Abstract
Wolbachia bacteria are maternally inherited symbionts that commonly infect terrestrial arthropods. Many Wolbachia reach high frequencies in their hosts by manipulating their reproduction, for example by causing reproductive incompatibilities between infected male and uninfected female hosts. However, not all strains manipulate reproduction, and a key unresolved question is how these non‐manipulative Wolbachia persist in their hosts, often at intermediate to high frequencies. One such strain, wSuz, infects the invasive fruit pest Drosophila suzukii, spotted‐wing drosophila. Here, we tested the hypothesis that wSuz infection provides a competitive benefit when resources are limited. Over the course of one season, we established population cages with varying amounts of food in a semi‐field setting and seeded them with a 50:50 mixture of flies with and without Wolbachia. We predicted that Wolbachia‐infected individuals should have higher survival and faster development than their uninfected counterparts when there was little available food. We found that while food availability strongly impacted fly fitness, there was no difference in development times or survival between Wolbachia‐infected and uninfected flies. Interestingly, however, Wolbachia infection frequencies changed dramatically, with infections either increasing or decreasing by as much as 30% in a single generation, suggesting the possibility of unidentified factors shaping Wolbachia infection over the course of the season.
Keywords: conditional mutualist, cytoplasmic incompatibility, gut microbe, insect symbiosis, larval competition, spotted wing drosophila, Wolbachia
We do not have a good understanding of how Wolbachia and other facultative inherited bacterial symbionts that do not manipulate host reproduction persist in their hosts; we addressed this question in a strain of Wolbachia that infects spotted‐wing drosophila, D. suzukii. To test the hypothesis that Wolbachia infection provides a competitive benefit when resources are limited, we established cages with varying amounts of food and seeded them with a 50:50 mixture of flies with or without Wolbachia. We found that although food availability did not affect Wolbachia prevalence, symbiont infection frequencies changed dramatically, either increasing or decreasing by as much as 30% in a single generation.
1. INTRODUCTION
Wolbachia bacteria are the most abundant host‐associated microbes on the planet, estimated to infect ~40% of terrestrial arthropod species (Zug & Hammerstein, 2012). This enormous host range is due to two distinct modes of transmission (Sanaei et al., 2021; Werren, 1997). Over ecological timescales, they are highly efficiently transmitted from females to their offspring, often in the egg cytoplasm, whereas over evolutionary timescales, they repeatedly colonize new species via mechanisms that are not well understood. In addition, many Wolbachia strains have evolved sophisticated strategies to manipulate host reproduction in order to increase the prevalence of infected females (Kaur et al., 2021; Werren et al., 2008). The most common of these strategies is cytoplasmic incompatibility (CI), whereby matings between infected males and uninfected females result in reduced viability of embryos. As a result, Wolbachia‐infected females are at a great advantage over their uninfected counterparts and can rapidly replace them (Hoffmann et al., 2011; Turelli & Hoffmann, 1991). There is currently great interest in using CI Wolbachia to control arthropod pests and the diseases they vector, either by using CI to rapidly drive desired traits, such as pathogen blocking, through a population (Gong et al., 2020; Hoffmann et al., 2011), or by releasing incompatible males in the wild, whereupon matings with uninfected females result in local population suppression (O'Connor et al., 2012; Zabalou et al., 2004).
Although not as well studied, many Wolbachia strains do not cause CI or other reproductive manipulations in their hosts. Yet, many of these strains are as prevalent and dynamic as their reproductive parasite counterparts. For example, a number of non‐CI strains of Wolbachia have recently invaded and spread through various Drosophila species (Kriesner et al., 2013; Turelli et al., 2018). In a powerful demonstration of the dynamic nature of non‐CI Wolbachia, Kriesner and Hoffmann (2018) used population cages to follow the prevalence of the wAu strain in D. simulans. Despite starting their experiment with ~35% wAu‐infected flies in each cage, the infection reached ~100% prevalence in only 30 generations, corresponding to an approximately 20% fitness benefit to carrying wAu. How and why wAu increases host fitness, at least under some conditions, is not known.
Indeed, condition‐dependent fitness benefits are critical in explaining the persistence of non‐CI strains, as the prevalence of maternally inherited symbionts that are not essential (i.e. obligate) for their hosts depends mainly on the fidelity of maternal transmission and the relative fitness of infected versus uninfected hosts (Hoffmann & Turelli, 1997). But the fitness benefits of non‐CI Wolbachia have remained largely elusive.
One potential benefit of infection with non‐CI Wolbachia is protection against natural enemies, with a number of Wolbachia strains shown to defend their hosts against pathogenic RNA viruses (Hedges et al., 2008; Teixeira et al., 2008). Interestingly, a recent study showed that wild D. melanogaster infected with Wolbachia were significantly less likely to harbour RNA viruses than Wolbachia‐free flies (Cogni et al., 2021); this is the first demonstration of strong protective effects in native host‐symbiont‐virus interactions outside of the lab.
Another possibility is that Wolbachia infection provides nutritional or metabolic benefits to its host, for example, by supplementing them with a limiting nutrient under stressful conditions (Brownlie et al., 2009). This was suggested as an explanation for the rapid increase in wAu in experimental population cages (Kriesner & Hoffmann, 2018), as flies likely experienced intense larval competition, with wAu‐infected larvae possibly receiving a fitness boost from their symbionts under these stressful conditions.
In this study, we used an experimental population cage approach to examine the dynamics of infection and conditional fitness benefits in wSuz, a non‐CI strain of Wolbachia that infects Drosophila suzukii (Figure 1), or spotted wing Drosophila, an invasive polyphagous pest of soft‐skinned fruits in both Europe and North America (Asplen et al., 2015). The wSuz strain does not cause CI or any other reproductive manipulations in its host (Cattel, Kaur, et al., 2016; Cattel, Martinez, et al., 2016; Hamm et al., 2014) and is imperfectly transmitted from mothers to offspring (Hamm et al., 2014). These factors alone should systematically reduce its prevalence; yet, wSuz infection rates are highly variable, they can be quite high in some populations but appear to average ~20% (from 7% to 57%) in North America (Hamm et al., 2014) and ~45% (from 0% to 100%) in Europe (Cattel, Kaur, et al., 2016; Cattel, Martinez, et al., 2016).
FIGURE 1.

Drosophila suzukii ovipositing on blueberry. Photo credit: Warren Wong.
We manipulated food availability in a semi‐field setting to test the hypothesis that resource competition differentially affects the fitness of Wolbachia‐infected D. suzukii, seeding population cages with a 50:50 mixture of flies with or without Wolbachia. Two specific predictions arise from the hypothesis that wSuz boosts host metabolism under stressful and limiting conditions. First, Wolbachia‐infected individuals should develop more quickly than their uninfected counterparts when there is little available food. Second, there should also be greater survival of Wolbachia‐infected individuals, which would result in an increase in Wolbachia frequencies in offspring compared to their parents under higher competition scenarios.
Manipulating food availability strongly affected fly fitness. Interestingly, however, while there was no effect of resource competition on Wolbachia prevalence, there were pronounced and dynamic changes in infection frequency, with infections either increasing or decreasing by as much as 30% in a single generation, suggesting the possibility of unidentified factors shaping Wolbachia infection over the course of the season.
2. MATERIALS AND METHODS
2.1. Drosophila suzukii lab cultures
We established two matched D. suzukii lines: one Wolbachia‐positive, the other Wolbachia‐negative. The Wolbachia‐positive line descended from a single D. suzukii female collected from Chilliwack, British Columbia, Canada (49°05′52.9″ N 121°55′28.0″ W) in August of 2018 from a Himalayan blackberry, Rubus armeniacus (Focke) (Rosaceae). The Wolbachia‐free line was established from a subsample of the Wolbachia‐positive line treated with tetracycline (Sigma‐Aldrich) mixed with prepared Instant Drosophila Medium (Carolina Biological Supply Company) at a concentration of 0.05% for three consecutive generations. The Wolbachia‐positive line received identical food and environmental conditions, except that tetracycline was not added. The infection status of each line was confirmed via PCR (see below) and checked every 3–4 generations. Wolbachia‐positive and negative fly lines were subsequently maintained in the Perlman laboratory (University of Victoria, Canada) in an incubator (24°C; 12:12 light: dark cycle), in vials with Instant Drosophila Medium provided as a food and reproduction substrate. All flies used in the following experiments were more than 75 generations post‐antibiotic treatment. This is expected to provide more than sufficient time to re‐acquire gut microbiota and recover from the adverse effects of antibiotic treatments (Ballard & Melvin, 2007; Li et al., 2014).
For the two generations preceding semi‐field experiments, flies were reared indoors in a non‐climate‐controlled room at an average temperature of ~21°C with natural June–August photoperiods (16.2–14.8 h) in Agassiz, British Columbia, Canada. A HOBO data logger (Onset) was used to measure the temperature at 5‐minute intervals. Wolbachia‐positive and negative lines of flies were reared separately in 125‐mL jars (Bernardin) with 60 mL of prepared instant Drosophila medium. Three frozen blueberries were added to each jar. The tops of the jars were covered with fine insect mesh and secured with an elastic band. Each rearing jar contained 10 males and 10 females, and flies were transferred to a new jar every 3 days.
Adult flies to be used in the experiment were collected on the day they emerged and placed in a vial (diameter: 2.6 cm, height: 9.2 cm; Diamed, Canada) secured with a cellulose plug. Each of these vials contained 30 flies, consisting of 15 males and 15 females of the same age and same Wolbachia status. Each vial contained 15 mL of prepared Instant Drosophila Medium. One frozen blueberry was added to each vial. Flies were transferred to a new vial daily until they were 7–9 days old, when they were used in the resource competition experiment. Vials that had >50% fly mortality were not used.
2.2. Resource competition experiment
This experiment was designed to examine the effect of resource competition on Wolbachia infection frequency changes over a single generation of D. suzukii, and to determine whether Wolbachia infection increases egg‐adult development rate under a range of resource competition scenarios in semi‐field conditions.
Experiments were done during August and September of 2020, part of the seasonal period during which D. suzukii is actively reproducing in British Columbia (Thistlewood et al., 2019). It took place outdoors on a covered porch in Agassiz, British Columbia, Canada, exposing insects to a realistic range of abiotic conditions (photoperiod, temperature, and humidity). Temperature was recorded hourly with a HOBO logger placed among the experimental units; hourly relative humidity and daily photoperiod data were retrieved from a nearby Environment Canada weather station (Environment Canada, 2020; see Table S1 for abiotic conditions recorded during the experiment). Fresh blueberries were used as the oviposition and larval development food substrate because they are a known fruit host for D. suzukii (Thistlewood et al., 2019) and they are readily available throughout the summer months in British Columbia. Blueberries were stored for 48 hours at 4°C prior to use to ensure that any pre‐existing D. suzukii eggs or larvae were killed. The berries were then washed twice, dried, and individually inspected to ensure only undamaged berries were used.
To create different levels of resource competition, we designed three competition treatments (low, medium, and high), each of which had a consistent number of adult D. suzukii flies (60; 30 males and 30 females) but a varying amount of food resources (fresh blueberries) available (low – 120; medium – 60; high – 20). Of the 60 flies, 30 were from the Wolbachia‐infected colony and the other 30 were from the uninfected colony. Of the 30 flies from each colony, 15 were males and 15 were females. The Wolbachia infection status of each fly from this ‘parent’ generation was confirmed later (see below). Ventilated plastic containers lined with paper towels were used as arenas for fly oviposition. A larger container (17.1 × 25.4 × 8.1 cm; 2.12 L) was used as an oviposition arena for the ‘low’ competition treatment than the ‘medium’ and ‘high’ competition treatments (15.6 × 15.6 × 8.6 cm; 1.18 L), to ensure all blueberries were in a single layer on the bottom of each container.
The groups of 60 flies were randomly assigned to oviposition arenas with different competition treatments and allowed to oviposit for 24 hours; they were then removed and preserved in 95% EtOH. Using a dissecting microscope, the number of D. suzukii eggs in each berry (identified as the egg's spiracles protruding from an oviposition scar) was counted. Once the eggs were counted, all of the blueberries were placed in identically ventilated development arenas in plastic containers (15.6 × 15.6 × 8.6 cm; 1.18 L) lined with paper towels. To ensure consistent development conditions for all of the competition treatments, berries from the low and medium treatments were subdivided into multiple development arenas containing 20 berries each. Every development arena was checked daily at the same time for D. suzukii offspring emergence and to remove any excess condensation. As offspring emerged, they were removed from their container, their sex was noted, and they were preserved in 95% EtOH for later Wolbachia screening. Each development arena was kept for at least 14 days after the last observed fly emergence.
Six separate temporal blocks, each containing two replicates of each of the three competition treatments, were conducted. However, due to time constraints, DNA extraction and Wolbachia screening were only performed for a randomly selected three out of the six blocks, and data for only these three blocks (6 total replicates per competition treatment) are presented here.
2.3. Determination of Wolbachia infection status of parents and offspring
DNA was extracted from parental mothers and all offspring in order to determine Wolbachia infection status. DNA was extracted by homogenizing individual flies in 50 μL of DNA extracting buffer (9.8 mL H2O, 100 μL 1 M Tris pH 8.0, 20 μL 0.5 M EDTA, 50 μL NaCl) and 0.5 μL of Proteinase K (BioLabs). After the flies were homogenized, they were incubated at 37°C for 20 min, followed by an incubation at 95°C for 2 min, and stored at 4°C. Using PCR, flies were screened for Wolbachia using the Wolbachia surface protein (wsp) specific primers (wsp_81F: 5′‐ TGGTCCAATAAGTGATGAAGAAAC‐3′, wsp_691R: 5′‐AAAAATTAAACGCTACTCCA‐3′; Zhou et al., 1998) using the following thermocycling conditions: 95°C × 3 min, (94°C × 30 s, 55°C × 30 s, 72°C × 45 s) × 30, 72°C × 10 min. PCR products were visualized on a 1% agarose gel (FroggaBio) following gel electrophoresis with the use of a 1 kb plus DNA ladder (Invitrogen). Wolbachia status was determined based on the presence/absence of a band. A Wolbachia‐positive control was included along with a DNA control for Wolbachia‐negative samples to ensure the extraction was successful. For the DNA control, we amplified either a 708 base pair fragment of cytochrome C oxidase I (COI), a mitochondrial gene, or a 552 base pair fragment of actin, a nuclear gene. COI primers used were LCO1490 (5′‐ GGTCAACAAATCATAAAGATATTGG ‐3′) and HCO 2198 (5′‐ TAAACTTCAGGGTGACCAAAAAATCA ‐3′) (Folmer et al., 1994), and actin primers used were Act42AF (5′‐ GCGTCGGTCAATTCAATCTT ‐3′) and Act42AR (5′‐ CTTCTCCATGTCGTCCCAGT ‐3′), using the same thermocycling conditions as above, but with an annealing temperature of 58°C.
2.4. Data analysis
All data analysis was conducted using R version 4.2.3 (R Core Team, 2023).
We first tested whether different ratios of adult flies to food resources (number of berries) increased the number of eggs laid per berry and whether resultant resource competition resulted in decreased survival levels of immature flies. The effects of the three resource competition treatments (low, medium, and high) on the number of eggs laid in each berry (i.e., averaged across all berries in each replicate) and the proportion of eggs emerging as adults (number of adult flies emerged/number of eggs counted) in each replicate were determined by fitting generalized linear models (GLMs) with competition treatment and temporal block as categorical predictors. Poisson and binomial error distributions were used for the models with egg count and proportion survival as response variables, respectively. For the model testing the effect of competition treatment on the number of eggs per berry, a likelihood ratio test was used to evaluate statistical significance using the Anova() function in the ‘car’ package in R (Fox & Weisberg, 2019). For the model testing the effect of competition treatment on proportion emergence, to account for model overdispersion, we rescaled the statistical model by a Pearson chi‐square statistic divided by the residual degrees of freedom and used an F‐test to evaluate statistical significance. Temporal block was not a significant predictor of the number of eggs per berry ( = 0.35, p = .35) or proportion emergence (F 2,13 = 2.23, p = .15) and was not retained in the final simplified statistical models. Post‐hoc multiple comparisons among treatments were done with Tukey contrasts implemented with the glht() function in the ‘multcomp’ package in R (Hothorn et al., 2008).
Next, we tested whether changes in Wolbachia infection frequencies of D. suzukii over the single generation of our experiment (proportion of mothers infected – proportion of emerging adult offspring infected) were associated with different levels of resource competition, using GLMs with Gaussian error distributions, after verifying that model fits met assumptions of normality and homoscedasticity. Here, because there was considerable within‐treatment variation in our two metrics of resource competition intensity (number of eggs per berry, proportion of survival to adulthood; see Figure 1), we conducted these analyses with the two metrics as continuous predictor variables. ‘Temporal block’ was also included in statistical models as a categorical predictor. F‐tests were used to evaluate the statistical significance of predictor variables.
Finally, to determine whether Wolbachia infection status affected egg‐adult development time of D. suzukii, accounting for offspring sex and temporal block, a linear mixed model was fit using the package ‘lmer’ (Bates et al., 2015) with the development time of each adult fly (the number of days between replicate set‐up and the emergence of the fly) as the response variable. The initial model contained Wolbachia infection status (infected, uninfected), sex (male, female) and temporal block as categorical main effects, and individual container as a random effect to account for the non‐independence of the Wolbachia‐infected and Wolbachia‐uninfected individuals emerging from the same container. Model assumptions were verified by inspecting a residuals plot, and the statistical significance of each model factor was determined using F‐tests.
3. RESULTS
3.1. Egg density and offspring survival differed among competition treatments
Increasing the ratio of adult D. suzukii to food resources reduced per‐offspring resource availability and resultant survival rates. Flies laid a greater average number of eggs in each berry ( = 42.47, p < .0001; Figure 2), and a lower proportion of offspring survived (F 2,15 = 6.09, p = .012) in the ‘high’ competition treatment compared to the ‘medium’ and ‘low’ competition treatments. Replicates with higher numbers of eggs per berry tended to subsequently have lower proportions of offspring surviving to adulthood (Pearson's correlation; r = −.51; t = −2.43, df = 16, p = .027).
FIGURE 2.
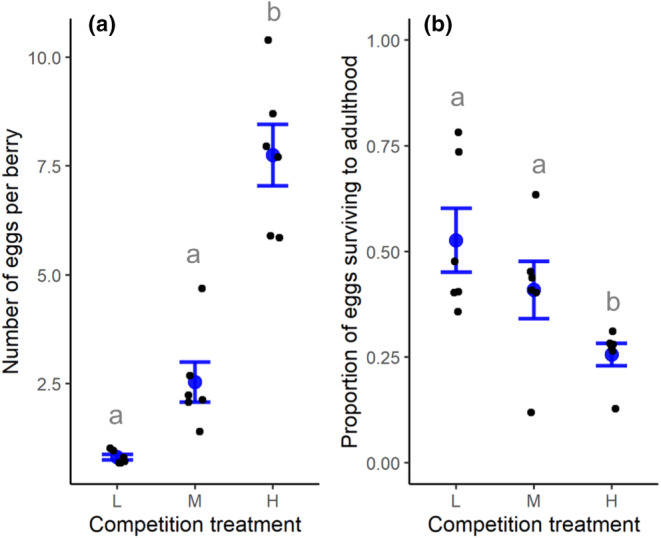
The effect of low (L), medium (M), and high (H) resource competition treatments (i.e., the relative ratio of food resources to the number of adult Drosophila suzukii) on: (a) the number of D. suzukii eggs laid per berry, and (b) the proportion of D. suzukii eggs surviving to adulthood. Black points represent individual replicates (n = 6 per treatment); blue points and error bars show treatment means with standard errors. Within panels, treatments labelled with different lower‐case grey letters are significantly different (p < .05; Tukey multiple comparisons on GLMs).
3.2. Wolbachia infection rates can change rapidly, but are not driven by variation in competition intensity
Changes in Wolbachia infection rates between D. suzukii parents and offspring varied widely, from increases of 32.9% to decreases of 34.8% (Figure 3). However, the direction and magnitude of these changes among replicates were not associated with the intensity of resource competition, measured either as the initial density of eggs on fruit (F 1,14 = 1.44, p = .25) or the proportion of eggs that survived to adulthood (F 1,14 = 2.05, p = .17; Figure 3). The strongest and only statistically significant predictor of changes in Wolbachia frequency was temporal block (F 2,15 = 20.52, p < .0001): infection rates tended to increase in the chronologically first temporal block (mean ± SE: 18.2 ± 0.06%) and decrease in the second (−13.5 ± 0.04%) and third (−20.7 ± 0.03%) blocks.
FIGURE 3.
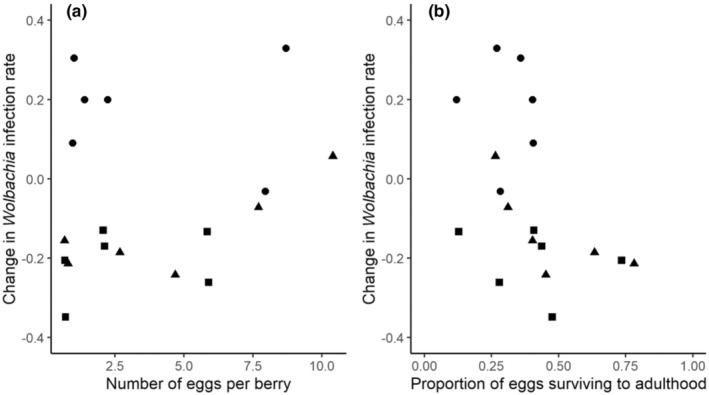
The proportional change in Wolbachia infection rate of Drosophila suzukii between parents and offspring did not vary under different levels of resource competition, measured as: (a) the initial number of eggs per berry in each replicate; and (b) the proportion of those eggs that survived to adulthood. In both panels, different symbols represent replicates belonging to different temporal blocks (circles – chronologically first block; triangles – second block; squares: third block).
3.3. Wolbachia infection does not change offspring development time
The egg to adult development time of D. suzukii offspring, which was marginally shorter for males than females (F 1,32 = 4.27, p = .047; Figure 4), was not associated with their Wolbachia infection status (F 1,31 = 2.58, p = .12). Development time of fly offspring varied among temporal blocks (F 2,32 = 35.30, p < .0001), with the chronologically first temporal block having, on average, shorter development times (global mean ± SE: 17.8 ± 0.2 days) than the second (20.0 ± 0.1) and third (18.9 ± 0.2) temporal blocks.
FIGURE 4.
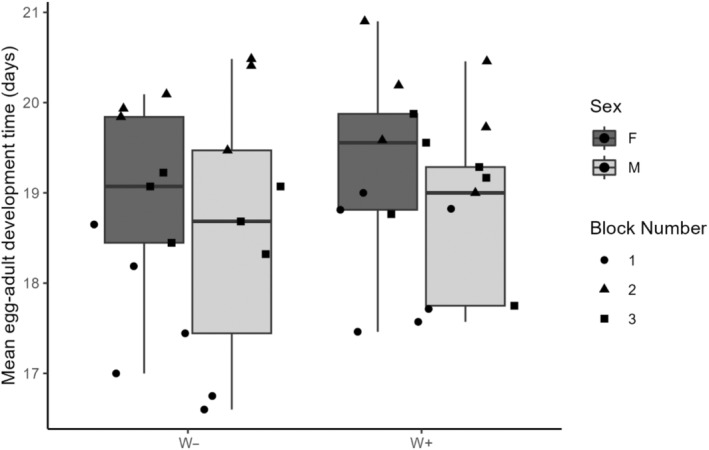
Mean Drosophila. suzukii offspring development times, which were marginally lower for males (light boxes) than females (dark boxes), were not associated with Wolbachia infection status (W+: infected; W−: not infected). Different symbols represent replicates belonging to different temporal blocks, which are numbered chronologically.
4. DISCUSSION
The prevalence of maternally inherited bacterial endosymbionts is determined by a combination of how faithfully they are transmitted from an infected mother to her offspring and the relative fitness of infected versus uninfected females (Hoffmann & Turelli, 1997). A long‐standing mystery is how symbionts that neither manipulate host reproduction (or only very weakly manipulate them) nor have horizontal routes of infection, such as the strain of Wolbachia that infects D. suzukii (wSuz), are maintained in host populations at intermediate to high frequencies. A likely explanation is that they benefit their hosts, but only under certain conditions – but the conditions under which these benefits become apparent are often difficult to identify (Cooper et al., 2017; Harcombe & Hoffmann, 2004; Hoffmann & Turelli, 1997; Zug & Hammerstein, 2015). In this study, we tested whether wSuz differentially impacts host fitness under conditions of varying resource competition. While the amount of available food for developing larvae had no effect on Wolbachia prevalence, our major result was that symbiont frequencies were highly dynamic, changing by over 30% in a single generation. Interestingly, there were major swings in frequency in both directions, with experiments performed earlier in the summer resulting in large decreases in Wolbachia infection and the opposite happening in experiments performed later in the season.
Other studies have found rapid and unexplained changes in the prevalence of facultative inherited symbionts, although we are not aware of any studies that have demonstrated this in a single generation. Our study and experimental approach were inspired by Kriesner and Hoffmann's (2018) work, where the wAu Wolbachia strain increased in prevalence in population cages of D. simulans from 35% to over 90% in as quickly as 10 generations, corresponding to an estimated ~20% fitness benefit. The wAu strain is similar to wSuz in that it does not cause cytoplasmic incompatibility (Hoffmann et al., 1996) and is not associated with an obvious host phenotype, although a recent study suggested that it may benefit flies breeding in rotting fruits that have extensive fungal growth (Cao et al., 2019). Another noteworthy example of rapid, unexplained changes in symbiont prevalence is found in a strain of Rickettsia infecting Bemisia tabaci whiteflies in the southern United States. In just 6 years, the prevalence of infected whiteflies increased from 1% to 97% (Himler et al., 2011), and just 6 years after that, the prevalence decreased to ~35% (Bockoven et al., 2020). At the time of the increase (but not the decline), infected females had higher fitness, both in the lab and in the field, although the reason behind this fitness difference is not known.
That Wolbachia frequencies changed in both directions over the course of our experiment rules out the possibility that the results are due to intrinsic fitness differences – that is, that this strain of Wolbachia, in this host genetic background, had relatively high or low fitness effects. The flies we used in this experiment were descended from a single isofemale line, so host genetic variation was not a factor here. While we cannot completely rule out the possibility of incomplete maternal transmission of wSuz in our experiment, this is unlikely to have been an important factor at play here, as the infection remained stable throughout the experiment, with virtually all parents from the Wolbachia‐positive line infected, and Wolbachia frequencies increased in one of the three blocks. The wSuz strain that we used has been stably maintained since 2018, demonstrating that, at least in this nuclear genetic background and under lab conditions, it exhibits high maternal transmission efficiency and little fitness costs.
So what explains the dramatic changes in the wSuz infection? We tested the hypothesis that resource competition affects Wolbachia‐infected individuals differently than uninfected ones. The effect of Wolbachia could occur in either direction. First, if harbouring bacterial symbionts is energetically costly, we might expect that Wolbachia‐infected hosts would be outcompeted by uninfected ones when resources are limited. For example, Wolbachia‐infected Aedes albopictus mosquito larvae performed worse than uninfected ones when reared together at high densities but better when reared together at low densities (Gavotte et al., 2010); this may perhaps be mediated by competition between Wolbachia and the mosquito host over amino acids or cholesterol (Caragata et al., 2013, 2014). Another example of reduced competitive ability was found in Trichogramma kaykai (Huigens et al., 2004) and T. dendrolimi (Zhou et al., 2023) parasitic wasps, with fewer Wolbachia‐infected individuals emerging as adults when both infected and uninfected female wasps lay eggs in the same moth host. Alternatively, it has been proposed that Wolbachia may supplement its hosts with essential nutrients (Brownlie et al., 2009; Newton & Rice, 2020), such as iron, heme, riboflavin, and nucleotides, in which case we might predict that Wolbachia‐infected hosts would have an advantage over uninfected ones under stressful conditions. While certain strains of Wolbachia, such as those infecting bedbugs, have a demonstrated role as nutritional symbionts that are essential for their hosts (Hosokawa et al., 2010), we are not aware of any studies that have demonstrated competitive benefits via nutrient supplementation in facultative strains of Wolbachia. Regardless, while our berry density manipulations had strong effects on the number of developing flies, the relative success of Wolbachia‐infected individuals was not affected by competition treatment, although it is possible that imposing even more extreme resource competition may have uncovered the effects of Wolbachia. It would also be informative to quantify key metabolites, as well as Wolbachia titres, under varying conditions of resource availability.
Having ruled out a role for resource competition, we are left to speculate on what drove the large swings in Wolbachia. If we first consider possible abiotic factors, an obvious place to look is temperature, which has been shown to affect Wolbachia titres, transmission efficiency, and fitness effects in a number of Drosophila species (Clancy & Hoffmann, 1998; Hague et al., 2020, 2022; Saeed et al., 2018). However, there were no consistent differences in temperature between the three experimental blocks (Table S1), with the average temperature during the egg‐adult development period being almost identical (~19°C). While the temperature at the time of oviposition was higher for the second block (~25°C), it was similarly low for the first and last blocks (~16–17°C). Likewise, there were no obvious differences in humidity among blocks that correlated with changes in Wolbachia infection frequencies (Table S1). One notable difference between temporal blocks that did correlate with the direction of changes in Wolbachia infection frequencies was photoperiod, with daylength shortening over the course of the experiment (from 14.8 to 12.8 h) (Table S1). Daylength serves as an important developmental cue for D. suzukii, triggering major physiological changes that culminate in reproductive dormancy and increase the fly's ability to survive the winter (Hamby et al., 2016; Toxopeus et al., 2016). While we are not aware of any studies that have directly tested whether daylength affects Wolbachia‐host interactions, simulating reproductive dormancy resulted in lower fitness in Wolbachia‐infected D. melanogaster (Kriesner et al., 2016). However, work on reproductive dormancy has focused on conditions that reflect later times in the growing season than when our experiment took place, such as much cooler temperatures, and we think it unlikely that daylength drove the large changes that we observed.
Alternatively, the sometimes large swings in Wolbachia that we observed could have been driven by biotic factors. Infections with facultative inherited symbionts that protect their hosts against natural enemies have been shown to increase rapidly in the presence of the enemy, both in experimental population cages (Oliver et al., 2008) and in the wild (Jaenike et al., 2010). If the symbiont is costly (Vorburger & Gouskov, 2011), it may be lost if the enemy is rare or absent. Like related Wolbachia in Drosophila (Hedges et al., 2008; Teixeira et al., 2008), wSuz has been found to protect against pathogenic positive‐sense RNA viruses, such as the Drosophila C Virus under laboratory conditions (Cattel, Kaur, et al., 2016; Cattel, Martinez, et al., 2016; Martinez et al., 2017). A recent study of wild Drosophila melanogaster found that flies that harboured Wolbachia carried on average 0.37 fewer viruses than uninfected ones (Cogni et al., 2021). They were also approximately 3 times less likely to be infected with Motts Mill virus, a close relative of Teise virus, which appears to be widespread in D. suzukii (Medd et al., 2018), although nothing is known about how either Motts Mill or Teise viruses are transmitted or whether they are pathogenic. However, unless these viruses are highly pathogenic and commonly acquired via the food substrate (i.e. were present in the blueberries used in our study), it seems unlikely that they are responsible for the dynamic changes in Wolbachia.
Finally, changes in Wolbachia frequency may have been driven by interactions with gut microbes. Here again, we look to the work that has been done primarily in D. melanogaster to inform what may be happening in D. suzukii. A number of studies have shown that the fly's gut microbiota is dynamic, affects host fitness, and has complex interactions with Wolbachia (Henry et al., 2022; Henry & Ayroles, 2021; Rudman et al., 2019). For example, Henry et al. (2022) established large field enclosures of either Wolbachia‐positive or negative flies and sampled the fly microbiome every 2 weeks, from July to November 2019. They found rapid changes in gut microbiota composition that were also dependent on whether hosts were infected with Wolbachia. They also found complex interactions between Wolbachia and certain gut microbiota that had major effects on host fitness. For example, later in the season, Wolbachia‐infected flies that had abundant Commensalibacter bacteria in their guts were less resistant to starvation. Like D. melanogaster, the D. suzukii microbiome is dynamic, affects host fitness and is primarily acquired from food (Bing et al., 2018; Hamby & Becher, 2016; Vacchini et al., 2017), and so we suspect that the changes in Wolbachia frequency in our experiment may have been shaped by the microbiota. It would therefore be interesting to perform controlled experiments that manipulate the composition and abundance of blueberry and D. suzukii gut microbes, as has been done in D. melanogaster (Rudman et al., 2019), and to then determine how this affects the prevalence of wSuz.
AUTHOR CONTRIBUTIONS
Audrey E. McPherson: Conceptualization (equal); formal analysis (equal); investigation (lead); visualization (equal); writing – original draft (equal); writing – review and editing (supporting). Paul K. Abram: Conceptualization (equal); data curation (lead); formal analysis (equal); funding acquisition (equal); project administration (equal); resources (equal); supervision (equal); visualization (equal); writing – original draft (lead); writing – review and editing (equal). Caitlin I. Curtis: Investigation (supporting); writing – review and editing (supporting). Erik R. Wannop: Investigation (supporting); writing – review and editing (supporting). Jan P. Dudzic: Methodology (supporting); writing – review and editing (supporting). Steve J. Perlman: Conceptualization (equal); funding acquisition (equal); project administration (equal); resources (lead); supervision (equal); writing – original draft (lead); writing – review and editing (lead).
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
Supporting information
Table S1
ACKNOWLEDGEMENTS
This work was supported by a Genome British Columbia Sector Innovation Grant to SJP and PKA (SIP‐017) and a Natural Sciences and Engineering Research Council of Canada Discovery Grant to SJP (RGPIN‐2020‐06996).
McPherson, A. E. , Abram, P. K. , Curtis, C. I. , Wannop, E. R. , Dudzic, J. P. , & Perlman, S. J. (2023). Dynamic changes in Wolbachia infection over a single generation of Drosophila suzukii, across a wide range of resource availability. Ecology and Evolution, 13, e10722. 10.1002/ece3.10722
DATA AVAILABILITY STATEMENT
Data files, R code, and a README file that describes them have been uploaded to Figshare and are currently available as private links (a single public link will be provided upon publication): Density data: https://figshare.com/s/418fc9d793c6b6b7ab10. Development time data: https://figshare.com/s/1751aec07548c7d2e9d5. R code for figures and statistics: https://figshare.com/s/d53e4b2daefa3440f09e. README file with metadata describing data and code: https://figshare.com/s/0998b81aacc25092a1ab.
REFERENCES
- Asplen, M. K. , Anfora, G. , Biondi, A. , Choi, D.‐S. , Chu, D. , Daane, K. M. , Gibert, P. , Gutierrez, A. P. , Hoelmer, K. A. , Hutchison, W. D. , IsaaTiscs, R. , Jiang, Z.‐L. , Karpati, Z. , Kimura, M. T. , Pascual, M. , Philips, C. R. , Plantamp, C. , Ponti, L. , Vetek, G. , … Desneux, N. (2015). Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. Journal of Pest Science, 88, 469–494. 10.1007/s10340-015-0681-z [DOI] [Google Scholar]
- Ballard, J. W. O. , & Melvin, R. G. (2007). Tetracycline treatment influences mitochondrial metabolism and mtDNA density two generations after treatment in Drosophila . Insect Molecular Biology, 16, 799–802. 10.1111/j.1365-2583.2007.00760.x [DOI] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bing, X. , Gerlach, J. , Loeb, G. , & Buchon, N. (2018). Nutrient‐dependent impact of microbes on Drosophila suzukii development. MBio, 9, e02199‐17. 10.1128/mBio.02199-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockoven, A. A. , Bondy, E. C. , Flores, M. J. , Kelly, S. E. , Ravenscraft, A. M. , & Hunter, M. S. (2020). What goes up might come down: The spectacular spread of an endosymbiont is followed by its decline a decade later. Microbial Ecology, 79, 482–494. 10.1007/s00248-019-01417-4 [DOI] [PubMed] [Google Scholar]
- Brownlie, J. C. , Cass, B. N. , Riegler, M. , Witsenburg, J. J. , Iturbe‐Ormaetxe, I. , McGraw, E. A. , & O'Neill, S. L. (2009). Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathogens, 5(4), e1000368. 10.1371/journal.ppat.1000368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, L.‐J. , Jiang, W. , & Hoffmann, A. A. (2019). Life history effects linked to an advantage for wAu Wolbachia in Drosophila . Insects, 10(5), 126. 10.3390/insects10050126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragata, E. P. , Rances, E. , Hedges, L. M. , Gofton, A. W. , Johnson, K. N. , O'Neill, S. L. , & McGraw, E. A. (2013). Dietary cholesterol modulates pathogen blocking by Wolbachia . PLoS Pathogens, 9(6), e1003459. 10.1371/journal.ppat.1003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragata, E. P. , Rances, E. , O'Neill, S. L. , & McGraw, E. A. (2014). Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti . Microbial Ecology, 67, 205–218. 10.1007/s00248-013-0339-4 [DOI] [PubMed] [Google Scholar]
- Cattel, J. , Martinez, J. , Jiggins, F. , Mouton, L. , & Gibert, P. (2016). Wolbachia‐mediated protection against viruses in the invasive pest Drosophila suzukii . Insect Molecular Biology, 25, 595–603. 10.1111/imb.12245 [DOI] [PubMed] [Google Scholar]
- Cattel, J. , Kaur, R. , Gibert, P. , Martinez, J. , Fraimout, A. , Jiggins, F. , Andrieux, T. , Siozios, S. , Anfora, G. , Miller, W. , Rota‐Stabelli, O. , & Mouton, L. (2016). Wolbachia in European populations of the invasive pest Drosophila suzukii: Regional variation in infection frequencies. PLoS One, 11(1), e0147766. 10.1371/journal.pone.0147766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy, D. , & Hoffmann, A. (1998). Environmental effects on cytoplasmic incompatibility and bacterial load in Wolbachia‐infected Drosophila simulans . Entomologia Experimentalis et Applicata, 86, 13–24. 10.1046/j.1570-7458.1998.00261.x [DOI] [Google Scholar]
- Cogni, R. , Ding, S. D. , Pimentel, A. C. , Day, J. P. , & Jiggins, F. M. (2021). Wolbachia reduces virus infection in a natural population of Drosophila . Communications Biology, 4, 1327. 10.1038/s42003-021-02838-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, B. S. , Ginsberg, P. S. , Turelli, M. , & Matute, D. R. (2017). Wolbachia in the Drosophila yakuba complex: Pervasive frequency variation and weak cytoplasmic incompatibility, but no apparent effect on reproductive isolation. Genetics, 205, 333–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environment Canada . (2020). Environment and Climate Change Canada Historical Climate Data web site. https://climate.weather.gc.ca/index_e.html
- Folmer, O. , Black, M. , Hoeh, W. , Lutz, R. , & Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–299. [PubMed] [Google Scholar]
- Fox, J. , & Weisberg, S. (2019). An R companion to applied regression (3rd ed.). Sage. https://socialsciences.mcmaster.ca/jfox/Books/Companion/ [Google Scholar]
- Gavotte, L. , Mercer, D. R. , Stoeckle, J. J. , & Dobson, S. L. (2010). Costs and benefits of Wolbachia infection in immature Aedes albopictus depend upon sex and competition level. Journal of Invertebrate Pathology, 105, 341–346. 10.1016/j.jip.2010.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, J.‐T. , Li, Y. , Li, T.‐P. , Liang, Y. , Hu, L. , Zhang, D. , Zhou, C.‐Y. , Yang, C. , Zhang, X. , Zha, S.‐S. , Duan, X.‐Z. , Baton, L. A. , Hong, X.‐Y. , Hoffmann, A. A. , & Xi, Z. (2020). Stable introduction of plant‐virus‐inhibiting Wolbachia into planthoppers for rice protection. Current Biology, 30, 4815–5066. 10.1016/j.cub.2020.09.033 [DOI] [PubMed] [Google Scholar]
- Hague, M. T. J. , Mavengere, H. , Matute, D. R. , & Cooper, B. S. (2020). Environmental and genetic contributions to imperfect wMel‐like Wolbachia transmission and frequency variation. Genetics, 215, 1117–1132. 10.1534/genetics.120.303330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague, M. T. J. , Shropshire, J. D. , Caldwell, C. N. , Statz, J. P. , Stanek, K. A. , Conner, W. R. , & Cooper, B. S. (2022). Temperature effects on cellular host‐microbe interactions explain continent‐wide endosymbiont prevalence. Current Biology, 32, 878–888.e8. 10.1016/j.cub.2021.11.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby, K. A. , & Becher, P. G. (2016). Current knowledge of interactions between Drosophila suzukii and microbes, and their potential utility for pest management. Journal of Pest Science, 89, 621–630. 10.1007/s10340-016-0768-1 [DOI] [Google Scholar]
- Hamby, K. A. , Bellamy, D. E. , Chiu, J. C. , Lee, J. C. , Walton, V. M. , Wiman, N. G. , York, R. M. , & Biondi, A. (2016). Biotic and abiotic factors impacting development, behavior, phenology, and reproductive biology of Drosophila suzukii . Journal of Pest Science, 89, 605–619. 10.1007/s10340-016-0756-5 [DOI] [Google Scholar]
- Hamm, C. A. , Begun, D. J. , Vo, A. , Smith, C. C. R. , Saelao, P. , Shaver, A. O. , Jaenike, J. , & Turelli, M. (2014). Wolbachia do not live by reproductive manipulation alone: Infection polymorphism in Drosophila suzukii and D. subpulchrella . Molecular Ecology, 23, 4871–4885. 10.1111/mec.12901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcombe, W. , & Hoffmann, A. A. (2004). Wolbachia effects in Drosophila melanogaster: In search of fitness benefits. Journal of Invertebrate Pathology, 87, 45–50. [DOI] [PubMed] [Google Scholar]
- Hedges, L. M. , Brownlie, J. C. , O'Neill, S. L. , & Johnson, K. N. (2008). Wolbachia and virus protection in insects. Science, 322, 702. 10.1126/science.1162418 [DOI] [PubMed] [Google Scholar]
- Henry, L. P. , & Ayroles, J. F. (2021). Meta‐analysis suggests the microbiome responds to evolve and Resequence experiments in Drosophila melanogaster . BMC Microbiology, 21, 108. 10.1186/s12866-021-02168-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, L. P. , Fernandez, M. , Wolf, S. , & Ayroles, J. F. (2022). Wolbachia interacts with the microbiome to shape fitness‐associated traits during seasonal adaptation in Drosophila melanogaster. Biorxiv . 10.1101/2022.05.31.494239 [DOI]
- Himler, A. G. , Adachi‐Hagimori, T. , Bergen, J. E. , Kozuch, A. , Kelly, S. E. , Tabashnik, B. E. , Chiel, E. , Duckworth, V. E. , Dennehy, T. J. , Zchori‐Fein, E. , & Hunter, M. S. (2011). Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science, 332, 254–256. 10.1126/science.1199410 [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. , Clancy, D. , & Duncan, J. (1996). Naturally‐occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity, 76, 1–8. 10.1038/hdy.1996.1 [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. A. , Montgomery, B. L. , Popovici, J. , Iturbe‐Ormaetxe, I. , Johnson, P. H. , Muzzi, F. , Greenfield, M. , Durkan, M. , Leong, Y. S. , Dong, Y. , Cook, H. , Axford, J. , Callahan, A. G. , Kenny, N. , Omodei, C. , McGraw, E. A. , Ryan, P. A. , Ritchie, S. A. , Turelli, M. , & O'Neill, S. L. (2011). Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature, 476, 454–457. 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. A. , & Turelli, M. (1997). Cytoplasmic incompatibility in insects. In O'Neill S. L., Werren J. H., & Hoffmann A. A. (Eds.), Influential passengers: Inherited mircoorganisms and arthropod reproduction (pp. 42–80). Oxford University Press. [Google Scholar]
- Hosokawa, T. , Koga, R. , Kikuchi, Y. , Meng, X.‐Y. , & Fukatsu, T. (2010). Wolbachia as a bacteriocyte‐associated nutritional mutualist. Proceedings of the National Academy of Sciences of the United States of America, 107, 769–774. 10.1073/pnas.0911476107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn, T. , Bretz, F. , & Westfall, P. (2008). Simultaneous inference in general parametric models. Biometrical Journal, 50(3), 346–363. [DOI] [PubMed] [Google Scholar]
- Huigens, M. , Hohmann, C. , Luck, R. , Gort, G. , & Stouthamer, R. (2004). Reduced competitive ability due to Wolbachia infection in the parasitoid wasp Trichogramma kaykai . Entomologia Experimentalis et Applicata, 110, 115–123. 10.1111/j.0013-8703.2004.00126.x [DOI] [Google Scholar]
- Jaenike, J. , Unckless, R. , Cockburn, S. N. , Boelio, L. M. , & Perlman, S. J. (2010). Adaptation via symbiosis: Recent spread of a Drosophila defensive symbiont. Science, 329, 212–215. 10.1126/science.1188235 [DOI] [PubMed] [Google Scholar]
- Kaur, R. , Shropshire, J. D. , Cross, K. L. , Leigh, B. , Mansueto, A. J. , Stewart, V. , Bordenstein, S. R. , & Bordenstein, S. R. (2021). Living in the endosymbiotic world of Wolbachia: A centennial review. Cell Host & Microbe, 29, 879–893. 10.1016/j.chom.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriesner, P. , Conner, W. R. , Weeks, A. R. , Turelli, M. , & Hoffmann, A. A. (2016). Persistence of a Wolbachia infection frequency cline in Drosophila melanogaster and the possible role of reproductive dormancy. Evolution, 70, 979–997. 10.1111/evo.12923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriesner, P. , & Hoffmann, A. A. (2018). Rapid spread of a Wolbachia infection that does not affect host reproduction in Drosophila simulans cage populations. Evolution, 72, 1475–1487. 10.1111/evo.13506 [DOI] [PubMed] [Google Scholar]
- Kriesner, P. , Hoffmann, A. A. , Lee, S. F. , Turelli, M. , & Weeks, A. R. (2013). Rapid sequential spread of two Wolbachia variants in Drosophila simulans . PLoS Pathogens, 9, e1003607. 10.1371/journal.ppat.1003607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.‐Y. , Floate, K. D. , Fields, P. G. , & Pang, B.‐P. (2014). Review of treatment methods to remove Wolbachia bacteria from arthropods. Symbiosis, 62, 1–15. 10.1007/s13199-014-0267-1 [DOI] [Google Scholar]
- Martinez, J. , Tolosana, I. , Ok, S. , Smith, S. , Snoeck, K. , Day, J. P. , & Jiggins, F. M. (2017). Symbiont strain is the main determinant of variation in Wolbachia‐mediated protection against viruses across Drosophila species. Molecular Ecology, 26, 4072–4084. 10.1111/mec.14164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medd, N. C. , Fellous, S. , Waldron, F. M. , Xuereb, A. , Nakai, M. , Cross, J. V. , & Obbard, D. J. (2018). The virome of Drosophila suzukii, an invasive pest of soft fruit. Virus Evolution, 4, vey009. 10.1093/ve/vey009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton, I. L. G. , & Rice, D. W. (2020). The Jekyll and Hyde symbiont: Could Wolbachia be a nutritional mutualist? Journal of Bacteriology, 202, e00589‐19. 10.1128/JB.00589-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, L. , Plichart, C. , Sang, A. C. , Brelsfoard, C. L. , Bossin, H. C. , & Dobson, S. L. (2012). Open release of male mosquitoes infected with a Wolbachia biopesticide: Field performance and infection containment. PLoS Neglected Tropical Diseases, 6, e1797. 10.1371/journal.pntd.0001797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, K. M. , Campos, J. , Moran, N. A. , & Hunter, M. S. (2008). Population dynamics of defensive symbionts in aphids. Proceedings of the Royal Society B: Biological Sciences, 275, 293–299. 10.1098/rspb.2007.1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2023). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Rudman, S. M. , Greenblum, S. , Hughes, R. C. , Rajpurohit, S. , Kiratli, O. , Lowder, D. B. , Lemmon, S. G. , Petrov, D. A. , Chaston, J. M. , & Schmidt, P. (2019). Microbiome composition shapes rapid genomic adaptation of Drosophila melanogaster . Proceedings of the National Academy of Sciences of the United States of America, 116, 20025–20032. 10.1073/pnas.1907787116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed, N. , Battisti, A. , Martinez‐Sañudo, I. , & Mori, N. (2018). Combined effect of temperature and Wolbachia infection on the fitness of Drosophila suzukii . Bulletin of Insectology, 71, 161–169. [Google Scholar]
- Sanaei, E. , Charlat, S. , & Engelstadter, J. (2021). Wolbachia host shifts: Routes, mechanisms, constraints and evolutionary consequences. Biological Reviews, 96, 433–453. 10.1111/brv.12663 [DOI] [PubMed] [Google Scholar]
- Teixeira, L. , Ferreira, A. , & Ashburner, M. (2008). The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster . PLoS Biology, 6, e2. 10.1371/journal.pbio.1000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thistlewood, H. M. A. , Rozema, B. , & Acheampong, S. (2019). Infestation and timing of use of non‐crop plants by Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the Okanagan Basin, Canada. Canadian Entomologist. 10.4039/tce.2018.47 [DOI] [Google Scholar]
- Toxopeus, J. , Jakobs, R. , Ferguson, L. V. , Gariepy, T. D. , & Sinclair, B. J. (2016). Reproductive arrest and stress resistance in winter‐acclimated Drosophila suzukii . Journal of Insect Physiology, 89, 37–51. 10.1016/j.jinsphys.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Turelli, M. , Cooper, B. S. , Richardson, K. M. , Ginsberg, P. S. , Peckenpaugh, B. , Antelope, C. X. , Kim, K. J. , May, M. R. , Abrieux, A. , Wilson, D. A. , Bronski, M. J. , Moore, B. R. , Gao, J.‐J. , Eisen, M. B. , Chiu, J. C. , Conner, W. R. , & Hoffmann, A. A. (2018). Rapid global spread of wRi‐like Wolbachia across multiple Drosophila . Current Biology, 28, 963–971.e8. 10.1016/j.cub.2018.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli, M. , & Hoffmann, A. A. (1991). Rapid spread of an inherited incompatibility factor in California Drosophila . Nature, 353, 440–442. 10.1038/353440a0 [DOI] [PubMed] [Google Scholar]
- Vacchini, V. , Gonella, E. , Crotti, E. , Prosdocimi, E. M. , Mazzetto, F. , Chouaia, B. , Callegari, M. , Mapelli, F. , Mandrioli, M. , Alma, A. , & Daffonchio, D. (2017). Bacterial diversity shift determined by different diets in the gut of the spotted wing fly Drosophila suzukii is primarily reflected on acetic acid bacteria. Environmental Microbiology Reports, 9(2), 91–103. [DOI] [PubMed] [Google Scholar]
- Vorburger, C. , & Gouskov, A. (2011). Only helpful when required: A longevity cost of harbouring defensive symbionts. Journal of Evolutionary Biology, 24, 1611–1617. 10.1111/j.1420-9101.2011.02292.x [DOI] [PubMed] [Google Scholar]
- Werren, J. (1997). Biology of Wolbachia . Annual Review of Entomology, 42, 587–609. 10.1146/annurev.ento.42.1.587 [DOI] [PubMed] [Google Scholar]
- Werren, J. H. , Baldo, L. , & Clark, M. E. (2008). Wolbachia: Master manipulators of invertebrate biology. Nature Reviews Microbiology, 6, 741–751. 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- Zabalou, S. , Riegler, M. , Theodorakopoulou, M. , Stauffer, C. , Savakis, C. , & Bourtzis, K. (2004). Wolbachia‐induced cytoplasmic incompatibility as a means for insect pest population control. Proceedings of the National Academy of Sciences, 101(42), 15042–15045. 10.1073/pnas.0403853101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. C. , Shang, D. , Liu, S. M. , Zhang, C. , Huo, L. X. , Zhang, L. S. , & Donga, H. (2023). Wolbachia‐infected Trichogramma dendrolimi is outcompeted by its uninfected counterpart in superparasitism but does not have developmental delay. Pest Management Science, 79, 1005–1017. [DOI] [PubMed] [Google Scholar]
- Zhou, W. , Rousset, F. , & O'Neill, S. (1998). Phylogeny and PCR–based classification of Wolbachia strains using wsp gene sequences. Proceedings of the Royal Society of London, Series B: Biological Sciences, 265, 509–515. 10.1098/rspb.1998.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug, R. , & Hammerstein, P. (2012). Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE, 7(6), e38544. 10.1371/journal.pone.0038544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug, R. , & Hammerstein, P. (2015). Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biological Reviews, 90, 89–111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Data files, R code, and a README file that describes them have been uploaded to Figshare and are currently available as private links (a single public link will be provided upon publication): Density data: https://figshare.com/s/418fc9d793c6b6b7ab10. Development time data: https://figshare.com/s/1751aec07548c7d2e9d5. R code for figures and statistics: https://figshare.com/s/d53e4b2daefa3440f09e. README file with metadata describing data and code: https://figshare.com/s/0998b81aacc25092a1ab.


