Abstract
Ovarian lesions represent a diagnostic challenge for the radiologist and should be approached according to the patient's age, menstrual cycle, and imaging characteristics. These lesions can be cystic, mixed, or solid-predominant structures. Generally, the occurrence of benign lesions surpasses that of malignant ones at a ratio of 3:1. However, within infantile and juvenile age groups, this becomes an infrequent occurrence, making up only about 5% of ovarian tumor cases. This case report sheds light on a unique scenario involving a pediatric patient who harbored 2 benign tumors simultaneously: a mature cystic teratoma and a serous cystadenoma.
Keywords: Teratoma, Dermoid cysts, Ovarian, Cystic, Serous, Tumor, Pediatric
Introduction
Ovarian tumors are not commonly found in infants, with a bimodal presentation between 2 and 3 years and another peak of presentation between 12 and 15 years. They are more prevalent within the 10 to 14-year age range, and older patients tend to have a greater risk of malignancy [1]. Clinical signs such as abdominal pain, vomiting, and abdominal distension are not specific. In the pediatric population, about 90% of ovarian tumors are derived from germ cells, with mature cystic teratoma (a dermoid tumor) being the most frequent subtype [2].
On the other hand, serous cystadenoma represents 25% of benign cystic neoplasms. These are usually unilateral and have the potential to transform into malignant subtypes in about 30% of cases [3]. Germ cell tumors constitute 47%-87.7% of tumors, of which 95% are benign mature teratomas [[2], [4]]. These tumors are typically unilateral and exhibit a fatty component and calcifications in 60% of instances [5]. Early diagnosis is crucial to establish appropriate treatment plans, rule out malignancies, and prevent complications associated with the tumor.
While diagnosis is sometimes incidental, ultrasound is the most commonly used imaging tool due to its high sensitivity, cost-effectiveness, and speed. It typically displays characteristic features in over half of the cases [[3], [6]]. This case report highlights an unusual and intriguing medical scenario where a mature cystic teratoma and a giant serous cystadenoma coexisted within a patient's ovary and this case underscores the importance of meticulous diagnostic evaluation and thorough surgical planning to address complex ovarian lesions comprehensively.
Case report
A 10-year-old female patient with two days of intermittent cramping pain in the hypogastrium without any related symptoms or previous medical issues. Initially, she visited an outpatient clinic where a transabdominal pelvic ultrasound was performed, and the results indicated a mixed mass of 13.6 × 13.8 × 12.7 cm with thick septa in its interior in the left adnexal region. Consequently, she was referred to our facility for urgent assessment under pediatric surgery to rule out the possibility of malignancy. To initiate the evaluation process, we carried out a contrast-enhanced abdominal and pelvic computed tomography (CT) scan on the patient (Fig. 1).
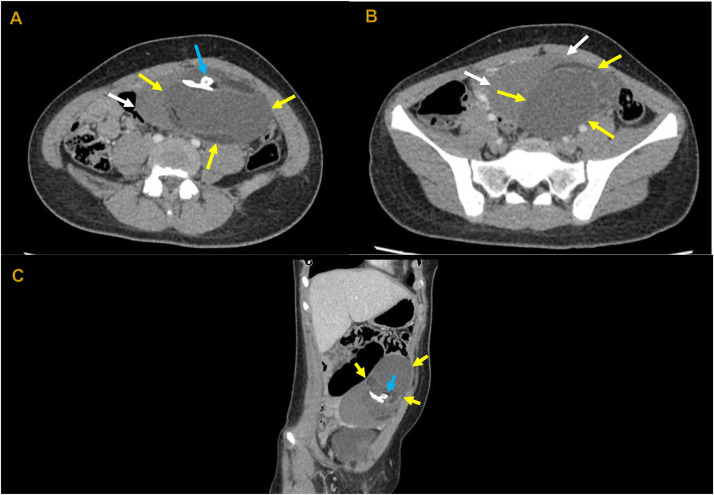
Fig. 1.
A, B (Axial view), and C (Oblique Sagittal Reformatted) reveal a complex adnexal mass on the left side (indicated by yellow arrows). This mass exhibits fatty content with a density of -10 Hounsfield Units (HU) and is characterized by multiple calcifications, including a tooth-shaped structure within it (highlighted by the blue arrow). Additionally, there is a region of higher attenuation adjacent to the inferior margin of the mass, raising suspicion of possible hemorrhagic conversion (as indicated by the white arrows).
The abdominopelvic CT scan revealed the existence of a significant mass on the left side adnexal, consistent with a mature teratoma undergoing hemorrhagic transformation (potentially due to a recent rupture). However, no blood in the abdominal cavity was observed. Based on these findings, the patient underwent laparoscopic surgery, during which it was discovered that the left ovary had been replaced by a sizable solid-cystic growth, making it difficult to distinguish between normal and abnormal tissue. No other abnormalities were identified in the surrounding area, leading to removing the left ovary (total left oophorectomy). The right ovary exhibited a typical appearance. The biopsy for a histopathological analysis confirmed the presence of a mature cystic teratoma and a serous cystadenoma, both of which showed no signs of malignancy (Fig. 2).
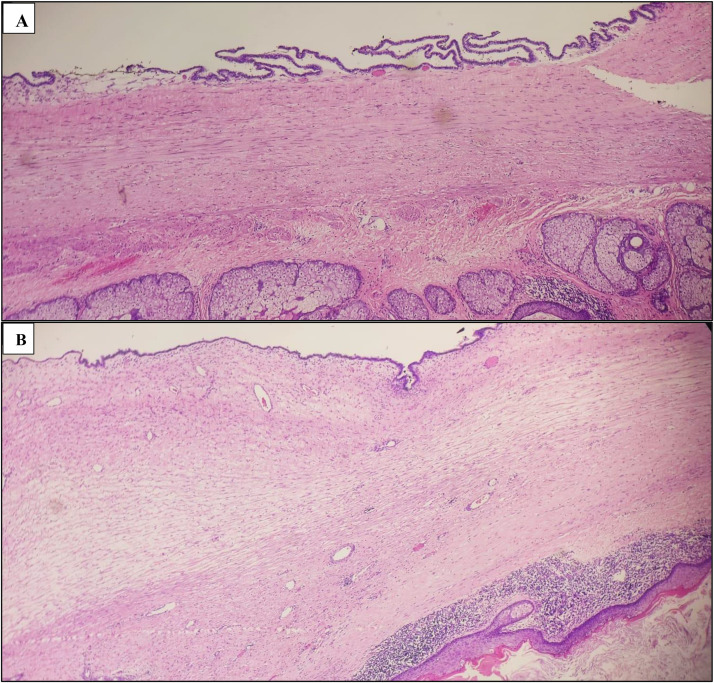
Fig. 2.
Microscopic histological description, A and B: The histological samples reveal a mixed tumor originating in germ cells, displaying numerous zones of diverse differentiation. This tumor exists alongside another benign tumor arising from the surface layer of epithelial cells. There is no indication of malignancy present.
The patient experienced a satisfactory postsurgery recovery, and subsequent tests for tumor markers such as beta subunit of chorionic gonadotropin, carcinoembryonic antigen, CA125 antigen, and alpha-fetoprotein all returned negative results. Consequently, the disease was considered benign, and an appointment with oncology was scheduled every 3 months with a follow-up transabdominal pelvic ultrasound.
Discussion
Ovarian tumors make up only 1% of all childhood tumors. Among these tumors, germ cell tumors account for 47%-87.7%, with 95% of them being benign mature cystic teratomas (MCT). Teratomas are the most common germ cell tumors in children and are categorized as mature, immature, or malignant [[2], [7]]. Among benign mature teratomas, MCT, often referred to as dermoid cysts, comprises 25% of all ovarian tumors [[2], [4], [8]]. Mature ovarian teratomas are more prevalent than immature ones, and they are typically composed of a cystic-solid structure with calcification present in 40% of cases [[4], [9]],. The incidence, histologic distribution, and clinical manifestations of ovarian masses in children differ from those in adults [9]. Bilateral cases account for 12% of cases [10], with unilateral cases being more frequent on the right side (72.2%) [11]. In this case report, the patient presented with a unilateral left ovarian mass and abdominal pain.
Additionally, these tumors may present with symptoms such as a palpable abdominal mass, pelvic pain, or signs of acute emergencies like torsion or rupture [[2], [12], [13]]. The incidence of malignant transformation of MCT is approximately 0.2%-2% [14]. Although malignant germ cell tumors are more common in children and adolescents compared to adults, MCT still predominates, constituting 61% of gonadal germ cell tumors (GCTs) in comparison to germ cell malignancies [15]. Furthermore, these tumors can be associated with various complications, including torsion (16% of ovarian teratomas), rupture (1%-4%), malignant transformation (1%-2%), infection (1%), and autoimmune hemolytic anemia (<1%) [16]. In some cases, patients may also develop N-methyl-D-aspartate (NMDA) encephalitis, a rare but severe neurological disorder [17]. Several studies have shown that young women or children with ovarian teratomas could develop encephalitis, leading to a wide range of neuropsychiatric symptoms such as psychosis, memory loss, and behavioral disorders, progressing to seizures, dyskinesias, and autonomic instability [15].
Ovarian cystadenomas are further classified based on cell type into serous cystadenomas, mucinous cystadenomas, and endometriomas [18]. Serous cystadenomas (SC) are benign, thin-walled cystic tumors containing fluid, or less commonly, blood or thin mucinous fluid. They are typically unilocular or paucilocular and may serve as precursors to serous borderline tumors, which can potentially progress to low-grade serous carcinomas [19]. These tumors are most commonly diagnosed during the fifth decade of life [20], and constitute 25% of benign cystic neoplasms. They are usually unilateral and have the potential to transform into malignant subtypes in about 30% of cases [3]. Generally, they contain clear serous fluid, or less commonly, hemorrhagic or thin mucinous fluid. Although the exact cause is not well understood, one proposed hypothesis suggests they may be embryological remnants of the urogenital apparatus with epithelial and mesothelial tissues. These cysts often reach considerable size before causing symptoms [21].
Treatment options for serous cystadenomas include unilateral salpingo-oophorectomy or ovarian cystectomy [22]. Recurrence risk can be increased by incomplete resection or the development of a new primary tumor [22]. Physical examination findings are similar to those of MCT with a soft, nontender, palpable mass, and serum levels of tumor markers such as carcinoembryonic antigen, CA19-9, CA-125, and CA15-3 typically fall within normal limits. Elevated CA-125 and CA19-9 levels have been rarely identified in clear cystic fluid [23]. Given the importance of tumor markers, they were requested for our patient, and the results were reported as within normal limits.
Both conditions (MCT and SC) can be readily identified through ultrasound imaging. In fact, ultrasound serves as the primary imaging modality in 95% of cases [[2], [12], [13]], as the typical sonographic features are rarely observed in malignancies. On occasion, a computed tomography scan (CT scan) or magnetic resonance imaging (MRI) can aid in diagnosis. Nevertheless, it is crucial to be vigilant for any signs of malignant changes, as their detection is paramount for both management and prognosis.
In terms of histological examination, MCTs exhibit distinctive pathological characteristics. They often display variability in size, typically averaging a diameter of 7 cm, and well-differentiated cells representing all germ layers [24]. These tumors commonly feature a squamous epithelium lining the inner wall, surrounded by ovarian stroma. In approximately 88% of cases, they are unilocular and may contain Rokitansky nodules with hair, teeth, and various tissues [25]. Sebaceous material, ectodermal, and mesodermal tissues are commonly found, while endodermal tissue is less frequent. Contents typically comprise sebum, keratin, hair, adipose tissue (67%-75%), and teeth (31%) [26]. In contrast, histological examination of SC typically reveals a lining of benign, tall, columnar epithelial cells with cilia and goblet cells. The stroma surrounding the cysts is typically smooth and unremarkable. These tumors are generally noninvasive and have a low potential for malignancy, classifying them as benign in nature [27].
The treatment approach for patients with both MCT and SC in the same ovary is multifaceted and depends on factors such as symptoms, imaging results, malignancy risk, and fertility preservation. Laparoscopy and laparotomy are considered in different cases, with the choice made after assessing the pros and cons [20]. Typically, laparoscopic surgery is regarded as the gold standard for managing benign ovarian masses in both children and adults. Laparoscopic cystectomy is a safe and effective option for preserving ovarian function (known as ovary-sparing surgery), offering benefits like reduced postoperative pain, less blood loss, and shorter hospital stays [[28], [29]]. In cases where there is a high risk of malignancy, laparotomy and oophorectomy should be considered [28], and postoperative follow-up is essential to monitor for recurrence, adhesions, and malignant transformation [30]. In this specific pediatric case, despite the initial inclination towards ovarian preservation, the decision was made to proceed with complete resection (left oophorectomy). This choice was influenced by the complexity of the mass, which had virtually replaced the entire ovary, consisting of a heterogeneous mass with both solid and liquid components. This presented a significant challenge in distinguishing normal from abnormal tissue.
Additionally, with the revised epidemiology, we are discussing two pathologies that are common in their respective groups, although they typically don't coexist within the same ovary but rather present individually or originate from different ovaries. When reviewing the scans, it's crucial to consider the density variations in imaging and differentiate between the compartments of the teratoma, which may have an increased wall thickness, skin teguments, and mural nodules, as opposed to the mucinous cystadenoma, which typically has a single compartment with lower density than the other mass and receives vascular input from the same group of ovarian vessels, indicating a common origin for both masses. Radiological findings are of critical importance due to the potential risk of peritoneal seeding resulting from the outflow of fluid from the mucinous cystadenoma during surgery. Peritoneal seeding can lead to the persistence of ascitic fluid over time, resulting in long-term complications. Radiologists play a pivotal role in providing essential information that guides clinical decisions and surgical planning, underscoring their significance in influencing patient management and prognosis [31].
Finally, it's essential to emphasize that the presence of both entities within the same ovary does not inherently indicate a higher overall risk of malignancy. Rather, it underscores the importance of conducting a comprehensive evaluation, ensuring an accurate diagnosis, and tailoring treatment strategies based on the unique characteristics of each tumor. Given the rarity of such a combination, individualized patient management is of utmost significance, often necessitating collaboration with oncologists or other specialists. This collaborative approach ensures that patients receive the most suitable and effective care, optimizing their chances of favorable outcomes.
Patient consent
We are pleased to inform you that we have obtained the written informed consent to publish our case report in Radiology Case Reports. This confirmation acknowledges that we had permission for the publication, adhering to the guidelines and principles of patient confidentiality and privacy.
Authors' contributions
JG and KS: Conceptualization, original draft, writing. DN: Project administration, review, writing & editing. JT: Conceptualization, review & editing.
Acknowledgments
Ethics approval and consent to participate
The reported case was reviewed and approved, and individual patient consent was obtained following institutional guidelines. Following our institutional policies, all protected health information was removed.
Availability of data and materials
Not applicable
Footnotes
Acknowledgments: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data sharing statement: The relevant anonymized patient-level data are available via request from the authors.
References
- 1.Hermans AJ, Kluivers KB, Janssen LM, Siebers AG, Wijnen MHWA, Bulten J, et al. Adnexal masses in children, adolescents and women of reproductive age in the Netherlands: A nationwide population-based cohort study. Gynecol Oncol. 2016;143(1):93–97. doi: 10.1016/j.ygyno.2016.07.096. [DOI] [PubMed] [Google Scholar]
- 2.Özcan R, Kuruoǧlu S, Dervişoǧlu S, Eliçevik M, Emir H, Büyükünal C, et al. Ovary-sparing surgery for teratomas in children. Pediatr Surg Int. 2013;29:233–237. doi: 10.1007/s00383-012-3228-x. [DOI] [PubMed] [Google Scholar]
- 3.Pereda J, Oliva C, Fernández C, Lobo M, Fernández V, Gonzalez J. Lesiones ováricas más frecuentes: Un reto por imagen. Seram. Recuperado a partir de <https://piper.espacio-seram.com/index.php/seram/article/view/1803> 2018 [accessed 30.06.23].
- 4.Terenziani M, D'Angelo P, Inserra A, Boldrini R, Bisogno G, Babbo GL, et al. Mature and immature teratoma: a report from the second Italian pediatric study. Pediatr Blood Cancer. 2015;62:1202–1208. doi: 10.1002/pbc.25423. [DOI] [PubMed] [Google Scholar]
- 5.Requena MJ, Gonzalez F, Guerrero R, García N, Beltrán M, Beltrán M. Mature cystic teratoma with strumal carcinoid tumor of the ovary. Report of a case. Rev Esp Patología. 2005;38:105–108. [Google Scholar]
- 6.Mlikoti A, McPhaul L, Hansen G, Sinow R. Significance of de solid component in predicting malignancy in ovarian cystic teratomas. J Ultrasound Med. 2001;20:859–866. doi: 10.7863/jum.2001.20.8.859. [DOI] [PubMed] [Google Scholar]
- 7.Raicevic M, Saxena AK. Review of laparoscopic management of mature cystic teratoma of ovaries in children. J Indian Assoc Pediatr Surg. 2019;24(2):92–96. doi: 10.4103/jiaps.JIAPS_246_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sait K, Simpson C. Ovarian teratoma diagnosis and management: Case presentations. J Obstet Gynaecol Can. 2004;26:137–142. doi: 10.1016/s1701-2163(16)30489-3. [DOI] [PubMed] [Google Scholar]
- 9.Heo SH, Kim JW, Shin SS, Jeong SI, Lim HS, Choi YD, et al. Review of ovarian tumors in children and adolescents: radiologic-pathologic correlation. Radiographics. 2014;34:2039–2055. doi: 10.1148/rg.347130144. [DOI] [PubMed] [Google Scholar]
- 10.Rouanet JP, Maubon A, Juhan V, Meny R, Salanon AP, Daclin PY. Imaging of benign ovarian tumors. J Radiol. 2000;81:1823–1830. [PubMed] [Google Scholar]
- 11.Ismail SR. An evaluation of the incidence of right-sided ovarian cystic teratoma visualized on sonograms. J Diagn Med Sonogr. 2005;21(4):336–342. [Google Scholar]
- 12.Péroux E, Franchi-Abella S, Sainte-Croix D, Canale S, Gauthier F, Martelli H, et al. Ovarian tumors in children and adolescents: a series of 41 cases. Diagn Interv Imaging. 2015;96:273–282. doi: 10.1016/j.diii.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Litz C, Danielson PD, Chandler NM. Single incision laparoscopic surgery for pediatric adnexal pathology. J Pediatr Surg. 2014;49:1156–1158. doi: 10.1016/j.jpedsurg.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Gadducci A, Guerrieri ME, Cosio S. Squamous cell carcinoma arising from mature cystic teratoma of the ovary: a challenging question for gynecologic oncologists. Crit Rev Oncol Hematol. 2019;133:92–98. doi: 10.1016/j.critrevonc.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Cong L, Wang S, Yeung SY, Lee JHS, Chung JPW, Chan DYL. Mature cystic teratoma: an integrated review. Int J Mol Sci. 2023;24(7):6141. doi: 10.3390/ijms24076141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SB, Kim JK, Kim KR, Cho KS. Imaging findings of complications and unusual manifestations of ovarian teratomas. Radiographics. 2008;28(4):969–983. doi: 10.1148/rg.284075069. [DOI] [PubMed] [Google Scholar]
- 17.Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feeley KM, Wells M. Precursor lesions of ovarian epithelial malignancy. Histopathology. 2001;38:87–95. doi: 10.1046/j.1365-2559.2001.01042.x. [DOI] [PubMed] [Google Scholar]
- 19.Cheng EJ, Kurman RJ, Wang M, Oldt R, Wang BG, Berman DM, et al. Molecular genetic analysis of ovarian serous cystadenomas. Lab Invest. 2004;84(6):778–784. doi: 10.1038/labinvest.3700103. [DOI] [PubMed] [Google Scholar]
- 20.Templeman CL, Fallat ME, Lam AM, Perlman SE, Hertweck SP, O'Connor DM. Managing mature cystic teratomas of the ovary. Obstet Gynecol Surv. 2000;55(12):738–745. doi: 10.1097/00006254-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Adams JT. In: Principles of surgery. 5th ed. Schwartz SI, Shires GT, Spencer FC, editors. McGraw Hill; New York, NY: 1989. Abdominal wall, omentum, mesentery and retroperitoneum; pp. 1491–1524. [Google Scholar]
- 22.Idris S, Daud S, Ahmad Sani N, Tee Mei Li S. A case of twisted ovarian cyst in a young patient and review of the literature. Am J Case Rep. 2021;22 doi: 10.12659/AJCR.933438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanefuji T. Retroperitoneal cystadenoma containing elevated concentrations of CA125 and CA19-9 in the cyst fluid: a case report. Hinyokika Kiyo. 2000;46:457–461. [PubMed] [Google Scholar]
- 24.Soslow RA, Tornos C. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2011. Diagnostic pathology of ovarian tumors. [Google Scholar]
- 25.Friedman AC, Pyatt RS, Hartman DS, Downey EF, Jr, Olson WB. CT of benign cystic teratomas. AJR Am J Roentgenol. 1982;138:659–665. doi: 10.2214/ajr.138.4.659. [DOI] [PubMed] [Google Scholar]
- 26.Caruso PA, Marsh MR, Minkowitz S, Karten G. An intense clinicopathologic study of 305 teratomas of the ovary. Cancer. 1971;27:343–348. doi: 10.1002/1097-0142(197102)27:2<343::aid-cncr2820270215>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.Prat J. Pathology of borderline and invasive cancers. Best Pract Res Clin Obstet Gynaecol. 2017;41:15–30. doi: 10.1016/j.bpobgyn.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Savasi I, Lacy JA, Gerstle JT, Stephens D, Kives S, Allen L. Management of ovarian dermoid cysts in the pediatric and adolescent population. J Pediatr Adolesc Gynecol. 2009;22(6):360–364. doi: 10.1016/j.jpag.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Spinelli C, Strambi S, Masoni B, Ghionzoli M, Bertocchini A, Sanna B, et al. Surgical management of ovarian teratomas in childhood: a multicentric study on 110 cases and a literature review. Gynecol Endocrinol. 2021;37(10):950–954. doi: 10.1080/09513590.2021.1948527. [DOI] [PubMed] [Google Scholar]
- 30.Coleman R, Westin SN, Ramirez PT, Salvo G, Gershenson DM. Malignant diseases of the ovary, fallopian tube, and peritoneum. Comprehensive gynecology. Elsevier; Texas: 2021. pp. 707–753.e7. [Google Scholar]
- 31.Łuczak J, Bagłaj M. Selecting treatment method for ovarian masses in children - 24 years of experience. J Ovarian Res. 2017;10(1):59. doi: 10.1186/s13048-017-0353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable