Abstract
Between November 2002 and April 2003, 244 bottles and cartons of commercially pasteurized cow's milk were obtained at random from retail outlets throughout the Czech Republic. During the same period, samples of raw milk and of milk that was subsequently subjected to a minimum of 71.7°C for 15 s in a local pasteurization unit were also obtained from two dairy herds, designated herds A and B, with low and high levels, respectively, of subclinical Mycobacterium avium subsp. paratuberculosis infection, and from one herd, herd C, without infection. Infection in individual cows in each herd was tested by fecal culturing. Milk samples were brought to the Veterinary Research Institute in Brno, Czech Republic, processed, inoculated onto Herrold's egg yolk slants, and incubated for 32 weeks. Colonies were characterized by morphology, Ziehl-Neelsen staining, mycobactin J dependency, and IS900 PCR results. M. avium subsp. paratuberculosis was cultured from 4 of 244 units (1.6%) of commercially pasteurized retail milk. M. avium subsp. paratuberculosis was also cultured from 2 of 100 (2%) cartons of locally pasteurized milk derived from infected herds A and B and from 0 of 100 cartons of milk from uninfected herd C. Raw milk from 1 of 10 (10%) fecal culture-positive cows in herd A and from 13 of 66 (19.7%) fecal culture-positive cows in herd B was culture positive for M. avium subsp. paratuberculosis. These findings confirm that M. avium subsp. paratuberculosis is present in raw milk from subclinically infected dairy cows. The culture of M. avium subsp. paratuberculosis in the Czech Republic from retail milk that had been pasteurized locally or commercially to the required national and European Union standards is in agreement with similar research on milk destined for consumers in the United Kingdom and the United States and shows that humans are being exposed to this chronic enteric pathogen by this route.
Mycobacterium avium subsp. paratuberculosis is an organism which causes chronic inflammation of the intestine in domestic and wild ruminants and other animals, including primates (5-7, 12, 14, 15, 22, 23, 31, 43). M. avium subsp. paratuberculosis can live in animals for years without necessarily causing clinical disease. Infection is widespread in domestic livestock in Europe and North America as well as elsewhere (28, 30, 47). Wildlife reservoirs contribute to the persistence and spread of infection (6, 7, 16, 38).
Subclinically infected cows secrete M. avium subsp. paratuberculosis in their milk (44, 46). M. avium subsp. paratuberculosis may also enter the milk by fecal contamination in the milking parlor (19, 37). This pathogen is more thermotolerant than Mycobacterium bovis (9, 34, 40) and may remain culturable after both experimental and commercial pasteurization (11, 17, 18, 20, 27, 29, 35, 36, 42, 45).
Research in Britain and the United States has shown that M. avium subsp. paratuberculosis can be cultured from about 2 to 3% of retail pasteurized milk units (19, 36; http://www.johnes.org/newsfiles/109216471862392.html) and that humans may be exposed to ingestion of this pathogen by this route. M. avium subsp. paratuberculosis is increasingly implicated in the causation of Crohn's disease, a chronic inflammatory condition of the intestine in humans (8, 10, 13, 24, 26, 32, 41). More data are needed from several countries on the potentially important public health issue of the residual presence of this chronic enteric pathogen in retail milk. The present studies were designed to determine whether live M. avium subsp. paratuberculosis may be entering the milk supply in the Czech Republic both in general retail pasteurized milk and in locally pasteurized milk from three specific dairy herds.
MATERIALS AND METHODS
Collection of milk cartons and bottles from retail outlets.
From November 2002 to April 2003, a total of 244 glass bottles and plastic cartons of whole cow's milk treated by commercial pasteurization at a minimum of 71.7°C for 15 s were obtained at random from shops and supermarkets throughout the Czech Republic. Samples were brought directly to the laboratory, with the temperature maintained at a maximum of 4°C by the use of insulated boxes with ice packs, and processed as described below.
Sampling from specific dairy herds.
Three separate farms which housed Holstein-Friesian dairy herds were enrolled in this study. Farm A had 268 cows and a low rate (3.0%) of M. avium subsp. paratuberculosis infection, farm B had 175 cows and a relatively high rate (12.0%) of infection, and farm C had 40 cows and no evidence of infection (3). To avoid any risk of cross-contamination, we performed fecal sampling and milk sampling from the animals on each farm on different days. Fecal samples were taken directly from the rectum of each animal by the use of disposable gloves and were transferred directly to screw-cap sterile plastic containers labeled with that animal's identification number. For milk sampling, the udder was washed and dried with a fresh towel. Two strips of milk were expressed from each teat and discarded. The teats were then cleaned with 96% ethanol. On farms A and C, an approximately equal amount of milk was taken from each teat and pooled to a final sample volume of up to 50 ml. On farm B, 15 ml of milk was taken separately from each teat into labeled sterile screw-cap plastic tubes. Samples were transferred to the laboratory and processed within 24 h. When this was not possible for a few samples, the samples were stored at −20°C for no more than 6 weeks.
Sampling of locally pasteurized milk.
The small-scale local pasteurization unit which received raw milk from farms A, B, and C exposed the milk to high-temperature, short-time (HTST) conditions of a minimum of 71.7°C for 15 s and packaged the treated milk in 1-liter cartons. Cartons of pasteurized milk originating from each individual farm were taken so that they corresponded with the raw milk already sampled directly from the animals on that farm on the same day. A total of 200 pasteurized milk samples destined for consumers were obtained.
Phosphatase test.
All commercially and locally pasteurized milk samples were subjected to the phosphatase test as previously described by Grant et al. (19).
Test controls.
A reference strain, M. avium subsp. paratuberculosis CAPM no. 6381 (Collection of Animal Pathogenic Microorganisms, Brno, Czech Republic), and distilled water were used as positive and negative controls, respectively, for PCR.
Sample processing.
Samples of feces and milk were processed in separate laboratories. Fecal samples were treated as previously described (39). Briefly, 1 g of feces was added to 30 ml of sterile distilled water and mixed by horizontal shaking for 30 min. Debris and course material were allowed to settle, and 5 ml of the supernatant was added to 25 ml of 0.75% hexadecylpyridinium chloride (HPC; Merck, Darmstadt, Germany) and incubated at 37°C for 72 h (39, 48). After centrifugation for 15 min at 2,500 × g, the pellet was resuspended in 800 μl of sterile distilled water. A 250-μl portion of the resuspended pellet was inoculated onto each of three cultures. For milk, 50 ml of raw or retail pasteurized milk was collected with a sterile pipette, transferred to a centrifuge tube, and centrifuged for 15 min at 2,500 × g. The resulting pellet was resuspended in 10 ml of 0.75% HPC. The 15-ml samples of raw milk taken from individual quarters of the udder were processed similarly. Decontamination was performed with 0.75% HPC for 5 h (19). After centrifugation as described above, the pellet was resuspended in 800 μl of sterile distilled water. A 250-μl portion of the resuspended pellet was inoculated onto each of three cultures.
Mycobacterial culture and colony characterization.
Culturing was performed on Herrold's egg yolk medium (HEYM) containing 2 μg of mycobactin J ml−1. For fecal samples, HEYM slants were sealed and incubated at 37°C for 4 months (39), after which the number of colonies on each slant was counted. The incubation period for raw and pasteurized milk samples was extended to 8 months. With the primary cultures, colonies resembling M. avium subsp. paratuberculosis were stained by the Ziehl-Neelsen (ZN) method (50) for the presence of acid-fast bacilli. To distinguish M. avium subsp. paratuberculosis from other mycobactin-independent M. avium species, colonies were picked, subcultured on four HEYM slants, with three slants containing and one slant not containing 2 μg of mycobactin J ml−1 (33, 39), and incubated at 37°C for 3 months.
DNA extraction and IS900 PCR.
DNAs were extracted from primary colonies. Briefly, a small loop of bacterial biomass was dissolved in 50 μl of distilled water in an Eppendorf tube and heated at 100°C for 20 min. After spinning down for 5 min at 16,000 × g, a 2-μl sample of the supernatant was added to the amplification mixture. PCR was performed with a Taq PCR master mix kit (QIAGEN, Hilden, Germany) by use of the primers IS900-P3N (5′-GGG TGT GGC GTT TTC CTT CG-3′) and IS900-P4N (5′-TCC TGG GCG CTG AGT TCC TC-3′) at a concentration of 10 μmol per reaction. The primers were designed by the program GeneBase (Applied Maths, Kortrijk, Belgium). For primary isolates with which only scarce colonies were obtained, the amplification was extended to 60 cycles (4) by use of a Hot-Start Taq PCR kit (QIAGEN). Electrophoresis was performed on a 2% agarose gel. The expected length of the amplification products was 257 bp. An internal standard with a length of 591 bp was used to control PCR inhibition.
RESULTS
Four of the 244 cartons and bottles of commercially pasteurized milk obtained from retail outlets throughout the Czech Republic yielded IS900 PCR-positive colonies of M. avium subsp. paratuberculosis, corresponding to an overall detection rate of 1.6% (Fig. 1; Table 1). From each positive milk sample, colonies appeared on at least one of the three slants after the ninth week of incubation. At their first appearance, the colonies were small (1 to 2 mm) and white. Towards the end of the incubation period, they typically became white to yellow with a somewhat rough surface and only one to two colonies per slant. Microscopically, all colonies were typical ZN-positive acid-fast bacilli showing characteristic clumping. Two of the four primary M. avium subsp. paratuberculosis isolates did not grow upon subculturing within the time incubated. Slants from 20 (8.2%) of the total of 244 commercially pasteurized milk cartons were overgrown with contaminants and could not be read. All commercially pasteurized retail milk samples tested phosphatase negative.
FIG. 1.
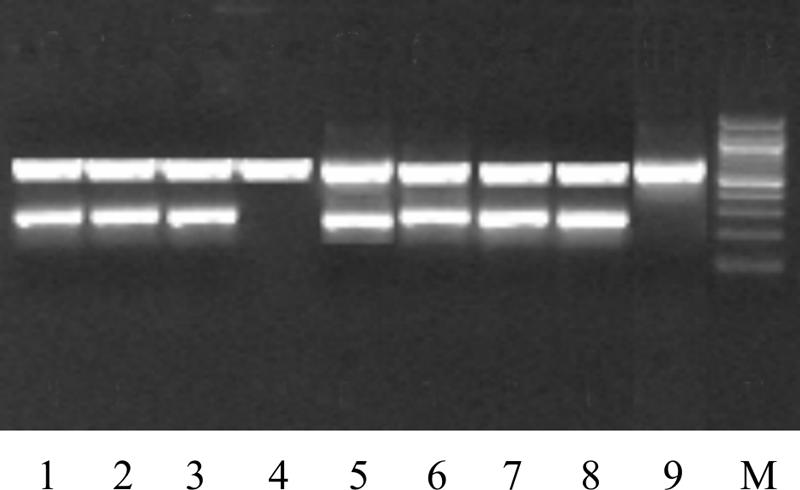
Detection of IS900 of M. avium subsp. paratuberculosis in six primary cultures of pasteurized milk by highly sensitive hot-start PCR. Lanes: 1, 2, 3, 5, 6, and 7, pasteurized milk samples of IS900-positive M. avium subsp. paratuberculosis; 4, one of the contaminated milk cultures; 8, positive control; 9, negative control; M, 100-bp molecular size marker (New England Biolabs).
TABLE 1.
Culture recovery of M. avium subsp. paratuberculosis from pasteurized cow's milk
| Originb | Package type | No. of primary culture isolates/no. of milk samples |
M. avium subsp. paratuberculosisa
|
|||
|---|---|---|---|---|---|---|
| Morphology | AFB | Dependence on M-J | IS900 PCR | |||
| Farms A and B | Plastic or carton | 2/100 | 2 | 2 | 0 | 2 |
| Farm C | Plastic or carton | 0/100 | 0 | 0 | 0 | 0 |
| Retail outlet | Glass bottle or carton | 4/244 | 4 | 4 | 2 | 4 |
| Total | 6/444 | 6 | 6 | 2 | 6 | |
The numbers in each column show the numbers of positive primary culture isolates by each given test. AFB, presence of acid-fast bacilli by ZN staining.
Milk from farms A, B, and C was pasteurized in a local pasteurization unit; milk from the retail outlet was treated by commercial pasteurization.
For the study of locally pasteurized milk from farm A, fecal samples from 10 of the 268 cows (3.7%) grew ZN-positive, IS900 PCR-positive M. avium subsp. paratuberculosis. These isolates all grew upon subculturing and were all mycobactin J dependent. Raw milk from 1 of these 10 (10%) demonstrably infected cows was also culture positive for M. avium subsp. paratuberculosis. In addition, milk from one cow that had a negative fecal sample was subsequently positive by culture. For farm B, fecal samples from 66 of the 175 cows (37.7%) grew ZN-positive, IS900 PCR-positive M. avium subsp. paratuberculosis. All isolates grew upon subculturing and were mycobactin J dependent. Raw milk from 13 of these 66 (19.7%) demonstrably infected cows was also culture positive for M. avium subsp. paratuberculosis. For the 13 cows with culture-positive milk on farm B, M. avium subsp. paratuberculosis was isolated from milk from two quarters of the udder for 1 animal and from only one quarter of the udder for the remaining 12 animals (Table 2). Two of the 100 (2%) cartons of locally pasteurized milk derived from farms A and B yielded IS900 PCR-positive colonies of M. avium subsp. paratuberculosis showing typical colony morphology and clumped ZN-positive mycobacteria (Table 1). Neither of these isolates grew upon subculturing. For farm C, fecal and raw milk samples from all 40 animals tested negative for M. avium subsp. paratuberculosis. All 100 cartons of locally pasteurized milk derived from farm C also tested negative. Slants from 19 (9.5%) of the total of 200 locally pasteurized milk cartons were overgrown with contaminants and could not be read (Table 3). All locally pasteurized retail milk samples tested were phosphatase negative.
TABLE 2.
Isolation of M. avium subsp. paratuberculosis from feces and raw milk of cows on three farms
| Farm | No. of M. avium subsp. paratuberculosis isolatesa
|
||||
|---|---|---|---|---|---|
| n | Feces
|
Milk
|
|||
| + | % | + | % | ||
| A | 268b | 10 | 3.7 | 2d | 10.0 |
| B | 175c | 66 | 37.7 | 13 | 19.7 |
| C | 40b | 0 | 0 | 0 | 0 |
| Total | 483 | 76 | 15.7 | 15 | 18.4 |
n, number of cows tested by fecal and/or milk culture; +, number of positive samples.
Pooled one-quarter milk samples from each cow.
Individual one-quarter milk samples. For one cow, two individual quarters tested positive, and for the remaining 12 cows, only one quarter tested positive.
One of the cows that harbored M. avium subsp. paratuberculosis in milk was negative by fecal culture.
TABLE 3.
Contamination and overgrowth of HEYM with nonmycobacterial organisms in pasteurized and raw milk cultures
| Milk type (n) | No. of contaminated slopesa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| One
|
Two
|
Three
|
Total
|
|||||
| n | % | n | % | n | % | n | % | |
| Commercially pasteurized (244) | 17 | 6.9 | 3 | 1.2 | 0 | 0 | 20 | 8.2 |
| Locally pasteurized (200) | 13 | 6.5 | 6 | 3.0 | 0 | 0 | 19 | 9.5 |
| Raw (1,008) | 62 | 6.2 | 29 | 2.9 | 70 | 6.9 | 161 | 16.0 |
| Total (1,452) | 90 | 6.2 | 40 | 2.8 | 70 | 4.8 | 200 | 13.8 |
Contamination of HEYM by fungi and other non-acid-fast bacteria staining blue by Z-N staining.
DISCUSSION
Paratuberculosis in farm animals is a notifiable disease in the Czech Republic, and a state-subsidized paratuberculosis monitoring and control program is in progress. Milk and milk products derived from cows with clinical or suspected paratuberculosis are not consumable even after pasteurization (1). Raw milk from infected herds is refused by milk processing plants. Because farmers are reluctant to reveal the paratuberculosis status of their herds or submit to voluntary inspection, our study of locally pasteurized milk was necessarily limited to three farms. Although the sizes of the herds on these farms were different, particularly in the small number of cows on farm C, no other suitable herds were available with a known paratuberculosis history, a sufficient number of lactating cows, and a retrievable milk supply record from a local small-scale pasteurizer.
In designing this work, we wished to address the following two principal questions. Do subclinically infected cows in herds with a low prevalence of clinical paratuberculosis shed M. avium subsp. paratuberculosis in milk in an abundance that is sufficient for detection by conventional culture? Given that the answer is yes, does standardized commercial milk pasteurization in the Czech Republic completely eliminate M. avium subsp. paratuberculosis from retail milk destined for consumers?
The culture of M. avium subsp. paratuberculosis from 10.0 and 19.7% of raw milk samples from fecal culture-positive, subclinically infected cows on farms A and B, respectively, in the present study agrees with the previous work of Sweeney et al. (46) and Streeter et al. (44). However, we were also able to isolate M. avium subsp. paratuberculosis from the milk of one fecal culture-negative cow (Table 2). This may have been due to the low sensitivity of conventional fecal culture or a result of intermittent shedding of the pathogen in feces (49), which may not necessarily coincide with the presence of M. avium subsp. paratuberculosis in the milk. Cows testing negative by a single fecal culture may still secrete the pathogen in their milk. Furthermore, our isolation of M. avium subsp. paratuberculosis from only a single quarter sample from most of the animals with positive raw milk is indicative of the uneven distribution of the pathogen within the udder. Thus, when individual cows are sampled to test for the presence of M. avium subsp. paratuberculosis in milk, samples should be collected from all quarters. Despite this observation, a comparison of fecal and milk shedding by apparently healthy infected cows on farms A and B showed that high-fecal-shedder cows release M. avium subsp. paratuberculosis in milk more often and in a higher concentration than apparent nonshedders. For farm C, the negative culture results for feces, raw milk, and pasteurized milk confirmed the herd's paratuberculosis-free status.
M. avium subsp. paratuberculosis may be cultured from milk after HTST pasteurization if the organism is present in raw milk in sufficient numbers (17-20, 35, 36). Our colony counts and the detection rates of M. avium subsp. paratuberculosis in Czech milk are likely underestimates because of the well-recognized reluctance of this organism to grow in culture and the need to include chemical decontamination during sample processing (0.75% HPC) to minimize overgrowth by other organisms. This inevitably inactivates some residual viable M. avium subsp. paratuberculosis (21). Despite decontamination, the results from 8 to 9% of our milk samples were lost due to the overgrowth of cultures by other organisms, which is in close agreement with the results of other studies (19). In addition, M. avium subsp. paratuberculosis in raw and retail pasteurized milk subjected to centrifugation segregates into both the pellet and cream fractions (36). Our need to contain the numbers of cultures by use of only the pellet fraction after centrifugation and by discarding the cream fraction may have been another source of underestimation.
It has been suggested that the majority of M. avium subsp. paratuberculosis in milk comes from contamination by fecal material in the milking parlor (20, 37). A theoretical modeling approach undertaken by Nauta and van der Giessen to evaluate human exposure to M. avium subsp. paratuberculosis concluded that subclinically infected animals make a negligible contribution to this exposure (37). However, the isolation of 4 to 20 CFU of M. avium subsp. paratuberculosis/50 ml of raw milk collected aseptically from the udder in the present study shows that direct shedding of this pathogen makes a significant contribution to their numbers in milk. Whitlock and Buergelt (49) have emphasized that clinical paratuberculosis is merely the tip of the iceberg in terms of the total number of infected animals. For every clinically infected animal born on a farm, a minimum of 25% of other animals on the same farm are probably infected, and <30% of these are detected by currently available tests. Our results are in agreement with this view, as we did not observe clinical paratuberculosis in any of the study herds. Our data suggest that subclinically infected cows constitute a substantial source of M. avium subsp. paratuberculosis in milk. Animals with clinical paratuberculosis are usually culled by farmers because of infertility or low milk yields, while the apparently healthy subclinically infected animals are allowed to persist.
The 2.0 and 1.6% culture detection rates of M. avium subsp. paratuberculosis in milk treated by small-scale and commercial pasteurization, respectively, in the Czech Republic are comparable to the results of Grant et al. (19) from the United Kingdom. Our study is also in agreement with a recent report of the culture of live M. avium subsp. paratuberculosis from 2.8% of 702 units of retail pasteurized milk in the United States (http://www.johnes.org/). Our detection of viable M. avium subsp. paratuberculosis in retail milk destined for consumers in the Czech Republic is further confirmatory evidence that the organism survives the minimum HTST pasteurization temperatures (71.7°C for 15 s and 71.2°C for 15 s, respectively) accepted by the legislation of the Czech Republic and the European Union (1, 2). Taken together, the results of research from several countries confirm that human populations are exposed to this chronic enteric pathogen in retail milk supplies. This may constitute a potential public health hazard, for which further research and the design and implementation of remedial measures are likely to prove necessary (25).
In conclusion, the results of this work demonstrate that cows with subclinical paratuberculosis shed M. avium subsp. paratuberculosis organisms that may occasionally survive HTST pasteurization. The particular economic significance of paratuberculosis in dairy enterprises combined with the risk and uncertainty related to the role of M. avium subsp. paratuberculosis in the development of Crohn's disease in humans highlights the need for further study of this organism.
Acknowledgments
This work was supported by SACROHN grant no. QLK2-CT-2000-00928 (Brussels, Belgium) and by Ministry of Agriculture of the Czech Republic grant no. QD1191.
We thank John Hermon-Taylor (St. George's Hospital Medical School, London, United Kingdom) for a critical reading of the manuscript. We thank Marcela Fisakova and Zdenka Rozsypalova for their skillful technical assistance. We are grateful to Lubomir Valicek for providing the reference strain CAPM 6381 from the Department of Collection of Animal Pathogenic Microorganisms, Veterinary Research Institute, Brno, Czech Republic.
REFERENCES
- 1.Anonymous. 1999. Milk pasteurization and processing in dairy plants. Law no. 166/1999, proclamation 287/99 collection. Section 94, p. 4633-4685. Ministry of Agriculture of the Czech Republic, Prague.
- 2.Anonymous. 1992. Laying down the health rules for the production and placing on the market of raw milk, heat-treated milk and milk-based products. Official Journal of the European Union, L268, Council directive 92/46/EEC, p. 1-32. European Commission, Brussels, Belgium.
- 3.Ayele, W. Y. 2003. Diagnostics, epidemiology and control of paratuberculosis in cattle. Ph.D. thesis. University of Veterinary and Pharmaceutical Sciences, Brno, Czech Republic.
- 4.Ayele, W. Y., M. Bartos, P. Svastova, and I. Pavlik. 2004. Distribution of Mycobacterium avium subsp. paratuberculosis in organs of naturally infected bull-calves and breeding bulls. Vet. Microbiol. 103:209-217. [DOI] [PubMed] [Google Scholar]
- 5.Ayele, W. Y., M. Machackova, and I. Pavlik. 2001. The transmission and impact of paratuberculosis infection in domestic and wild ruminants. Vet. Med.-Czech. 46:205-224. [Google Scholar]
- 6.Beard, P. M., M. J. Daniels, D. Henderson, A. Pirie, K. Rudge, D. Buxton, S. Rhind, A. Greig, M. R. Hutchings, I. McKendrick, K. Stevenson, and J. M. Sharp. 2001. Paratuberculosis infection of non-ruminant wildlife in Scotland. J. Clin. Microbiol. 39:1517-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beard, P. M., D. Henderson, M. J. Daniels, A. Pirie, D. Buxton, A. Greig, M. R. Hutchings, I. McKendrick, S. Rhind, K. Stevenson, and J. M. Sharp. 1999. Evidence of paratuberculosis in fox. Vet. Rec. 145:612-613. [Google Scholar]
- 8.Bull, T. J., E. J. McMinn, K. Sidi-Boumedine, A. Skull, D. Durki, P. Neild, G. Rhodes, R. Pickup, and J. Hermon-Taylor. 2003. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn's disease. J. Clin. Microbiol. 41:2915-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerf, O., and M. W. Griffiths. 2000. Mycobacterium paratuberculosis heat resistance. Lett. Appl. Microbiol. 30:341-342. [DOI] [PubMed] [Google Scholar]
- 10.Chiodini, R. J. 1989. Crohn's disease and the mycobacterioses: a review and comparison of two disease entities. Clin. Microbiol. Rev. 2:90-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiodini, R. J., and J. Hermon-Taylor. 1993. The thermal resistance of M. paratuberculosis in raw milk under conditions simulating pasteurization. J. Vet. Diagn. Investig. 5:629-631. [DOI] [PubMed] [Google Scholar]
- 12.Chiodini, R. J., H. J. van Kruiningen, and R. S. Merkal. 1984. Ruminant paratuberculosis/Johne's disease: the current status and future prospects. Cornell Vet. 74:218-262. [PubMed] [Google Scholar]
- 13.Chiodini, R. J., H. J. Van Kruiningen, W. R. Thayer, and J. A. Coutu. 1986. Spheroplastic phase of mycobacteria isolated from patients with Crohn's disease. J. Clin. Microbiol. 24:357-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke, C. J. 1997. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J. Comp. Pathol. 116:217-261. [DOI] [PubMed] [Google Scholar]
- 15.Collins, M. T. 1994. Clinical approach to control of bovine paratuberculosis. J. Am. Vet. Med. Assoc. 204:208-210. [PubMed] [Google Scholar]
- 16.Daniels, M. J., M. R. Hutchings, and A. Greig. 2003. The risk of disease transmission to livestock posed by contamination of farm stored feed by wildlife excreta. Epidemiol. Infect. 130:561-568. [PMC free article] [PubMed] [Google Scholar]
- 17.Grant, I. R., H. J. Ball, and M. T. Rowe. 1998. Effect of high-temperature, short-time (HTST) pasteurization on milk containing low number of Mycobacterium paratuberculosis. Lett. Appl. Microbiol. 26:166-170. [DOI] [PubMed] [Google Scholar]
- 18.Grant, I. R., H. J. Ball, S. D. Neill, and M. T. Rowe. 1996. Inactivation of Mycobacterium paratuberculosis in cow's milk at pasteurization temperatures. Appl. Environ. Microbiol. 62:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant, I. R., H. J. Ball, and M. T. Rowe. 2002. Incidence of Mycobacterium paratuberculosis in bulk raw and commercially pasteurized cows' milk from approved dairy processing establishments in the United Kingdom. Appl. Environ. Microbiol. 68:2428-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant, I. R., E. I. Hitchings, A. McCartney, F. Ferguson, and M. T. Rowe. 2002. Effect of commercial-scale high-temperature, short-time pasteurization on the viability of Mycobacterium paratuberculosis in naturally infected cows' milk. Appl. Environ. Microbiol. 68:602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant, I. R., and M. T. Rowe. 2004. Effect of chemical decontamination and refrigerated storage on the isolation of Mycobacterium avium subsp. paratuberculosis from heat-treated milk. Lett. Appl. Microbiol. 38:283-288. [DOI] [PubMed] [Google Scholar]
- 22.Greig, A., K. Stevenson, V. Perez, A. Pirie, J. M. Grant, and J. M. Sharp. 1997. Paratuberculosis in wild rabbits (Oryctolagus cuniculus). Vet. Rec. 140:141-143. [DOI] [PubMed] [Google Scholar]
- 23.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. J. Clin. Microbiol. Rev. 14:489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermon-Taylor, J. 1993. Causation of Crohn's disease: the impact of clusters. Gastroenterology 104:643-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermon-Taylor, J., and T. J. Bull. 2002. Crohn's disease caused by Mycobacterium avium subsp. paratuberculosis: a public health tragedy whose resolution is long overdue. J. Med. Microbiol. 51:3-6. [DOI] [PubMed] [Google Scholar]
- 26.Hermon-Taylor, J., T. J. Bull, J. M. Sherdian, J. Cheng, M. L. Stellakis, and N. Sumar. 2000. Causation of Crohn's disease by Mycobacterium avium subsp. paratuberculosis. Can. J. Gastroenterol. 14:521-539. [DOI] [PubMed] [Google Scholar]
- 27.Hope, A. F., P. A. Tulk, and R. J. Condron. 1996. Pasteurization of Mycobacterium paratuberculosis in whole milk, p. 377-382. In R. J. Chiodini, M. E. Hines II, and M. T. Collins (ed.), M. paratuberculosis in foods and the public health implications. Proceedings of the 5th International Colloquium on Paratuberculosis. International Association for Paratuberculosis, Madison, Wis.
- 28.Kennedy, D. J., R. Hood, and M. B. Allworth. 2002. Directions for the future control of Johne's disease caused by cattle types of M. paratuberculosis, p. 429-434. In R. A Juste, M. Geijo, and J. M. Garrido (ed.), Epidemiology and control. Proceedings of the 7th International Colloquium on Paratuberculosis. International Association for Paratuberculosis, Madison, Wis.
- 29.Keswani, J., and J. F. Frank. 1998. Thermal inactivation of Mycobacterium paratuberculosis in milk. J. Food Prot. 61:974-978. [DOI] [PubMed] [Google Scholar]
- 30.Manning, E. J. B., and M. T. Collins. 2001. Mycobacterium avium subsp. paratuberculosis: pathogen, pathogenesis and diagnosis. Rev. Sci. Tech. Off. Int. Epiz. 20:133-150. [DOI] [PubMed] [Google Scholar]
- 31.McClure, H. M., R. J. Chiodini, D. C. Anderson, R. B. Swenson, W. R. Thayer, and J. A. Coutu. 1987. Mycobacterium paratuberculosis infection in a colony of stump-tail macaques (Macaca arctoides). Infect. Dis. 155:1011-1019. [DOI] [PubMed] [Google Scholar]
- 32.McFadden, J. J., P. D. Butcher, R. J. Chiodini, and J. Hermon-Taylor. 1987. Crohn's disease-isolated mycobacteria are identical to Mycobacterium paratuberculosis, as determined by DNA probes that distinguish between mycobacterial species. J. Clin. Microbiol. 25:796-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merkal, R. S. 1984. Paratuberculosis: advances in cultural, serologic and vaccination methods. J. Am. Vet. Med. Assoc. 184:939-943. [PubMed] [Google Scholar]
- 34.Merkal, R. S., P. Lyle, and D. L. Whipple. 1981. Heat inactivation of in vivo-grown and in vitro-grown mycobacteria in meat products. Appl. Environ. Microbiol. 41:1484-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meylan, M., D. M. Rings, W. P. Shulaw, J. J. Kowalski, S. Bech-Nielsen, and G. F. Hoffsis. 1996. Survival of Mycobacterium paratuberculosis and preservation of immunoglobulin G in bovine colostrum under experimental conditions simulating pasteurization. Am. J. Vet. Res. 57:1580-1585. [PubMed] [Google Scholar]
- 36.Millar, D., J. Ford, J. Sanderson, S. Withey, M. Tizard, T. Doran, and J. Hermon-Taylor. 1996. IS900 PCR to detect Mycobacterium paratuberculosis in retail supplies of whole pasteurized cows' milk in England and Wales. Appl. Environ. Microbiol. 62:3446-3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nauta, M. J., and J. W. van der Giessen. 1998. Human exposure to Mycobacterium paratuberculosis via pasteurized milk: a modeling approach. Vet. Rec. 143:293-296. [DOI] [PubMed] [Google Scholar]
- 38.Pavlik, I., J. Bartl, L. Dvorska, P. Svastova, R. Du Maine, M. Machackova, W. Y. Ayele, and A. Horvathova. 2000. Epidemiology of paratuberculosis in wild ruminants studied by restriction fragment length polymorphism in the Czech Republic during the period 1995-1998. Vet. Microbiol. 77:231-251. [DOI] [PubMed] [Google Scholar]
- 39.Pavlik, I., L. Matlova, J. Bartl, P. Svastova, L. Dvorska, and R. Whitlock. 2000. Parallel fecal and organ Mycobacterium avium subsp. paratuberculosis culture of different productivity types of cattle. Vet. Microbiol. 77:309-324. [DOI] [PubMed] [Google Scholar]
- 40.Schulzerobbecke, R., and K. Buchholtz. 1992. Heat susceptibility of aquatic mycobacteria. Appl. Environ. Microbiol. 58:1869-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz, D., I. Shafran, C. Romero, C. Piromalli, J. Biggerstaff, N. Naser, W. Chamberlain, and S. A. Naser. 2000. Use of short-term culture for identification of Mycobacterium avium subsp. paratuberculosis in tissue from Crohn's disease patients. Clin. Microbiol. Infect. 6:303-307. [DOI] [PubMed] [Google Scholar]
- 42.Stabel, J. R., E. M. Steadham, and C. A. Bolin. 1997. Heat inactivation of Mycobacterium paratuberculosis in raw milk: are current pasteurization conditions effective? Appl. Environ. Microbiol. 63:4975-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stehman, S. M. 1996. Paratuberculosis in small ruminants, deer, and South American camelids. Vet. Clin. N. Am. Food Anim. Pract. 12:441-455. [DOI] [PubMed] [Google Scholar]
- 44.Streeter, R. N., G. F. Hoffsis, S. Bechnielsen, W. P. Shulaw, and M. Rings. 1995. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am. J. Vet. Res. 56:1322-1324. [PubMed] [Google Scholar]
- 45.Sung, N., and M. T. Collins. 1998. Thermal tolerance of Mycobacterium paratuberculosis. Appl. Environ. Microbiol. 64:999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sweeney, R. W., R. H. Whitlock, and A. E. Rosenberger. 1992. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J. Clin. Microbiol. 30:166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wells, S. J., and B. A. Wagner. 2000. Herd-level risk factors for infection with Mycobacterium paratuberculosis in U.S. dairies and association between familiarity of the herd manager with the disease or prior diagnosis of the disease in that herd and use of preventive measures. J. Am. Vet. Med. Assoc. 216:1450-1457. [DOI] [PubMed] [Google Scholar]
- 48.Whipple, D. L., D. R. Callihan, and J. L. Jarnagin. 1991. Cultivation of M. paratuberculosis from bovine fecal specimens and a suggested standardized procedure. J. Vet. Diagn. Investig. 3:368-373. [DOI] [PubMed] [Google Scholar]
- 49.Whitlock, H. R., and C. Buergelt. 1996. Preclinical and clinical manifestations of paratuberculosis (including pathology). Vet. Clin. N. Am. Anim. Pract. 12:345-356. [DOI] [PubMed] [Google Scholar]
- 50.Zimmer, K., K. G. Drager, W. Klawonn, and R. G. Hess. 1999. Contribution to the diagnosis of Johne's disease in cattle. Comparative studies on the validity of Ziehl-Neelsen staining, faecal culture and a commercially available DNA-Probe test in detecting Mycobacterium paratuberculosis in faeces from cattle. J. Vet. Med. B 46:137-140. [DOI] [PubMed] [Google Scholar]


