cancer of the gastric cardia and oesophagus. Br J Surg 1995;82:352–354. [DOI] [PubMed] [Google Scholar]
- 90.Kriplani AK, Kapur BM. Laparoscopy for pre-operative staging and assessment of operability in gastric carcinoma. Gastrointest Endosc 1991;37:441–443. [DOI] [PubMed] [Google Scholar]
- 91.Shandall A, Johnson C. Laparoscopy or scanning in oesophageal and gastric carcinoma? Br J Surg 1985;72:449–451. [DOI] [PubMed] [Google Scholar]
- 92.Xie D, Wang Y, Shen J, et al. Detection of carcinoembryonic antigen in peritoneal fluid of patients undergoing laparoscopic distal gastrectomy with complete mesogastric excision. Br J Surg 2018;105:1471–1479. [DOI] [PubMed] [Google Scholar]
- 93.Pak LM, Coit DG, Eaton AA, et al. Percutaneous peritoneal lavage for the rapid staging of gastric and pancreatic cancer. Ann Surg Oncol 2017;24:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Menon KV, Dehn TCB. Multiport staging laparoscopy in esophageal and cardiac carcinoma. Dis Esophagus 2003;16:295–300. [DOI] [PubMed] [Google Scholar]
- 95.Schlag PM, Hünerbein M, Rau B. The importance of staging laparoscopy for the treatment of gastric cancer. Onkologie 1998;21:486–491. [Google Scholar]
- 96.Romijn MG, Van Overhagen H, Spillenaar Bilgen EJ, et al. Laparoscopy and laparoscopic ultrasonography in staging of oesophageal and cardial carcinoma. Br J Surg 1998;85:1010–1012. [DOI] [PubMed] [Google Scholar]
- 97.Burke EC, Karpeh MS, Jr, Conlon KC, et al. Laparoscopy in the management of gastric adenocarcinoma. Ann Surg 1997;225:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderson DN, Campbell S, Park KGM. Accuracy of laparoscopic ultrasonography in the staging of upper gastrointestinal malignancy. Br J Surg 1996;83:1424–1428. [DOI] [PubMed] [Google Scholar]
- 99.Madsen MR, Bau Mortensen M, Hovendal C. Explorative laparotomy or laparoscopy in patients with carcinoma of the stomach and pancreas? Minimally Invasive Ther 1994;3:267–270. [Google Scholar]
- 100.Watt I, Stewart I, Anderson D, et al. Laparoscopy, ultrasound and computer tomography in cancer of the oesophagus and gastric cardia: a prospective comparison for detecting intra-abdominal metastases. Br J Surg 1989;76:1036–1039. [DOI] [PubMed] [Google Scholar]
- 101.Gross E, Bancewicz J, Ingram G. Assessment of gastric cancer by laparoscopy. Br Med J 1984;288:1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Allen CJ, Newhook TE, Vreeland TJ, et al. Yield of peritoneal cytology in staging patients with gastric and gastroesophageal cancer. J Surg Oncol 2019;120:1350–1357. [DOI] [PubMed] [Google Scholar]
- 103.Bae GE, Kim SH, Choi MK, et al. Targeted sequencing of ascites and peritoneal washing fluid of patients with gastrointestinal cancers and their clinical applications and limitations. Front Oncol 2021;11:712754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bentrem D, Wilton A, Mazumdar M, et al. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol 2005;12:347–53. [DOI] [PubMed] [Google Scholar]
- 105.Broll R, Weschta M, Windhoevel U, et al. Prognostic significance of free gastrointestinal tumor cells in peritoneal lavage detected by immunocytochemistry and polymerase chain reaction. Langenbeck’s Arch Surg 2001;386:285–292. [DOI] [PubMed] [Google Scholar]
- 106.Burke EC, Karpeh MS, Jr, Conlon KC, et al. Peritoneal lavage cytology in gastric cancer: an independent predictor of outcome. Ann Surg Oncol 1998;5:411–415. [DOI] [PubMed] [Google Scholar]
- 107.Carboni F, Federici O, Giofrè M, et al. An 18-year experience in diagnostic laparoscopy of peritoneal carcinomatosis: results from 744 patients. J Gastrointest Surg 2020;24:2096–2103. [DOI] [PubMed] [Google Scholar]
- 108.Carneiro FP, Muniz-Junqueira MI, Carneiro MD, et al. Anti-EpCAM antibodies for detection of metastatic carcinoma in effusions and peritoneal wash. Oncol Lett 2019;18:2019–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chang-Qing F, Yi L, De-Guang W, et al. Immune clearance gastric carcinoma cells in ascites by activating caspase-9-induced apoptosis. APMIS 2011;119:173–179. [DOI] [PubMed] [Google Scholar]
- 110.Dalal KM, Woo Y, Kelly K, et al. Detection of micrometastases in peritoneal washings of gastric cancer patients by the reverse transcriptase polymerase chain reaction. Gastric Cancer 2008;11:206–213. [DOI] [PubMed] [Google Scholar]
- 111.Davidson B, Totsch M, Wohlschlaeger J, et al. The diagnostic role of BAP1 in serous effusions. Hum Pathol, 092018;79:122–126. [DOI] [PubMed] [Google Scholar]
- 112.Del Monte SR, Ranieri D, Mazzetta F, et al. Free peritoneal tumor cells detection in gastric and colorectal cancer patients. J Surg Oncol 2012;106:17–23. [DOI] [PubMed] [Google Scholar]
- 113.Elkeleny MR, Ahmed K. Impact of diagnostic laparoscopy in the management of gastric cancer in Egyptian patients. Egypt J Surg 2019;38:394–398. [Google Scholar]
- 114.Fujimura T, Ohta T, Kitagawa H, et al. Trypsinogen expression and early detection for peritoneal dissemination in gastric cancer. J Surg Oncol 1998;69:71–75. [DOI] [PubMed] [Google Scholar]
- 115.Fukumoto Y, Ikeguchi M, Matsumoto S, et al. Detection of cancer cells and gene expression of cytokines in the peritoneal cavity in patients with gastric cancer. Gastric Cancer 2006;9:271–276. [DOI] [PubMed] [Google Scholar]
- 116.Garofalo A, Valle M. Laparoscopy in the management of peritoneal carcinomatosis. Cancer J 2009;15:190–195. [DOI] [PubMed] [Google Scholar]
- 117.Han TS, Kong SH, Lee HJ, et al. Dissemination of free cancer cells from the gastric lumen and from perigastric lymphovascular pedicles during radical gastric cancer surgery. Ann Surg Oncol 2011;18:2818–2825. [DOI] [PubMed] [Google Scholar]
- 118.Hara M, Nakanishi H, Jun Q, et al. Comparative analysis of intraperitoneal minimal free cancer cells between colorectal and gastric cancer patients using quantitative RT-PCR: possible reason for rare peritoneal recurrence in colorectal cancer. Clin Exp Metastasis 2007;24:179–189. [DOI] [PubMed] [Google Scholar]
- 119.Hasbahceci M, Akcakaya A, Guler B, et al. Use of peritoneal washing cytology for the detection of free peritoneal cancer cells before and after surgical treatment of gastric adenocarcinoma. J Cancer Res Ther 2018;14:1225–1229. [DOI] [PubMed] [Google Scholar]
- 120.Hesamifard B, Sharifi A, Saffar H, et al. The role of open diagnostic peritoneal lavage in the evaluation of peritoneal cytology for advanced gastric cancer: an old diagnostic modality with new usage. Middle East J Cancer 2021;12:249–254. [Google Scholar]
- 121.Hiraki M, Kitajima Y, Koga Y, et al. Aberrant gene methylation is a biomarker for the detection of cancer cells in peritoneal wash samples from advanced gastric cancer patients. Ann Surg Oncol 2011;18:3013–3019. [DOI] [PubMed] [Google Scholar]
- 122.Hiroi S, Nakanishi K, Kawai T. Expressions of human telomerase mRNA component (hTERC) and telomerase reverse transcriptase (hTERT) mRNA in effusion cytology. Diagn Cytopathol 2003;29:212–216. [DOI] [PubMed] [Google Scholar]
- 123.Hu XY, Ling ZN, Hong LL, et al. Circulating methylated THBS1 DNAs as a novel marker for predicting peritoneal dissemination in gastric cancer. J Clin Lab Anal 2021;35:e23936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Iida T, Iwahashi M, Katsuda M, et al. Prognostic significance of IL-17 mRNA expression in peritoneal lavage in gastric cancer patients who underwent curative resection. Oncol Rep 2014;31:605–612. [DOI] [PubMed] [Google Scholar]
- 125.Juhl H, Kalthoff H, Kruger U, et al. Immunocytological detection of micrometastatic cells in gastrointestinal cancer-patients. Zentralbl Chir 1995;120:116–122. [PubMed] [Google Scholar]
- 126.Juhl H, Stritzel M, Wroblewski A, et al. Immunocytological detection of micrometastatic cells: comparative evaluation of findings in the peritoneal cavity and the bone marrow of gastric, colorectal and pancreatic cancer patients. Int J Cancer 1994;57:330–335. [DOI] [PubMed] [Google Scholar]
- 127.Jung M, Jeung HC, Lee SS, et al. The clinical significance of ascitic fluid CEA in advanced gastric cancer with ascites. J Cancer Res Clin Oncol 2010;136:517–526. [DOI] [PubMed] [Google Scholar]
- 128.Kanamaru R, Ohzawa H, Miyato H, et al. Low density neutrophils (LDN) in postoperative abdominal cavity assist the peritoneal recurrence through the production of neutrophil extracellular traps (NETs). Sci Rep 2018;8:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Katsuragi K, Yashiro M, Sawada T, et al. Prognostic impact of PCR-based identification of isolated tumour cells in the peritoneal lavage fluid of gastric cancer patients who underwent a curative R0 resection. Br J Cancer 2007;97:550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kitayama J, Emoto S, Yamaguchi H, et al. Flow cytometric quantification of intraperitoneal free tumor cells (FTC) in patients with peritoneal metastasis. Cytometry B Clin Cytom 2013;86:56–62. [DOI] [PubMed] [Google Scholar]
- 131.Kitayama J, Emoto S, Yamaguchi H, et al. Flow cytometric quantification of intraperitoneal free tumor cells is a useful biomarker in gastric cancer patients with peritoneal metastasis. Ann Surg Oncol 2015;22:2336–2342. [DOI] [PubMed] [Google Scholar]
- 132.Kodera Y, Nakanishi H, Ito S, et al. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: analysis of real time reverse transcriptase-polymerase chain reaction after 5 years of followup. J Am Coll Surg 2006;202:231–236. [DOI] [PubMed] [Google Scholar]
- 133.Kodera Y, Nakanishi H, Ito S, et al. Detection of disseminated cancer cells in linitis plastica-type gastric carcinoma. Jpn J Clin Oncol 2004;34:525–531. [DOI] [PubMed] [Google Scholar]
- 134.Kodera Y, Nakanishi H, Ito S, et al. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: detection of cytokeratin 20 mRNA in peritoneal washes, in addition to detection of carcinoembryonic antigen. Gastric Cancer 2005;8:142–148. [DOI] [PubMed] [Google Scholar]
- 135.Kodera Y, Nakanishi H, Ito S, et al. Quantitative detection of disseminated cancer cells in the greater omentum of gastric carcinoma patients with real-time RT-PCR: a comparison with peritoneal lavage cytology. Gastric Cancer 2002;5:69–76. [DOI] [PubMed] [Google Scholar]
- 136.Kodera Y, Nakanishi H, Ito S, et al. Quantitative detection of disseminated free cancer cells in peritoneal washes with real-time reverse transcriptase-polymerase chain reaction: a sensitive predictor of outcome for patients with gastric carcinoma. Ann Surg 2002;235:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kodera Y, Nakanishi H, Yamamura Y, et al. Prognostic value and clinical implications of disseminated cancer cells in the peritoneal cavity detected by reverse transcriptase-polymerase chain reaction and cytology. Int J Cancer 1998;79:429–433. [DOI] [PubMed] [Google Scholar]
- 138.Koemans WJ, Houwink A, van der Kaaij RT, et al. Perioperative management of gastric cancer patients treated with (sub)total gastrectomy, cytoreductive surgery, and hyperthermic intraperitoneal chemotherapy (HIPEC): lessons learned. Ann Surg Oncol 2021;28:4647–4654. [DOI] [PubMed] [Google Scholar]
- 139.Koyama S. Coordinate cell-surface expression of matrix metalloproteinases and their inhibitors on cancer-associated myofibroblasts from malignant ascites in patients with gastric carcinoma. J Cancer Res Clin Oncol 2005;131:809–814. [DOI] [PubMed] [Google Scholar]
- 140.Kundu UR, Krishnamurthy S. Use of the monoclonal antibody MOC-31 as an immunomarker for detecting metastatic adenocarcinoma in effusion cytology. Cancer Cytopathol 2011;119:272–278. [DOI] [PubMed] [Google Scholar]
- 141.Li XJ, Li FQ, Han JK, et al. Ascites metabolism measurement enhanced the diagnostic value and accuracy of prognostic evaluation in F-18-FDG PET/CT studies in malignant ascites patients. Nucl Med Commun 2013;34:544–550. [DOI] [PubMed] [Google Scholar]
- 142.Li ZY, Li ZM, Jia SQ, et al. Depth of tumor invasion and tumor-occupied portions of stomach are predictive factors of intra-abdominal metastasis. Chin J Cancer Res 2017;29:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lorenzen S, Panzram B, Rosenberg R, et al. Prognostic significance of free peritoneal tumor cells in the peritoneal cavity before and after neoadjuvant chemotherapy in patients with gastric carcinoma undergoing potentially curative resection. Ann Surg Oncol 2010;17:2733–2739. [DOI] [PubMed] [Google Scholar]
- 144.Lu J, Li XF, Kong LX, et al. Expression and significance of cyclooxygenase-2 mRNA in benign and malignant ascites. World J Gastroenterol 2013;19:6883–6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marutsuka T, Shimada S, Shiomori K, et al. Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: an ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res 2003;9:678–685. [PubMed] [Google Scholar]
- 146.Mensch LS, Weller L, Simmons-Arnold L, et al. GLUT1 antibody staining in thin-layer specimens of benign and malignant body cavity effusions. Acta Cytol 2002;46:813–818. [DOI] [PubMed] [Google Scholar]
- 147.Mezhir JJ, Posner MC, Roggin KK. Prospective clinical trial of diagnostic peritoneal lavage to detect positive peritoneal cytology in patients with gastric cancer. J Surg Oncol 2013;107:794–798. [DOI] [PubMed] [Google Scholar]
- 148.Mezhir JJ, Shah MA, Jacks LM, et al. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol 2010;17:3173–3180. [DOI] [PubMed] [Google Scholar]
- 149.Mezhir JJ, Shah MA, Jacks LM, et al. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Indian J Surg Oncol 2011;2:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mori K, Aoyagi K, Ueda T, et al. Highly specific marker genes for detecting minimal gastric cancer cells in cytology negative peritoneal washings. Biochem Biophys Res Commun 2004;313:931–937. [DOI] [PubMed] [Google Scholar]
- 151.Mori K, Suzuki T, Uozaki H, et al. Detection of minimal gastric cancer cells in peritoneal washings by focused microarray analysis with multiple markers: clinical implications. Ann Surg Oncol 2007;14:1694–1702. [DOI] [PubMed] [Google Scholar]
- 152.Mori T, Fujiwara Y, Sugita Y, et al. Application of molecular diagnosis for detection of peritoneal micrometastasis and evaluation of preoperative chemotherapy in advanced gastric carcinoma. Ann Surg Oncol 2004;11:14–20. [DOI] [PubMed] [Google Scholar]
- 153.Muntean V, Oniu T, Lungoci C, et al. Staging laparoscopy in digestive cancers. J Gastrointest Liver Dis 2009;18:461–467. [PubMed] [Google Scholar]
- 154.Nakanishi H, Kodera Y, Torii A, et al. Detection of carcinoembryonic antigen-expressing free tumor cells in peritoneal washes from patients with gastric carcinoma by polymerase chain reaction. Jpn J Cancer Res 1997;88:687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nakanishi H, Kodera Y, Yamamura Y, et al. Rapid quantitative detection of carcinoembryonic antigen-expressing free tumor cells in the peritoneal cavity of gastric-cancer patients with real-time RT-PCR on the lightcycler. Int J Cancer 2000;89:411–417. [DOI] [PubMed] [Google Scholar]
- 156.Nakanishi H, Kodera Y, Yamamura Y, et al. Molecular diagnostic detection of free cancer cells in the peritoneal cavity of patients with gastrointestinal and gynecologic malignancies. Cancer Chemother Pharmacol 1999;43(Suppl):S32–S36. [DOI] [PubMed] [Google Scholar]
- 157.Nath J, Moorthy K, Taniere P, et al. Peritoneal lavage cytology in patients with oesophagogastric adenocarcinoma. Br J Surg 2008;95:721–726. [DOI] [PubMed] [Google Scholar]
- 158.Oh CA, Bae JM, Oh SJ, et al. Long-term results and prognostic factors of gastric cancer patients with only positive peritoneal lavage cytology. J Surg Oncol 2012;105:393–399. [DOI] [PubMed] [Google Scholar]
- 159.Ohzawa H, Kumagai Y, Yamaguchi H, et al. Exosomal microRNA in peritoneal fluid as a biomarker of peritoneal metastases from gastric cancer. Ann Gastroenterol Surg 2020;4:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Okada K, Fujiwara Y, Nakamura Y, et al. Oncofetal protein, IMP-3, a potential marker for prediction of postoperative peritoneal dissemination in gastric adenocarcinoma. J Surg Oncol 2012;105:780–785. [DOI] [PubMed] [Google Scholar]
- 161.Oyama K, Terashima M, Takagane A, et al. Prognostic significance of peritoneal minimal residual disease in gastric cancer detected by reverse transcription-polymerase chain reaction. Br J Surg 2004;91:435–443. [DOI] [PubMed] [Google Scholar]
- 162.Park CK, Malinowski DP, Cho NH. Diagnostic algorithm for determining primary tumor sites using peritoneal fluid. PLoS One 2018;13:e0199715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pomjanski N, Grote HJ, Doganay P, et al. Immunocytochemical identification of carcinomas of unknown primary in serous effusions. Diagn Cytopathol 2005;33:309–315. [DOI] [PubMed] [Google Scholar]
- 164.Ribeiro U, Jr, Gama-Rodrigues JJ, Safatle-Ribeiro AV, et al. Prognostic significance of intraperitoneal free cancer cells obtained by laparoscopic peritoneal lavage in patients with gastric cancer. J Gastrointest Surg 1998;2:244–249. [DOI] [PubMed] [Google Scholar]
- 165.Ribeiro U, Jr, Gama-Rodrigues JJ, Bitelman B, et al. Value of peritoneal lavage cytology during laparoscopic staging of patients with gastric carcinoma. Surg Laparosc Endosc 1998;8:132–135. [PubMed] [Google Scholar]
- 166.Sakakura C, Hagiwara A, Shirasu M, et al. Polymerase chain reaction for detection of carcinoembryonic antigen-expressing tumor cells on milky spots of the greater omentum in gastric cancer patients: a pilot study. Int J Cancer 2001;95:286–289. [DOI] [PubMed] [Google Scholar]
- 167.Sakakura C, Takemura H, Hagiwara A, et al. Overexpression of dopa decarboxylase in peritoneal dissemination of gastric cancer and its potential as a novel marker for the detection of peritoneal micrometastases with real-time RT-PCR. Br J Cancer 2004;90:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sasaki M, Wakasa K, Sakurai M, et al. Serosal balls detected immunocytochemically in peritoneal lavage obtained during surgery. Diagn Cytopathol 1995;13:124–127. [DOI] [PubMed] [Google Scholar]
- 169.Schmidt P, Thiele M, Rudroff C, et al. Detection of tumor cells in peritoneal lavages from patients with gastrointestinal cancer by multiplex reverse transcriptase PCR. Hepatogastroenterology 2001;48:1675–1679. [PubMed] [Google Scholar]
- 170.Schuhmacher C, Becker KF, Reich U, et al. Rapid detection of mutated E-cadherin in peritoneal lavage specimens from patients with diffuse-type gastric carcinoma. Diagn Mol Pathol 1999;8:66–70. [DOI] [PubMed] [Google Scholar]
- 171.Shim WY, Park KH, Jeung HC, et al. Quantitative detection of telomerase activity by real-time TRAP assay in the body fluids of cancer patients. Int JMol Med 2005;16:857–863. [PubMed] [Google Scholar]
- 172.Shimomura K, Sakakura C, Takemura M, et al. Combination of L-3-phosphoserine phosphatase and CEA using real-time RT-PCR improves accuracy in detection of peritoneal micrometastasis of gastric cancer. Anticancer Res 2004;24(2C):1113–1120. [PubMed] [Google Scholar]
- 173.Strandby RB, Svendsen LB, Ambrus R, et al. The incidence of free peritoneal tumor cells before and after neoadjuvant chemotherapy in gastroesophageal junction cancer. J Cytol 2020;37:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun XM, Dong WG, Yu BP, et al. Detection of type IV collagenase activity in malignant ascites. World J Gastroenterol 2003;9:2592–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Suzuki T, Ochiai T, Hayashi H, et al. Peritoneal lavage cytology findings as prognostic factor for gastric cancer. Semin Surg Oncol 1999;17:103–107. [DOI] [PubMed] [Google Scholar]
- 176.Takebayashi K, Murata S, Yamamoto H, et al. Surgery-induced peritoneal cancer cells in patients who have undergone curative gastrectomy for gastric cancer. Ann Surg Oncol 2014;21:1991–1997. [DOI] [PubMed] [Google Scholar]
- 177.Tamai M, Tanimura H, Yamaue H, et al. Expression of carcinoembryonic antigen in fresh human gastric cancer cells assessed by flow cytometry. J Surg Oncol 1993;52:176–180. [DOI] [PubMed] [Google Scholar]
- 178.Tamura N, Iinuma H, Takada T. Prospective study of the quantitative carcinoembryonic antigen and cytokeratin 20 mRNA detection in peritoneal washes to predict peritoneal recurrence in gastric carcinoma patients. Oncol Rep 2007;17:667–672. [PubMed] [Google Scholar]
- 179.Tamura S, Fujiwara Y, Kimura Y, et al. Prognostic information derived from RT-PCR analysis of peritoneal fluid in gastric cancer patients: results from a prospective multicenter clinical trial. J Surg Oncol 2014;109:75–80. [DOI] [PubMed] [Google Scholar]
- 180.To EMC, Chan WY, Chow C, et al. Gastric cancer cell detection in peritoneal washing: cytology versus RT-PCR for CEA transcripts. Diagn Mol Pathol 2003;12:88–95. [DOI] [PubMed] [Google Scholar]
- 181.Tokuhisa M, Ichikawa Y, Kosaka N, et al. Exosomal miRNAs from peritoneum lavage fluid as potential prognostic biomarkers of peritoneal metastasis in gastric cancer. PLoS One 2015;10:e0130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Toyota Y, Okamoto K, Tanaka N, et al. Conversion surgery of Stage IV gastric cancer with peritoneal dissemination after nivolumab. Int Cancer Conf J 2021;10:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tuzun Y, Yilmaz Ş, Canoruc F, et al. How to increase the diagnostic value of malignancy-related ascites: discriminative ability of the ascitic tumour markers. J Int Med Res 2009;37:87–95. [DOI] [PubMed] [Google Scholar]
- 184.van Dijkum E, Sturm PD, de Wit LT, et al. Cytology of peritoneal lavage performed during staging laparoscopy for gastrointestinal malignancies: is it useful? Ann Surg 1998;228:728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vogel P, Rüschoff J, Kümmel S, et al. Prognostic value of microscopic peritoneal dissemination: comparison between colon and gastric cancer. Dis Colon Rectum 2000;43:92–100. [DOI] [PubMed] [Google Scholar]
- 186.Wang JY, Lin SR, Lu CY, et al. Gastric cancer cell detection in peritoneal lavage: RT-PCR for carcinoembryonic antigen transcripts versus the combined cytology with peritoneal carcinoembryonic antigen levels. Cancer Lett 2005;223:129–135. [DOI] [PubMed] [Google Scholar]
- 187.Wang T, Wang L, Qian X, et al. Relationship between gene expression of 5-fluorouracil metabolic enzymes and 5-fluorouracil sensitivity in primary cancer cells isolated from malignant ascites. Cancer Invest 2011;29:130–136. [DOI] [PubMed] [Google Scholar]
- 188.Wang Z, Zhang X, Xu H, et al. Detection of peritoneal micrometastasis by reverse transcriptase-polymerase chain reaction for heparanase mRNA and cytology in peritoneal wash samples. J Surg Oncol 2005;90:59–65. [DOI] [PubMed] [Google Scholar]
- 189.Wong J, Kelly KJ, Mittra A, et al. Rt-PCR increases detection of submicroscopic peritoneal metastases in gastric cancer and has prognostic significance. J Gastrointest Surg 2012;16:889–896; discussion 896. [DOI] [PubMed] [Google Scholar]
- 190.Yamaguchi H, Satoh Y, Ishigami H, et al. Peritoneal lavage CEA mRNA levels predict conversion gastrectomy outcomes after induction chemotherapy with intraperitoneal paclitaxel in gastric cancer patients with peritoneal metastasis. Ann Surg Oncol 2017;24:3345–3352. [DOI] [PubMed] [Google Scholar]
- 191.Yamamura Y, Ito S, Mochizuki Y, et al. Distribution of free cancer cells in the abdominal cavity suggests limitations of bursectomy as an essential component of radical surgery for gastric carcinoma. Gastric Cancer 2007;10:24–28. [DOI] [PubMed] [Google Scholar]
- 192.Yamashita K, Kuba T, Shinoda H, et al. Detection of K-ras point mutations in the supernatants of peritoneal and pleural effusions for diagnosis complementary to cytologic examination. Am J Clin Pathol 1998;109:704–711. [DOI] [PubMed] [Google Scholar]
- 193.Yonemura Y, Endou Y, Fujimura T, et al. Diagnostic value of preoperative RT-PCR-based screening method to detect carcinoembryonic antigen-expressing free cancer cells in the peritoneal cavity from patients with gastric cancer. ANZ J Surg 2001;71:521–528. [DOI] [PubMed] [Google Scholar]
- 194.Yonemura Y, Fujimura T, Ninomiya I, et al. Prediction of peritoneal micrometastasis by peritoneal lavaged cytology and reverse transcriptase-polymerase chain reaction for matrix metalloproteinase-7 mRNA. Clin Cancer Res 2001;7:1647–1653. [PubMed] [Google Scholar]
- 195.Yun J, Han SB, Kim HJ, et al. Exosomal miR-181b-5p downregulation in ascites serves as a potential diagnostic biomarker for gastric cancer-associated malignant ascites. J Gastric Cancer 2019;19:301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang F, Feng Z, Zhang Y, et al. Determination of the optimal volume of ascitic fluid for the precise diagnosis of malignant ascites. Saudi J Gastroenterol 2019;25:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang W, Tong Q, Wang X, et al. T lymphocyte subsets determination and DNA ploidy analysis in the differential diagnosis between benign and malignant ascites. Cancer Invest 2009;27:67–69. [DOI] [PubMed] [Google Scholar]
- 198.Zhang X, Liu X, Sun F, et al. Greater omental milky spot examination for diagnosis of peritoneal metastasis in gastric cancer patients. J Laparoendosc Adv Surg Tech A 2017;27:106–109. [DOI] [PubMed] [Google Scholar]
- 199.Zhang YS, Xu J, Luo GH, et al. Detection of carcinoembryonic antigen mRNA in peritoneal washes from gastric cancer patients and its clinical significance. World J Gastroenterol 2006;12:1408–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang YZ, Zhao JH, Yu HB, et al. Detection and isolation of free cancer cells from ascites and peritoneal lavages using optically induced electrokinetics (OEK). Sci Adv 2020;6:eaba9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gross E, Bancewicz J, Ingram G. Assessment of gastric cancer by laparoscopy. Br Med J (Clin Res Ed) 1984;288:1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Oba A, Inoue Y, Ono Y, et al. Staging laparoscopy for pancreatic cancer using intraoperative ultrasonography and fluorescence imaging: the SLING trial. Br J Surg 2021;108:115–118. [DOI] [PubMed] [Google Scholar]
- 203.Rawicz-Pruszynski K, Sedlak K, Pelc Z, et al. Staging LaParoscopy to Assess Lymph NOde InvoLvement in Advanced GAstric Cancer (POLA)-Study protocol for a single-arm prospective observational multicenter study. PLoS One 2023;18:e0285758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kaushik N, Khalid A, Brody D, et al. Endoscopic ultrasound compared with laparoscopy for staging esophageal cancer. Ann Thorac Surg 2007;83:2000–2002. [DOI] [PubMed] [Google Scholar]
- 205.De Rosa A, Cameron IC, Gomez D. Indications for staging laparoscopy in pancreatic cancer. HPB (Oxford) 2016;18:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vaz SC, Oliveira F, Herrmann K, et al. Nuclear medicine and molecular imaging advances in the 21st century. Br J Radiol 2020;93:20200095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Panagiotopoulou PB, Courcoutsakis N, Tentes A, et al. CT imaging of peritoneal carcinomatosis with surgical correlation: a pictorial review. Insights Imaging 2021;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li K, Cannon JGD, Jiang SY, et al. Diagnostic staging laparoscopy in gastric cancer treatment: a cost-effectiveness analysis. J Surg Oncol 2018;117:1288–1296. [DOI] [PubMed] [Google Scholar]
- 209.Marchet A, Mocellin S, Ambrosi A, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg 2007;245:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Degiuli M, De Manzoni G, Di Leo A, et al. Gastric cancer: current status of lymph node dissection. World J Gastroenterol 2016;22:2875–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ma J, Li X, Zhao S, et al. Robotic versus laparoscopic gastrectomy for gastric cancer: a systematic review and meta-analysis. World J Surg Oncol 2020;18:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet 2020;396:635–648. [DOI] [PubMed] [Google Scholar]
- 213.Chang L, Stefanidis D, Richardson WS, et al. The role of staging laparoscopy for intraabdominal cancers: an evidence-based review. Surg Endosc 2009;23:231–241. [DOI] [PubMed] [Google Scholar]
- 214.Ajani JA, D’Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw Oct 2016;14:1286–1312. [DOI] [PubMed] [Google Scholar]
- 215.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021;24:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fukagawa T. Role of staging laparoscopy for gastric cancer patients. Ann Gastroenterol Surg 2019;3:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ramos RF, Scalon FM, Scalon MM, et al. Staging laparoscopy in gastric cancer to detect peritoneal metastases: a systematic review and meta-analysis. Eur J Surg Oncol 2016;42:1315–1321. [DOI] [PubMed] [Google Scholar]
- 218.Leake PA, Cardoso R, Seevaratnam R, et al. A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer. Gastric Cancer 2012;15(Suppl 1):S38–S47. [DOI] [PubMed] [Google Scholar]
- 219.Leake PA, Cardoso R, Seevaratnam R, et al. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer 2012;15(Suppl 1):S27–S37. [DOI] [PubMed] [Google Scholar]
- 220.Schnelldorfer T, Jenkins RL, Birkett DH, et al. Laparoscopic narrow band imaging for detection of occult cancer metastases: a randomized feasibility trial. Surg Endosc 2016;30:1656–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Baiocchi GL, Molfino S, Molteni B, et al. Fluorescence-guided lymphadenectomy in gastric cancer: a prospective western series. Updates Surg 2020;72:761–772. [DOI] [PubMed] [Google Scholar]
- 222.Belia F, Biondi A, Agnes A, et al. The use of Indocyanine green (ICG) and near-infrared (NIR) fluorescence-guided imaging in gastric cancer surgery: a narrative review. Front Surg 2022;9:880773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Boggi U. Resection for pancreatic cancer with arterial involvement: a paradigm shift away from unresectable to “how to do it”. Surgery 2021;169:1036. [DOI] [PubMed] [Google Scholar]
- 224.Takeda FR, Tustumi F, Cecconello I. ASO author reflections: Evaluation of tumor regression after neoadjuvant chemoradiotherapy in esophageal carcinoma – how to do it? Ann Surg Oncol 2020;27:1248–1249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supplementary tables summarize the study search strategy and result in retrieval comprehensively. The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials, Supplemental Digital Content 4, http://links.lww.com/JS9/A857.


