Abstract
Background:
The Portico transcatheter aortic heart valve is a self-expandable, fully resheathable bioprosthetic valve with a nitinol frame and porcine pericardial sealing cuff. It has been used among symptomatic severe aortic stenosis (AS) who are at high or extreme surgical risk. However, till date very few studies has been reported with inconclusive evidence for its postprocedure safety outcomes.
Objective:
The authors aim to evaluate the safety of the Portico transcatheter aortic valve replacement system among patients with AS.
Methodology:
The authors conducted a systematic literature search on PubMed, Embase, and Scopus from inception till 10th April 2023 by using predefined MESH terms using ‘AND’ and ‘OR’. The following search terms were used: ‘Aortic Stenosis’ AND ‘Transcatheter aortic valve replacement’ OR ‘Portico valve’. Finally, descriptive statistics were used to summarize the data in this paper. The mean and SD were adopted to describe continuous variables, whereas frequencies and percentages were used for dichotomous data.
Results:
A total of 7 studies with 2782 patients were included in the analysis. The mean age of patients was 82.3 years, and 54.63% were female. The most common comorbidity was hypertension (65.21%) and diabetes mellitus (26.45%). Among patients of AS with Portico valve implants, postprocedural outcomes including 30-day mortality (2.32%), cardiovascular mortality (2.37%), stroke (2.23%), myocardial infarction (0.94%), major bleeding (3.97%), major vascular complications (4.91%), acute kidney injury (1.37%), and permanent pacemaker implantations in 15.73% patients were reported. Overall, device success was observed in 95.82% of patients.
Conclusion:
Transcatheter aortic valve replacement with the repositionable Portico valve, a new bioprosthesis, appears to have a low postprocedural mortality rate and other clinical outcomes in high-risk patients with severe AS.
Keywords: aortic stenosis, outcomes, Portico valve implantation, transcatheter aortic valve replacement
Introduction
Highlights
The transcatheter aortic valve is recommended to significantly reduce the mortality and symptoms among patients with severe symptomatic aortic stenosis. However, no systematic analysis has been done to summarize all the limited literature evaluating the safety of Portico valves.
This very first systematic review shows that transcatheter aortic valve replacement with the Portico valve appears to be safe and effective with low mortality and clinical outcomes in high-risk patients with severe aortic stenosis.
Most studies do not have long-term follow-up data with echocardiographic outcomes, encouraging further research and comparison with different valve types.
Transcatheter aortic valve implantation (TAVI) is a minimally invasive procedure that offers a less invasive approach to replace a dysfunctional aortic valve, commonly due to severe aortic stenosis (AS). TAVI has emerged as a well-established and effective treatment option for patients with severe AS who are at high surgical risk. Due to its minimally invasive nature, TAVI offers a safe alternative to surgical aortic valve replacement, with reduced death and disability as evidenced by the PARTNER 1 and PARTNER 2 trials1,2. TAVI has significantly evolved since its first introduction in 2002, with newer and innovative devices being developed and refined over the years. The Portico device is one of the latest TAVI devices to enter the market and has shown promise in clinical trials. It was first awarded the CE Mark in 2012 and received FDA approval in 2021 for use with the FlexNav Delivery System3. It is a self-expandable bioprosthetic, self-expanding and repositionable valve system made up of three bovine pericardial leaflets and a nitinol frame, as well as a porcine pericardial sealing cuff. Its outflow stent frame is designed with retention tabs that secure the crimped valves during deployment. The device is available in four sizes based on inflow measurements: 23 mm, 25 mm, 27 mm, and 29 mm and is typically inserted through transfemoral or trans-subclavian routes using a delivery catheter. The catheter is equipped with a soft, tapered nose cone, and an 18 Fr capsule that contains the compressed valve4. Overall, the Portico device offers a unique and innovative approach to TAVI that has shown promising outcomes in clinical trials5,6. The advantages of the Portico valve are its flexibility and low profile, making it easier to use in potentially challenging cases of torturous, calcified vessels7. Furthermore, retrieving and repositioning the Portico valve adds to the advantageous feature of this valve system4.
Despite promising outcomes in clinical trials, there have been limited studies with the Portico device and conflicting results regarding the incidence of postoperative complications and permanent pacemaker implantation (PPI)8,9. Thus, we sought to perform a systematic review to describe short-term safety and clinical outcomes post-TAVR by using the Portico valve.
Methods
This systematic review was conducted and reported in conformity with the Cochrane and PRISMA (Preferred reporting items for systematic review and Meta-analysis), Supplemental Digital Content 1, http://links.lww.com/JS9/A889, Supplemental Digital Content 2, http://links.lww.com/JS9/A890 2020 and AMSTAR (Assessing the methodological quality of systematic reviews), Supplemental Digital Content 3, http://links.lww.com/JS9/A891 guidelines as described previously10–12. The prespecified protocol has been registered on Prospero (CRD42023411524).
Search strategy
We conducted a systematic literature search in MEDLINE, Embase, Scopus, Web of Science using predefined MESH terms by using ʻANDʼ and ʻORʼ. The following search terms were used: (((((((Aortic Stenosis [MeSH Terms]) OR (TAVI[OtherTerm])) OR (Transcatheter aortic valve replacement [OtherTerm])) AND (Portico Valve[Other Terms])) OR (valve [Other Term])) AND (outcomes[Other Term]) OR (Mortality[Other Term]) OR (Stroke [Other Term]).
Eligibility criteria
Studies were included if they fulfilled the following criteria: patients 18 years of age with a definitive diagnosis of AS, studies with Portico Valve use, and postimplantation safety outcomes. Studies such as prospective and retrospective were sought to be eligible. Studies that involved animal testing, any other valve type, no desired outcomes, and case report, case series, and review articles were excluded.
Study selection
We queried databases from inception till 10th April 2023 without language restriction. The studies were carefully screened and exported to the Endnote 2020 library (X9). Two reviewers (J.C. and V.J.) reviewed the titles and abstract. Discrepancies regarding the inclusion of studies were arbitrated by the senior author (J.W.). The same reviewers also performed the full-text screening independently to decide which articles fulfilled the inclusion criteria. The senior author arbitrated discrepancies regarding the inclusion of studies.
Data extraction and statistical analysis
The following data were extracted from the studies: demographic data (age and sex), study design, publication year, patient comorbidities, complications, and outcomes. Two authors (J.C., H.A.) assembled all available information in a shared Excel 2019 spreadsheet. For missing, incorrect, or unreported data, the corresponding authors of the respective papers were contacted via e-mail for clarification. Supplementary material related to the main article was also investigated in such cases. Finally, descriptive statistics were used to summarize the data in this paper. The mean and SD were adopted to describe continuous variables, whereas frequencies and percentages were used for dichotomous data. Two investigators (S.N. and K.R.) independently appraised the potential risk of bias using the Newcastle–Ottawa (NOS) scale for observational studies. We then classified studies as low, moderate, or high quality based on the scores after evaluation. All statistical analyses were conducted using the software R (v4.1.2; R Core Team 2023) (available at https://www.R-project.org/).
Results
Study selection
The preliminary search using a predetermined search strategy yielded 578 articles, of which 187 studies were excluded as duplicates. Three hundred eighty three articles were subsequently excluded through title and abstract screening based on the inclusion criteria determined. A full-text review was done for the remaining nine studies identified during the search period. Furthermore, two studies were removed as they had no outcomes or were review articles. Finally, seven studies were included in the review, of which all are clinical cohort-based studies conducted between 2011 and 2022. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram is depicted in Figure 1. The quality assessment of the observational studies was a low risk of bias on NOS for all observational studies. (Supplementary Table 1, Supplemental Digital Content 4, http://links.lww.com/JS9/A892).
Figure 1.
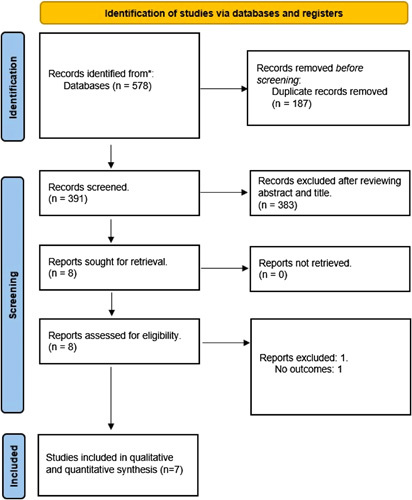
Preferred Reporting Items for Systematic Review and Meta-analysis flow of the search strategy for systematic review and meta-analysis.
Baseline characteristics of the included patients
A total of seven3,4,8,13–15 studies with 2782 patients were included in this systematic review. The mean age of the patients was 82.3 years, with 1520 (54.63%) female patients. All patients included were diagnosed with severe symptomatic AS. The STS (Society of Thoracic Surgeons) Score was calculated in five studies, with a mean value of 6.08±1.6. The most common comorbidity include hypertension in 65.21% (n=1663/2550) patients, diabetes mellitus in 26.45% (n=736/2782) patients, followed by, myocardial infarction in 9.63% (n=267/2772) of patients, 6.42% (n=178/2772) having a past history of stroke, 10.19% (n=272/2668) with history of peripheral artery disease, and 31.94% (n=849/2658) with history of atrial fibrillation. 23.92% (n=636/2658) patients underwent percutaneous coronary intervention (PCI), and 11.58% (n=309/2668) patients had PPI. The mean LVEF was 51.04±15.76 while the mean aortic valve area (AVA) was 0.67±0.04. The mean transaortic gradient (in mmHg) recorded was 44.26±2.6. Moderate to severe mitral regurgitation was present in 5.55% (n=139/2503) of patients (Table 1).
Table 1.
Baseline characteristics of patients included in the studies.
| Variables | Blumenstein et al., 20228 | Corcione et al., 202013 | Perlman et al.4 | Willson et al.14 | Mollmann et al.3 (PorticoTM DS) | Mollmann et al.3 (FlexNavTM DS) | Möllmann et al.15 | Raj R Makkar et al. |
|---|---|---|---|---|---|---|---|---|
| Sample size | 344 | 114 | 57 | 10 | 501 | 500 | 222 | 1034 |
| Study design | Nonrandomized cohort | Observational cohort | Observational cohort | Observational cohort | Observational cohort | Observational cohort | Prospective, nonrandomized, multicenter study | RCT |
| Age, years (Mean) | 82.92 | 82.4 | 80.8 | 82.4 | 81.7 | 82.3 | 83.0 | 83±7 |
| Female, n (%) | 206 (59.9) | 70 (61) | 47 (82.5) | 10 (100%) | 320 (63.70%) | 307 (61.40%) | 165 (74.32%) | 395 (52·7%) |
| NYHA Class III/IV | 290 | 85/1 | 43 | 8/0 | 295 / 32 | 293/18 | 166/ 9 | 229 (60·1%)/43 (11·3%) |
| STS score | – | – | 7.7±5.7 | 8.1 3.2 | 4.2±2.9 | 4.2±2.7 | 5.8±3.3 | 6·5 ±3·4 |
| Diabetes, n (%) | 120 | 21 | 20 | 5 | 176 | 182 | 69 | 143 (37·5%) |
| Hypertension, n (%) | 308 | 80 | 46 | – | 440 | 431 | – | 358 (94·0%) |
| Previous MI, n (%) | 37 | 10 | 10 | – | 68 | 61 | 26 | 55 (14·4%) |
| Previous stroke, n (%) | 31 | 2 | 7 | – | 53 | 38 | 18 | 29 (7·6%) |
| Previous peripheral artery disease, n (%) | 48 | – | 14 | 1 | 60 | 59 | 18 | 72 (18·9%) |
| Previous atrial fibrillation, n (%) | 135 | – | 20 | – | 246 | 238 | 85 | 125 (32·8%) |
| Mean AVA, cm2 | – | 0.6 | 0.7 | 0.62 | 0.71 | 0.72 | – | 0·68±0·17 |
| LVEF,% | 59.25 | 55 | 58.4 | 57.3 | – | – | >20 | 57·3 (11·5) |
| Moderate to severe mitral regurgitation, n | 5 | 50 | – | 5 | 1 | 1 | – | 78 (20·5%) |
| Mean Transaortic gradient, mmHg | 42.75 | 50 | 41.8 | 44.5 | 43.4 | 42.2 | 43.3 | 46·2 (11·2) |
Postprocedural outcomes and device success
Among patients of AS with Portico valve, postprocedural outcomes including 30-day mortality (2.32%, n=62/2668), in-hospital mortality (0.75%, n=11/1459), cardiovascular mortality (2.37%, n=29/1223), stroke (2.23%, n=61/2725), myocardial infarction (0.94%, n=16/1691), major bleeding (3.97%, n=108/2715), major vascular complications (4.91%, n=134/2725), acute kidney injury (1.37%, n=36/2611), PPIs (15.73%, n=266/1691), and 0.36% (n=6/1624) patients had coronary obstruction after the procedure. Overall device success was seen in 95.82% (n=1675/1748) patients, 0.245% (n=3/1223) patients reported to have an annular rupture, and 1.78% (n=6/336) patients reported undergoing valve-in-valve procedures (Table 2).
Table 2.
Clinical outcomes postprocedure, and at follow-up post-Portico valve implantation.
| Variables | Blumenstein et al., 20228 | Corcione et al., 202013 | Perlman et al.4 | Willson et al.14 | Mollmann3 (PorticoTM DS) | Mollmann3 (FlexNavTM DS) | Möllman et al.15 | Raj R Makkar et al. |
|---|---|---|---|---|---|---|---|---|
| Sample size | 344 | 114 | 57 | 10 | 501 | 500 | 222 | 1034 |
| All-cause mortality, n | – | 16 | 9 | – | – | 10 | – | 53 (14·3%) |
| In-hospital mortality, n | 10 | 0 | – | – | 1 | 0 | – | – |
| Cardiovascular Mortalitya, n | – | – | – | – | 15 | 6 | 8 | – |
| 30 Days mortality, n | 13 | – | 2 | 0 | 16 | 10 | 8 | 13 (3·5%) |
| Strokea, n (in hospital/ follow-up) | 13/– | 0/0 | –/4 | 1/– | 13/– | 16/– | 12/– | 6 |
| MIa, n (in hospital/ follow-up) | 6/– | 0/0 | –/1 | 0/– | 2/– | 1/– | 7/– | – |
| Major bleedinga, n (in hospital/ follow-up) | 2/– | 0/0 | –/4 | – | 26/– | 33/– | 25/– | 22 |
| Major vascular complicationsa, n (in hospital/ follow-up) | 9/– | 0/0 | –/5 | 0/– | 32/– | 41/– | 16/– | 36 |
| AKIa, n (in hospital/ follow-up) | 12/– | – | –/1 | 0/– | 7/– | 4/– | 9/– | 4 |
| PPIa, n (in hospital/ follow-up) | 52/– | 13/14 | –/5 | 0/– | 87/– | 84/– | 30/– | – |
| Coronary Obstruction, n | 0 | – | 0 | – | 2 | 3 | 1 | – |
| Major adverse eventa, n (in hospital/ follow-up) | – | 0/16 | – | – | – | – | – | – |
| Device success, n | 318 | 112 | 43 | 10 | 488 | 488 | 216 | – |
| Annular rupture, n | – | – | – | – | 2 | 1 | 0 | – |
| Valve-in-valve procedure, n | – | 2 | – | – | 0 | 0 | 4 | – |
| AVA, cm2 postprocedure | – | 1.8 (2.2) | 1.27±0.31 | 1.3±0.2 | – | – | 1.9±0.5 | 1·85±0·46 |
Data arranged in form of postprocedure outcomes/outcomes at follow-up.
Discussion
To our knowledge, this is the very first and most comprehensive systematic review to date, with the highest sample size evaluating the clinical outcomes after transcatheter valve implantation with a Portico valve. In our study, postimplantation 30 days mortality was 2.32%, less than reported by Linke et al. (3.6%) and Perlman et al. (3.5%)4,16. Furthermore, our analysis showed that the 30-day-mortality due to cardiovascular causes was 2.37%, which was also less than reported by Linke et al. (3.6%)16. Our study reported post-Portico valve implantation in-hospital mortality was 0.75%, stroke was 2.23%, myocardial infarction was 0.94%, major bleeding was 3.97%, the major vascular complications were 4.91%, and acute kidney injury was 1.37% (Fig. 2).
Figure 2.
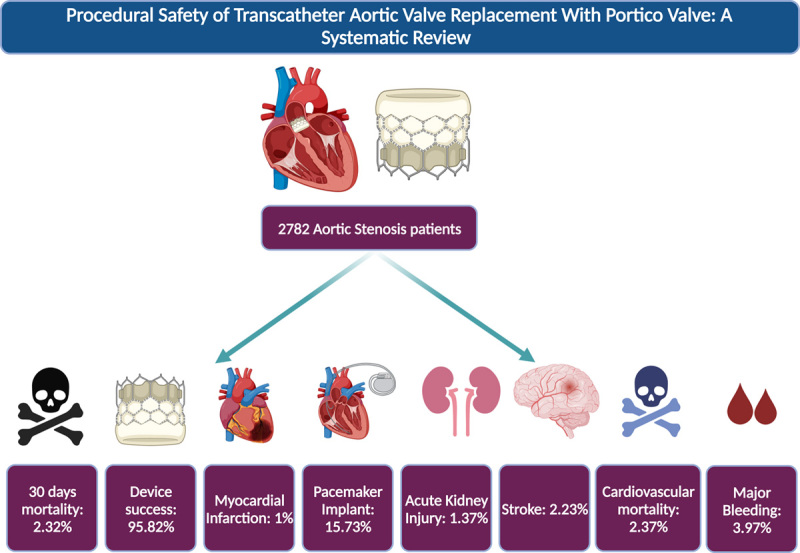
Central illustration highlighting the clinical outcomes post-Portico valve implantation.
The new generation devices such as Portico and Evolut were compared by Giordano et al. and Corcione et al., and they reported that no significant differences were found in procedural and in-hospital stroke, myocardial infarction, major bleeding, major vascular complications, and all-cause mortality13,17. However, follow-up data by Giordano et al. showed significantly higher all-cause mortality (14.3 vs. 5.3%) and major adverse events (14.3 vs. 5.3%) post-Evolut interventions in comparison to those patients who underwent transcatheter valve implantation with the Portico valve17. Similarly, Evolut was associated with significantly higher pacemaker implantations and lower peak and mean aortic gradients compared to the Portico valve17.
All five devices (Portico, Evolut, Acurat, Lotus, and Sapien3) were compared by Giordano et al.18, and they reported that the Portico valve was associated with significantly reduced major adverse events, major vascular complications, renal failure, and pericardial effusion in comparison to the other four-valve devices. However, no significant differences were found in terms of all-cause mortality, cardiovascular death, stroke, and myocardial infarction among all five devices18. Another study conducted by Trigo et al.19 compared the Portico valve with Sapien XT and found that the Portico valve was associated with a lower rate of myocardial infarction, pacemaker implantation, and conversion to open heart surgery while a higher rate of stroke and major bleeding, although the result was nonsignificant (P>0.05). Blumenstein et al.8 compared the Portico valve with the Acurate Neo and no significant differences were reported in terms of stroke, major bleeding, myocardial infarction, renal failure, and in-hospital mortality in both the groups. They reported rare instances of urgent conversion to sternotomy, which was prompted by complications such as coronary impairment, THV embolization, pericardial effusion, and severe mitral regurgitation due to wire perforation. Willson et al.14 demonstrated successful recapture and repositioning of the Portico valve in four cases of suboptimal implantation. Similarly, Möllmann et al.15 demonstrated successful valve resheathing and repositioning in one third of the procedures to address suboptimal implantation depth and paravalvular leakage. The study conducted by Mollman H et al.3 revealed instances of unsuccessful valve implantation, resulting in the use of alternative nonstudy valves. Additionally, cases of annular rupture, coronary obstruction, and the requirement for a secondary valve within 30 days were documented3. Notably, among the subjects, two individuals (0.2%, 2/1001) necessitated a second valve due to paravalvular leakage3. One subject received a second Portico valve, while the other subject underwent implantation with a nonstudy valve3.
The Portico Valve consists of a self-expandable frame with a pericardial sealing cuff and three pericardial leaflets20. It has large cells with more tissue and less metal that can conform better around the native valve leaflets, improving valve apposition and reducing the risk of leaks from the aorta to the ventricle21. The valve is fully repositionable and retrievable and indicated for patients with high-risk for open-heart surgery and hemodynamically unstable patients during the procedure21. Recently the FDA approved this valve for use in severe AS patients at high-risk for SAVR22. However, this procedure is not risk-free, and life-threatening bleeding, acute renal injury, stroke, the need for permanent pacemaker replacement, and death has been reported in the literature23.
The Portico valve is the choice of valve for patients having large annulus size (>27 mm), in patients having severe aortic calcifications, in patients having a bicuspid aortic valve, and in patients using small vessels as access routes of entry24. The leaflet material used for Portico valve is of bovine tissue as compared to Evolut and ACCURATE, which are made up of porcine tissue24. The Portico valve and Evolut valves are repositionable, retrievable, and resheathable while SAPIEN 3 and ACCURATE valves are not repositionable and retrievable24. The access routes for Portico valve are transvascular and transaortic with an 18 or 19 Fr delivery system, while for SAPIEN access routes are transvascular, transaortic, and transapical with 14–16 Fr inner diameter of sheath, for Evolut R and PRO access routes are transvascular and transaortic with an inline sheath diameter of 14 Fr, and for ACCURATE neo access routes are transapical and transvascular with a 14 Fr inner diameter of the sheath24. Recently, with the introduction of the Newer FlexNav delivery system, the results are more promising and have further reduced all-cause mortality, cardiovascular mortality, acute kidney injuries, myocardial infarction events, and new PPIs as compared to the old delivery system of the Portico valve3. However, a large trial needs to be conducted on these newer FlexNav delivery systems compared to the older delivery system and other valve types to get more significant results.
Limitations
This review includes only observational studies, so they are subject to publication bias. Secondly, most studies did not have reported long-term follow-up data, which can be something to research further and evaluate the outcomes. Lastly, we cannot evaluate the incidence rate because of the limited number of events and study sample size.
Conclusion
Transcatheter aortic valve replacement with the Portico valve appears safe and effective, with low mortality and clinical outcomes in high-risk patients with severe AS. However, most studies do not have long-term follow-up data, including echocardiographic outcomes, which encourage further research and comparison with different valve types.
Ethical approval
Since this is a review article of previously published studies, hence ethical approval is not required.
Consent
None.
Sources of funding
None.
Author contribution
V.J.: contributed to the conception or design of the work; V.J., V.S., Z.W.: contributed to the acquisition, analysis, or interpretation of data for the work; V.J., E.E., V.S., M.H., K.R., S.N.: drafted the manuscript. All authors critically revised the manuscript. All gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Conflicts of interest disclosure
V.J. serves as an Associate Editor in the European Journal of Medical Research in Cardiology section (Unpaid).
Research registration unique identifying number (UIN)
This paper was registered in Prospero registration: CRD42023411524.
Guarantor
Vikash Jaiswal.
Data availability statement
The data underlying this article are available in the article and its online supplementary material.
Provenance and peer review
Not commissioned, externally peer reviewed.
Supplementary Material
Acknowledgements
None.
Footnotes
Z.W. and V.J. contributed equally and are joined first author.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 15 August 2023
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Contributor Information
Vikash Jaiswal, Email: vikash29jaxy@gmail.com.
Zarghoona Wajid, Email: hj2051@wayne.edu.
Vinay Suresh, Email: vinay4607.19@kgmcindia.edu.
Muhammed Hanif, Email: hanifafridi273@gmail.com.
Kripa Rajak, Email: rjkripa@gmail.com.
Anupam Halder, Email: anupam.2666@gmail.com.
Evbayekha Endurance, Email: greatomri@gmail.com.
Henry Aiwuyo, Email: olam4eva@gmail.com.
Jinal Choudhari, Email: drjinalc1110@gmail.com.
Sidra Naz, Email: sidrasss582@gmail.com.
Song P. Ang, Email: hestonang23@gmail.com.
Abhigan B. Shrestha, Email: abigan17@gmail.com.
References
- 1.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. New Eng J Med 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 2.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. New Eng J Med 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 3.Mollmann H, Linke A, Nombela-Franco L, et al. Procedural safety and device performance of the PorticoTM valve from experienced TAVI centers: 30-day outcomes in the multicenter CONFIDENCE registry. J Clin Med 2022;11:4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perlman GY, Cheung A, Dumont E, et al. Transcatheter aortic valve replacement with the Portico valve: one-year results of the early Canadian experience. EuroIntervention 2017;12:1653–1659. [DOI] [PubMed] [Google Scholar]
- 5.Manoharan G, Linke A, Moellmann H, et al. Multicentre clinical study evaluating a novel resheathable annular functioning self-expanding transcatheter aortic valve system: safety and performance results at 30 days with the Portico system. EuroIntervention 2016;12:768–774. [DOI] [PubMed] [Google Scholar]
- 6.Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016;387:2218–2225. [DOI] [PubMed] [Google Scholar]
- 7.Giulia Costa, Lars Søndergaard. Portico with FlexNav TAVI system: enhancing innovative technology to optimize patient outcomes and physician experience. Portico with FlexNav TAVI system: enhancing innovative technology to optimize patient outcomes and physician experience. Published May 5, 2020. Accessed 14 April 2023. https://www.pcronline.com/Cases-resources-images/Industry-partner-perspectives/Sponsored-content/2020/Portico-with-FlexNav-TAVI-system
- 8.Blumenstein J, Eckel C, Husser O, et al. Multi-center comparison of two self-expanding transcatheter heart valves: a propensity matched analysis. J Clin Med 2022;11:4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walther T, Manoharan G, Linke A, et al. Incidence of new-onset left bundle branch block and predictors of new permanent pacemaker following transcatheter aortic valve replacement with the PorticoTM valve. Eur J Cardiothorac Surg 2018;54:467–474. [DOI] [PubMed] [Google Scholar]
- 10.Jaiswal V, Hanif M, Ang SP, et al. Racial disparity between the post-procedural outcomes among patients with peripheral artery disease: a systematic review and meta-analysis. Curr Probl Cardiol 2023;48:101595. [DOI] [PubMed] [Google Scholar]
- 11.Jaiswal V, Nepal G, Dijamco P, et al. Cerebral venous sinus thrombosis following covid-19 vaccination: a systematic review. J Prim Care Community Health 2022;13:21501319221074450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaiswal V, Ang SP, Ishak A, et al. Comparison of outcome among type 2 vs type 1 myocardial infarction: a systematic review and meta-analysis. J Investig Med 2023;71:223–234. [DOI] [PubMed] [Google Scholar]
- 13.Corcione N, Biondi-Zoccai G, Ferraro P, et al. Long-term follow-up of transcatheter aortic valve implantation with Portico versus evolut devices. Am J Cardiol 2020;125:1209–1215. [DOI] [PubMed] [Google Scholar]
- 14.Willson AB, Rodès-Cabau J, Wood DA, et al. Transcatheter aortic valve replacement with the St. Jude Medical Portico valve: first-in-human experience. J Am Coll Cardiol 2012;60:581–586. [DOI] [PubMed] [Google Scholar]
- 15.Möllmann H, Linke A, Holzhey DM, et al. Implantation and 30-Day follow-up on all 4 valve sizes within the portico transcatheter aortic bioprosthetic family. JACC Cardiovasc Interv 2017;10:1538–1547. [DOI] [PubMed] [Google Scholar]
- 16.Linke A, Holzhey D, Möllmann H, et al. Treatment of aortic stenosis with a self-expanding, resheathable transcatheter valve: one-year results of the international multicenter portico transcatheter aortic valve implantation system study. Circ Cardiovasc Interv 2018;11:e005206. [DOI] [PubMed] [Google Scholar]
- 17.Giordano A, Corcione N, Ferraro P, et al. Propensity-score-adjusted comparison of Evolut vs. Portico devices for transcatheter aortic valve implantation. J Cardiovasc Med (Hagerstown) 2019;20:351–357. [DOI] [PubMed] [Google Scholar]
- 18.Giordano A, Corcione N, Ferraro P, et al. Comparative one-month safety and effectiveness of five leading new-generation devices for transcatheter aortic valve implantation. Sci Rep 2019;9:17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Trigo M, Dahou A, Webb JG, et al. Self-expanding Portico valve versus balloon-expandable SAPIEN XT valve in patients with small aortic annuli: comparison of hemodynamic performance. Rev Esp Cardiol (Engl Ed) 2016;69:501–508. [DOI] [PubMed] [Google Scholar]
- 20.Tzikas A, Amrane H, Bedogni F, et al. Transcatheter aortic valve replacement using the portico system: 10 things to remember. J Interv Cardiol 2016;29:523–529. [DOI] [PubMed] [Google Scholar]
- 21.Tzikas A, Chrissoheris M, Halapas A, Spargias K. PorticoTM transcatheter heart valve. Hellenic J Cardiol 2015;56 Suppl A:15–19. [PubMed] [Google Scholar]
- 22.Jared M, O’Leary FDA. Approves PORTICO with FlexNav TAVR System for Use in Severe Aortic Stenosis Patients at High or Extreme Risk for Surgical Aortic Valve Replacement | SCAI. Accessed 26 April 2023https://scai.org/fda-approves-portico-flexnav-tavr-system-use-severe-aortic-stenosis-patients-high-or-extreme-risk
- 23.Reardon MJ, Chehab B, Smith D, et al. 30-day clinical outcomes of a self-expanding transcatheter aortic valve: the international PORTICO NG study. JACC Cardiovasc Interv 2023;16:681–689. [DOI] [PubMed] [Google Scholar]
- 24.Renker M, Kim WK. Choice of transcatheter heart valve: should we select the device according to each patient’s characteristics or should it be “one valve fits all”? Ann Transl Med 2020;8:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article and its online supplementary material.


