Abstract
Diffuse midline glioma (DMG), H3 K27-altered, is a newly defined “pediatric-type,” diffuse, high-grade glioma under current WHO classifications (updated in 2021). An essential diagnostic criteria of DMG is its occurrence in the midline structures; most intracranial DMG occurs in the brainstem or thalamus but can also occur in other midline structures. We experienced 2 adult cases of intracranial DMGs in areas other than the brainstem and thalamus that were initially difficult to diagnose. Case 1 was a 49-year-old man with extensive T2 high-signal lesions in the bilateral frontal lobes and corpus callosum on brain MRI. A Gd-based contrast medium partially enhanced the lesion and showed marked diffusion restriction, mimicking malignant lymphoma. Case 2 was a 24-year-old man who presented with paroxysmal olfactory abnormalities. The tumor extended mainly to the right temporal lobe, the right basal forebrain, and the bilateral hypothalamus, showing a T2/FLAIR mismatch sign suggestive of IDH-mutant astrocytoma without 1p/19q co-deletion. After a biopsy, both cases were properly diagnosed as DMG, H3 K27-altered (K27M-mutant). Diagnosing adult cases involving atypical midline structures is sometimes challenging before surgery; we discuss this phenomenon with both case details and a literature review.
Keywords: Corpus callosum, Adult diffuse midline glioma, H3 K27-altered, H3 K27M, Hypothalamus
Introduction
Diffuse midline glioma (DMG), H3 K27-altered is a newly defined, infiltrative brain tumor under current WHO classification guidelines for central nervous system (CNS) tumors (updated in 2021) [1], also referred to as “DMG, H3 K27M-mutant” in a previous edition [2]. Although DMG is categorized as a “pediatric-type,” diffuse, high-grade glioma, it also occurs in adults, especially young adults [3].
An essential diagnostic criterion for DMG is its occurrence in the midline structures. Most intracranial DMG occurs in the brainstem or thalamus but can also occur in other midline structures such as the cerebellum, pineal region, or sellar region [[4], [5], [6], [7], [8], [9]–10]. Therefore, diagnosing cases in relatively rare age groups (ie, adults) with nontypical midline sites is sometimes challenging.
This report describes the diagnosis, treatment, and outcomes of 2 difficult-to-differentiate adult cases of intracranial DMG that occurred in areas other than the brainstem and thalamus.
Case presentation
Case 1
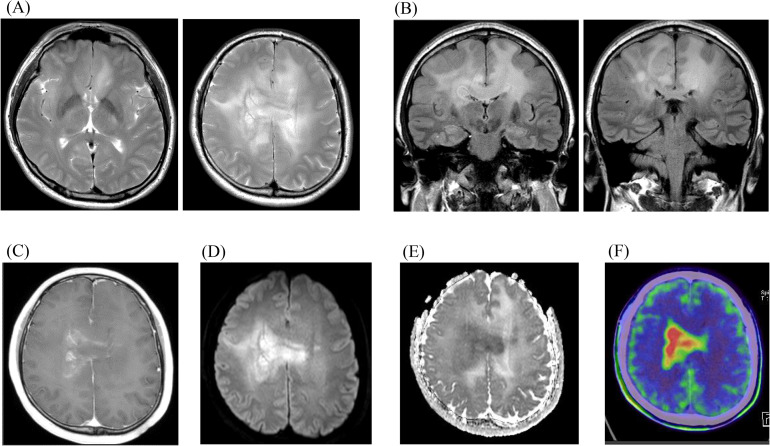
A 49-year-old man presented with headache and left hemiparesis; a brain MRI revealed extensive T2 high-signal lesions in the bilateral frontal lobes and corpus callosum (CC) (Fig. 1). There were no lesions in the thalamus or brainstem. The lesion had an indistinct boundary and showed a partially patchy contrast effect with a slight increase in relative cerebral blood volume (rCBV). Methionine (MET) uptake was markedly elevated on positron-emission tomography (PET), consistent with the enhanced area, while the CC showed increased signal intensity on diffusion-weighted imaging (DWI) with restricted diffusion. From these findings, preoperative imaging led to suspicion of a primary central nervous system lymphoma (PCNSL) that primarily affected or secondarily extended across the CC and a rigid endoscope biopsy was performed based on contrast and hyper-accumulated methionine.
Fig. 1.
Imaging findings of Case 1. Preoperative images are shown. The tumor, demonstrating bilateral widespread high signal intensity area on MRI T2WI (A) and FLAIR coronal sections (B), with heterogeneous and pale contrast enhancement on contrast enhanced T1WI (C). The tumor, partially showing a very restricted diffusion, especially in the corpus callosum on DWI (D) and ADC-map (E). A hyperaccumulation of methionine is seen on MET PET (F). Abbreviations: ADC, apparent diffusion coefficient; DWI, diffusion weighted image; FLAIR, fluid-attenuated inversion recovery; MET PET, methionine positron-emission tomography; MRI, magnetic resonance imaging; WI, weighted image.
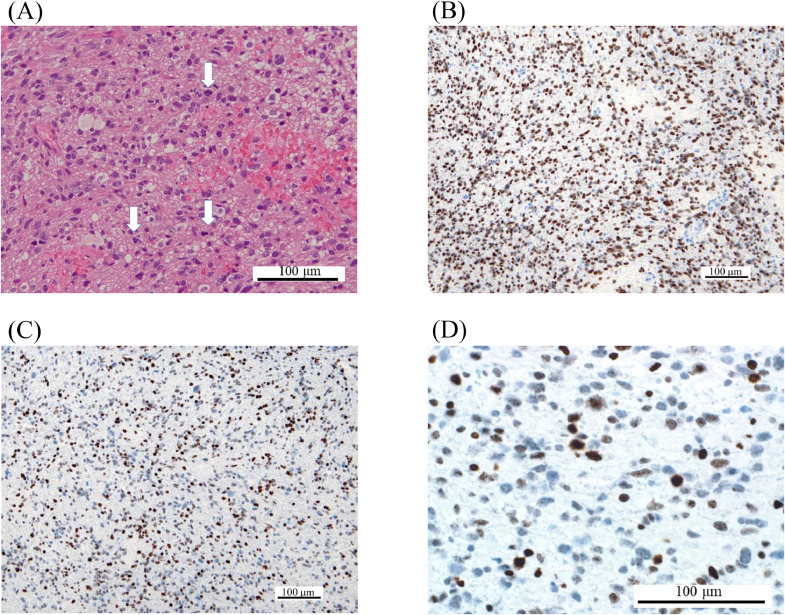
Histologically, glioma cells with prominent nuclear atypia were confirmed, with no evidence of necrosis or microvascular proliferation. This finding was equivalent to anaplastic astrocytoma, grade III in a previous classification (Fig. 2). Molecular information was as follows: mIDH1(R132H) (-), p53 (<5%), H3 K27M (+), H3 K27me3 (1/2 loss), ATRX (partial loss), and Ki-67 index (10%) by immunohistochemistry; MGMT hypermethylation (-) by quantitative PCR; and IDH1 (wildtype), IDH2 (wildtype), H3F3A K27 (K27M-mutant), H3F3A G34 (wildtype), HIST1H3 K27 (wildtype), and TERT promoter hotspot mutation (-) by sequence. Hence, the integrated diagnosis was diffuse midline glioma, H3 K27-altered, CNS WHO grade 4. The tumor was too large to remove and radiotherapy (60 Gy/30 fractions) with temozolomide (TMZ) and bevacizumab was offered. Refusing further treatment, the patient was transferred to another hospital for palliative care, where he gradually lost consciousness and died with an overall survival (OS) of 9 months.
Fig. 2.
Pathological findings of Case 1. H&E staining reveals glioma cells with prominent nuclear atypia (A). Although there is no evidence of necrosis or microvascular proliferation, multiple mitoses are confirmed (blank arrows). The tumor is positive for H3 K27M (B) and half negative for H3 K27Mme3 (C and D). Abbreviations: H&E, hematoxylin and eosin.
Case 2
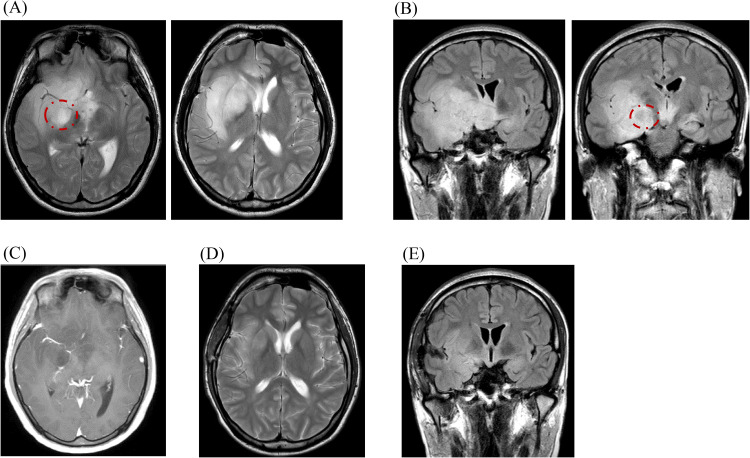
A 24-year-old man presented with paroxysmal olfactory abnormalities and was found with a brain tumor, mainly in the right temporal lobe but also extended to the right basal forebrain and bilateral hypothalamus (Fig. 3). The lesion did not extend to the thalamus or brainstem. An MRI revealed low signal intensity on T1WI and high on T2WI without diffusion restriction. The tumor's intensity was partially suppressed on fluid-attenuated inversion recovery (FLAIR), indicating a T2/FLAIR mismatch. There was neither contrast enhancement nor MET hyperaccumulation. We suspected astrocytoma (IDH-mutant and 1p/19q co-deleted) and performed a biopsy because radical resection was unavailable. We resected the anterior part of the superior temporal gyrus (Fig. 4).
Fig. 3.
Imaging findings of Case 2. Pre- and 6-month-postoperative images are shown. The tumor, demonstrating hyperintensity on MRI T2WI (A) and FLAIR coronal sections (B) without contrast enhancement on T1WI (C), spreading from the right temporal lobe to the right frontal lobe and bilateral hypothalamic areas. Areas enclosed by dotted circles suggesting of T2/FLAIR mismatch. Six months postoperative T2WI (D) and FLAIR coronal sections (E), revealing that the lesion has responded well to treatment and has shrunk markedly. Abbreviations: FLAIR, fluid-attenuated inversion recovery; MRI, magnetic resonance imaging; WI, weighted image.
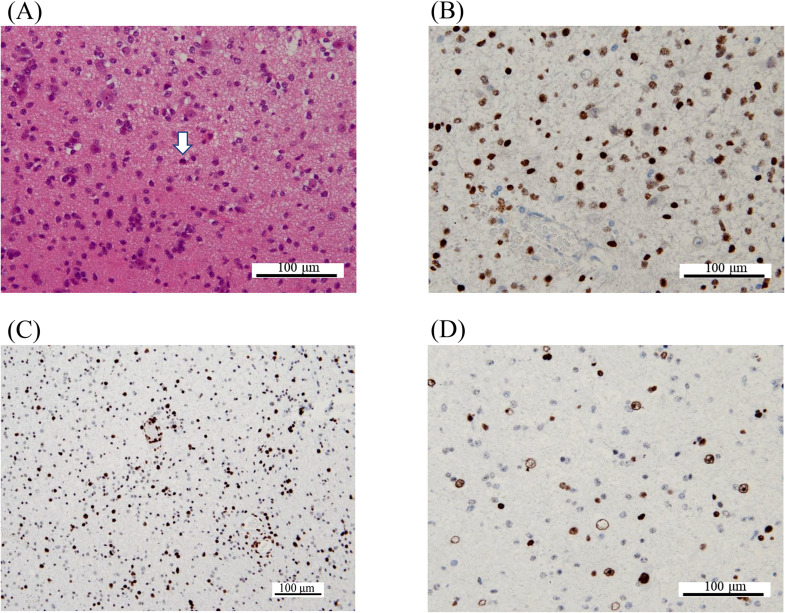
Fig. 4.
Pathological findings of Case 2. H&E staining reveals glioma cells with prominent nuclear atypia invading existing brain structures (A). Although there is no evidence of necrosis or microvascular proliferation, areas of nuclear atypia and high cell density are conspicuous, and little mitosis is seen (blank arrow). The tumor cells are positive for H3 K27M (B). Tumor cells showing nuclear atypia are negative for H3 K27me3 (C and D), whereas cells in existing brain tissue are positive. Abbreviations: H&E, hematoxylin and eosin.
Histological findings suggested lower-grade, adult-type, diffuse glioma, but the H3 K27M mutation was confirmed by sequencing. Other molecular information was as follows: mIDH1(R132H) (-), p53 (-), H3 K27M (+), H3 K27me3 (loss), ATRX (loss), and Ki-67 index (10%) by immunohistochemistry; MGMT hypermethylation (-) by quantitative PCR; and IDH1 (wildtype), IDH2 (wildtype), H3F3A G34 (wildtype), HIST1H3 K27 (wildtype), and TERT promoter hotspot mutation (-) by sequence. From these results, we diagnosed it as diffuse midline glioma, H3 K27-altered, CNS WHO grade 4. The patient underwent radiation therapy (60 Gy/30 fractions) with concomitant TMZ and is on maintenance TMZ chemotherapy as an outpatient (6 months at this time).
Discussion
We experienced 2 rare cases of DMG arising from the hypothalamus or CC. There were no apparent lesions in the distinctive areas of the DMG (ie, brainstem or thalamus), which made the preoperative diagnosis difficult; we initially suspected other tumor types before surgery but reached an accurate pathological diagnosis of DMG from molecular information. DMG, H3 K27-altered, is a new disease concept changed in 2021 that includes epidermal growth factor receptor (EGFR) mutation/amplification, enhancer of Zeste homologs inhibitory protein (EZHIP) overexpression, and H3 K27M mutation [1,11]. The exact incidence rate of H3 K27-altered DMG is unclear since there are still few existing reports, but the H3 K27M mutation rate among adult infiltrating glioma is reported to be 2.3%-3% [7,11,12]. About 80%-85% of DMGs occur within any of the 3 most frequent locations (ie, brainstem, thalamus, and spinal cord) and the brainstem is most common in children while thalamus localization is dominant in adults [4–10]. Although reports focusing on DMG arising outside these distinctive areas are scarce, reported frequencies of DMGs from the hypothalamus and CC are 1.2%-3.3% and 4.5%-4.9%, respectively [5,7–9]. The mean age at diagnosis in adults is older among DMG with nondistinctive areas vs distinctive areas [9]; however, the mechanisms and predictors of DMG development in unusual sites are not yet known. In any case, we must consider DMG as a differential diagnosis in cases of diffuse glioma with nontypical midline structure involvement, even in adults.
In addition to the scarcity of the site of occurrence, preoperative imaging findings were also confusing in the presented cases. In Case 1, the tumor spread bilaterally with heterogeneous contrast enhancement and showed CC involvement with evidently restricted diffusion. In adult cases, such findings usually infer PCNSL because it extends along white matter fibers, including the CC, and exhibits lower ADC values than gliomas [13,14]. As for Case 2, IDH-mutant astrocytoma was initially suspected due to the presence of a T2/FLAIR mismatch sign, a particular MRI feature strongly indicating IDH-mutant astrocytoma without 1p/19q codeletion [15]. In recent years, some reported H3 K27-altered DMG cases with T2/FLAIR mismatch, suggesting a T2/FLAIR mismatch sign is not necessarily unique for 1p/19q noncodel astrocytoma [[16], [17]–18].
Generally, DMG shows hypointensity on T1WI and hyperintensity on T2WI, with varied contrast effects by Gd-based medium ranging from no enhancements to partial/heterogeneous, diffuse, nodular, or rim enhancements [6,7,11,19]. Leptomeningeal involvement or subependymal dissemination at presentation are reported at 4.8%-4.9% [4,6] and become more prevalent later in the disease course [20]. Bilateral or whole brain extension can occur in DMG patients [7,8,21] and rCBV values are variable but generally increased in the enhanced area [19,22]. DMG generally shows restricted diffusion, but the apparent diffusion coefficient (ADC) value alone is not decisive in delineating DMG from others [19,20]. MET hyperaccumulation was seen in 81.8%-85.7% of DMG and most recurrence occurs within the initial MET accumulated areas; however, MET-PET imaging features are reported unhelpful in differentiating H3 K27M-mutations [20,23,24]. In summary, if multidisciplinary imaging studies are combined, no definitive findings would differentiate DMG.
There is no established treatment method for DMG and the reality is that treatment is considered on a case-by-case basis. Safe maximal resection should be the first consideration and up to 35% of DMG patients undergo surgical resection (from gross total resection to partial removal) [3,11]. However, the impact of tumor resection on survival is still controversial in H3 K27M-mutant tumors [10,25,26]. In the presented cases, safe maximal resection was difficult due to the vast tumor extension, and we chose to perform a biopsy followed by radiation plus chemotherapy with TMZ. Radiation therapy for DMG is indispensable and a conventional high dose of 54-60 Gy fractionated external beam radiotherapy is commonly used [10,11,26]. As for chemotherapy, no treatment drugs or regimens have been proven to be effective for DMG [10,11]. Nevertheless, TMZ is considered a first-line therapy in adult DMG patients in accordance with other high-grade gliomas because there are no other treatment options [3,4,11,26,27]. In recent years, a small number of cases have been reported suggesting the effectiveness of convection-enhanced delivery (CED) for DMG and further development is expected [28].
The median OS period of DMG is reported to be 10.5-19.6 months, considered as poor as glioblastoma [4,6,9], and, generally, adult DMG patients have a better prognosis than children [6]. This age-related difference may be partly caused by the anatomical tendencies of involved midline structures (eg, brainstem vs others). Some reports suggest that anatomical location may impact the prognosis of DMG since brainstem DMGs have a poorer prognosis than those of the thalamus or spinal cord [9,10,29] and DMGs with non-distinctive midline structure (ie, CC or cerebellum) involvement seem to confer an extended survival period versus typical locations [9]. On the contrary, as some reports indicate that anatomical location does not affect prognosis [8,25], a consensus has yet to be reached. Additionally, the H3 K27M mutation may not necessarily indicate a poor prognosis, especially in adults. In 3 reports on thalamic DMGs in adults, tumors with H3 K27M mutations showed similar [3,29] or even better [27] prognoses than those without mutations. Taken together, adult DMG with K27-alterations arising outside the brainstem may expect a relatively long OS prognosis. Further accumulation of cases is awaited.
Here, we report 2 adult cases of intracranial DMG with H3 K27-alterations that occurred in nondistinctive areas of the hypothalamus and CC that were challenging to differentiate from other tumors before surgery. The imaging findings of DMG are varied; we must consider DMG as a differential diagnosis in case of diffuse glioma with midline structure involvement, even in adults.
Patient consent
We obtained informed consent concerning publication and handled clinical information anonymously in accordance with the principles of the Declaration of Helsinki and the Act on the Protection of Personal Information in Japan.
Footnotes
Acknowledgments: We thank Dr Bryan J. Mathis (International Medical Center, University of Tsukuba Hospital) for language revision. The corresponding author (N.S.) also would like to extend his indebtedness to Yuka Sugii for her endless love, understanding, support, encouragement, and sacrifice throughout his work.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 3.Aihara K, Mukasa A, Gotoh K, Saito K, Nagae G, Tsuji S, et al. H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro Oncol. 2014;16:140–146. doi: 10.1093/neuonc/not144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyronet D, Esteban-Mader M, Bonnet C, MO Joly, Uro-Coste E, Amiel-Benouaich A, et al. Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol. 2017;19:1127–1134. doi: 10.1093/neuonc/now274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon DA, Wood MD, Tihan T, Bollen AW, Gupta N, Phillips JJ, et al. Diffuse midline gliomas with histone H3-K27M Mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 2016;26:569–580. doi: 10.1111/bpa.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng L, Gong J, Yu T, Zou Y, Zhang M, Nie L, et al. Diffuse midline gliomas with histone H3 K27M mutation in adults and children: a retrospective series of 164 cases. Am J Surg Pathol. 2022;46:863–871. doi: 10.1097/pas.0000000000001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu T, Chanchotisatien A, Qin Z, Wu J, Du Z, Zhang X, et al. Imaging characteristics of adult H3 K27M-mutant gliomas. J Neurosurg. 2019;133:1–9. doi: 10.3171/2019.9.Jns191920. [DOI] [PubMed] [Google Scholar]
- 8.Li HN, Shan CG, Fan CZ, Cheng LN, Wu SG, Liu MT, et al. Clinicopathological characteristics and prognosis of diffuse midline gliomas with histone H3K27M mutation: an analysis of 30 cases. Zhonghua Bing Li Xue Za Zhi. 2019;48:192–198. doi: 10.3760/cma.j.issn.0529-5807.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Li Z, Zhang M, Piao Y, Chen L, Liang H, et al. H3 K27M-mutant diffuse midline gliomas in different anatomical locations. Hum Pathol. 2018;78:89–96. doi: 10.1016/j.humpath.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Vuong HG, Le HT, Jea A, McNall-Knapp R, Dunn IF. Risk stratification of H3 K27M-mutant diffuse midline gliomas based on anatomical locations: an integrated systematic review of individual participant data. J Neurosurg Pediatr. 2022:1–8. doi: 10.3171/2022.3.Peds2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Pérez CA, Franco-Mojica X, Villanueva-Gaona R, Díaz-Alba A, Rodríguez-Florido MA, Navarro VG. Adult diffuse midline gliomas H3 K27-altered: review of a redefined entity. J Neurooncol. 2022;158:369–378. doi: 10.1007/s11060-022-04024-5. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimoto K, Hatae R, Sangatsuda Y, Suzuki SO, Hata N, Akagi Y, et al. Prevalence and clinicopathological features of H3.3 G34-mutant high-grade gliomas: a retrospective study of 411 consecutive glioma cases in a single institution. Brain Tumor Pathol. 2017;34:103–112. doi: 10.1007/s10014-017-0287-7. [DOI] [PubMed] [Google Scholar]
- 13.Nilles C, Delgadillo D, Sarazin M, Nichelli L, Mokhtari K, Mathon B, et al. Primary CNS lymphoma of the corpus callosum: presentation and neurocognitive outcomes. J Neurooncol. 2022;158:99–109. doi: 10.1007/s11060-022-04014-7. [DOI] [PubMed] [Google Scholar]
- 14.Horger M, Fenchel M, Nägele T, Moehle R, Claussen CD, Beschorner R, et al. Water diffusivity: comparison of primary CNS lymphoma and astrocytic tumor infiltrating the corpus callosum. AJR Am J Roentgenol. 2009;193:1384–1387. doi: 10.2214/ajr.09.2486. [DOI] [PubMed] [Google Scholar]
- 15.Patel SH, Poisson LM, Brat DJ, Zhou Y, Cooper L, Snuderl M, et al. T2-FLAIR mismatch, an imaging biomarker for IDH and 1p/19q status in lower-grade gliomas: a TCGA/TCIA Project. Clin Cancer Res. 2017;23:6078–6085. doi: 10.1158/1078-0432.Ccr-17-0560. [DOI] [PubMed] [Google Scholar]
- 16.Johnson DR, Kaufmann TJ, Patel SH, Chi AS, Snuderl M, Jain R. There is an exception to every rule-T2-FLAIR mismatch sign in gliomas. Neuroradiology. 2019;61:225–227. doi: 10.1007/s00234-018-2148-4. [DOI] [PubMed] [Google Scholar]
- 17.Valentino WL, Okada D, Bhanu S. A curious case of T2-FLAIR mismatch in H3K27M mutant glioma. Radiol Case Rep. 2022;17:2930–2935. doi: 10.1016/j.radcr.2022.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurokawa R, Kurokawa M, Baba A, Ota Y, Kim J, Capizzano A, et al. Dynamic susceptibility contrast-MRI parameters, ADC values, and the T2-FLAIR mismatch sign are useful to differentiate between H3-mutant and H3-wild-type high-grade midline glioma. Eur Radiol. 2022;32:3672–3682. doi: 10.1007/s00330-021-08476-7. [DOI] [PubMed] [Google Scholar]
- 19.Lasocki A, Abdalla G, Chow G, Thust SC. Imaging features associated with H3 K27-altered and H3 G34-mutant gliomas: a narrative systematic review. Cancer Imaging. 2022;22:63. doi: 10.1186/s40644-022-00500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovibond S, Gewirtz AN, Pasquini L, Krebs S, Graham MS. The promise of metabolic imaging in diffuse midline glioma. Neoplasia. 2023;39 doi: 10.1016/j.neo.2023.100896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.La Rocca G, Sabatino G, Altieri R, Signorelli F, Ricciardi L, Gessi M, et al. Significance of H3K27M mutation in “Nonmidline” high-grade gliomas of cerebral hemispheres. World Neurosurg. 2019;131:174–176. doi: 10.1016/j.wneu.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Kathrani N, Chauhan RS, Kotwal A, Kulanthaivelu K, Bhat MD, Saini J, et al. Diffusion and perfusion imaging biomarkers of H3 K27M mutation status in diffuse midline gliomas. Neuroradiology. 2022;64:1519–1528. doi: 10.1007/s00234-021-02857-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X, Li D, Qiao Z, Wang K, Chen Q, Pan C, et al. (11)C-methionine PET imaging characteristics in children with diffuse intrinsic pontine gliomas and relationship to survival and H3 K27M mutation status. Eur J Nucl Med Mol Imaging. 2023;50:1709–1719. doi: 10.1007/s00259-022-06105-z. [DOI] [PubMed] [Google Scholar]
- 24.Tinkle CL, Duncan EC, Doubrovin M, Han Y, Li Y, Kim H, et al. Evaluation of (11)C-Methionine PET and anatomic MRI associations in diffuse intrinsic pontine glioma. J Nucl Med. 2019;60:312–319. doi: 10.2967/jnumed.118.212514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karremann M, Gielen GH, Hoffmann M, Wiese M, Colditz N, Warmuth-Metz M, et al. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol. 2018;20:123–131. doi: 10.1093/neuonc/nox149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon HI, Wee CW, Kim YZ, Seo Y, Im JH, Dho YS, et al. The Korean Society for Neuro-Oncology (KSNO) guideline for adult diffuse midline glioma: version 2021.1. Brain Tumor Res Treat. 2021;9:1–8. doi: 10.14791/btrt.2021.9.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimaldi S, Harlay V, Appay R, Bequet C, Petrirena G, Campello C, et al. Adult H3K27M mutated thalamic glioma patients display a better prognosis than unmutated patients. J Neurooncol. 2022;156:615–623. doi: 10.1007/s11060-022-03943-7. [DOI] [PubMed] [Google Scholar]
- 28.Spinazzi EF, Argenziano MG, Upadhyayula PS, Banu MA, Neira JA, Higgins DMO, et al. Chronic convection-enhanced delivery of topotecan for patients with recurrent glioblastoma: a first-in-patient, single-centre, single-arm, phase 1b trial. Lancet Oncol. 2022;23:1409–1418. doi: 10.1016/s1470-2045(22)00599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng J, Hao S, Pan C, Wang Y, Wu Z, Zhang J, et al. The H3.3 K27M mutation results in a poorer prognosis in brainstem gliomas than thalamic gliomas in adults. Hum Pathol. 2015;46:1626–1632. doi: 10.1016/j.humpath.2015.07.002. [DOI] [PubMed] [Google Scholar]