Abstract
Bacterial biofilms have great significance for public health, since biofilm-associated microorganisms exhibit dramatically decreased susceptibility to antimicrobial agents and treatments. To date most attention has focused on biofilms that arise from the colonization of solid-liquid or solid-air interfaces. It is of interest that colonization of the interface between air and liquid, which can be selectively advantageous for aerobic or facultative aerobic bacteria, has been rarely studied, although it may present a major problem in industrial aquatic systems. In this work we investigated the role of a biofilm at the interface between air and liquid (pellicle) in the susceptibility of Salmonella enterica serovar Typhimurium to stress conditions. For a control we used a mutant that had lost its ability to synthesize cellulose and thin aggregative fimbriae and thus did not produce the pellicle. Resistance of bacteria from the pellicle to heat, acidification, and chlorination was compared to resistance of planktonic cells from the logarithmic and stationary phases of growth. Pellicle cells were significantly more resistant to chlorination, and thus the surrounding matrix conferred protection against the reactive sodium hypochlorite. However, the stress management of pellicle cells in response to heat and low pH was not enhanced compared to that of stationary-phase cells. A long-period of incubation resulted in endogenous hydrolysis of the pellicle matrix. This phenomenon provides a potential new approach to combat microbial cells in biofilms.
Bacterial biofilms are defined as microbially derived sessile communities characterized by cells that are attached to a substratum, to an interface, or to each other. Biofilm cells are embedded in a matrix of extracellular polymeric substances that they have produced and exhibit an altered phenotype with respect to growth rate and gene transcription (8). Biofilms have developed into a significant issue for public health, since biofilm-associated microorganisms are less susceptible to antimicrobial agents and treatments. Bacteria in biofilms can be up to 1,000-fold more resistant to antibacterial agents than the same organisms grown planktonically (7, 12). Such resistance is demonstrated not only towards antibiotics and antiseptics, but also towards highly reactive chemicals, including isothiazolones, halogens, and quaternary ammonium compounds (12). Several factors have been suggested to account for biofilm resistance: (i) slow growth, (ii) the presence of an exopolysaccharide matrix that can slow the diffusion of chemicals or inactivate the antibacterial agents, and (iii) phenotypic changes in bacteria resulting in resistance occurring within the biofilm environment. Although each of these explanations is supported by evidence, no single mechanism can account for the general observation of resistance (1, 6, 7, 12).
Attachment to the surface is the first stage in the formation of a biofilm, and subsequent growth often occurs along with the production of an extensive network of extracellular polymers that facilitate dense adherence of bacteria to daughter cells and to the surface (12, 17, 31). The close physical association of cells within the matrix results in a structure with significant physical properties and leads to the development of new bacterial phenotypes (6, 17, 31, 33). Biofilm contents and architectures are highly heterogenous and variable, suggesting that bacterial phenotypes and physiology are also variable. They depend not only on the bacterial strains that form the biofilm but also on the material of the surface and on the growth and environmental conditions. The contents of biofilms of Salmonella spp., for instance, vary depending on the surface. In biofilms formed on glass, cellulose was shown to be the primary exopolysaccharide (28, 36), but cellulose was not a major component in biofilm production on gallstone (22). The resistance of salmonellae to treatment with sanitizers also varied depending on the surface. Biofilm cells on stainless steel were significantly more sensitive than those on plastic (15).
To date most attention has focused on biofilms that arise from the colonization of solid-liquid or solid-air interfaces, but several other kinds of surfaces and interfaces also provide ecological opportunities for bacteria. One of these is the interface between air and liquid, which, when colonized, provides bacteria with access to both the gaseous (oxygen) and liquid (nutrient and water) phases (29). Most bacteria propagated in liquid culture grow within the broth phase, sink to the bottom, form aggregates, or are attached to the walls. However, several bacteria, such as Salmonella enterica serovars, Escherichia coli, Pseudomonas fluorescens, and Vibrio cholerae, are able to colonize the air-liquid interface and to form either fragile or rigid pellicles that block the surface of the standing culture (2, 23, 24, 28, 30, 35, 36). Colonization of the air-liquid interface in water-filled environments can be selectively advantageous for aerobic or facultatively aerobic bacteria. It is of interest that colonization of the air-liquid interface has rarely been studied.
The ability to produce a pellicle on standing cultures is a common phenotype of salmonellae. It was found that 71% of Salmonella enterica serovar Enteritidis isolates from different origins (environment, food, animals, and clinical) produced pellicles on Luria-Bertani (28). A very strong correlation between pellicle formation and the ability to form a biofilm on polyvinyl chloride (28) and to generate distinctive rough, dry, and wrinkled colonies (termed rdar colonies) on agar plates was observed (2, 24, 25). In Salmonella spp. as well as P. fluorescens, colonization of the air-liquid niche is largely due to overproduction of a cellulosic polymer (28, 29, 36). Knocking out agfA (coding for thin aggregative fimbriae, also termed curli) resulted in the lack of the ability to form a pellicle, indicating that thin aggregative fimbriae are also important compounds of the extracellular matrix of salmonellae (24, 26). However, cellulases hydrolyzed the biofilm matrix formed on glass but not the biofilm formed at the air-liquid interface (36), suggesting that cellulose in the pellicle is less accessible to the enzymes.
Are pellicle cells more resistant to killing than stationary-phase planktonic bacteria? A comparison of susceptibility to killing between pellicle cells and logarithmic- and stationary-phase planktonic cells was undertaken in this study. We found that cells in the pellicle and stationary-phase planktonic cells had very similar tolerance to stress conditions such as low pH. However, cells in the pellicle were significantly more resistant to chlorine, suggesting that the stress management of stationary-phase planktonic cells was not improved in the pellicle, while the surrounding matrix in the pellicle conferred protection against the reactive sodium hypochlorite.
MATERIALS AND METHODS
Strains and plasmids.
Salmonella enterica serovar Typhimurium UMR1 (ATCC 14028-1s, Nalr) and its mutants MAE52 and MAE190 used in this study were described previously (36). In MAE52 the highly regulated rdar morphotype of UMR1 was converted to a semiconstitutive rdar morphotype by an individual single point mutation in the agfD promoter region. This mutation abolished temperature regulation, allowing expression of agfD at 37°C (11, 25). AgfD (also called CsgD, for curli subunit gene D) is a transcriptional regulator that positively controls the production of cellulose and thin aggregative fimbriae, the two main extracellular compounds of the biofilm of salmonellae (11). Consequently, MAE52 cells produce biofilms on glass walls and pellicle on the air-liquid interface at both 37°C and 28°C, while UMR1 produces the biofilm and the pellicle only at 28°C (36). MAE190 does not express the gene products of bcsA (bacterial cellulose synthesis A) and agfBA (also called csgBA). Thus, it does not produce a biofilm or a pellicle (36). Bacteria were transformed with the pGFP plasmid (Clontech, Palo Alto, Calif.) to obtain green fluorescent protein (GFP)-labeled cells or pUC19 (New England Biolabs, Beverly, Mass.) as a control.
Growth conditions and production of pellicle at the air-liquid interface.
Bacterial starters were prepared by inoculation of a colony on Luria-Bertani (LB) broth without NaCl and incubation overnight at 37°C with aeration. For pellicle production, cells were diluted (1:100) in fresh medium and incubated at 28 or 37°C without shaking in 24-well microplates (1.5 ml) or petri dishes (5 ml) for 1 to 7 days. For growth of cells that carry plasmid pGFP or pUC19, ampicillin (100 μg ml−1) was added.
Mixed pellicles were formed by incubation of equal volumes (45 μl; optical density at 600 nm, 0.8) of MAE52 and MAE190 that carry plasmids pUC19 and pGFP, respectively, in 1.5 ml of LB for 24 h at 37°C. The pellicles were washed twice with 4.5 ml of saline (0.85% NaCl) before fluorescent microscope scanning. For a control we used MAE52 carrying plasmid pGFP.
Experiments in anaerobic atmospheres were performed in sealed sterile tubes which contained 4.5 ml of salt-free LB. The air was drained out by pumping N2 through the tube's rubber cap. Overnight cultures of UMR1 or MAE52 (optical density at 600 nm, 0.8, 45 μl) were injected into the tubes and incubated at 28 and 37°C without shaking.
Enumeration of cells in the pellicle.
Pellicles were gently removed from the surface of the broth with sterile tweezers, washed twice with 4.5 ml of sterile saline for 5 min, and placed in tubes containing 4.5 ml of saline and about 30 (3-mm) glass beads. The pellicles were disrupted for 40 s by vortexing at the highest speed, serially diluted, and plated to enumerate the cells.
Growth conditions for susceptibility testing.
Cultures for all susceptibility experiments were obtained as follows. Pellicles were prepared by growing MAE52 cells in LB broth without NaCl at 37°C in 24-well microplates for 24 h without agitation. Pellicles were gently removed with sterile tweezers and washed twice with saline. Planktonic cells of MAE52 were prepared by collecting the broth under the pellicle; 1 ml of overnight culture was centrifuged and resuspended in 2 ml of saline to a final concentration of ca 108 CFU ml−1. Stationary-phase cultures of MAE190 were prepared at the same growth conditions. Logarithmic-phase cultures were obtained by growing cells to optical density of 0.6 at 37°C. Cells were then centrifuged and resuspended in saline to a final concentration of ca 108 CFU ml−1. All experiments were carried out at least twice in triplicate. The average of six measurements is shown.
Susceptibility to heat treatment.
Twenty-four-hour-old MAE52 pellicles were washed with sterile saline and placed in 4.5 ml of heated saline (60, 70, and 80°C) for 0, 1, 5, 10, 20, 30, and 40 min. After rapid cooling in ice, the pellicles were grained with glass beads. The stationary-phase cells and logarithmic-phase cells were resuspended in 4.5 ml of heated saline and incubated for 0 to 40 min at 60, 70, and 80°C. After cooling in ice, all samples were serially diluted and enumerated by standard plating.
Susceptibility to acidic conditions.
Twenty-four-hour-old MAE52 pellicles were washed twice with sterile saline and placed in 2 ml of citrate-phosphate buffer (40 mM citric acid and 20 mM dibasic sodium phosphate, pH 3) for 0, 15, 30, 45, 60, 75, and 90 min. The pellicles were then transferred to 5 ml of neutralizing solution (0.1 M Na2S2O3) for 30 s, rinsed with sterile saline, and disrupted with glass beads. The stationary-phase cells and logarithmic-phase cells (ca. 108 CFU) were centrifuged, and the cell pellets were resuspended in 1 ml of sterile citrate-phosphate buffer. Aliquots were taken at 0 to 90 min, serially diluted, and plated.
Susceptibility to chlorine.
Twenty-four-hour-old MAE52 pellicles were washed with sterile saline and placed for 15 min in different concentrations of 2 ml of sodium hypochlorite solution (50, 100, 150, 200, and 250 ppm). The pellicles were then transferred to 5 ml of neutralizing solution (0.1 M Na2S2O3) for 30 s, rinsed with sterile saline, and grained. The stationary-phase cells and logarithmic-phase cells (ca. 108 CFU) were centrifuged, and the cell pellets were resuspended in different concentrations of 1 ml of sodium hypochlorite solution (50 to 250 ppm). Aliquots were taken at 15 min, serially diluted, and plated.
Statistic analysis.
The statistical significance of the differences in mortality between cultures was determined by a Tukey-Kramer test with one-way analysis of variance. A P value of ≤0.01 was accepted as indicating significance.
RESULTS
Formation and disposal of surface pellicle by salmonellae.
When S. enterica serovar Typhimurium UMR1 inoculated in LB medium without salt and without shaking at 28°C, the bacteria grew exponentially in the planktonic phase of the culture. After 48 to 72 h of growth, a thin pellicle was built up at the air-liquid interface. MAE190 did not form a pellicle, although the cultures were incubated for 7 days. Cultures of MAE52 produced a distinctive pellicle visible to the naked eye within less than 15 h following incubation at either 28 or 37°C. The turbidity of the cells in the broth was constant after 6 h of incubation, indicating that cells were in the stationary phase of growth. After 24 h of incubation, the pellicle had acquired rigid properties. Neither UMR1 nor MAE52 incubated in an anaerobic atmosphere produced pellicles.
The first stage of pellicle production at the air-liquid interface involved attachment to the walls (either glass or plastic) at the interface. Subsequent growth occurred concomitantly with the production of an extensive network of extracellular polymers on the surface of the liquid towards the center. Finally, a thick pellicle that covered the surface of the culture was formed. The time needed to cover the surface was not dependent on the radius of the container at a range of 0.35 to 4.2 cm (a well in a 96-well microplate and a petri dish, respectively). A pellicle that was gently detached from the walls with sharp tweezers sank immediately, indicating that attachment to the walls supports the pellicle on the surface (i.e., it is not buoyant). About 48 h after the appearance of the pellicle, a biofilm disintegration process was initiated and progressed until the pellicle was almost completely eliminated and turned into aggregates floating on the medium.
Quantification of cells in the pellicle.
Quantification of cells was conducted by a standard plating method following dismantling of the pellicle with glass beads. Cell density in a 24-h-old MAE52 pellicle, obtained in 30 independent experiments, was in the range of 4 × 107 to 1 × 108 CFU cm−2.
Incorporation of mutants in the pellicle.
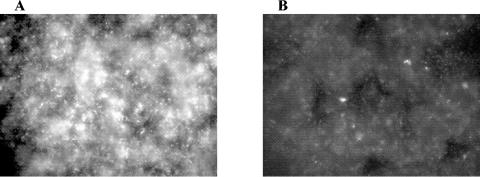
MAE190 mutants are not able to produce cellulose and thin aggregative fimbriae and do not form a pellicle (36). However, when a mixed population of MAE52(pUC19) and MAE190(pGFP) cells was cultured, almost half of the cells in the pellicle (>40%) were fluorescent green (Fig. 1). The density of cells in the heterogenous and homogenous pellicles was very similar, with an average of 4 × 107 CFU cm−2 versus 7 × 107 CFU cm−2 respectively. The GFP-expressing cells (MAE190) were found to be embedded all over the biofilm (Fig. 1).
FIG. 1.
Fluorescent microscope images of the pellicle formed by Salmonella enterica serovar Typhimurium strain MAE52(pGFP) (A) and a 1:1 mixture of MAE52(pUC19) and MAE190(pGFP) (B). Magnification, ×40.
Susceptibility to stress conditions.
To learn whether cells in the pellicle are more resistant to inactivation processes and therefore are able to stay alive in water supplies and food-processing chains, we carried out survival experiments with two Salmonella enterica serovar Typhimurium mutants, MAE52 and MAE190. Cultures of logarithmic- and stationary-phase cells of MAE190 (which do not produce the main components of the pellicle matrix) were used as a control.
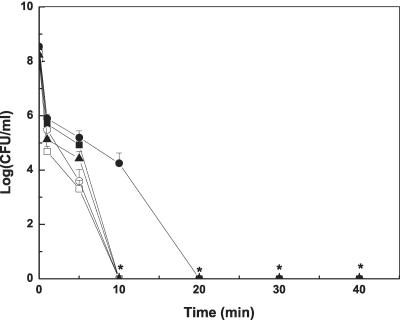
The results of heat treatments (at 70°C) are shown in Fig. 2. At this temperature, logarithmic growing cells were more susceptible to heat treatment than stationary-phase cells (P < 0.01), and stationary-phase planktonic cells were slightly more resistant than pellicle cells. The highest tolerance was observed in stationary-phase MAE190 cells. Cells in the pellicle were slightly more sensitive to heat compared to stationary-phase planktonic cells also at 60°C (data not shown). However this mild heat treatment resulted in less than 5-log reduction after 40 min. Treatment at 80°C killed all cells (8-log reduction) within 5 min or less.
FIG. 2.
Survival of pellicle cells and free cells following heat treatment (70°C). MAE52 pellicle cells (▴), MAE52 logarithmic-phase cells (□) and MAE52 stationary-phase cells (▪), MAE190 logarithmic-phase cells (○), and MAE190 stationary-phase cells (•). *, Points on the x axis indicate that bacteria were not detected at the minimum level of sensitivity (<10 CFU ml−1).
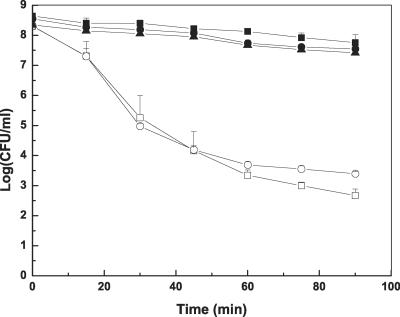
The results of acid treatment (pH 3) are shown in Fig. 3. All stationary-phase cells, including pellicle cells, cells from broth under the pellicle, and MAE190 had the same high resistance to acidic stress compared to logarithmically growing cells. A 90-min exposure to acid stress resulted in about a 1-log reduction of stationary cells and cells in the pellicles but more than 4-log reduction of logarithmic cells. Most of the logarithmic cells were killed within the first 60 min, and a longer treatment did not have a significant effect.
FIG. 3.
Survival of pellicle cells and free cells upon a 90-min exposure to citrate-phosphate buffer (pH = 3). Pellicle cells (▴), MAE52 logarithmic-phase cells (□), MAE52 stationary-phase cells (▪), MAE190 logarithmic-phase cells (○), and MAE190 stationary-phase cells (•).
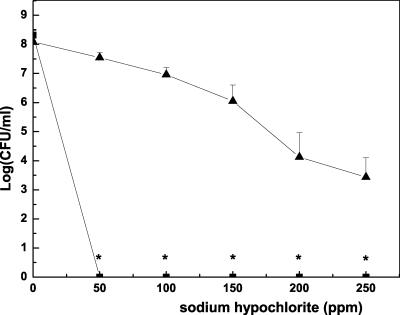
The results of chlorination are shown in Fig. 4. All of the planktonic cells, including MAE190 and MAE52 (approximately 108 CFU ml−1) were killed by chlorination (50 ppm) within 15 min. In contrast, about half of the cells in the pellicle survived exposure to 50 ppm. Treatment with 250 ppm resulted in only a 4-log reduction, and about 0.01% survived.
FIG. 4.
Survival of pellicle cells (▴) and planktonic cells (logarithmic and stationary phase) of MAE52 (▪) following a 15-min exposure to sodium hypochlorite. MAE190 cells in the logarithmic phase and stationary phase were as sensitive to this treatment as free MAE52 cells. *, Points on the x axis indicate that bacteria were not detected at the minimum level of sensitivity (<10 CFU ml−1).
DISCUSSION
In water-filled environments, colonization of the interface between liquid and gas phases can be selectively advantageous to survival in the environment outside the host (23). P. fluorescens, V. cholerae, E. coli, and serovars of S. enterica are known to be able to colonize the air-liquid interface and to form fragile or rigid pellicles (2, 23, 24, 28, 30, 35, 36). It seems that the higher oxygen concentrations at the air-liquid interface have an important role in pellicle formation. We observed that the pellicle built up by Salmonella enterica serovar Typhimurium was not formed under anaerobic atmosphere, probably because the production of its components, cellulose and thin aggregative fimbriae, is regulated by oxygen (11).
Production of the microbial film is usually a process that takes several days. Three to 7 days is the time needed for S. enterica serovar Enteritidis isolates (28), Salmonella enterica serovar Typhimurium DT104 strain 11601 (2), P. fluorescens SBW25 (29), and V. cholerae O1 E1 Tor (35) to produce visible pellicles in laboratory growth conditions. However, some mutants, such as Salmonella enterica serovar Typhimurium MAE52 and P. fluorescens B52 (1), form pellicles rapidly, within less than a day. The characteristics of the pathogens in these pellicles have rarely been studied, although colonization of this niche should have attracted the attention of scientists in different areas, such as the medical field, aquatic environment, and food-processing industries, and although it provides a novel model system for studying a variety of aspects of biofilm production, permeability, and survival.
In order to quantify cells in the pellicle and to estimate the efficacy of inactivation treatments against cells in the pellicle, a method for recovery and measurement is needed. Many techniques that allow quantification of biofilm cells on solid surfaces are available (8). Very few methods are suitable for bacterial pellicles formed at the air-liquid interface, since the pellicle matrix of salmonellae is usually elastic, not fragile, and sticky. We tried several methods to optimize breakage of the pellicle and removal of the viable cells. The methods we used were gentle sonication, crushing with a glass stick, passage through a syringe needle, and vortexing with glass beads (data not shown). Although none of the techniques completely removed all aggregates and cell clusters, dismantling of the pellicle by vortexing with glass beads was found to be the most reliable method for removal of cells from the matrix, and it also gave the highest number of CFU per square centimeter. Hence, this method was chosen to count the cells following the killing treatments.
Using MAE190 expressing GFP, we found that while this mutant was not able to produce the pellicle, it was able to join in when the pellicle was created by another strain. The incorporation of a high percentage (>40%) of mutants in the pellicle area indicates that cells do not need to produce the matrix components in order to be encased in it. It seems that adherence of cells from the broth plays a major role in pellicle expansion, which is not limited to replication of cells in the film. Attachment is not dependent on the ability to produce cellulose or thin aggregative fimbriae. This observation may supply clues regarding the formation process of the biofilm. During the production of the film, cells from the broth are probably stuck to the tenacious polymers and are integrated into the matrix that is produced by the cells or their neighbors and eventually envelops them.
Evidence from the literature supports the assumption that cells from the liquid are attracted to the biofilm and that motility is important for movement of cells from the broth to the interface. Romling and Rohde (24) found that MAE52 deficient of flagella formed individual clumps of bacteria instead of a tight mat of cells (pellicle). However, flagella were not important for production of the extracellular matrix since scanning electron microscopy studies showed that the matrix around cells in the pellicle and around the mutants in the aggregates was similar (24). Kearns and his group (16) speculated that phosphatidylethanolamine-directed twitching motility may be involved in biofilm formation in Pseudomonas spp. In E. coli and Salmonella enterica serovar Typhimurium, starved cells appear to form aggregates as a result of a self-generated and secreted attractant that is sensed by the chemotaxis machinery (3, 34). It is not clear whether the incorporation of cells from the broth into the pellicle is incidental or by design, and hence it is worth investigating if attractants are secreted by cells in the pellicle and the role of chemotaxis, if any, in pellicle formation.
In this work we investigated the role of the biofilm at the air-liquid interface in susceptibility to stress conditions. Three different treatments were chosen: heat, acidification, and sanitation with oxidizing agents such as chlorine. Those are the main antimicrobial barriers usually used by the food industries, water supply authorities, and agriculture to inactivate pathogens in water, food, and the environment. The susceptibility of pellicle cells was compared to the susceptibility of planktonic cells from the broth under the pellicle (which are in the stationary phase, based on absorbance measurements) and to logarithmically growing cells. The stationary-phase planktonic cells may have physiological properties which are similar to those of pellicle cells, because soluble factors secreted by pellicle cells might also affect the expression of genes by the cells in the broth. Moreover, cells in the broth may produce the same protected matrix and assemble in the suspension to form small aggregates or granules that also protect them. It is also not clear if these planktonic cells originated from the cells which were never associated with the pellicle or whether these cells originated from the pellicle and fell into the broth. Thus, cultures of logarithmic- and stationary-phase free cells of mutant MAE190 were used as a control in all experiments.
We found that the pellicle did not provide any benefit during heat or acidification compared to stationary-phase planktonic cells. However, both stationary-phase and biofilm cells were significantly more resistant than logarithmic-phase cells (Fig. 2 and 3). Chavant et al. also observed that acid treatment acted in a similar way on both sessile and stationary-phase Listeria monocytogenes cells (4). Other researchers compared the acid tolerance of biofilm cells of Streptococcus mutans to that of logarithmically growing cells and found that the cells in the biofilm are highly resistant to low pH (21). The ways in which planktonic Salmonella enterica serovar Typhimurium cells respond to inimical processes have been studied extensively. Numerous researchers have shown that stationary-phase cells are more resistant than exponential-phase cells to a range of environmental stresses, such as extremes of temperature, pH, and osmolarity (10, 20). These differences have been ascribed to an alternative sigma factor, σs, encoded by rpoS, that is active in the stationary phase of many bacteria and induces a number of mechanisms during prolonged starvation that ensure resistance to environmental stresses (5, 14, 18, 27). Based on our results, the stress management of pellicle cells in response to low pH was not different from that of stationary-phase cells.
In contrast to heat and acidification, the tolerance of pellicle cells to chlorination was significant (Fig. 4). Increased resistance of salmonellae in biofilms on solid surfaces to chlorination has been observed recently. The efficiency of the treatment varied depending on the surface. Biofilm cells on stainless steel, for instance, were more sensitive than those on plastic (15). The resistance of biofilm and pellicle cells to chlorine treatment is not limited to salmonellae. Yildiz and his group (35) found that the exopolysaccharides formed in a biofilm of V. cholerae are directly responsible for resistance to chlorine and other oxidants (32).
Sodium hypochlorite has been the principal means of preventing water-borne infectious diseases since the first decade of the 20th century (19). We used a concentration that is 10-fold higher than the free chlorine concentrations typically obtained in municipal water supplies (according to Israeli standards) and is in the concentration range used as a sanitizer for food plants. The Food and Drug Administration allows the use of sodium hypochlorite as a sanitizing agent for the food contact surfaces of food processing equipment in concentrations up to 200 ppm (Code of Federal Regulations). We have shown that cells in biofilms formed on liquids are much more resistant to chlorination than planktonic cells. Such biofilm cells, which are not removed during normal chlorination procedures, could be a source of contamination for water and foods.
The observation that the pellicle cells showed higher resistance to chlorine but not to acid or heat, suggests that the matrix polymers might react chemically with chlorine and directly neutralize the reactive molecules. Alternatively, the polymers might act as an ion exchange resin that actively removes strongly charged molecules. Anriany et al. observed that pellicle-forming Salmonella enterica serovar Typhimurium DT104 were completely surrounded by capsular material, which bound cationic ferritin, demonstrating that it contained an acidic (anionic) component (2). The film's negative charge may contribute to the reduction of the active chlorine ions. Solano et al. demonstrated that cellulose is directly responsible for the chlorine resistance of salmonellae in the biofilm, since cellulose-minus mutants did not survived under low concentrations of NaCl (28). Another case in which biofilm acts as a diffusion barrier was described for hydrogen peroxide. Unlike planktonic cells of P. aeruginosa, which were very sensitive to killing by 50 mM H2O2, biofilm cells that actually had lower levels of catalase (KatA) were effectively protected (9, 13).
Proposed explanations for the observed resistance of biofilm communities include diffusional resistance of the extracellular matrix, augmented by chemical/enzymatic modification of the agent, physiological changes due to slow growth rate and starvation responses, and the induction of biofilm-specific drug resistance physiologies. We found that starvation responses of cells in the pellicle were not advantageous compared to stationary-phase cells. However, diffusional resistance or other biofilm-specific mechanisms may play an important role in resistance to oxidative agents. Our studies were performed with synthetic surfaces and media and with one mutant strain of salmonellae only. Testing wild-type strains under typical environmental conditions would be important to our understanding of survival of bacteria.
One of the most significant outcomes of our study is the observation that a few days after the appearance of the biofilm on the air-liquid interface, a process of self-disintegration begins, releasing the embedded cells into the liquid. Endogenous, probably enzymatic hydrolysis of the pellicle was also observed in P. fluorescens (1). The genetic basis for this process warrants further investigation, since it may contribute to the development of novel methods to remove the biofilm matrix and combat the resistant microorganisms in the biofilms.
Acknowledgments
We thank Anat Lapidot for technical assistance.
This work was partially supported by Gabriel and Matilda Barnett funds and partially by Elite Food Engineering Research funds.
REFERENCES
- 1.Allison, D. G., B. Ruiz, C. SanJose, A. Jaspe, and P. Gilbert. 1998. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. FEMS Microbiol. Lett. 167:179-184. [DOI] [PubMed] [Google Scholar]
- 2.Anriany, Y. A., R. M. Weiner, J. A. Johnson, C. E. De Rezende, and S. W. Joseph. 2001. Salmonella enterica serovar Typhimurium DT104 displays a rugose phenotype. Appl. Environ. Microbiol. 67:4048-4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budrene, E. O., and H. C. Berg. 1991. Complex patterns formed by motile cells of Escherichia coli. Nature 349:630-633. [DOI] [PubMed] [Google Scholar]
- 4.Chavant, P., B. Gaillard-Martinie, and M. Hebraud. 2004. Antimicrobial effects of sanitizers against planktonic and sessile Listeria monocytogenes cells according to the growth phase. FEMS Microbiol. Lett. 236:241-248. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C. Y., L. Eckmann, S. J. Libby, F. C. Fang, S. Okamoto, M. F. Kagnoff, J. Fierer, and D. G. Guiney. 1996. Expression of Salmonella Typhimurium rpoS and rpoS-dependent genes in the intracellular environment of eukaryotic cells. Infect. Immun. 64:4739-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 7.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 8.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkins, J. G., D. J. Hassett, P. S. Stewart, H. P. Schweizer, and T. R. McDermott. 1999. Protective role of catalase in Pseudomonas aeruginosa biofilm resistance to hydrogen peroxide. Appl. Environ. Microbiol. 65:4594-4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster, J. W., and M. P. Spector. 1995. How Salmonella survive against the odds. Annu. Rev. Microbiol. 49:145-174. [DOI] [PubMed] [Google Scholar]
- 11.Gerstel, U., and U. Romling. 2001. Oxygen tension and nutrient starvation are major signals that regulate agfD promoter activity and expression of the multicellular morphotype in Salmonella Typhimurium. Environ. Microbiol. 3:638-648. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert, P., D. G. Allison, and A. J. McBain. 2002. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J. Appl. Microbiol. 92:98S-110S. [PubMed] [Google Scholar]
- 13.Hassett, D. J., J. F. Ma, J. G. Elkins, T. R. McDermott, U. A. Ochsner, S. E. West, C. T. Huang, J. Fredericks, S. Burnett, P. S. Stewart, G. McFeters, L. Passador, and B. H. Iglewski. 1999. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol. Microbiol. 34:1082-1093. [DOI] [PubMed] [Google Scholar]
- 14.Hengge-Aronis, R. 1999. Interplay of global regulators and cell physiology in the general stress response of Escherichia coli. Curr. Opin. Microbiol. 2:148-152. [DOI] [PubMed] [Google Scholar]
- 15.Joseph, B., S. K. Otta, and I. Karunasagar. 2001. Biofilm formation by Salmonella spp. on food contact surfaces and their sensitivity to sanitizers. Int. J. Food Microbiol. 64:367-372. [DOI] [PubMed] [Google Scholar]
- 16.Kearns, D. B., J. Robinson, and L. J. Shimkets. 2001. Pseudomonas aeruginosa exhibits directed twitching motility up phosphatidylethanolamine gradients. J. Bacteriol. 183:763-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuchma, S. L., and G. A. O'Toole. 2000. Surface-induced and biofilm-induced changes in gene expression. Curr. Opin. Biotechnol. 11:429-433. [DOI] [PubMed] [Google Scholar]
- 18.Loewen, P. C., and R. Hengge-Aronis. 1994. The role of the sigma factor sigma S (KatF) in bacterial global regulation. Annu. Rev. Microbiol. 48:53-80. [DOI] [PubMed] [Google Scholar]
- 19.Margolin, A. B. 1997. Control of microorganisms in source water and drinking water, p. 195-202. In C. J. Hurst et al. (ed.), Manual of environmental microbiology. ASM Press, Washington, D.C.
- 20.Matin, A. 1991. The molecular basis of carbon-starvation-induced general resistance in Escherichia coli. Mol. Microbiol. 5:3-10. [DOI] [PubMed] [Google Scholar]
- 21.McNeill, K., and I. R. Hamilton. 2003. Acid tolerance response of biofilm cells of Streptococcus mutans. FEMS Microbiol. Lett. 221:25-30. [DOI] [PubMed] [Google Scholar]
- 22.Prouty, A. M., and J. S. Gunn. 2003. Comparative analysis of Salmonella enterica serovar Typhimurium biofilm formation on gallstones and on glass. Infect. Immun. 71:7154-7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rainey, P. B., and M. Travisano. 1998. Adaptive radiation in a heterogeneous environment. Nature 394:69-72. [DOI] [PubMed] [Google Scholar]
- 24.Romling, U., and M. Rohde. 1999. Flagella modulate the multicellular behavior of Salmonella Typhimurium on the community level. FEMS Microbiol. Lett. 180:91-102. [DOI] [PubMed] [Google Scholar]
- 25.Romling, U., M. Rohde, A. Olsen, S. Normark, and J. Reinkoster. 2000. AgfD, the checkpoint of multicellular and aggregative behaviour in Salmonella Typhimurium regulates at least two independent pathways. Mol. Microbiol. 36:10-23. [DOI] [PubMed] [Google Scholar]
- 26.Romling, U., W. D. Sierralta, K. Eriksson, and S. Normark. 1998. Multicellular and aggregative behaviour of Salmonella Typhimurium strains is controlled by mutations in the agfD promoter. Mol. Microbiol. 28:249-264. [DOI] [PubMed] [Google Scholar]
- 27.Schellhorn, H. E., J. P. Audia, L. I. Wei, and L. Chang. 1998. Identification of conserved, RpoS-dependent stationary-phase genes of Escherichia coli. J. Bacteriol. 180:6283-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solano, C., B. Garcia, J. Valle, C. Berasain, J. M. Ghigo, C. Gamazo, and I. Lasa. 2002. Genetic analysis of Salmonella Enteritidis biofilm formation: critical role of cellulose. Mol. Microbiol. 43:793-808. [DOI] [PubMed] [Google Scholar]
- 29.Spiers, A. J., J. Bohannon, S. M. Gehrig, and P. B. Rainey. 2003. Biofilm formation at the air-liquid interface by the Pseudomonas fluorescens SBW25 wrinkly spreader requires an acetylated form of cellulose. Mol. Microbiol. 50:15-27. [DOI] [PubMed] [Google Scholar]
- 30.Spiers, A. J., S. G. Kahn, J. Bohannon, M. Travisano, and P. B. Rainey. 2002. Adaptive divergence in experimental populations of Pseudomonas fluorescens. I. Genetic and phenotypic bases of wrinkly spreader fitness. Genetics 161:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutherland, I. W. 2001. The biofilm matrix-an immobilized but dynamic microbial environment. Trends Microbiol. 9:222-227. [DOI] [PubMed] [Google Scholar]
- 32.Wai, S. N., Y. Mizunoe, A. Takade, S. I. Kawabata, and S. I. Yoshida. 1998. Vibrio cholerae O1 strain TSI-4 produces the exopolysaccharide materials that determine colony morphology, stress resistance, and biofilm formation. Appl. Environ. Microbiol. 64:3648-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wimpenny, J., W. Manz, and U. Szewzyk. 2000. Heterogeneity in biofilms. FEMS Microbiol. Rev. 24:661-671. [DOI] [PubMed] [Google Scholar]
- 34.Woodward, D. E., R. Tyson, M. R. Myerscough, J. D. Murray, E. O. Budrene, and H. C. Berg. 1995. Spatio-temporal patterns generated by Salmonella Typhimurium. Biophys. J. 68:2181-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yildiz, F. H., and G. K. Schoolnik. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. USA 96:4028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zogaj, X., M. Nimtz, M. Rohde, W. Bokranz, and U. Romling. 2001. The multicellular morphotypes of Salmonella Typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 39:1452-1463. [DOI] [PubMed] [Google Scholar]