Abstract
Electronic waste recycling is a strategy that contributes to implement a circular economy model which include reuse, component and raw material recovery and minimum final deposition. Given the importance of reincorporating the components of electronic devices into the productive chain and a correct recovery for some hazardous metals such as lead contained in such residues. This study is focused on the effect of maximum available content (MAC) of metal, sulfuric acid initial concentration, agitation velocity, and oxidising agent on the recovery of copper, lead and iron from electronic waste through acid leaching. A solid-state characterization before and after treatment and electrochemical analysis was carried out to analyse MCA effects and surface chemistry. It was found that sub-millimetric particles show a better available extraction percentage in case of copper and iron, being opposite for lead. Presence of hydrogen peroxide enhance the extraction efficiency, however, this cause iron and lead precipitation, therefore it is inefficient for metals recovery as well as for reagent consumption. The presence of calcium salts reacts producing gypsum, which reduces the extraction yield of copper at particle size below 250 μm.
Keywords: Circular economy, Metal recovery, WEEE, Recycling, Hydrometallurgy
1. Introduction
E-waste is comprised of a range of valuable materials that can be recycled, including noble and base metals, plastics, and glass [1]. The process associated to recycling is well known as urban mining [2]. The global e-waste generated in 2019 was estimated to be USD 57 billion worthy, being the waste printed circuit boards (WPCBs) the most valuable component of e-waste [3]. WPCBs only account for 3–6% of the total volume of e-waste globally and 1–3% of total weight [4]; however, they are highly valuable due to the high precious and base metals content regardless chips and electronic components [5]. The worldwide formal recycling in 2019 was reported to be 9.3 Mt (metric megaton), approximately 17.4 % of e-waste generated. Recycling has shown an annual growth of almost 0.4 Mt since 2014, compared with an annual growth of almost 2 Mt for generated e-waste. Thus, recycling growth is below the growth of e-waste generation [4]. In Latin America countries, recycling is below the values reported for the world, with only 9.8 % of e-waste recycling in the case of Mexico in 2017 [6]. In terms of environmental impact, e-waste contains many hazardous substances, including heavy metals such as lead, particularly in welding, organic pollutants (POP) and brominated flame retardants (BFR) within plastics [7]. Inappropriate management of WPCB can generate pollution in soil deposits in landfills; in water by the natural leaching process; and in air, from non-controlled burn of the WPCBs, generating dioxins and brominated subproducts and the possible bioaccumulation of contaminants in the food chain [[8], [9], [10]]. Besides, the effects on human health which has been documented [11]. Among these effects, poor foetal development in early life, sensory integration difficulty, reduced cognitive scores related to blood lead levels and disrupted thyroid function related to exposition to e-waste toxic chemical such as lead which is not recovered but freely released to the environment. On another hand, the high demand on the noble and base metals for new technologies and electronic devices can be unsatisfied in the current production chain due to geopolitics problems, social protests and wars developed in some regions where metals of interest are obtained. Other factor that affects the completion of the demand is given by the current technologies used to extract metals by mines [12,13]. Moreover, the mining activities have an inherent high environmental impact [14,15]. Because of their potential positive environmental, economic, and social impacts, WPCBs are focus for e-waste recycling. However, new technologies must be developed to allow recovering most of metal and decreasing environmental impact.
For an adequate treatment proposal is important to understand the nature of WPCBs, which are generally highly heterogeneous depending on their previous functions, models and even the country of origin, use and specially, the metal nature and composition variation related to year of production [16,17]. PCBs are composed of a mixture of metals (40–60 %), plastics (30–40 %), and ceramics (10–30 %) and can contain over 60 different elements [16,18]. Typically, WPCBs are classified by its gold content into low-grade (<100 ppm Au), medium-grade (100–400 ppm Au), and high-grade (>400 ppm Au) WPCBs [19]. There are several principal routes to recover metal from WPCBs, such as pyrometallurgical processes, hydrometallurgical processes, biological processes, and possible combinations of these. The pyrometallurgical process has 2 problems: the first one is associated with the generation of organic dioxins derived from Br-based flame retardants (BFR) [20]: the other problem is the high energetic requirements for metal melting and the subsequent separation of the produced amalgam [21]. Bioprocesses are based on biological organism such as bacteria and fungus strain, or combination of them to leach some metals of interest, having as advantage its selectivity, but the long treatment time (>5 days) and the large space demand make prohibitive these kind of processes for large-scale implementation [21,22]. Hydrometallurgical processes are based on use of acids or basic solutions and complexing agents to leach metals, having as disadvantage the low selectivity, implying the need for successive separation stages [2,22,23]. Some acid leaching solutions have been evaluated, within which HNO3, HCl, and H2SO4+H2O2 solutions have been proved as effective leaching processes at large WPCB particle size. However, most of the studies are focused in yield and pseudo first order kinetic fitting [24,25] and coarse particle size [22]. The efficiency is low at small particle size requiring a high reagent consumption due possibly to the conditions are inadequately stablished in terms of nature of WPCBs and metals content [22] which affect surface interaction and subsequently leaching process, especially electrochemical equilibria [26].
Korf et al. [16] did a comparative study among characterization techniques such as inductively coupled plasma mass spectrometry (ICP-MS) and atomic absorption spectroscopy (AAS), and an additional review of the content of metals of interest reported on previous works from 2000 to 2018. This compilation was focused on noble metals such Au, Ag, Pd, Pt, rare earth, and base metals such as Cu, Al and Fe and heavy metals such as Pb, As, Hg. The review shows a high variability of metal content,consequently, a metal content characterization is necessary and how this can affect the treatment output like the mineral nature in traditional mining [27,28]. Additionally, some metals such as Ca are poorly considered, and how this affect the leaching process is not discussed, but this can represent a problem in acid leaching process, like the research by Jadhav et al. [22], who found a low leaching yield with sulfuric acid at large particle of WPCB with no further explanation [16]. Kumar et al. [29] reported gypsum generation during leaching process using H2SO4 at large particle size (>1 mm) but not the effects on acid leaching process and how vary depending on calcium content or its liberation at different particle size. Other variable of interest is the particle size of WPCB for recycling. Zhao et al. [30] reported the physical separation of Cu having the particle size as variable, finding the best size is near to 1 mm; however, at smaller size the physical process become inefficient. This result is opposite to the data from Oliveira et al. [31] who reported an analysis over the effect of particle sizes on the leaching process, being beneficial at submillimetric particle size for zinc.
Current recycling strategies are not enough to hold the processing of the increasingly larger amount of e-waste, this is of particular concern in the current circular economy model within the agenda 2030 of the United Nations (UN). One of the ways to trigger new recycling technologies implies understanding the hydrometallurgical processes’ behaviour with mineral acids e.g., sulfuric acid, implying understanding the effect of the operating variables and/or the feed variable, as particle size can affect the process.
Therefore, this study is focused on evaluate the effects of submillimetric particle size of WPC, agitation velocity, sulfuric acid concentration, and use of H2O2 as oxidising agent in Cu, Pb and Fe leaching process, MAC related to particle size and a further electrochemical discussion on metal behaviour in leaching process, along with the characterization of solid subproducts to explain the leaching yields for a better understanding of process and generalization of leaching process proposals. To the authors' knowledge, the effect of calcium content variation and MAC content related to particle size variation on the performance of metals recovery process has not been analysed. Moreover, the effect of the treatment variables for the recovery of multiple metals has not been previously studied.
2. Materials and methods
2.1. Materials
Electronic wastes were medium range smartphones WPCB from 2015 to 2018, donated by OMI Soluciones Tecnológicas (Mexico). Reagents such as: sulfuric acid, hydrogen peroxide and nitric acid were purchased from J. K. Baker. Chlorohydric acid and Standard solution for AAS was purchased from Sigma Aldrich. Reagents were analytical grade, and they were used with no further purification.
2.2. Physical pre-treatment
First stage was dismantling WPCBs from lower-medium range smartphones and electronic tablets. Most of electronic components such as chips, transistors, electronic resistance, and other common electronic component, were retired by electronic pliers, heating the WCPB at 180–200 °C to avoid toxic smoke generation. Second stage was comminution with intercalated blender Kitchenaid RSB 1575 model and disc mill Retsch DM 200 model to obtain the granulometric distribution shown in Fig. 1. Granulometric distribution of WPCB after conminution.
Fig. 1.
Granulometric distribution of WPCB after conminution.
2.3. Leaching
MAC of metals by leaching was determined by aqua regia leaching [16]. Agitation velocity was evaluated at 2 mol/L H2SO4 at near values reported by Demir [32]. Oxidising agent used was hydrogen peroxide to obtain piranha solution. Leaching time was 24 h at ambient temperature according to Kumar [29], only for leaching with aqua regia the last 6 h were at 80 °C adapting Korf's work [16]. The leaching apparatus was a three neck round bottom flask. One neck was used for temperature measurement, central neck was coupled with a condenser and the third neck was used as reagent input and sealed. Liquid-solid ratio was 20:1 as optimum ratio [33]. H2O2 concentration defined by previous works based on [29]. Values of variables evaluated by multifactorial experimental design are presented in Table 1.
Table 1.
Variables and evaluated values.
| Variables | Var | Values | ||||
|---|---|---|---|---|---|---|
| Particle size (μm) | [PS] | 1000 | 500 | 250 | 105 | |
| Agitation velocity (rpm)a | [v] | 350 | 500 | 700 | 1000 | |
| [sulfuric acid] (mol/L) | [Ac] | 1 | 2 | 3 | 4 | |
| [hydrogen peroxide] v/va | [PO] | 0 | 0.025 | |||
| Measured response | mass metal/tWPCB | |||||
These levels were only evaluated with [H2SO4] 2 mol/L.
2.4. Characterization
All metal concentration measurements in solution were performed by atomic absorption spectroscopy (AAS) equipment AAnalyst 200, PerkinElmer. Solids were characterized by field emission scanning electronic microscopy (FESEM) Jeol JSM-7601F before and after of acid treatment for morphological surface analysis and X-Ray diffraction (XRD) Bruker D8 Advance with Cu K 1 tube at 40 kV 30 mA. Precipitated solids and solid residues after aqua regia leaching were no characterized.
3. Results and discussion
3.1. Granulometric analysis
Granulometric distribution is shown in Fig. 1. Almost 80 % of processed solid has a particle size lower than 250 , found as an ideal size to metal exposition to leaching agents. This result is like those obtained by Oliveira et al. [31] for submillimetric size.
3.2. Quantification of maximum available content (MAC) of metal by aqua regia leaching
Available Cu, Fe, Pb and Ca, obtained by leaching with aqua regia, is shown in Fig. 2 a, b, c, and d, respectively.
Fig. 2.
WPCB metal content at diferent particle size of: a. Cu b. Fe. c. Pb d. Ca.
At smaller particle size there is a higher proportion of copper, iron, and calcium, indicating these metals are embedded within polymeric matrix. In comparison of smaller particle size (105 μm) Cu increase in almost 27 %, Fe increases sevenfold and Ca doubles which varies expected outputs and yields as can be seen in Pourbaix diagram and distribution diagram (support information for further discussion). With respect to larger particle size (1000 m). For Iron, a physical separation process is recommended to avoid reagent consumption. In Cu and Fe evaluation, the obtained values are in the range reported in other works [16]. At larger particle size, Pb concentration in leaching solution is higher, due to lead presence in welding associated with Sn and Ag at the outside layers [34]. Therefore, a further milling process is not necessary for an appropriate metal exposition to leaching agents [22].
3.3. Effect of agitation velocity
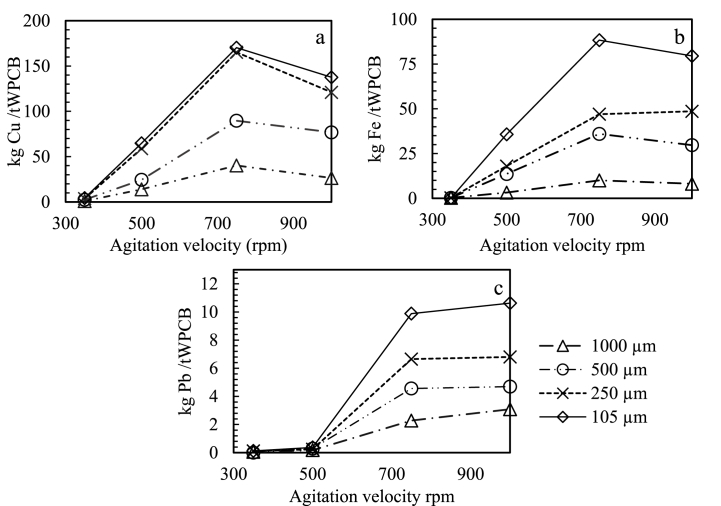
During the experimental procedure some phenomena were observed, which are described in this section. A thin hydrophobic layer in the upper part of solution was formed when mixing solid with leaching with no agitation, it was more visible when particle size was lower than 500 , possibly due to the polymeric and silica submicrometric particles observed in Fig. 5A.c and A.d which can form a kind of colloid. In another hand, in all solution, agglomerates of solids were formed due to high hydrophobicity. The agglomerates were of size proportional at particle size of WPCB, but the cohesion strength was inversely proportional to particle size of WPCB. The amount of Cu, Fe, and Pb extracted from WPCB with respect to agitation velocity are shown in Fig. 3.
Fig. 5.
Micrograph of WPCB A. before leaching, after leaching with B.AS2, and C. with SP at a. 1000 m, b. 500 m, c. 250 m d. 105 m (Micrographies are in SI).
Fig. 3.
Effect of agitation velocity on metal leaching of a. Cu, b. Fe, and c. Pb.
An agitation velocity higher or equal to 750 rpm was found necessary to assure a correct mixing of species in all cases greater than 500 reported by Ref. [33], who studied a maximum agitation velocity of 500 rpm used in less complex WPCB cell phones from before 2010. Nevertheless, at higher speeds such as 1000 rpm, the yield of leaching decreased, this effect can be due to reactive consumption or passivation species generation [29] as well as physic effects, such vortex generation, avoiding a correct contact between leaching agent and metal. In the case of piranha solution, the agitation velocity was stablished at 750 rpm.
3.4. Acid leaching of Cu, Fe, and Pb
Yield extraction (% at right) respect to AR analysis, and g of metal extracted (left) for each particle size are shown in Fig. 4 A-D. Sulfuric acid concentration in leaching solution 1, 2, 3 and 4 mol/L are tagged as AS1, AS2, AS3 and AS4 respectively, and piranha solution as SP. Piranha solution only was prepared with [H2SO4] 2 mol/L, and H2O2 2.5 % v/v.
Fig. 4.
Extracted metal from WPCB of PS A. 1000 m B. 500 m C. 250 m D. 105 m with different leaching solution of Cu (left), Fe (center), and Pb (right).
In the case of lead at H2SO4 concentrations greater than 2 mol/L, pH is strongly acid and there is a precipitation of lead species. The calcium has a strong variation in gypsum generation depending on calcium MCA, which depend on particle size. The tendendence observed in Pourbaix diagram (SI) showed at higher Ca MCA the equilibria line between CaHSO4+ (soluble) and CaSO4 (insoluble) shift to left (more acid enviromment) which explain a greater generation of gypsum. A high gypsum generation preventing metal leaching as is discussed in section 3, 3.5.6.
For Cu recovery, the best acid leaching system from studied ones was SP, due to H2O2 which facilitate the copper oxidation [35] and as observed in Pourbaix diagram of Cu (SI). With SP the recovery of Cu were reached 96 % and 92 %. which grow as a passivation layer that inhibited the interaction of leaching agent and Cu.
At lower particle size, the same behaviour was observed, nevertheless, the Cu recovery yield decay abruptly at 105 to 13 %. Experimentally a gray foam was observed, being the foam generation more intense at 105 , as a kind of physical barrier avoiding the correct contact between metals and leaching agents resulting be gypsium related to more calcium is exposed at smaller particle size as is shown in Fig. 2 d. Despite at lower particle size the yield of recovery was smaller, the maximum copper mass recovery was obtained at 250 with 50 % of recovery, near to 200 kg t/WPCB, which is high in comparison to 50–55 kg/tWPC in other evaluated systems, which is not observed in H2SO4 system. A general observation is that at lower particle size the recovery of Cu is worst with H2SO4 leaching systems; despite, the MAC is higher at lower particle size, contrary to the reported by Ref. [33] This difference can be explained by gypsum generation and the changing nature of WPCB analysed which depend strongly on model, brand and country of fabrication. That is further discussed in 3.5 Scanning Electron Microscopy Analysisand 3.6 X-Ray Diffraction Analysis sections.
For Fe, likewise for Pb, the best system is AS2 representing the pH and electrochemical potential necessary to leach these metals. H2O2 promote Fe and Pb salt precipitaiton for high oxidative potential at acid pH such a lead jarosite [36] which can explain the lower yield leaching of Fe and Pb when H2O2 (oxidising agent) is used.
3.4.1. Scanning Electron microscopy analysis
Micrograph of solid WPCB before treatment, after AS2 and SP are shown in Fig. 5A, B, and 5C respectively, at different particle size of 1000, 500, 250, and 105 in a, b, c, and d.
Grain size is proportional to particle size as expected, At larger particle size metal exposition is only superficial, at lower particle size metallic grain isolated are observed instead. The exposition of Cu, Fe and Ca (and others) and smaller (10–50) grain size facilitate the metal leaching explaining the higher MAC at lower particle size. The silica wire were expected embedded in polymeric matrix indicating at larger particle size, while is clearly exposed at smaller particle observed at in c and d. The silica wire form is the principal source of physical resistance and the difficulty to get smaller than 1 mm particle size in comparison with usual polymers. Additionaly the use if SP system promote the Fenton reaction:
| Fe+2 + H2O2→ Fe+3 + •OH + OH− | [37] |
Where radical •OH can react with polymeric matrix, which can lead to oxidation and chemical cracking of polymeric [38] visible in a more eroded surface and a green color of solution instead of gray color with only sulfuric acid solutions.
After AS2 leaching, metal is available yet, corresponding principally to residual copper in WPCB which cannot be recovered. Grains do not suffer a visible change, except for decrease in metal content, so gypsum is observed as a coating onto the surface, indicating possible passivation layer which prevents the correct contact between leching agents and metals.
Metal content seems in grains, especially in particle size larger than 250 , but the frost like overlay clearly observed in a. is gypsum but thicker in comparison with AS2. Gypsum layer acts as a passive layer, preventing contact between metal and leaching agents more noticeable at particle size below 250 , as is clearly observed in the diffractograms shown in Fig. 7 a-b.
Fig. 7.
WPCB difractogram after a. AS2 leaching process b. SP leaching process at 105 m, 250 m, 500 m, and 1000 m.
3.5. X-Ray Diffraction Analysis
The diffractograms (Fig. 6) show good crystallinity, where main peaks were related to 1 Cu, 2 Sn, 3 Ba2Cu3O5x, 4 SiO2, 5 Fe2O3. 6 Al(OH)3, 7 Pb, being Cu more intense for a higher concentration with a high purity, Sn and Pb are associated to welding [34]. A Ba2Cu3O5x is present in some chips and LEDs which could not be separated in physical pretreatment.
Fig. 6.
WPCB difractogram before leaching process at 105 m, 250 m, 500 m, and 1000 m 05 m RS140, 250 m RS60, 500 m 35, and 1000 m RS18.
Additional peaks were observed in Fig. 7 a-b, as: 8 CaSO4 2H2O, 9 CuO2, 10 CuSO4 5H2O. BS BaSO4, z peaks related to Cu7S4 and Cu8S5 only appear after SP leaching (Fig. 7b) mainly due to high oxidative potential which promote Fenton reaction [37] and probably is related to generation of this CuxSy species, SP was sufficiently strong to recover Cu from Ba2Cu3O5x for the BaSO4 formation. Cu peaks (1) is noticeable in Fig. 7 a, which indicate a residual copper after AS2 leaching system, related to low recovery yield in comparison with SP. A strong peak of CaSO4 2H2O after both AS2 and SP leaching systems was observed, specially at 105 μm indicating a high exposition of calcium at this particle size, and equally observable in larger particle size (1000 μm) with lesser intensity.
4. Conclusion
Maximum available content (MAC) of base metal within analysed WPCB from smartphones were 406 kg Cu/tWPCB, 95.5 kg Fe/tWPCB, and 8 kg Pb/tWPCB. Variables such as agitation velocity and particle size have a high effect on metal recovery using hydrometallurgical processes, being better for Cu and Fe recovery smaller particle size, being opposite for Pb, since was found at the outside surface. MCA have a special effect in gypsum effect. The acid leaching was effective on lead recovery being an adequate treatment for lead free release in landfills. Agitation velocity is better at values of 750 rpm, lower velocity is not enough to an adequate contact between metals and leaching agent in aqueous solution, and higher velocities can represent a greater reagent consumption and lesser recovery. The most efficient process for Cu recovery was H2SO4 concentration of 2 mol/L with H2O2 as oxidising agent for particle size of 1000 , but greater leached Cu mass leached was with the same solution at a particle size of 250 with 200 kg tWPC-1 and a yield near to 50 % nevertheless at smaller particle size the yield fall abruptally, in spite of electrochemical behaviour of Cu at acid pH and greater MCA, there was a generation of CaSO4. For Fe and Pb leaching, the use of H2O2 oxidising agent was detrimental, due to precipitation for oxidising potential, being more efficient with H2SO4 concentration of 2 mol/L treatment developing yields near to 100 % at every evaluated particle size. Presence of Ca dramatically decreased the process yields for gypsum generation as a passivation layer that prevents contact between metal and leaching agents, being necessary to quantify the Ca content before acid treatments. This work has been focused on better understanding the phenomena and interaction between the metals and the leaching solution during the leaching process at submillimetric particle size. Nevertheless, it is important to mention that other metals such as Ni, Zn and Pt, can affect the process, but they were not included in the study. Moreover, smaller particle sizes may also have effect on the performance of the process and must be studied in the future. Moreover, the behaviour of the polymeric matrix during Fenton reaction is other aspect to be analysed.
Data availability
The additional data that support the findings of this study are available on request from the corresponding author.
CRediT authorship contribution statement
Jaime A. Gómez Duran: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. Zeferino Gamiño Arroyo: Conceptualization. Fernando Israel Gómez Castro: Conceptualization, Formal analysis, Supervision, Writing – original draft, Writing – review & editing. Patricia Quintana Owen: Data curation, Investigation, Validation, Writing – review & editing, Funding acquisition, Resources, Validation, Writing – review & editing. Lorena Eugenia Sánchez Cadena: Formal analysis, Resources, Writing – review & editing. Mónica V. Ayala Gómez: Formal analysis, Funding acquisition, Investigation, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing, Resources.
Declaration of competing interest
The authors of the manuscript entitled “Evaluation of the effect of physical and chemical factors in the recovery of Cu, Pb and Fe from waste PCB through acid leaching”: Jaime Andrés Gómez-Durán, Zeferino Gamiño-Arroyo, Fernando Israel Gómez-Castro, Patricia Quintana-Owen, Lorena Eugenia Sánchez-Cadena, and Mónica Virginia Ayala-Gómez declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Authors acknowledge the support of Dr. V. Rejon and M-sc D Aguilar, from Lanbio, CINVESTAV-Mérida, for the characterization by FESEM and XRD. J.A. Gomez-Duran thanks to Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCyT before CONACyT), Mexico, for the scholarship granted for the development of his doctoral degree studies.
References
- 1.Kaya M. vol. XXIX. Springer International Publishing; 2019. Electronic Waste and Printed Circuit Board Recycling Technologies; p. 326. [DOI] [Google Scholar]
- 2.Kaya M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manage. (Tucson, Ariz.) 2016;57:64–90. doi: 10.1016/j.wasman.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Sivakumar P., Prabhakaran D., Thirumarimurugan M. Optimization studies on recovery of metals from printed circuit board waste. Bioinorg. Chem. Appl. 2018;2018 doi: 10.1155/2018/1067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forti V.B.C., Kuehr R., Bel G. United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR) – co-hosted SCYCLE Programme, ITU & ISWA; Bonn/Geneva/Rotterdam: 2020. The Global E-Waste Monitor 2020, Quantities, Flows, and the Circular Economy Potential; pp. 978–992. 808-9114-0. [Google Scholar]
- 5.Flandinet L., et al. Metals recovering from waste printed circuit boards (WPCBs) using molten salts. J. Hazard Mater. 2012;213–214:485–490. doi: 10.1016/j.jhazmat.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 6.Basura I.N.E.G.I. 2021. Cuéntame de México 2018 15-08.http://cuentame.inegi.org.mx/territorio/ambiente/basura.aspx?tema=T Available from: [Google Scholar]
- 7.Duan H., et al. Examining the technology acceptance for dismantling of waste printed circuit boards in light of recycling and environmental concerns. J. Environ. Manag. 2011;92(3):392–399. doi: 10.1016/j.jenvman.2010.10.057. [DOI] [PubMed] [Google Scholar]
- 8.Cruz Sotelo S., et al. From Pollution to Resource. 2016. Electronic waste in Mexico – challenges for sustainable management. 978-953-51-2499-3. [DOI] [Google Scholar]
- 9.Cui J., Zhang L. Metallurgical recovery of metals from electronic waste: a review. J. Hazard Mater. 2008;158(2):228–256. doi: 10.1016/j.jhazmat.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Lee J., Kim Y., Lee J.-c. Disassembly and physical separation of electric/electronic components layered in printed circuit boards (PCB) J. Hazard Mater. 2012;241–242:387–394. doi: 10.1016/j.jhazmat.2012.09.053. [DOI] [PubMed] [Google Scholar]
- 11.Parvez S.M., et al. Health consequences of exposure to e-waste: an updated systematic review. Lancet Planet. Health. 2021;5(12):e905–e920. doi: 10.1016/S2542-5196(21)00263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Shafy H.I., Mansour M.S.M. Solid waste issue: sources, composition, disposal, recycling, and valorization. Egypt. J. Petrol. 2018;27(4):1275–1290. doi: 10.1016/j.ejpe.2018.07.003. [DOI] [Google Scholar]
- 13.Månberger A., Johansson B. The geopolitics of metals and metalloids used for the renewable energy transition. Energy Strategy Rev. 2019;26 doi: 10.1016/j.esr.2019.100394. [DOI] [Google Scholar]
- 14.Yahaya N. IJEE; 2012. Environmental Impact of Electricity Consumption in Crushing and Grinding Processes of Traditional and Urban Gold Mining by Using Life Cycle Assessment (LCA) [DOI] [Google Scholar]
- 15.Xue M., et al. In: REWAS 2016: towards Materials Resource Sustainability. Kirchain R.E., et al., editors. Springer International Publishing; Cham: 2016. Waste management of printed wiring boards: a life cycle assessment of the metals recycling chain from liberation through refining; pp. 287–288. [DOI] [Google Scholar]
- 16.Korf N., et al. Multi-element chemical analysis of printed circuit boards – challenges and pitfalls. Waste Manage. (Tucson, Ariz.) 2019;92:124–136. doi: 10.1016/j.wasman.2019.04.061. [DOI] [PubMed] [Google Scholar]
- 17.Charles R.G., et al. An investigation of trends in precious metal and copper content of RAM modules in WEEE: implications for long term recycling potential. Waste Manage. (Tucson, Ariz.) 2017;60:505–520. doi: 10.1016/j.wasman.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Li J., Zeng X. In: Waste Electrical and Electronic Equipment (WEEE) Handbook. Goodship V., Stevels A., editors. Woodhead Publishing; 2012. 13 - recycling printed circuit boards; pp. 287–311. 978-0-85709-089-8. [DOI] [Google Scholar]
- 19.Goosey M., Kellner R. Recycling technologies for the treatment of end of life printed circuit boards (PCBs) Circ. World. 2003;29(3):33–37. doi: 10.1108/03056120310460801. [DOI] [Google Scholar]
- 20.Van Yken J., et al. Hydrometallurgy; 2020. Potential of Metals Leaching from Printed Circuit Boards with Biological and Chemical Lixiviants. [DOI] [Google Scholar]
- 21.Jenkin G.R.T., et al. The application of deep eutectic solvent ionic liquids for environmentally-friendly dissolution and recovery of precious metals. Miner. Eng. 2016;87:18–24. doi: 10.1016/j.mineng.2015.09.026. [DOI] [Google Scholar]
- 22.Jadhav U., Hocheng H. Hydrometallurgical recovery of metals from large printed circuit board pieces. Sci. Rep. 2015;5 doi: 10.1038/srep14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serpe A. In: Waste Electrical and Electronic Equipment Recycling. Vegliò F., Birloaga I., editors. Woodhead Publishing; 2018. 11 - green chemistry for precious metals recovery from WEE; pp. 271–332. 978-0-08-102057-9. [DOI] [Google Scholar]
- 24.Rajahalme J., et al. Effective recovery process of copper from waste printed circuit boards utilizing recycling of leachate. JOM. 2021;73(4):980–987. doi: 10.1007/s11837-020-04510-z. [DOI] [Google Scholar]
- 25.Sohrab Hossain M., et al. Selective recovery of Copper from waste mobile phone printed circuit boards using Sulphuric acid leaching. Mater. Today: Proc. 2018;5(10):21698–21702. doi: 10.1016/j.matpr.2018.07.021. [DOI] [Google Scholar]
- 26.Fleming C.A. Hydrometallurgy of precious metals recovery. Hydrometallurgy. 1992;30(1):127–162. doi: 10.1016/0304-386X(92)90081-A. [DOI] [Google Scholar]
- 27.Nicol M.J., Schalch E., Balestra P., amp, Hegedus H. A modern study of the kinetics and mechanism of the cementation of gold. J. South. Afr. Inst. Min. Metall. 1979;79(7):191–198. doi: 10.10520/AJA0038223X_935. [DOI] [Google Scholar]
- 28.Gabra G. In: Precious Metals: Mining, Extraction, and Processing. Kudryk D.A.C.V., Liang W.W., editors. Metallurgical Society of AIME and the Precious Metals Institute; New York: 1984. A kinetic study of the leaching of gold from pyritic concentrate using acidified thiourea; pp. 145–172. [Google Scholar]
- 29.Kumar M., et al. Leaching of metals from waste printed circuit boards (WPCBs) using sulfuric and nitric acids. Environ Eng Manag J. 2014;13:2601–2607. doi: 10.30638/eemj.2014.290. [DOI] [Google Scholar]
- 30.Zhao Y., et al. Recovery of copper from waste printed circuit boards. Min. Metall. Explor. 2004;21(2):99–102. doi: 10.1007/BF03403310. [DOI] [Google Scholar]
- 31.Oliveira P., et al. The effect of shredding and particle size in physical and chemical processing of printed circuit boards waste. Mater. Sci. Forum. 2012;730–732:653–658. doi: 10.4028/www.scientific.net/MSF.730-732.653. [DOI] [Google Scholar]
- 32.Demir H., et al. Determination of a semi empirical kinetic model for dissolution of metallic copper particles in HNO 3 solutions. Chem. Eng. Process. 2004;43:1095–1100. doi: 10.1016/j.cep.2003.11.002. [DOI] [Google Scholar]
- 33.Hao J., et al. Optimizing the leaching parameters and studying the kinetics of copper recovery from waste printed circuit boards. ACS Omega. 2022;7(4):3689–3699. doi: 10.1021/acsomega.1c06173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X., et al. Selective desoldering separation of tin–lead alloy for dismantling of electronic components from printed circuit boards. ACS Sust. Chem. Eng. 2015;3(8):1696–1700. doi: 10.1021/acssuschemeng.5b00136. [DOI] [Google Scholar]
- 35.Birloaga I., et al. Study on the influence of various factors in the hydrometallurgical processing of waste printed circuit boards for copper and gold recovery. Waste Manage. (Tucson, Ariz.) 2013;33(4):935–941. doi: 10.1016/j.wasman.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Dutrizac J.E., Dinardo O., Kaiman S. Factors affecting lead jarosite formation. Hydrometallurgy. 1980;5(4):305–324. doi: 10.1016/0304-386X(80)90022-5. [DOI] [Google Scholar]
- 37.Weiss J. Reaction mechanism of oxidation-reduction processes. Nature. 1934;133(3365):648–649. doi: 10.1038/133648c0. [DOI] [Google Scholar]
- 38.Hu K., et al. Degradation of microplastics by a thermal fenton reaction. ACS ES&T Engineering. 2022;2(1):110–120. doi: 10.1021/acsestengg.1c00323. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The additional data that support the findings of this study are available on request from the corresponding author.