Abstract
Soil samples were collected from around fresh and year-old bison carcasses and areas not associated with known carcasses in Wood Buffalo National Park during an active anthrax outbreak in the summer of 2001. Sample selection with a grid provided the most complete coverage of a site. Soil samples were screened for viable Bacillus anthracis spores via selective culture, phenotypic analysis, and PCR. Bacillus anthracis spores were isolated from 28.4% of the samples. The highest concentrations of B. anthracis spores were found directly adjacent to fresh carcasses and invariably corresponded to locations where the soil had been saturated with body fluids escaping the carcass through either natural body orifices or holes torn by scavengers. The majority of positive samples were found within 2 m of both year-old and fresh carcasses and probably originated from scavengers churning up and spreading the body fluid-saturated soil as they fed. Trails of lesser contamination radiating from the carcasses probably resulted from spore dissemination through adhesion to scavengers and through larger scavengers dragging away disarticulated limbs. Comparison of samples from minimally scavenged and fully necropsied carcass sites revealed no statistically significant difference in the level of B. anthracis spore contamination. Therefore, the immediate area around a suspected anthrax carcass should be considered substantially contaminated regardless of the condition of the carcass.
Sporadic outbreaks of anthrax have occurred in the wild bison (Bison bison) herds in and around Wood Buffalo National Park in northern Canada since at least 1962 and have resulted in the death of thousands of animals (2). In 2000, a large anthrax outbreak occurred within the park, resulting in the death of over 100 bison. The deaths occurred in the Davidson Tower and Lake Claire Delta regions of the park. Because of the remoteness of the affected regions, a shortage of resources, and no direct threat to tourist areas, Parks Canada flew surveillance flights to monitor the situation and determine the extent of the outbreak but did not attempt any clean-up operations.
A collaboration was initiated between Defense R&D Canada-Suffield, the U.S. Army Medical Research Institute of Infectious Diseases and Parks Canada, whereby in the summer of 2001 the former two agencies would collect samples of environmental interest to Parks Canada with which to test their anthrax detection technologies. Another anthrax outbreak occurred in the park just weeks before the arrival of Defence R&D Canada-Suffield and U.S. Army Medical Research Institute of Infectious Diseases personnel, allowing the team the opportunity to collect soil samples from fresh and year-old bison carcass sites. The 2001 outbreak was limited to the Lake Claire Delta region and resulted in the deaths of 92 bison and one moose. As with the 2000 outbreak, no carcass clean-up operations were conducted. The untreated carcass sites provided an ideal setting to investigate the natural dispersion of anthrax spores.
This paper details the field collection and analysis of soil samples with selective polymyxin, lysozyme, EDTA, and thallium acetate (PLET) medium (6) combined with phenotypic analysis and PCR.
MATERIALS AND METHODS
Soil specimens were collected in Wood Buffalo National Park under Parks Canada research permit WB01-1027. All personnel involved in the collection and handling of samples were vaccinated with the anthrax vaccine absorbed (Bioport Corporation). When handling samples, personnel wore protective clothing comprising rubber boots, disposable Tyvek overalls, double latex gloves, and full-face HEPA-filtered powered air-purifying respirators. Afterwards, equipment and protective clothing were sprayed down with 10% bleach. All disposable materials were collected and bagged for incineration.
Three different sample selection methods were used: the central peg method, the transect method, and the grid method. The central peg method involved placing a metal peg at the center of the carcass remains. Random soil core samples were collected from around the site, recording the distance and bearing of each from the peg with a knotted cord and compass (Fig. 1). For the transect method, a 15-m cord was laid out in a straight line, and its bearing was recorded with a compass. Surface soil samples were collected every 3 m along the cord (Fig. 2). For the grid method, a 4-m by 4-m grid of chain was centered over a carcass. The sides of the grid were aligned with the cardinal compass points, and four 1-m-wide east-west lanes, marked at 1-m intervals, were formed with cord within the grid to serve as collection guides. Surface soil samples were collected from the center of each square meter of the grid. Additional grids were constructed adjacent to the faces of the center grid to collect samples at greater distances from the carcass (Fig. 3).
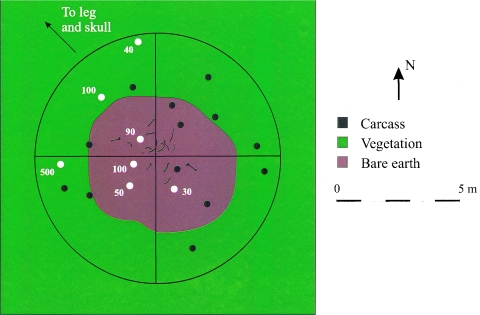
FIG. 1.
Distribution of viable B. anthracis spores around carcass site 10 from the 2000 anthrax outbreak. The black circles represent samples from which no anthrax spores could be isolated, and the white circles represent anthrax spore-positive samples. The numbers beside the white circles represent the number of viable anthrax spores per gram at that location.
FIG. 2.
Distribution of viable B. anthracis spores along winter road transects in an area affected by the 2000 anthrax outbreak.
FIG. 3.
Distribution of viable B. anthracis spores around carcasses from the 2001 anthrax outbreak on the Lake Claire Delta. A. Necropsied carcass 44. B. Carcass 49. The numbers represent the number of viable anthrax spores per gram at the center of each square.
For surface sampling, disposable plastic scoops were employed, with a new scoop used every four samples. To collect core samples, a metal core sampler was used. The core sampler was not cleaned between samples but was cleaned with 10% bleach between sites.
Selective culture of the samples was conducted in the Defence R&D Canada—Suffield biocontainment level 3 laboratories. Samples were extracted via a high-buoyant-density flotation method detailed previously (4). Representative colonies displaying a B. anthracis-like morphology on PLET medium were subcultured to sheep blood agar and overlaid with 10-U penicillin disks to check for hemolysis and penicillin susceptibility, respectively. Identification of B. anthracis isolates was confirmed by SYBR Green fluorescence-based PCR assays of crude culture lysates on the Smart Cycler (Cepheid). The PCR primers targeted a unique sequence in the B. anthracis chromosome and the lethal factor gene (lef) on the pX01 plasmid.
RESULTS
A total of 250 soil samples were processed and analyzed (Fig. 4). Colonies displaying B. anthracis-like morphology on PLET medium were observed in 160 (64.0%) of the soil samples. One thousand forty-seven B. anthracis-like isolates were randomly picked for subculture to SBA and overlaid with penicillin disks. Of these isolates, 259 (24.7%) displayed the classic B. anthracis phenotype (nonhemolytic and penicillin sensitive). All nonhemolytic, penicillin-susceptible isolates and a limited number of isolates from the other hemolysis and penicillin groupings were screened by PCR for confirmatory identification. Two hundred two (78.0%) of the nonhemolytic, penicillin-susceptible isolates were PCR positive for the chromosomal and pX01 targets, while all other isolates were negative for both targets. The B. anthracis-positive isolates originated from 71 (28.4%) of the soil samples.
FIG. 4.
Analysis of 2001 Wood Buffalo National Park soil samples for the presence of viable B. anthracis spores.
Soil cores were collected from around two carcass sites with the central peg method. Both animals died during the 2000 outbreak in locations where several carcasses had been found in close proximity. Carcass site 3 was situated in the middle of a small, flat meadow. Only a few bones remained on the site, but the dead vegetation and body fluid-darkened soil that had been under the carcass were still visible. Only one soil sample on the periphery of the site was positive for anthrax spores, with an estimated concentration of 40 spores/g. Site 10 was located in an old bison wallow. Most of the bones remained in the wallow, but the skull and right rear leg bones had been dragged from the site in a northwest direction by a large scavenger. No soil samples were collected from around the skull or leg bones. Bacillus anthracis spores were recovered from seven (35.0%) of the soil samples collected at the site (Fig. 1).
Surface soil samples were collected along transects at a number of locations. Two transects were set up on one side of the meadow in which carcass site 3 was located. The transects ran in parallel across well-trampled and grazed ground. No B. anthracis spores were recovered along the meadow transects. A second pair of transects were set up across a winter road creek crossing close to where several bison had died during the 2000 outbreak. One transect was set up along the center of the road as it descended to the creek. The second transect ran perpendicular to the road near the bottom of the incline. Three (25.0%) of the samples collected along the winter road transects were positive for anthrax spores (Fig. 2). Two transects were also set up along well-used bison trails leading to a river crossing near where approximately 20 bison died during each outbreak. The transects were set up along the center of the trails and terminated at the muddy shore. No B. anthracis spores were recovered from the river-crossing transects.
Surface soil samples were collected around carcasses 44 and 49 with the grid method. Both animals died during the 2001 outbreak within 5 weeks of sampling. Both carcasses were lying on level open prairie. The grass around each carcass had been trampled to a distance of approximately 2 m from the body. Carcass 44 had been opened for a full necropsy 34 days prior to sampling. At the time of soil collection, scavenging was well advanced. Conversely, carcass 49 had not been disarticulated, and damage from scavengers was minimal, although small openings were observed at the rump, neck, and belly and the eye on the exposed side had been removed. Thirty-five (44.3%) and 25 (26.3%) of the samples collected at carcass 44 (Fig. 3A) and carcass 49 (Fig. 3B) were positive for B. anthracis, respectively. The distribution of spore concentrations at both sites failed a normality test, and in a Wilcoxon rank sum test, there was no significant difference between the distribution of anthrax spores found at each site. There was also no significant difference in the frequency of positive samples obtained from the two sites according to a Yates corrected chi-squared test.
DISCUSSION
The anthrax spore concentration of each positive sample was calculated from the number of B. anthracis-like colonies formed on PLET medium multiplied by the fraction of isolates screened in confirmatory assays that proved to be B. anthracis. The theoretical sensitivity limit of the isolation protocol based on sample dilution was 10 spores/g, but this assumes that the flotation procedure was able to isolate every spore in a given sample and that all anthrax spores germinated equally on PLET medium. Both assumptions have been shown to be false (3). Therefore, the anthrax spore concentrations presented herein can only be considered rough estimates of the true concentrations.
It is unlikely that anthrax spores are distributed uniformly throughout the soil at a given location. Only a portion of the sample collected was processed for culture on PLET medium. If viable anthrax spores were heterogeneously distributed in the sample, they may have been missed or diluted through sample mixing to a concentration below the sensitivity limit of the isolation protocol. This was probably the case where core samples were collected. The cores were placed in tubes that were slighter wider than the core. By the time the samples were processed, the cores had collapsed, mixing the top and bottom strata. Thus, none of the negative samples can be conclusively declared to be free of B. anthracis spores. However, the results can still be used to delineate the general distribution of anthrax spore contamination around the sampled sites.
Of the sample selection methods employed, the grid method provided the most complete coverage and best illustrated spore distribution at a site. Coverage with the central peg method tended to be good near the peg but became patchy with increasing distance from the center. The transect method provided the poorest coverage and was insufficient to provide context to the positive samples obtained.
The highest concentrations of anthrax spores at carcasses 44 and 49 were found directly adjacent to the carcass and invariably corresponded to locations where the soil had been saturated with body fluids escaping the carcass through either the natural orifices or holes torn by scavengers (Fig. 3). The majority of positive samples were found within 2 m of both old and fresh carcasses in the area of the trample zone mentioned previously and probably originated from scavengers' churning and spreading the B. anthracis-infected soil created by the escaping body fluids as they fed. Although water runoff may have moved anthrax spores downhill from a carcass site to the west of the winter road transects (Fig. 2), dispersion of spores beyond 2 m from a carcass at other sites was probably due to scavengers. The positive samples on the southwest side of carcass 49 are in direct line with the closest vegetative cover and delineate the probable track taken by scavengers if disturbed from the carcass (Fig. 3B). Similarly, the positive samples in the northwest quadrant of carcass site 10 may reflect the deposition of spores by the large scavenger that dragged the head and leg away (Fig. 1). It is interesting that the personnel who necropsied carcass 44 arrived and departed from the east, which may explain the extended dissemination of spores in this direction (Fig. 3A).
In contrast to the necropsy, during environmental sample collection care was taken to minimize the movement of personnel over a site. Personnel avoided stepping in any puddles, mud, viscera, or fecal matter and collected samples as they moved towards the center of the site. While redistribution of anthrax spores by environmental sampling personnel remains a possibility, it does not appear to have confounded the spore dissemination patterns obtained. There was also some concern that the reuse of scoops and the core sampler during collection could result in cross-contamination. However, the order of samples collected at each site was recorded, and there was no correlation between the order of collection and anthrax spore dispersion patterns determined in this analysis.
Based on this study, scavengers are very effective at releasing and spreading contaminated fluids and viscera around the immediate area of the carcass. Spore dissemination via adherence to fur and feathers or by dragging of carcass parts by larger scavengers may contribute to the spread of spores beyond the carcass, but this route is probably minimal compared to dissemination via intestinal carriage and fecal deposition (1, 4, 5). Water runoff from a carcass site may carry anthrax spores away, but the extent of movement remains unknown and would be dependent on the topography around each site. It appears that the death sites of animals infected with anthrax remain the areas of highest anthrax spore contamination in northern Canada and are the most likely sites for future infection and initiation of subsequent outbreaks.
Although the levels of disarticulation, evisceration, and scavenging at carcasses 44 and 49 were very different, there was no significant difference in the resultant level of anthrax spore contamination at these sites. It is apparent that even light scavenging of carcasses is adequate to release sufficient concentrations of B. anthracis vegetative cells from the body to microenvironments conducive to the formation of spores, resulting in substantial contamination of the immediate environment. Anyone approaching a suspected anthrax-infected carcass should consider the area immediately around the carcass substantially contaminated regardless of the condition of the carcass and should take appropriate safety precautions.
Acknowledgments
We thank U.S. Army Medical Research Institute of Infectious Diseases field technicians Wes Carter, Elizabeth Brock, and Andy Worden for their logistic support and the U.S. Army Medical Research Institute of Infectious Diseases for providing helicopter and base camp support. We acknowledge Warden Paul Sargent as well as John Nishi of the Northwest Territories Department of Resources, Wildlife and Economic Development for logistic support. We thank Lori McLaws, Laurel Negrych, Glen Fisher, and Nicole Stady of Defence R&D Canada—Suffield for technical assistance. We also thank Pam Coker for allowing us to collect samples at carcass sites from the 2000 outbreak that she had marked and sampled the previous year.
The PCR assays used in this study were developed under DND contract W7702-9-R767 by Infectio Diagnostic Inc. (Québec) and the Infectious Diseases Research Center (Université Laval, Québec, Canada).
REFERENCES
- 1.Choquette, L. P. E. 1970. Anthrax, p. 256-266. In J. W. Davis, L. H. Karstad, and D. O. Trainer (ed.), Infectious diseases of wild mammals. Iowa State University Press, Ames, Iowa.
- 2.Dragon, D. C., and B. T. Elkin. 2001. An overview of early anthrax outbreaks in northern Canada: field reports of the Health of Animals Branch, Agriculture Canada, 1962-1971. Arctic 54:32-40. [Google Scholar]
- 3.Dragon, D. C., and R. P. Rennie. 2001. Evaluation of spore extraction and purification methods for selective recovery of viable Bacillus anthracis spores. Lett. Appl. Microbiol. 33:100-105. [DOI] [PubMed] [Google Scholar]
- 4.Dragon, D. C., R. P. Rennie, and B. T. Elkin. 2001. Detection of anthrax spores in endemic regions of northern Canada. J. Appl. Microbiol. 91:435-441. [DOI] [PubMed] [Google Scholar]
- 5.Gates, C. C., B. T. Elkin, and D. C. Dragon. 1995. Investigation, control and epizootiology of anthrax in a geographically isolated, free-roaming bison population in northern Canada. Can. J. Vet. Res. 59:256-264. [PMC free article] [PubMed] [Google Scholar]
- 6.Knisely, R.F. 1966. Selective medium for Bacillus anthracis. J. Bacteriol. 92:784-786. [DOI] [PMC free article] [PubMed] [Google Scholar]