Abstract
Background
Stroke prevention is complicated in patients with atrial fibrillation (AF) and coronary artery disease (CAD). We compared the risk of major bleeding among Japanese patients with AF and CAD commencing warfarin, dabigatran, or rivaroxaban.
Methods
This study included adults with AF and CAD who were newly prescribed the non-vitamin K antagonist oral anticoagulants (NOACs) dabigatran or rivaroxaban, or warfarin, and registered between 18 April 2011 through 31 December 2020 in the Medical Data Vision hospital-based clinical database. The primary outcome was major bleeding, and the secondary outcome was a composite of stroke, systemic embolism, myocardial infarction, all-cause inpatient mortality, major bleeding, major gastrointestinal bleeding, and intracerebral hemorrhage. Cox proportional hazard models with stabilized inverse probability treatment weighting were used to estimate hazard ratios (HRs) with 95 % CIs via a two-step approach; first between warfarin and each NOAC, then between NOACs if sample size conditions were met.
Results
Dabigatran, rivaroxaban, and warfarin groups included 6712, 20,329, and 12,316 patients, respectively. Major bleeding risk was lower in NOACs versus warfarin (dabigatran: HR 0.50, 95 % CI: 0.40–0.62; rivaroxaban: HR 0.78, 95 % CI: 0.69–0.90); this risk was lower with dabigatran compared with rivaroxaban (HR 0.64, 95 % CI: 0.51─0.79). Net clinical benefit was superior to warfarin in both NOACs (dabigatran: HR 0.78, 95 % CI: 0.71–0.85; rivaroxaban: HR 0.83, 95 % CI: 0.78–0.88).
Conclusions
Among real-world Japanese patients with AF and CAD, NOACs were associated with better clinical outcomes than warfarin. Treatment with dabigatran had a lower risk of major bleeding than rivaroxaban.
Clinical trial registration: NCT05051904 (ClinicalTrials.gov)
Keywords: Asian, Atrial fibrillation, Coronary artery disease, Major bleeding, Non-vitamin K antagonist oral anticoagulant, Warfarin
1. Introduction
The incidence and prevalence of atrial fibrillation (AF) in Asia is on the rise, with the total number of cases projected to be higher than that for Europe or North America due to the greater overall and aging population [1], [2], [3], [4]. Potentially 17–50 % of patients with AF may also have concomitant coronary artery disease (CAD) [5], [6], [7], and patients with both diseases have been associated with increased risk of cerebrovascular and cardiovascular morbidities than either disease alone [5], [8]. In order to address stroke prevention and risk of cardiovascular events, the management of AF and concomitant CAD requires anticoagulation and antiplatelet therapies [4], [9], [10], [11]; however, the therapeutic benefits of co-administrating both therapies may be complicated due to increased bleeding risk.
Oral anticoagulants (OACs), including vitamin K antagonists and non-vitamin K antagonist oral anticoagulants (NOACs), are a central feature of AF management. Many studies, including clinical trials, have shown that NOACs are at least as effective as warfarin in stroke/systemic embolism with lower bleeding risk [12], [13]. This has also been demonstrated in patients with AF and concomitant CAD through post-hoc subanalyses of several key Phase III NOAC trials [6], [7] and a meta-analysis [14] in AF. While the efficacy and safety of NOACs over warfarin have also been demonstrated in Asian patients with AF [15], these studies do not directly inform the use of NOACs in those with concomitant CAD. There are known differences in the risk–benefit profile for antithrombotic therapy in Asian patients with AF compared with the Western population, such as a higher risk of intracranial hemorrhage (ICH) in East Asian patients versus Caucasian patients receiving warfarin [16], [17], [18]. Nevertheless, the use of warfarin in routine clinical practice remains dominant in Asia.
Currently, data regarding the safety and efficacy of anticoagulants among Asian patients with AF and concomitant CAD in routine clinical practice are limited. Using a large Japanese hospital-based database that includes acute care hospitals, we aimed to assess the risk of major bleeding and net clinical benefit of warfarin, dabigatran (approved in Japan in 2011), or rivaroxaban (approved in Japan in 2012), among Japanese patients with AF and concomitant CAD. These results will provide evidence to support clinical decisions in the management of patients with AF and concomitant CAD.
2. Methods
2.1. Study design
This study was a non-interventional cohort study based on existing data from the Medical Data Vision (MDV; Tokyo, Japan) database (Fig. 1). The database collects data from over 460 hospitals with ≥40 million patients, including several categories of information, such as medical records and pharmacy claims. Each patient is assigned an identification number to which all inpatient and outpatient data are linked. In the database, information pertinent to exposure records included the date of dispensing, drug quantity, dose, number of days of prescription supply, and prescriber. Covariates were recorded with the International Statistical Classification of Diseases and Related Health Problems 10th revision (ICD-10) codes or local disease codes for disease diagnosis, local procedure codes for medical procedures, and MDV Receipt Codes for medications. Covariates collected at baseline included demographic, ischemic and hematologic characteristics, comorbidities, co-medication, and medical procedures before or on the index date (Supplementary Table S1).
Fig. 1.
Patient disposition.
The study period (data collection period) was from 18 April 2011 to 31 December 2020, where eligible patients were categorized into three groups comprising new users of: warfarin (Group 1), dabigatran (Group 2), and rivaroxaban (Group 3).
For entry into the study population, patients were required to have had a lookback period of at least 365 days of enrollment prior to the index date (defined as the first date of prescription for warfarin, dabigatran, or rivaroxaban during the study period). The follow-up period was defined as the time between the cohort index date and the earliest occurrence of any of the following: 1) discontinuation of the index OAC (defined as a continuous gap of ≥45 days between the expected refill date and the actual refill date); 2) switching to another OAC; 3) loss to follow-up; 4) occurrence of outcomes of interest (for primary outcome: major bleeding; for secondary outcome: onset of the first occurring individual component event of the composite outcome; for further outcomes: the respective onset of component events); 5) death (except for analyses for the composite outcome and all-cause mortality as a component outcome); and 6) end of the study.
2.2. Participants
Japanese patients with AF and concomitant CAD, who were newly prescribed with warfarin, dabigatran, or rivaroxaban, were eligible for the study. CAD was defined based on ICD-10 and local diagnosis codes (MDV database disease codes) (Supplementary Table S2).
The inclusion criteria were: 1) aged ≥18 years; 2) had lookback period of 1 year prior to the index date; 3) new users of dabigatran, rivaroxaban, or warfarin (defined as patients without historic use of any OACs during the lookback period); 4) had at least one diagnosis of AF during the lookback period prior to, or on the index date; and 5) had at least one diagnosis of CAD during the lookback period prior to, or on the index date.
The exclusion criteria were: 1) diagnosed with end-stage renal disease, or underwent hemodialysis, or experienced pregnancy during the study period; 2) initiated warfarin, dabigatran, or rivaroxaban, due to valvular AF, AF associated with mechanical valve malfunction or mechanical complication of heart valve prosthesis, or rheumatic AF; 3) underwent joint replacement procedures or diagnosed with venous thromboembolism during the lookback period prior to, or on the index date; 4) prescribed more than one OAC on the index date; 5) prescribed more than two antiplatelet drugs per prescription (i.e., triple or quadruple antiplatelet use), or prescribed any antiplatelet injection in lookback period prior to or at the index date; and 6) patients with missing or ambiguous age or sex information.
2.3. Outcomes
The primary outcome was fatal or non-fatal major bleeding (defined as any blood transfusion and/or any hospitalization with associated bleeding) in all three patient groups. Secondary outcome was the composite outcome of stroke, systemic embolism, myocardial infarction (MI), all-cause mortality (inpatient), major bleeding, major gastrointestinal (GI) bleeding (hospitalization due to GI bleeding), or ICH in all three patient groups.
Further outcomes included the individual components of the composite outcome, including stroke, systemic embolism, MI, all-cause mortality (inpatient), major GI bleeding, and ICH in all three patient groups.
2.4. Statistics
Sample size was calculated based on a recent study that was also based on the MDV database, which reported that the mean (standard deviation; SD) treatment duration of different NOACs among new user groups ranged from 265 (263.8) to 868 (725) days, with the dabigatran group having the longest treatment exposure [19]. The same study also calculated that the major bleeding rate for patients with AF on all NOACs was 1.6 per 100 patient-years [19]. However, for patients with AF and concomitant CAD, the rate for major bleeding has been reported to increase approximately 2–3 times [6], [7]. Based on these data, the major bleeding rate was assumed to be 3–4.5 per 100 patient-years in Japanese patients with AF and concomitant CAD in the MDV database.
For dabigatran versus warfarin, assuming the hazard ratio (HR) to be 0.66 and the average exposure time to be 1 year per patient, an event rate of 3 per 100 patient-years with 80 % power required a total of 6050 and 3025 patients for each arm; an event rate of 4.5 per 100 patient-years required a total of 4040 and 2020 patients for each arm. To be conservative, 3025 patients as the lower bound was needed for each arm. For rivaroxaban versus warfarin, assuming the HR to be 0.74 and the average exposure time to be 1 year per patient, an event rate of 3 per 100 patient-years with 80 % power required a total of 9690 and 4845 patients for each arm. If sample loss due to the inverse probability of treatment weighting (IPTW) truncation is assumed to be 20 %, sample sizes for dabigatran and rivaroxaban groups were required to be more than 3781 and 6056 patients, respectively.
Descriptive and comparative analyses were performed for all groups; comparative analyses followed a two-step approach. In the first step, a multinominal logistic regression model was used to estimate the generalized propensity score (GPS) to account for confounding effects and ensure that patient characteristics were balanced between the treatment groups. All covariate variables listed in Supplementary Table S1 except for “Anti-platelet drugs” and “The number of anti-platelet drugs per prescription” were independent variables in the multinomial logistic regression model, as the two variables can be fully derived from the variable “Anti-platelet use duration”. The IPTW method using the calculated GPS was applied, and stabilized IPTW (s-IPTW) was used to avoid the inflation of type I error and extremely large weight [19]. The calculated s-IPTW was simultaneously applied to the treatment groups and the covariates balance between the two pairs of NOAC/warfarin cohorts was assessed through absolute standardized differences (ASD) using a threshold of 0.1. ASD > 0.1 may indicate a meaningful imbalance of covariates between paired treatment groups [20]. HRs and 95 % confidence intervals (CIs) between NOAC and warfarin were estimated using a s-IPTW Cox proportional hazards regression model. A patient was censored if the index OAC was discontinued, switched to another OAC, experienced death, or was lost to follow-up before an outcome of interest occurred. The second step utilized the estimated results from step one, which were HRs of outcomes between Groups 1 and 2, as well as Groups 1 and 3, to determine the required sample size for comparing outcomes between dabigatran- and rivaroxaban-treated groups with 80 % power. If the database fulfilled the sample size requirement, comparative analysis of that outcome was performed between the two NOAC groups. The covariates balance between the two NOAC groups after s-IPTW was also assessed using ASD. Kaplan–Meier curves after s-IPTW were plotted for non-fatal major bleeding and composite outcomes.
Sensitivity analysis was conducted using a conventional 1:1 propensity score (PS)-matching method to assess the robustness of the s-IPTW method. As in the main analysis, the ASD threshold of 0.1 was used for confirming the covariance balance between the matched cohorts, and HRs and 95 % CIs were estimated using Cox proportional hazards models. Sensitivity analysis was also performed by restricting the follow-up period to 3 years for Cox regression models on primary and secondary outcomes.
The cohort selection, outcome and covariates derivation were performed with the Instant Health Data tool, and all the analyses were conducted with SAS v9.4, Cary, NC.
3. Results
3.1. Patient baseline characteristics and incidence rates
During the study period, 78,949, 200,098, and 424,606 patients were prescribed with dabigatran, rivaroxaban, and warfarin, respectively, in the MDV database. Of these, 6712, 20,329, and 12,316 patients met the eligibility criteria and were included in the study (Fig. 1).
The number of patients censored due to reasons including discontinuation of index OAC, switching to another OAC, death, or lost to follow-up during the study period were similar across all three treatment groups (Supplementary Table S3).
After s-IPTW, 6682, 20,422, and 12,231 patients were in the dabigatran, rivaroxaban, and warfarin groups, respectively; baseline characteristics of patients were well balanced between the three cohorts (ASD < 0.1; Table 1). Briefly, for the dabigatran, rivaroxaban, and warfarin groups, respectively, mean age (SD) at index date was 74.3 (9.8), 74.8 (10.7), and 74.8 (11.1) years; 31.1 %, 32.4 %, and 32.1 % were female; mean CHA2DS2-VASc scores (SD) were 4.2 (1.5), 4.3 (1.6), and 4.3 (1.5); and mean HAS-BLED scores (SD) were 2.4 (1.1), 2.4 (1.1), and 2.4 (1.0). Prior stroke/transient ischemic attack/systemic embolism were reported in 17.1 %, 17.6 %, and 17.8 % of patients in the dabigatran, rivaroxaban, and warfarin groups, respectively. Crude and PS-matched patient baseline demographics and clinical characteristics are shown in Supplementary Tables S4 and S5, respectively. The majority (64.1 %) of patients were on a reduced dabigatran dose of 110 mg twice daily, and 23.2 % of patients were on a standard dose of 150 mg twice daily. For rivaroxaban, more than half (50.5 %) of patients received 15 mg daily, and 46.4 % received 10 mg daily (Supplementary Table S6).
Table 1.
Baseline patient demographics and clinical characteristics (s-IPTW-adjusted).
| Variable | Demographics and clinical characteristics |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Listing 1 |
Listing 2 |
Listing 3 |
|||||||
| Dabigatran | Warfarin | ASD | Rivaroxaban | Warfarin | ASD | Dabigatran | Rivaroxaban | ASD | |
| Age (years), mean (SD) | 74.3 (9.8) | 74.8 (11.1) | 0.041 | 74.8 (10.7) | 74.8 (11.1) | 0.005 | 74.3 (9.8) | 74.8 (10.7) | 0.047 |
| Female, n (%) | 2081 (31.1 %) | 3931 (32.1 %) | 0.021 | 6615 (32.4 %) | 3931 (32.1 %) | 0.005 | 2081 (31.1 %) | 6615 (32.4 %) | 0.027 |
| CHA2DS2-VASc score, mean (SD) | 4.2 (1.5) | 4.3 (1.5) | 0.032 | 4.3 (1.6) | 4.3 (1.5) | 0.004 | 4.2 (1.5) | 4.3 (1.6) | 0.028 |
| HAS-BLED score, mean (SD) | 2.4 (1.1) | 2.4 (1.0) | 0.021 | 2.4 (1.1) | 2.4 (1.0) | 0.014 | 2.4 (1.1) | 2.4 (1.1) | 0.007 |
| Comorbidities*, n (%) | |||||||||
| Heart failure | 4114 (61.6) | 7518 (61.5) | 0.002 | 12,679 (62.1) | 7518 (61.5) | 0.013 | 4114 (61.6) | 12,679 (62.1) | 0.010 |
| Peripheral arterial disorder | 787 (11.8) | 1414 (11.6) | 0.007 | 2355 (11.5) | 1414 (11.6) | 0.001 | 787 (11.8) | 2355 (11.5) | 0.008 |
| Hypertension | 4887 (73.1) | 8987 (73.5) | 0.008 | 14,849 (72.7) | 8987 (73.5) | 0.017 | 4887 (73.1) | 14,849 (72.7) | 0.010 |
| Diabetes | 1930 (28.9) | 3563 (29.1) | 0.005 | 5871 (28.8) | 3563 (29.1) | 0.008 | 1930 (28.9) | 5871 (28.8) | 0.003 |
| Prior stroke/TIA/SE | 1144 (17.1) | 2179 (17.8) | 0.018 | 3586 (17.6) | 2179 (17.8) | 0.007 | 1144 (17.1) | 3586 (17.6) | 0.012 |
| Acute coronary syndrome | 92 (1.4) | 155 (1.3) | 0.009 | 243 (1.2) | 155 (1.3) | 0.008 | 92 (1.4) | 243 (1.2) | 0.016 |
| Myocardial infarction | 1515 (22.7) | 2779 (22.7) | 0.001 | 4530 (22.2) | 2779 (22.7) | 0.013 | 1515 (22.7) | 4530 (22.2) | 0.012 |
| Unstable angina | 721 (10.8) | 1354 (11.1) | 0.009 | 2240 (11.0) | 1354 (11.1) | 0.003 | 721 (10.8) | 2240 (11.0) | 0.006 |
| Bleeding history | 300 (4.5) | 516 (4.2) | 0.013 | 858 (4.2) | 516 (4.2) | 0.001 | 300 (4.5) | 858 (4.2) | 0.014 |
| Renal dysfunction | 676 (10.1) | 1283 (10.5) | 0.012 | 2198 (10.8) | 1283 (10.5) | 0.009 | 676 (10.1) | 2198 (10.8) | 0.021 |
| Hepatic dysfunction | 29 (0.4) | 48 (0.4) | 0.007 | 81 (0.4) | 48 (0.4) | 0.001 | 29 (0.4) | 81 (0.4) | 0.006 |
| Cancers | 1092 (16.3) | 1988 (16.3) | 0.002 | 3299 (16.2) | 1988 (16.3) | 0.003 | 1092 (16.3) | 3299 (16.2) | 0.005 |
| Peptic ulcer disease | 1490 (22.3) | 2723 (22.3) | 0.001 | 4535 (22.2) | 2723 (22.3) | 0.001 | 1490 (22.3) | 4535 (22.2) | 0.002 |
| Cerebrovascular disease | 1527 (22.9) | 2910 (23.8) | 0.022 | 4859 (23.8) | 2910 (23.8) | 0.00 | 1527 (22.9) | 4859 (23.8) | 0.022 |
| Obesity | 23 (0.3) | 44 (0.4) | 0.003 | 77 (0.4) | 44 (0.4) | 0.003 | 23 (0.3) | 77 (0.4) | 0.006 |
| Concomitant medication†, n (%) | |||||||||
| Antiplatelet drugs (include aspirin§, clopidogrel, ticagrelor, prasugrel) |
2986 (44.7) | 5534 (45.2) | 0.011 | 9028 (44.2) | 5534 (45.2) | 0.021 | 2986 (44.7) | 9028 (44.2) | 0.010 |
| Number of antiplatelet drugs per prescription | |||||||||
| Single antiplatelet | 2225 (33.3) | 4122 (33.7) | 0.011 | 6799 (33.3) | 4122 (33.7) | 0.024 | 2225 (33.3) | 6799 (33.3) | 0.015 |
| Dual antiplatelet | 761 (11.4) | 1412 (11.5) | 2229 (10.9) | 1412 (11.5) | 761 (11.4) | 2229 (10.9) | |||
| None | 3696 (55.3) | 6696 (54.8) | 11,393 (55.8) | 6696 (54.8) | 3696 (55.3) | 11,393 (55.8) | |||
| Antiplatelet use duration | |||||||||
| Single antiplatelet use: 6–12 months | 724 (10.8) | 1336 (10.9) | 0.016 | 2235 (10.9) | 1336 (10.9) | 0.030 | 724 (10.8) | 2235 (10.9) | 0.019 |
| Single antiplatelet use: 1–6 months | 169 (2.5) | 304 (2.5) | 501 (2.5) | 304 (2.5) | 169 (2.5) | 501 (2.5) | |||
| Single antiplatelet use: <1 month | 1333 (19.9) | 2483 (20.3) | 4063 (19.9) | 2483 (20.3) | 1333 (19.9) | 4063 (19.9) | |||
| Dual antiplatelet use: 6–12 months | 193 (2.9) | 341 (2.8) | 579 (2.8) | 341 (2.8) | 193 (2.9) | 579 (2.8) | |||
| Dual antiplatelet use: 1–6 months | 72 (1.1) | 136 (1.1) | 224 (1.1) | 136 (1.1) | 72 (1.1) | 224 (1.1) | |||
| Dual antiplatelet use: <1 month | 496 (7.4) | 934 (7.6) | 1426 (7.0) | 934 (7.6) | 496 (7.4) | 1426 (7.0) | |||
| None | 3696 (55.3) | 6696 (54.8) | 11,393 (55.8) | 6696 (54.8) | 3696 (55.3) | 11,393 (55.8) | |||
| Nonsteroidal anti-inflammatory drugs | 1272 (19.0) | 2287 (18.7) | 0.008 | 3773 (18.5) | 2287 (18.7) | 0.006 | 1272 (19.0) | 3773 (18.5) | 0.014 |
| Gastric secretion inhibitors | 865 (12.9) | 1593 (13.0) | 0.002 | 2638 (12.9) | 1593 (13.0) | 0.003 | 865 (12.9) | 2638 (12.9) | 0.001 |
| Statins | 2449 (36.7) | 4545 (37.2) | 0.011 | 7405 (36.3) | 4545 (37.2) | 0.019 | 2449 (36.7) | 7405 (36.3) | 0.008 |
| Heparins | 3045 (45.6) | 5615 (45.9) | 0.007 | 9231 (45.2) | 5615 (45.9) | 0.014 | 3045 (45.6) | 9231 (45.2) | 0.008 |
| Proton pump inhibitor | 3218 (48.2) | 5951 (48.7) | 0.010 | 9900 (48.5) | 5951 (48.7) | 0.004 | 3218 (48.2) | 9900 (48.5) | 0.006 |
| Antihypertensive drugs | 5931 (88.8) | 10,898 (89.1) | 0.011 | 18,120 (88.7) | 10,898 (89.1) | 0.012 | 5931 (88.8) | 18,120 (88.7) | 0.001 |
| Medical procedures‖, n (%) | |||||||||
| Cardioversion | 127 (1.9) | 252 (2.1) | 0.011 | 377 (1.8) | 252 (2.1) | 0.015 | 127 (1.9) | 377 (1.8) | 0.004 |
| Ablation | 69 (1.0) | 146 (1.2) | 0.015 | 205 (1.0) | 146 (1.2) | 0.018 | 69 (1.0) | 205 (1.0) | 0.003 |
| Percutaneous coronary intervention or coronary artery bypass grafting | 338 (5.1) | 575 (4.7) | 0.016 | 946 (4.6) | 575 (4.7) | 0.003 | 338 (5.1) | 946 (4.6) | 0.020 |
Abbreviations: ASD = absolute standardized difference; CHA2DS2-VASc = congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category (female); HAS-BLED = hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly (>65 years), drugs/alcohol concomitantly; OTC, over the counter; INR = international normalized ratio; SD = standard deviation; SE = systemic embolism; s-IPTW = stabilized inverse probability of treatment weighting; TIA = transient ischemic attack.
Had at least one diagnosis before or on the index date during the lookback period.
Had used the drug before or on the index date during the lookback period.
OTC aspirin use not captured.
Underwent the procedure before or on the index date during the lookback period.
3.2. Primary and secondary outcomes
During the follow-up period after s-IPTW adjustment, 130 (1.95 %), 614 (3.01 %), and 455 (3.72 %) fatal or non-fatal bleeding events occurred in 6682, 20,422, and 12,231 patients in the dabigatran, rivaroxaban, and warfarin groups, respectively. Additionally, incidence rate of the primary outcome for these three groups was 26/1000 person-years (95 % CI: 22.00–31.00), 41/1000 person-years (95 % CI: 38.00–44.00), and 52/1000 person-years (95 % CI: 47.00–57.00), respectively (Table 2).
Table 2.
Rates and risks of primary and secondary outcomes in the follow-up period with s-IPTW adjustment.
| Dabigatran | Rivaroxaban | Warfarin | |
|---|---|---|---|
| Fatal or non-fatal major bleeding events | |||
| Number of patients | 6682 | 20,422 | 12,231 |
| Number of events | 130 | 614 | 455 |
| Number of person-years | 5010 | 14,940 | 8770 |
| Incidence rate per 1000 person-years (95 % CI) | 26 (22.00–31.00) | 41 (38.00–44.00) | 52 (47.00–57.00) |
| Incidence proportion, n (%) |
130 (1.95 %) | 614 (3.01 %) | 455 (3.72 %) |
| Composite outcome of stroke, SE, MI, all-cause mortality (inpatient), major bleeding, major GI bleeding (hospitalization due to GI bleeding), or ICH | |||
|---|---|---|---|
| Number of patients | 6682 | 20,422 | 12,231 |
| Number of events | 922 | 2963 | 2055 |
| Number of person-years | 4323 | 12,986 | 7461 |
| Incidence rate per 1000 person-years (95 % CI) | 213 (200.00–228.00) | 228 (220.00–237.00) | 275 (264.00–288.00) |
| Incidence proportion, n (%) |
922 (13.8 %) | 2963 (14.51 %) | 2055 (16.8 %) |
Abbreviations: CI = confidence interval; GI = gastrointestinal; ICH = intracranial hemorrhage; MI = myocardial infarction; SE = systemic embolism; s-IPTW = stabilized inverse probability of treatment weighting.
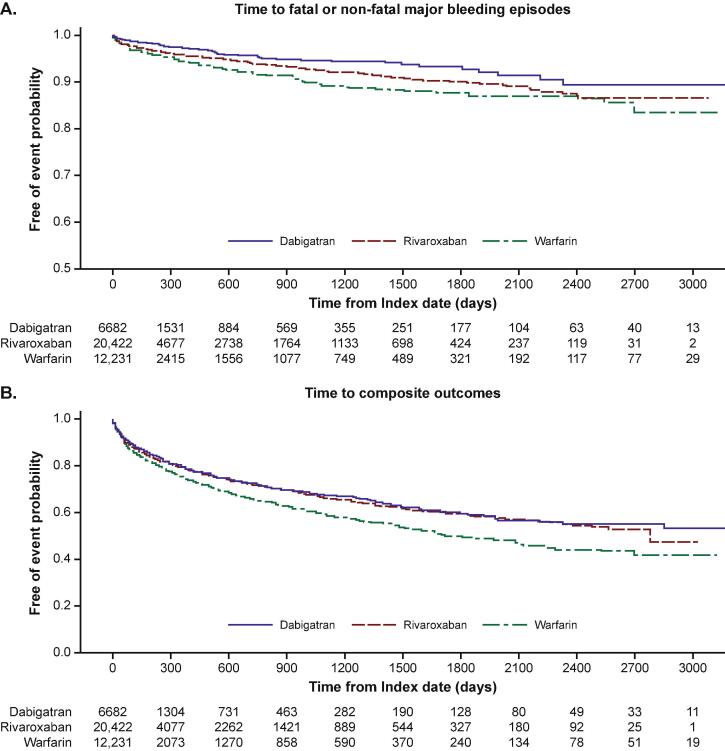
The risk of fatal or non-fatal major bleeding events was significantly lower with dabigatran (HR 0.50, 95 % CI: 0.40–0.62; P < 0.0001) and rivaroxaban (HR 0.78, 95 % CI: 0.69–0.90; P = 0.0004) compared with warfarin (Table 2, Fig. 2, Fig. 3A). The database met the sample size required for the analysis of dabigatran- and rivaroxaban-treated groups (minimum of 2827 patients for each arm to achieve 80 % power). The comparative analysis showed that dabigatran was associated with a significantly lower risk of fatal or non-fatal major bleeding events compared with rivaroxaban (HR 0.64, 95 % CI: 0.51–0.79; P < 0.0001).
Fig. 2.
Forest plot depicting the risk of primary and secondary outcomes for NOACs versus warfarin with s-IPTW adjustment.
Fig. 3.
Kaplan–Meier curves for free of primary and secondary outcome probability with s-IPTW adjustment at follow-up until end of study.
For the secondary outcome, 922 (13.80 %), 2963 (14.51 %), and 2055 (16.80 %) events occurred in 6682, 20,422, and 12,231 patients in the dabigatran, rivaroxaban, and warfarin groups, respectively, during the follow-up period after s-IPTW adjustment. The incidence rate of the secondary outcome for these three groups was 213/1000 person-years (95 % CI: 200.00–228.00), 228/1000 person-years (95 % CI: 220.00–237.00), and 275/1000 person-years (95 % CI: 264.00–288.00), respectively (Table 2).
Compared with warfarin, dabigatran (HR 0.78, 95 % CI: 0.71–0.85; P < 0.0001) and rivaroxaban (HR 0.83, 95 % CI: 0.78–0.88; P < 0.0001) were associated with a significantly lower risk of the composite outcome of stroke, systemic embolism, MI, all-cause mortality (inpatient), major bleeding, major GI bleeding, or ICH events (Table 2, Fig. 2, Fig. 3B).
3.3. Further outcomes
For stroke events, rivaroxaban was associated with a significantly lower risk compared with warfarin; no statistically significant difference was observed between the dabigatran and warfarin groups with s-IPTW adjustment (Supplementary Table S7).
Compared with warfarin, dabigatran was associated with a significantly lower risk of systemic embolism, all-cause mortality (inpatient), major GI bleeding (hospitalization due to GI bleeding), and ICH events with s-IPTW adjustment (Supplementary Table S7).
Rivaroxaban was also associated with a significantly lower risk of systemic embolism, all-cause mortality (inpatient), major GI bleeding, and ICH events with s-IPTW adjustment, compared with warfarin (Supplementary Table S7).
For MI, dabigatran and rivaroxaban groups did not show statistically significant differences in the risk of MI events with s-IPTW adjustment, compared with the warfarin group (Supplementary Table S7).
3.4. Sensitivity analysis
During the main follow-up period, the PS-matched HRs for fatal or non-fatal bleeding comparing dabigatran or rivaroxaban with warfarin were consistent with the s-IPTW-adjusted HRs. During the 3-year follow-up, the PS-matched and s-IPTW-adjusted HRs for fatal or non-fatal bleeding were also consistent with the main results (Supplementary Table S8). In addition, no notable differences for each comparison between the main follow-up period and the 3-year follow-up were observed (Fig. 3, Fig S1).
For the secondary outcome, the PS-matched HRs for dabigatran or rivaroxaban versus warfarin were consistent with the s-IPTW-adjusted analysis for risk of the secondary outcome in the follow-up period (Supplementary Table S8 and Fig. S2). During the 3-year follow-up period, the s-IPTW and PS-matched HRs for the secondary outcome were consistent with those in the main follow–up period for each comparison group (Supplementary Table S8).
Compared with warfarin, rivaroxaban significantly reduced the risk of stroke; both dabigatran and rivaroxaban significantly reduced the risk of systemic embolism, all-cause mortality (inpatient), major GI bleeding, and ICH events (Supplementary Table S8, Fig. S2, Fig. S3).
4. Discussion
In this large, retrospective, observational study, we examined the safety and efficacy of anticoagulants in Japanese patients with AF and concomitant CAD in real-world clinical practice. Overall, dabigatran and rivaroxaban were associated with a lower risk of fatal or non-fatal major bleeding events than warfarin in this population. Dabigatran treatment had a lower risk of fatal or non-fatal major bleeding events compared with rivaroxaban treatment. Both NOACs investigated in this study were also associated with a favorable net clinical benefit compared with warfarin, namely, lower risk of the composite outcome of stroke, systemic embolism, MI, all-cause mortality (inpatient), major bleeding, major GI bleeding (hospitalization due to GI bleeding), or ICH events.
The current analysis demonstrated that both dabigatran and rivaroxaban had a lower risk of major bleeding and better overall net clinical benefit compared with warfarin in this Japanese population of AF and concomitant CAD. These findings are consistent with pivotal clinical trials of AF that have shown NOACs to be at least as effective as warfarin in stroke/systemic embolism, and demonstrated reductions in major bleeding risk [21], [22]. Since CAD was present in a proportion of patients in the pivotal NOAC randomized controlled trials (RCTs) for dabigatran and rivaroxaban (31 % and 17 %, respectively), post-hoc analyses of AF and CAD patient subgroups were conducted [6], [7]. Both NOACs showed a better net clinical benefit compared with warfarin in AF and CAD patient populations, suggesting that treatment effects remained consistent regardless of whether CAD/MI was present [6], [7]. A trial-level meta-analysis of four NOAC RCTs demonstrated a general trend toward improved risk of major bleeding with standard-dose NOACs versus warfarin in patients with AF and concomitant CAD, except for rivaroxaban [14]. These findings have also been demonstrated in larger populations with more diverse clinical profiles and treatment management practices. In a large real-world Medicare population of patients diagnosed with AF and concomitant CAD, we found consistent trends showing that dabigatran was associated with a non-significantly lower rate of major bleeding compared with warfarin, although a significantly higher rate of major bleeding was reported in patients treated with rivaroxaban [23]. This was similar to a meta-analysis of 28 studies that included real-world and Phase IV studies, where both dabigatran and rivaroxaban showed similar risks of major bleeding versus warfarin [13]. While there may be some differences in RCT versus real-world results, which are likely due to study design and population type, the safety profile among patients with AF and concomitant CAD are generally consistent with that established in patients with AF.
Previous research has found different OAC risk profiles among Asian versus non-Asian patients. In particular, a greater propensity for bleeding has been found in Asian patients treated with warfarin than non-Asian patients [17], [24]. Therefore, the administered doses of OACs for AF treatment tend to be lower in Asian versus Caucasian patients to minimize the risk of bleeding, especially for those using warfarin [25]. A more updated and comprehensive meta-analysis of NOACs, which included RCTs and real-world data of OAC use in Asian patients with AF, showed that standard-dose NOACs have better efficacy and safety outcomes versus warfarin, while low-dose NOACs reduced safety risks with comparable efficacy outcomes compared with warfarin [15]. Asian guidelines recommend the use of standard-dose NOACs among OACs where eligible [4], [26]; however, lower doses of NOACs are more readily available in the region.
Subtle differences remain in bleeding risk profiles between different NOACs; the subgroup analysis of Asian patients with AF enrolled in the RE-LY trial found significantly lower rates of major bleeding regardless of a 150 or 110 mg dose of dabigatran versus warfarin [24], whilst a trend for lower rate of major bleeding was observed for rivaroxaban versus warfarin in the ROCKET-AF East Asian cohort [27]. In terms of overall clinical outcomes, a meta-analysis of the key Phase III NOAC RCTs demonstrated that standard-dose NOACs had better efficacy and safety outcomes than warfarin; however, the clinical benefit of NOACs was more profound in Asian compared with non-Asian patients [18]. In a prospective, multicenter registry, the rates of major bleeding with NOACs were lower than with warfarin in Asian patients with AF and concomitant CAD [28], which is aligned with results from this study, and largely consistent with results from a larger US observational analysis [23].
The net clinical benefit outcome (i.e., a measure of the overall benefit and risk) was in favor of NOACs, including both dabigatran and rivaroxaban, compared with warfarin. This difference may largely be due to a lower risk in individual components, including systemic embolism, all-cause mortality (inpatient), major GI bleeding, and ICH for dabigatran, and additionally lower stroke risk for rivaroxaban. Comparison between dabigatran and rivaroxaban was not performed for the secondary outcome because sample sizes did not provide sufficient power for the analysis. Meanwhile, the lack of significant difference in stroke risk for dabigatran versus warfarin could be due to at least two-thirds of patients with AF and concomitant CAD being on the lower dose of dabigatran (110 mg). However, prior research has demonstrated that the lower dabigatran dose is superior to warfarin with respect to major bleeding, but non-inferior for stroke [21], [24], whilst the subgroup analysis of patients with CAD or previous MI from the RE-LY study demonstrated a significant net clinical benefit for dabigatran 150 mg over warfarin [7]. Overall, AF and CAD subgroup analyses of NOAC RCTs have found that NOACs collectively demonstrate a trend towards benefit, with lower rates of stroke/systemic embolism compared with warfarin [14]. A real-world study of AF and CAD, which included standard and low-dose NOACs, showed that rivaroxaban is associated with a lower rate of stroke/systemic embolism compared with warfarin [23]. No additional risk in MI was reported for either NOAC versus warfarin in this analysis, which is consistent with previous RCT meta-analyses of AF and concomitant CAD subgroup studies [14], [29].
These analyses were performed on the MDV database, which is a claims database that includes all insurance types and has a large population size and low selection bias. The MDV data is one of the largest databases in Japan; the hospitals that participate in the database accounts for around 26 % of advanced treatment hospitals in Japan. From a design perspective, we applied an IPTW method to adjust for confounding in this observational study, and the s-IPTW was used to avoid inflation of type I error and extremely large weights. We also applied a 1:1 PS-matching method to confirm the robustness of results with the s-IPTW approach. In addition, ASD was used to indicate the balance condition of covariates between paired treatment groups. Patients had a follow-up of up to 9 years in the analyses and showed consistent primary and secondary outcomes within a follow-up period of 3 years. This study confirmed these findings in a relatively shorter time period, where fewer patients were censored due to loss to follow-up.
4.1. Limitations
Despite the strengths of this study, it also has several limitations. Since the MDV database was mostly based on large hospitals that specialize in acute care, a large proportion of patients were likely in poorer health conditions, with more comorbidities and higher risk of stroke/adverse events, as well as bleeding, compared with the average population requiring hospitalization. The study also did not include measurements for certain vital signs or laboratory test (e.g., blood pressure, international normalized ratio values, international normalized ratio titration level, time in therapeutic range, renal function parameters). As a result, we were unable to include these variables when calculating the propensity score and may not be matched across cohorts after s-IPTW. Although the MDV database did not collect reasons for discontinuation of OAC, switching to another OAC, death, or lost to follow-up, the proportion of patients who were censored from the analyses due to the reasons mentioned above were similar across the three treatment groups. Over-the-counter aspirin use was not captured by the MDV database, which could potentially result in unmeasured confounding. In addition to potential unmeasured confounders, we also recognize the common limitations in comparative effectiveness studies of NOACs conducted among patients with AF in real-world settings, including channeling bias [30]. We were unable to perform comparative analyses with stratification by dose due to insufficient sample sizes, although the distribution of different doses is provided in Supplementary Table S6. In addition, the lack of data in follow-up and death in an outpatient setting is an inherent limitation of the MDV database; therefore, the mortality rates of this study only referred to those collected at an inpatient setting. While this study did not include apixaban, prior analysis of patients with AF and CAD from a large US Medicare population have shown that the risk of bleeding is lowest with apixaban than either dabigatran or rivaroxaban [23]. Considering that the patient pool of AF and CAD may be limited since NOACs were only available in Japan since 2011, we prioritized the investigation of dabigatran and rivaroxaban versus warfarin. Finally, this study included patients with both stable and unstable CAD, with the majority having mild and relatively stable CAD; future studies could investigate different subtypes of CAD to provide insight on the bleeding risk differences.
5. Conclusions
Among Japanese patients with AF and concomitant CAD in a real-world setting, both dabigatran and rivaroxaban were associated with lower risks of fatal or non-fatal major bleeding events, compared with warfarin. Treatment with dabigatran or rivaroxaban was associated with a lower risk of the composite outcome of stroke, systemic embolism, MI, all-cause mortality (inpatient), major bleeding, major GI bleeding (hospitalization due to GI bleeding), or ICH events, compared with warfarin. In addition, dabigatran was associated with a lower risk of major bleeding compared with rivaroxaban. This study is one of the first to provide real-world evidence that NOACs have superior benefits to warfarin in Asian patients with AF and concomitant CAD, and the findings may inform the benefit and risk considerations when weighing the balance between thromboembolic and bleeding risks in the management of stroke prevention during routine practice. It is an area that warrants further research to gain deeper insights into the effects of NOACs and warfarin in this group of Asian patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Writing and editorial support was provided by Rebecca Yao, PhD, and Xu Hu, PhD, of MediTech Media, which was contracted and funded by Boehringer Ingelheim (China). Yinan Zhu is acknowledged for providing scientific support at the initiation of the study. The study was funded by Boehringer Ingelheim (China).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2023.101285.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Chiang C.E., Wang K.L., Lip G.Y. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb. Haemost. 2014;111:789–797. doi: 10.1160/TH13-11-0948. [DOI] [PubMed] [Google Scholar]
- 2.Kornej J., Börschel C.S., Benjamin E.J., et al. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ. Res. 2020;127:4–20. doi: 10.1161/CIRCRESAHA.120.316340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tse H.F., Wang Y.J., Ahmed Ai-Abdullah M., et al. Stroke prevention in atrial fibrillation – an Asian stroke perspective. Heart Rhythm. 2013;10:1082–1088. doi: 10.1016/j.hrthm.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Chao T.F., Joung B., Takahashi Y., et al. 2021 Focused update of the 2017 consensus guidelines of the Asia Pacific Heart Rhythm Society (APHRS) on stroke prevention in atrial fibrillation. J. Arrhythm. 2021;37:1389–1426. doi: 10.1002/joa3.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotalczyk A., Guo Y., Fawzy A.M., et al. Outcomes in elderly Chinese patients with atrial fibrillation and coronary artery disease. A report from the Optimal Thromboprophylaxis in Elderly Chinese Patients with Atrial Fibrillation (ChiOTEAF) registry. J. Arrhythm. 2022;38:580–588. doi: 10.1002/joa3.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahaffey K.W., Stevens S.R., White H.D., et al. Ischaemic cardiac outcomes in patients with atrial fibrillation treated with vitamin K antagonism or factor Xa inhibition: results from the ROCKET AF trial. Eur. Heart J. 2014;35:233–241. doi: 10.1093/eurheartj/eht428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hohnloser S.H., Oldgren J., Yang S., et al. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Circulation. 2012;125:669–676. doi: 10.1161/CIRCULATIONAHA.111.055970. [DOI] [PubMed] [Google Scholar]
- 8.Miao B., Hernandez A.V., Roman Y.M., et al. Four-year incidence of major adverse cardiovascular events in patients with atherosclerosis and atrial fibrillation. Clin. Cardiol. 2020;43:524–531. doi: 10.1002/clc.23344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin R.I.R., Bates M.G.D. Management of atrial fibrillation and concomitant coronary artery disease. Contin. Cardiol. Educ. 2017;3:47–55. [Google Scholar]
- 10.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 11.January C.T., Wann L.S., Calkins H., et al. 2019 AHA/ACC/HRS Focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019;74:104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Ruff C.T., Giugliano R.P., Braunwald E., et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 13.Ntaios G., Papavasileiou V., Makaritsis K., et al. Real-world setting comparison of nonvitamin-K antagonist oral anticoagulants versus vitamin-K antagonists for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke. 2017;48:2494–2503. doi: 10.1161/STROKEAHA.117.017549. [DOI] [PubMed] [Google Scholar]
- 14.Zelniker T.A., Ruff C.T., Antman E.M., et al. The efficacy and safety of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and coronary artery disease: a meta-analysis of randomized trials. Eur. Heart J. Acute Cardiovasc. Care. 2019;8:554–561. doi: 10.1177/2048872618796990. [DOI] [PubMed] [Google Scholar]
- 15.Xue Z., Zhang H. Non-vitamin K antagonist oral anticoagulants versus warfarin in Asians with atrial fibrillation: meta-analysis of randomized trials and real-world studies. Stroke. 2019;50:2819–2828. doi: 10.1161/STROKEAHA.119.026054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You J.H., Chan F.W., Wong R.S., et al. Is INR between 2.0 and 3.0 the optimal level for Chinese patients on warfarin therapy for moderate-intensity anticoagulation? Br. J. Clin. Pharmacol. 2005;59:582–587. doi: 10.1111/j.1365-2125.2005.02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen A.Y., Yao J.F., Brar S.S., et al. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J. Am. Coll. Cardiol. 2007;50:309–315. doi: 10.1016/j.jacc.2007.01.098. [DOI] [PubMed] [Google Scholar]
- 18.Wang K.L., Lip G.Y., Lin S.J., et al. Non-vitamin K antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation: meta-analysis. Stroke. 2015;46:2555–2561. doi: 10.1161/STROKEAHA.115.009947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohsaka S., Katada J., Saito K., et al. Safety and effectiveness of non-vitamin K oral anticoagulants versus warfarin in real-world patients with non-valvular atrial fibrillation: a retrospective analysis of contemporary Japanese administrative claims data. Open Heart. 2020;7 doi: 10.1136/openhrt-2019-001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mamdani M., Sykora K., Li P., et al. Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ. 2005;330:960–962. doi: 10.1136/bmj.330.7497.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connolly S.J., Ezekowitz M.D., Yusuf S., et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 22.Patel M.R., Mahaffey K.W., Garg J., et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 23.Lopes R.D., Steffel J., Di Fusco M., et al. Effectiveness and safety of anticoagulants in adults with non-valvular atrial fibrillation and concomitant coronary/peripheral artery disease. Am. J. Med. 2018;131:1075–1085. doi: 10.1016/j.amjmed.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Hori M., Connolly S.J., Zhu J., et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke. 2013;44:1891–1896. doi: 10.1161/STROKEAHA.113.000990. [DOI] [PubMed] [Google Scholar]
- 25.Johnson J.A. Ethnic differences in cardiovascular drug response: potential contribution of pharmacogenetics. Circulation. 2008;118:1383–1393. doi: 10.1161/CIRCULATIONAHA.107.704023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C.Z.S., Hua W. Current knowledge and management of atrial fibrillation: consensus of Chinese experts 2021. Chin. J. Cardiac. Arrhyth. 2022;26:15–88. [Google Scholar]
- 27.Wong K.S., Hu D.Y., Oomman A., et al. Rivaroxaban for stroke prevention in East Asian patients from the ROCKET AF trial. Stroke. 2014;45:1739–1747. doi: 10.1161/STROKEAHA.113.002968. [DOI] [PubMed] [Google Scholar]
- 28.Fukamachi D., Okumura Y., Yokoyama K., et al. Adverse clinical events in Japanese atrial fibrillation patients with and without coronary artery disease – findings from the SAKURA AF Registry. Curr. Med. Res. Opin. 2019;35:2053–2062. doi: 10.1080/03007995.2019.1650014. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H., Xue Z., Yi D., et al. Non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation with coronary or peripheral artery disease. Int. Heart J. 2020;61:231–238. doi: 10.1536/ihj.19-202. [DOI] [PubMed] [Google Scholar]
- 30.Bunge E.M., Bv H., Haas S., et al. Critical appraisal and issues regarding generalisability of comparative effectiveness studies of NOACs in atrial fibrillation and their relation to clinical trial data: a systematic review. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-042024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.