Abstract
Neurolymphomatosis is an uncommon presentation of lymphoma caused by the infiltration of the peripheral nervous system by lymphoid cells. Here, we describe a case of neurolymphomatosis of the sciatic nerve in 41-year-old woman, which presented by acute onset pain and progress to paresthesia and weakness. Magnetic resonance imaging (MRI) revealed lobulated mass involving the right sciatic nerve with central necrosis and mild surrounding edema, which was isointense on T1-weighted images, hyperintense on short tau inversion recovery (STIR). Positron emission tomography and computed tomography (PET-CT) showed centrally necrotic mass with avid fluorodeoxyglucose (FDG) uptake in the right sciatic nerve. Partial resection of the tumor was done, and the diagnosis of the diffuse large B-cell lymphoma was made and confirmed by bone marrow biopsy. Patient was treated with R-CHOP chemotherapy (regimen consisting of cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine) and radiotherapy.
Keywords: Chemotherapy, Diffuse large B-cell, Lymphoma, Radiotherapy
Introduction
Neurolymphomatosis is the infiltration of the peripheral nervous system (PNS) by neoplastic lymphomatous cells, which may occur in about 5% of patients with lymphoma. Although it is rare, but neurolymphomatosis may sometimes be the preceding presentation of the underlying lymphoma, which nearly always is B-cell non-Hodgkin's lymphoma (NHL). An early diagnosis is vital to proceed with the best treatment and deliver the best patient care and management [[1], [2]–3].
Here, we describe a rare case of neurolymphomatosis diagnosed by the primary diffuse large B-cell lymphoma (DLBCL) with progressive sciatic nerve involvement.
Case presentation
A 41-year-old woman with a 5-year history of chronic idiopathic neutropenia, which was treated with G-CSF/Neupogen, presented with subacute onset of right leg sensory and motor symptoms. Her symptoms started with spontaneous right calf pain and soreness during running and progressed to rest pain and paresthesia of the right lower extremity. She developed pitting edema in her right foot, pins/needles sensation in the L5 and S1 distributions, and weakness, with a following foot drop. The patient described the pain as continuous, sharp, and jabbing, which was aggravated by activities like walking and revealed by oxycodone. The average pain intensity score was 5-6/10, which sometimes increased to 7/10. On physical examination, the strength of hip flexion, knee flexion and extension were 5/5 on the right side. The dorsiflexion, plantar flexion, inversion, and eversion were all absent on the right side and the toes only wiggled slightly. Sensory exam shows decreased sensation in the entire right foot which continues to the calf, more severe on the fibular side. The sensory and motor examination of the left lower extremity were both normal.
Deep vein thrombosis was ruled out with color-Doppler ultrasound. An electromyogram obtained with nerve conduction studies indicated a neuropathic process involving the right tibialis anterior, gastrocnemius, peroneus longus, posterior tibialis, and flexor digitorum longus muscles.
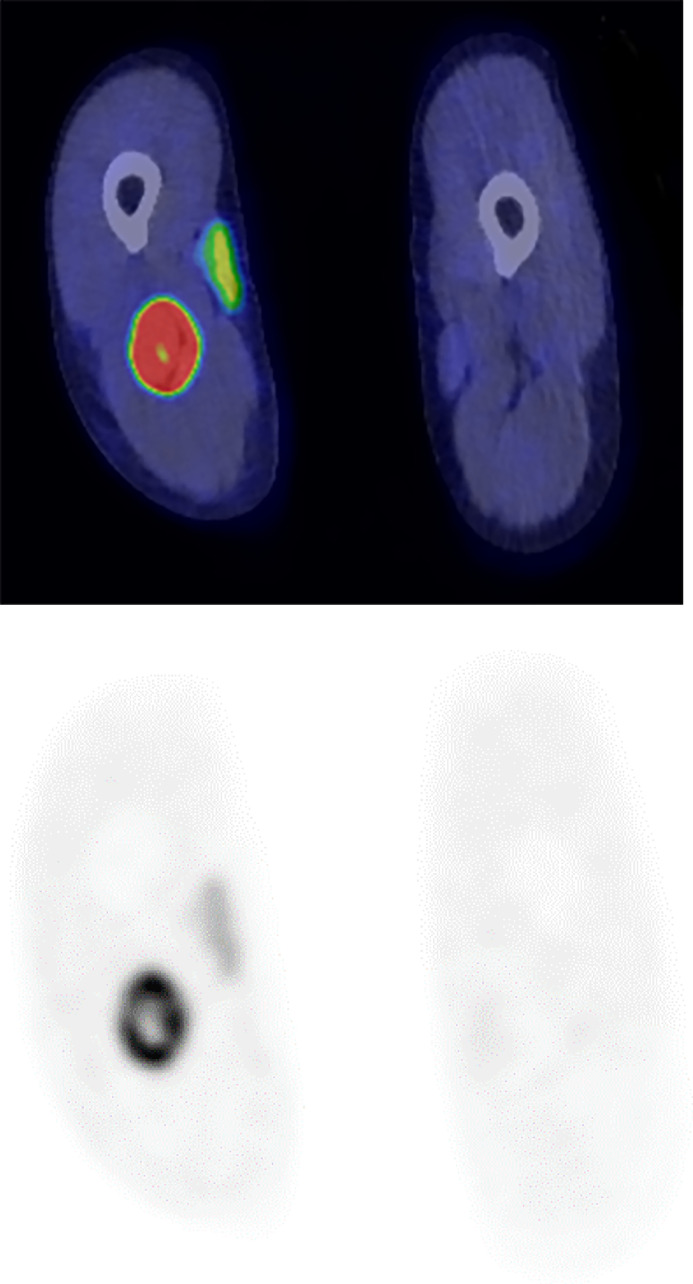
Magnetic resonance imaging revealed a lobulated mass involving the right sciatic nerve with central necrosis and mild surrounding edema (Fig. 1). The tumor displayed isointense signal on T1-weighted images (T1-WI), hyperintense signal on short tau inversion recovery (STIR) images and avid postcontrast enhancement (Fig. 2). The mass displaced the right peroneal artery anteriorly without gross occlusion or invasion (Fig. 3). There was no evidence of invasion of surrounding musculature. A preoperative radiological diagnosis of peripheral nerve sheath tumor was made. Positron emission tomography-computed tomography (PET-CT) showed centrally necrotic mass with avid fluorodeoxyglucose (FDG) uptake in the right sciatic nerve at the level of mid femur. There was an additional small focus of FDG uptake inferior to the larger mass, likely representing an additional lesion (Fig. 4).
Fig. 1.
Pretreatment coronal T1-weighted MR image illustrating fusiform enlargement of right sciatic nerve at the level of mid-thigh. Tumor extension into adjacent structures is not seen.
Fig. 2.
Pretreatment coronal postcontrast T1-weighted MR image illustrating avid post contrast enhancement of right sciatic nerve lesion at the level of mid-thigh. Mild surrounding edema is also appreciated.
Fig. 3.
Pretreatment axial postcontrast T1-weighted MR image illustrating avid postcontrast enhancement of right sciatic nerve lesion at the level of mid-thigh (arrow). Mild surrounding edema is also appreciated. The mass anteriorly displaces and narrows the peroneal artery without gross evidence of occlusion or invasion.
Fig. 4.
Pretreatment PET-CT (Fusion and attenuation correction images) showing a centrally necrotic FDG avid mass in the right sciatic nerve at the level of the mid femur.
Partial resection of the tumor confirmed the diagnosis of the extra nodal DLBCL apparently isolated to the right sciatic nerve and surrounding tissue. Treatment was started with R-CHOP chemotherapy (regimen consisting of cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine) and radiotherapy.
Post-treatment right thigh neurogram (Fig. 5) showed markedly decreased size of sciatic nerve mass. The post-treatment MRI (Fig. 6) revealed a focal T2-weighted images (T2-WI) hyperintense signal and equivocal contrast enhancement with surrounding edema in the fascicles. There was a new denervation signal abnormality in the posterior lateral muscle compartments of the distal thigh, predominantly within the short head of the biceps femoris.
Fig. 5.
Post-treatment PET-CT (Fusion and attenuation correction images) showing no evidence of metabolically active residual/recurrent lymphoma in right mid-thigh or adenopathy. Deauville score 1.
Fig. 6.
Post-treatment axial T1-weighted (A) and fat-saturated (B) MR image at the level of previous mid sciatic nerve mass showed no evidence of new disease or nodular enhancement. Normal femoral musculature signal and bulk. No suspicious osseous abnormality. No acute fracture. No new lymphadenopathy.
One year follow-up PET-CT showed no evidence of metabolically active residual/recurrent lymphoma in the right mid-thigh or adenopathy. Deauville score was 1. Stable post-treatment changes including mild thickening of the sciatic nerve were appreciated on a 2-year follow-up MR examination in order enhancement to suggest recurrence.
Discussion
Neurolymphomatosis (NL) is a rare manifestation of lymphoma especially NHL, which defined as infiltration of the PNS by proliferative lymphomatous cells. DLBCL is the most common type of NHL with incidence rate of 4.68 cases per 100,000 per year in the United States [1–4]. Various peripheral nervous structures could be affected by NL, that commonly mimics compressive mononeuropathy or disc-related nerve root pathology [5]. Although it is rare, NL should always be considered as the differential diagnosis to neurological complications of lymphomas [1,4,5]. The timing of onset is heterogeneous and can occur at any stages of disease course, including at onset. Spontaneous pain is the major complaint in patients with NL and should evolve further investigation [1,4,5]. Accordingly, our patient was presented with sudden and progressive neuropathic pain that, together with sensory and motor symptoms, strongly suggested the possibility of direct neoplastic invasion [1,3,6,7].
Without systemic involvement, primary NL happens to be extremely rare and tends to have a predilection for the sciatic nerve [8–10]. Other affected peripheral nerves included brachial plexus, the radial, median, and ulnar nerves [9]. The pathogenesis of sciatic nerve's preferential involvement has been largely debated. Baehring et al. [11] theorized that specific adhesion receptors on lymphoma cells being analogous to normal lymphoid cells might be the cause of involvement of specific tissues. Quiñones-Hinojosa et al. [12] proposed that the original lymphoma might be derived from B cells from or around the sciatic nerve. Its clinical presentation is similar to lumbar radiculopathy, which causes progressive paresthesia, numbness, and weakness in the posterior thigh and lower leg, including the plantar and lateral aspects of the foot. There is often a delay between the onset of numbness and weakness and the onset of pain. Foot drop, poor knee flexion and weak hip extension are visible motor signs.
Imaging manifestations include diffuse enlargement of the peripheral nerve and mass formation along the course of the involved nerve with or without contrast enhancement [13,14]. Wadhwa and Lee et al. [13,15] reported that on T2-WI, NL frequently exhibits patches of sporadic minor heterogeneous hypo intensity (black dots) within the mass. This appearance may be explained by the features of lymphoma, which tend to surround nearby tissues or maintain the shape or contour of the affected structure rather than invading or destroying the tissue. The popliteal artery and vein, which were encased in the mass, can also provide information about lymphoma. Therefore, it is crucial to distinguish lymphoma from other diseases like malignant peripheral nerve sheath tumors on MRI, which typically show distinctive destruction of the nerve architecture within the lesion and heterogeneous features due to their complex components. These findings suggest relatively preserved nerve fascicles and vascular structures [16].
Studies show that FDG PET/CT is the most sensitive imaging modality for detecting suspected NL, outperforming gadolinium-enhanced MRI [17,18]. Jeong et al. [19] evaluated 9 cases of NL and reported the sensitivities of FDG PET/CT and MRI for detecting NL 100% and 78%, respectively.
There have been several therapeutic approaches used in previous studies including nerve resection, radiotherapy, and chemotherapy, but the outcomes have been poor [1,8]. Our patient was treated with R-CHOP chemotherapy along with local radiation. As a result, a satisfying remission was attained.
Patient consent
Written informed consent for the publication of this case report was obtained from the patient.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Majid Chalian: RSNA R&E Scholar Grant and Boeing Technology Development Grant.
References
- 1.Briani C, Visentin A, Campagnolo M, Salvalaggio A, Ferrari S, Cavallaro T, et al. Peripheral nervous system involvement in lymphomas. J Peripher Nerv Syst. 2019;24(1):5–18. doi: 10.1111/jns.12295. [DOI] [PubMed] [Google Scholar]
- 2.Sideras PA, Matthews J, Sakib SN, Ofikwu F, Spektor V. Neurolymphomatosis of the peripheral nervous system: a case report and review of the literature. Clin Imaging. 2016;40(6):1253–1256. doi: 10.1016/j.clinimag.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Campagnolo M, Cacciavillani M, Cavallaro T, Ferrari S, Gasparotti R, et al. Neurolymphomatosis, a rare manifestation of peripheral nerve involvement in lymphomas: Suggestive features and diagnostic challenges. J Peripher Nerv Syst. 2020;25(3):312–315. doi: 10.1111/jns.12401. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi S. Diffuse large B-cell lymphoma (DLBCL): epidemiology. Medscape. 2021 Accessed August 4, 2022. [Google Scholar]
- 5.Gan HK, Azad A, Cher L, Mitchell PL. Neurolymphomatosis: diagnosis, management, and outcomes in patients treated with rituximab. Neuro Oncol. 2010;12:212–215. doi: 10.1093/neuonc/nop021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baehring JM, Batchelor TT. Diagnosis and management of neurolymphomatosis. Cancer J. 2012;18(5):463–468. doi: 10.1097/PPO.0b013e31826c5ad5. [DOI] [PubMed] [Google Scholar]
- 7.Tomita M, Koike H, Kawagashira Y, Iijima M, Adachi H, Taguchi J, et al. Clinicopathological features of neuropathy associated with lymphoma. Brain. 2013;136(Pt 8):2563–2578. doi: 10.1093/brain/awt193. [DOI] [PubMed] [Google Scholar]
- 8.Kahraman S, Sabuncuoglu H, Gunhan O, Gurses MA, Sirin S. A rare reason of foot drop caused by primary diffuse large b-cell lymphoma of the sciatic nerve: case report. Acta Neurochir (Wien) 2010;152:125–128. doi: 10.1007/s00701-009-0339-9. [DOI] [PubMed] [Google Scholar]
- 9.Descamps MJ, Barrett L, Groves M, Yung L, Birch R, Murray NM, et al. Primary sciatic nerve lymphoma: a case report and review of the literature. J Neurol Neurosurg Psychiatry. 2006;77:1087–1089. doi: 10.1136/jnnp.2006.087577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Facchinelli D, Ciliberti E, Stüssi G, Ceriani L, Zucca E. Sciatic pain by neurolymphomatosis as initial presentation of disseminated diffuse large B cell lymphoma involving the testis and the CNS. Hematol Oncol. 2020;38:197–200. doi: 10.1002/hon.2698. [DOI] [PubMed] [Google Scholar]
- 11.Baehring JM, Damek D, Martin EC, Betensky RA, Hochberg FH. Neurolymphomatosis. Neuro Oncol. 2003;5:104–115. doi: 10.1215/S1522-8517-02-00017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quiñones-Hinojosa A, Friedlander RM, Boyer PJ, Batchelor TT, Chiocca EA. Solitary sciatic nerve lymphoma as an initial manifestation of diffuse neurolymphomatosis: case report and review of the literature. J Neurosurg. 2000;92:165–169. doi: 10.3171/jns.2000.92.1.0165. [DOI] [PubMed] [Google Scholar]
- 13.Wadhwa V, Thakkar RS, Maragakis N, Höke A, Sumner CJ, Lloyd TE, et al. Sciatic nerve tumor and tumor-like lesions - uncommon pathologies. Skelet Radiol. 2012;41:763–774. doi: 10.1007/s00256-012-1384-7. [DOI] [PubMed] [Google Scholar]
- 14.Muslimani AA, Spiro TP, Daw HA, Chan V, Bambakidis P. Neurolymphomatosis: the challenge of diagnosis and treatment. Commun Oncol. 2008;5:339–341. [Google Scholar]
- 15.Lee JE, An JY, Park JS, Ryu KN, Moon SK. Primary neurolymphoma of the tibial nerve: a case report with characteristic MRI findings. J Korean Soc Radiol. 2016;75(5):399–403. [Google Scholar]
- 16.Li CS, Huang GS, Wu HD, Chen WT, Shih LS, Lii JM, et al. Differentiation of soft tissue benign and malignant peripheral nerve sheath tumors with magnetic resonance imaging. Clin Imaging. 2008;32:121–127. doi: 10.1016/j.clinimag.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Grisariu S, Avni B, Batchelor TT, van den Bent MJ, Bokstein F, Schiff D, et al. Neurolymphomatosis: an international primary CNS Lymphoma Collaborative Group report. Blood. 2010;115:5005–5011. doi: 10.1182/blood-2009-12-258210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah HJ, Lele VR, Keraliya AR, Aland PS. Peripheral nerves and muscles involvement by non-Hodgkin's lymphoma seen on FDG PET/CT scan. Neurol India. 2014;62:83–85. doi: 10.4103/0028-3886.128341. [DOI] [PubMed] [Google Scholar]
- 19.Jeong J, Kim SW, Sung DH. Neurolymphomatosis: a single-center experience of neuromuscular manifestations, treatments, and outcomes. J Neurol. 2021;268:851–859. doi: 10.1007/s00415-020-10202-0. [DOI] [PubMed] [Google Scholar]