Abstract
FBXL19 is a member of the Skp1-Cullin-F-box family of E3 ubiquitin ligases and is linked to a variety of vital biological processes, such as cell proliferation, migration, and differentiation. Previous studies have identified it as an oncogene in breast cancer and glioma. However, its role in hepatocellular carcinoma (HCC) remains unclear. To comprehensively elucidate its role in tumour biology and its underlying mechanisms, a variety of sophisticated methods, including bioinformatics analysis, RNA-sequencing technique, and in vitro cell biology experiments, were used. Here, we found that FBXL19 was upregulated in patients with HCC and correlated with poor prognosis. In in vitro experiments, the specific targeting of short hairpin RNAs via lentiviruses successfully induced the knockdown of FBXL19, resulting in notable inhibition of the proliferation, migration, and invasion of HCC cells. Furthermore, FBXL19 downregulation resulted in significant induction of G0/G1 phase cell cycle arrest. Importantly, FBXL19 knockdown inhibited tumour malignant behaviour primarily by inactivating extracellular signal-regulated protein kinase 1/2 and p38 mitogen-activated protein kinases. In conclusion, this study revealed that FBXL19 was upregulated in patients with HCC, and that its expression was negatively correlated with prognosis. Thus, FBXL19 displays oncogenic properties in HCC by activating mitogen-activated protein kinase signalling.
Keywords: Hepatocellular carcinoma, FBXL19, Cell proliferation, Migration
1. Introduction
Hepatocellular carcinoma (HCC) is the most prevalent form of primary liver cancer and the third leading cause of cancer-related deaths worldwide in 2020 [1]. Several risk factors contribute to HCC development, including hepatitis B and C viral infections, exposure to carcinogens/toxins, and genetic factors [2]. Increasing evidence suggests that HCC pathogenesis involves the activation of oncogenic drivers such as VEGFA, FGF19/CNND1, TERT, TP53, and CTNNB1 [3]. However, the mechanisms underlying the acceleration of HCC initiation and progression mediated by these oncogenes remain unclear.
FBXL19 belongs to the Skp1-Cullin-F-box family of E3 ubiquitin ligases and mainly consists of a CXXC-PHD domain, an F-box domain, and a leucine-rich repeat [4,5]. This protein plays a critical role in the ubiquitin–proteasome system by promoting the ubiquitinated degradation of various proteins, ultimately contributing to the regulation of cellular biological functions. For example, in mouse lung epithelial cells, FBXL19 regulates cell proliferation by mediating the stability of RhoA [6], inhibits cell migration by promoting the ubiquitination and degradation of Rac1 [7,8], and attenuates IL-33-induced apoptosis by mediating the ubiquitination degradation of ST2L [9]. Previous studies have suggested that FBXL19 functions in conjunction with other F-box proteins to promote Ku80 ubiquitination, which is integral for regulating the nonhomologous end-joining double-strand break repair pathway [10]. Additionally, FBXL19 plays a role in Rnf20-mediated H2B mono-ubiquitination in embryonic stem cells [11]. However, aberrant FBXL19 expression has also been linked to numerous non-neoplastic ailments, including psoriasis, lung inflammation, type I diabetes, Lewy body dementia, Alzheimer's disease, and Parkinson's disease [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21]]. FBXL19 plays a critical role in tumourigenesis and cancer development, making it a subject of intense scrutiny in cancer research. Specifically, studies have suggested that high FBXL19 expression is strongly associated with a poor prognosis in breast cancer and is a major contributor to glioma progression, resulting in poor outcomes in affected patients [[22], [23], [24]]. However, the effect of FBXL19 in HCC remains largely unexplored and requires further investigation.
In this study, we merged RNA sequencing (RNA-seq) data on the expression of members of the F-Box and Leucine Rich Repeat Protein (FBXL) family with important clinical factors to perform differential expression analysis, Cox regression modeling, and Kaplan–Meier survival analysis. Our results indicated that the gene with the most significant correlation with poor outcomes was FBXL19, which showed high expression levels. Thus, our hypothesis suggests that FBXL19 acts as an oncogenic driver of HCC. To verify this hypothesis, we examined the role and potential mechanism of FBXL19 in vitro and proposed novel clinical interventions to develop new treatments for HCC.
2. Materials and methods
2.1. Bioinformatics analysis
The transcriptome sequencing data and clinical characteristics of HCC were acquired from The Cancer Genome Atlas (TCGA) database using the cBioPortal website (http://www.cbioportal.org/). The RNA-seq data from the HCC cohort encompassed 371 patients and 424 samples, comprising 374 tumours and 50 normal tissues, thus facilitating differential expression analysis of 371 cancer tissues and 50 normal tissues. Univariate and multivariate Cox regression analyses focused on 365 participants, excluding six patients who lacked survival data. RNA sequencing (RNA-Seq, Metware, Wuhan, China) was performed to investigate the potential mechanism of action of FBXL19 in HCC. The screening criteria for differentially expressed genes (DEGs) were |log2Fold Change| ≥ 1 and false discovery rate <0.05. Gene set enrichment analysis (GSEA) was performed for DEGs.
2.2. Human samples
Human HCC samples were collected from patients at the First Affiliated Hospital of Guangxi Medical University. All the participants provided written informed consent. This study was approved by the institutional review board of the First Affiliated Hospital of Guangxi Medical University.
2.3. Cell culture and transfection
Five human HCC cell lines were used in this study: SNU-449, Li7, MHCC-LM3, MHCC97-H, and Huh7. Cell lines with short tandem repeat reports were purchased from Cellcook Biotech (Guangzhou, China). RPMI 1640 medium (C11875500BT, Gibco) was used to culture SNU-449, Li7, MHCC-LM3, and MHCC97-H cells, whereas Huh7 cells were grown in Dulbecco's modified Eagle medium (DMEM, C11995500BT, Gibco). Each medium was supplemented with 10 % fetal bovine serum (Gibco, USA) and 1 % penicillin–streptomycin (Biological Industries, Shanghai, China). All cells were incubated at 37 °C with 5 % CO2 in a humidified incubator. Lentiviral infection (Ctrl, shFBXL19-1, or shFBXL19-2) was performed according to the manufacturer's instructions to knock down FBXL19 expression in SNU-449 cells. The lentivirus was purchased from GeneChem (Shanghai, China). Target sequences for FBXL19 knockdown were TGCTTCTCAAGGACAGCTA and CCCAGCATGGTTACTACGAAA, and the control sequence was TTCTCCGAACGTGTCACGT. The sequences were cloned into a vector containing hU6-MCS-CMV-puro using EcoRI and AgeI restriction enzymes.
2.4. Cell proliferation assay
Cell Counting Kit 8 (CCK-8) assay (CK04, Dojindo, Japan) was used to assess cell proliferation according to the manufacturer's instructions. Cells were seeded in 96-well culture plates (Corning, New York, USA) at 1, 000 cells/well. CCK-8 reagent was added to each well and incubated at 37 °C for 1 h; the absorbance at 450 nm (OD450) was then measured to assess cell viability. The experiment was performed for 7 days, spanning from day 0 to day 6. Cells were recorded on days 0 and 6 h after seeding.
2.5. Plate clone formation assay
The cells were seeded at a density of 500 cells per well in three replicate wells of six-well culture plates (Corning, New York, USA). The cells were maintained under standard culture conditions for approximately 14 d. Subsequently, the colonies were gently washed with a phosphate buffer solution, fixed with 4 % formaldehyde for 30 min, and stained with 0.1 % crystal violet. The number of colonies was counted and recorded for further analysis.
2.6. Cell cycle analysis
The cells were exposed to a synchronisation process, namely starvation for 2 h, followed by 24 h of culture. Subsequently, the cells were trypsinised without ethylenediaminetetraacetic acid, and approximately 2 million cells were collected from each sample. According to the protocol of the Cell Cycle Staining Kit (CCS012, MultiSciences, Hangzhou, China), the cells were resuspended in 1 mL of DNA Staining Solution and 10 μL of Permeabilization Solution, and the samples were gently vortexed for 5–10 s to ensure proper mixing and incubated in the dark for 30 min at 25 °C. The cells were then subjected to cell cycle analysis using flow cytometry (Beckman Coulter, Indiana, USA).
2.7. Wound healing assay
The cells were seeded in six-well culture plates (Corning, New York, USA) with three replicate wells. Once the cells reached 80–90 % confluence, wounds were created using a 200-μL micropipette tip. To remove any shed cells or debris, the cells were washed with PBS and cultured in a serum-free medium. The wounds were photographed at the same positions at 0, 24, and 48 h to assess wound progression.
2.8. Transwell assay
Transwell chambers (Corning, New York, USA) were used for cell migration and invasion assays. For the migration assays, approximately 2 × 104 cells in the upper chamber were cultured in serum-free DMEM, and the lower chamber was filled with RPMI 1640 medium containing 10 % serum. To perform invasion assays, approximately 2 × 104 cells were seeded onto Matrigel-coated (1:30 dilution, 354,248, BD Biosciences) membrane inserts. Serum-free RPMI 1640 medium containing 0.1 % bovine serum albumin was added to the upper chamber. The chambers were then placed into the cell culture plate and incubated at 37 °C for 48 h. Afterwards, the cells that had migrated or invaded across the transwell membrane were fixed using 4 % paraformaldehyde for 30 min, followed by staining with 0.1 % crystal violet for 20 min. The migratory and invasive cells were meticulously examined and counted under a microscope.
2.9. Immunohistochemical (IHC) staining
HCC and paired paracancerous tissues were collected and fixed in 10 % formalin and embedded in paraffin, and 4-μm thick sections were made for IHC staining. Sections were dewaxed and dehydrated before rehydration and antigen retrieval in citrate buffer. Endogenous peroxidase activity was blocked with 3.0 % hydrogen peroxide for 10 min. To prevent nonspecific binding, sections were blocked for 30 min with 10 % goat serum. Subsequently, they were separately incubated at 4 °C overnight with primary antibodies targeted toward FBXL19 (Bioss, bs-16201R) at a dilution of 1:300. Primary antibodies were detected using a horseradish peroxidase-polymer anti-rabbit IHC kit (PV-9001, Zsbio, Beijing, China) according to the manufacturer's instructions. The sections were visualised using diaminobenzidine, stained with haematoxylin, dehydrated in alcohol, hyalinised in xylene, and covered with coverslips. IHC staining results were graded as previously described [25].
2.10. Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA from individual cells was extracted using the TRIzol reagent (Invitrogen, Carlsbad, USA), followed by reverse transcription and cDNA synthesis using the iScript cDNA Synthesis Kit (Bio-Rad, California, USA). qRT-PCR was performed using the iQ SYBR Green SuperMix (Bio-Rad, California, USA). Primers for the target genes were purchased from Tsingke (Beijing, China). The 2−ΔΔCt method was used for the analysis of gene expression levels. All primers used for qRT-PCR are listed in Table 1.
Table 1.
Primers sequences for qRT-PCR.
| Target genes | Sequences |
|---|---|
| FBXL19 | Forward primers: 5′-CTGGCGAGAAGGAGAACCGT-3′ |
| Reverse primers: 5′-GCTTGTCATAGCACCAGCGG-3′ | |
| CDK4 | Forward primers: 5′-GAGCATGTAGACCAGGACCTAAG-3′ |
| Reverse primers: 5′-GTTCCACCACTTGTCACCAGAAT-3′ | |
| CDK6 | Forward primers: 5′-TGACCAGCAGCGGACAAATA-3′ |
| Reverse primers: 5′-GACTTCGGGTGCTCTGTACC-3′ | |
| Cyclin B | Forward primers: 5′-CTGTTGGTTTCTGCTGGGTGTAG-3′ |
| Reverse primers: 5′-GCCATGTTGATCTTCGCCTTATT-3′ | |
| Vimentin | Forward primers: 5′-GGGAGAAATTGCAGGAGGAGATG-3′ |
| Reverse primers: 5′-TTGGACATGCTGTTCCTGAATCT-3′ | |
| Twist1 | Forward primers: 5′-CATGTCCGCGTCCCACTAG-3′ |
| Reverse primers: 5′-AGTTATCCAGCTCCAGAGTCTCT-3′ | |
| GAPDH | Forward primers: 5′-ACTCCACTCACGGCAAATTC-3′ |
| Reverse primers: 5′-TCTCCATGGTGGTGAAGACA-3′ |
2.11. Western blot assay
Equal numbers of cells were lysed in the lysate (R0100, Solarbio) for protein extraction. Protein was denatured with 5 × sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (P1040, Solarbio) at 95–100 °C for 5 min. The extracted protein was separated on 10 % SDS–PAGE gels and transferred onto 0.45-μm polyvinylidene fluoride membrane (IPVH0001, Millipore). The membranes were blocked with 5 % non-fat milk for 1 h at room temperature. The membranes were probed with rabbit antibodies specific for FBXL19, extracellular signal-regulated protein kinase 1/2 (ERK1/2), p-ERK1/2, p38, p-p38, JNK, p-JNK, and GAPDH in 0.5 % milk–Tris–buffered saline with Tween solution overnight at 4 °C. The membranes were washed in TBST and incubated with the horseradish peroxidase-conjugated anti-rabbit IgG antibody for 1–2 h at room temperature. The protein bands were visualised using a hypersensitive chemiluminescence kit (SQ201, Epizyme). Details of the antibodies used for the western blotting assays are listed in Table 2.
Table 2.
Antibodies for Western blot assay.
| Antibodies | Incorporation | Catalog number | Dilution |
|---|---|---|---|
| FBXL19 | Abcam | ab172961 | 1:1,000 |
| ERK1/2 | CST | 4695T | 1:1,000 |
| p-ERK1/2 | CST | 4370T | 1:1,000 |
| p38 | CST | 8690T | 1:1,000 |
| p-p38 | CST | 4511T | 1:1,000 |
| JNK | CST | 9252T | 1:1,000 |
| p-JNK | CST | 4668T | 1:1,000 |
| GAPDH | Proteintech | 10494-1-AP | 1:5,000 |
| HRP-conjugated anti-rabbit IgG antibody | Proteintech | SA00001-2 | 1:10,000 |
2.12. Statistical analysis
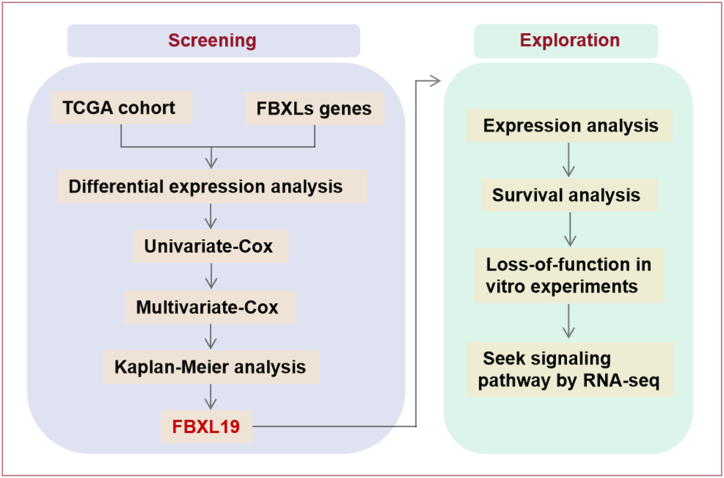
Quantitative data are presented as mean ± standard deviation. Student's t-test was used for comparison between two groups. For multigroup comparisons, analysis of variance was applied, after which the Bonferroni correction was applied. A Cox regression model was used to perform univariate and multivariate analyses. Log-rank tests were used to compare groups on the basis of overall survival (OS) estimated by the Kaplan–Meier method. For RNA-seq data, DEseq2 was used to determine differential expression. ClusterProfiler 3.10.1 was used to perform the GSEA. Statistical analyses were performed using IBM SPSS (Version 27.0). Graphs were visualised using the GraphPad Prism 9 software. Statistical significance was set at p < 0.05. The design concept of this study is illustrated in Fig. 1.
Fig. 1.
Workflow of the study design.
3. Results
3.1. High FBXL19 expression screened from FBXL family members was closely associated with poor prognosis in patients with HCC
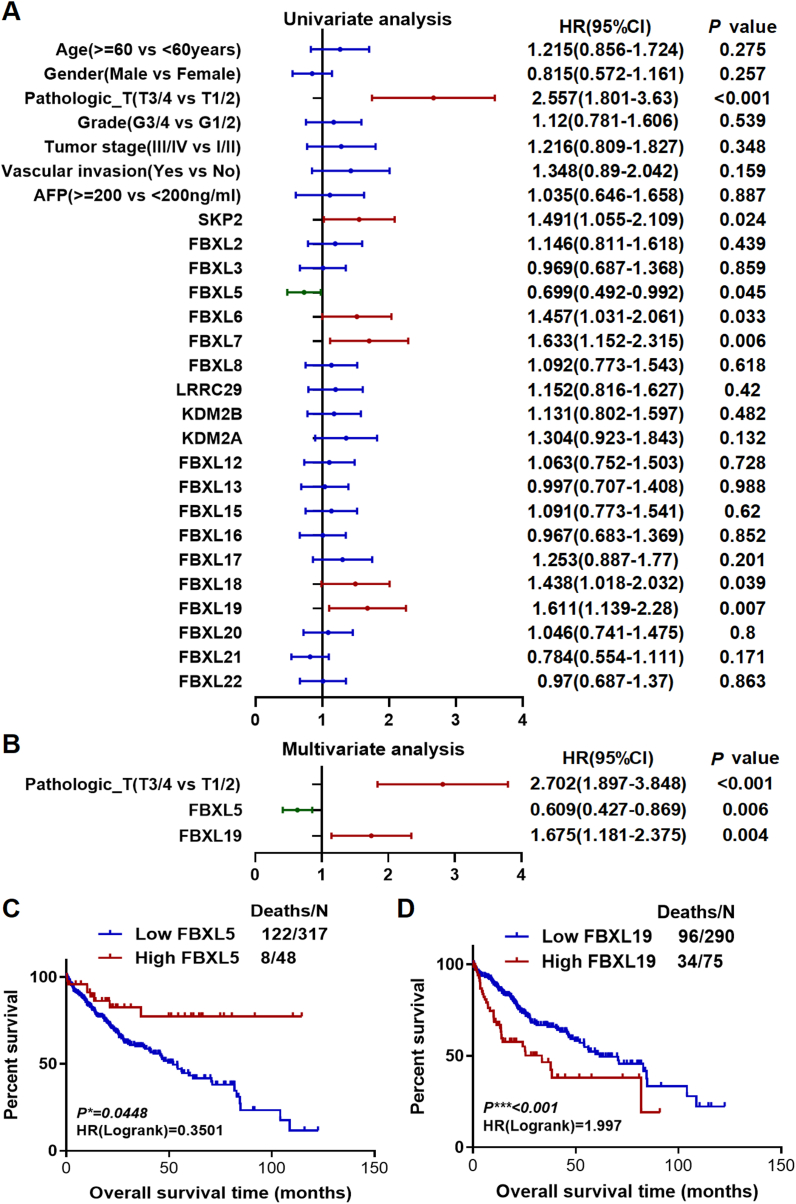
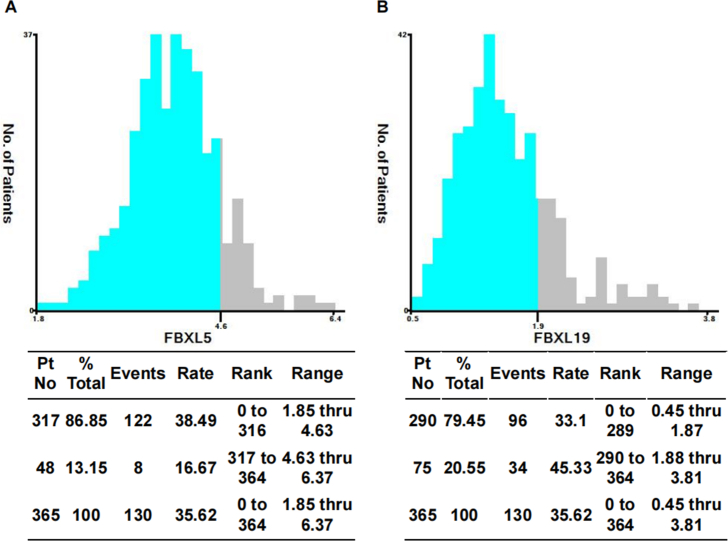
Initially, we performed a screening process to identify any DEGs within the F-box and leucine-rich repeat protein family, including 22 members of the family, known as F-box with leucine-rich amino acid repeat proteins (FBXLs). Our screening process involved comparison of 371 HCC tissues with 50 normal tissues. We successfully identified 20 DEGs, consisting of 19 upregulated genes and 1 downregulated gene (Fig. S1), which showed significant differential expression. The present study used identified DEGs and several widely observed clinical features to develop a univariate Cox regression model using data from 365 patients. Notably, this study found that certain factors, including pathological staging (T3/4 versus T1/2) and high-versus low-level expression of SKP2, FBXL5, FBXL6, FBXL7, FBXL18, and FBXL19 (determined as the top 50 % versus bottom 50 %), were significantly associated with the prognosis of patients with HCC [ (Risk factors with p < 0.05 for the outcome: pathological-T3/4 or T1/2, the top 50 % of gene expression of SKP2, FBXL5, FBXL6, FBXL7, FBXL18, and FBXL19. Risk factors with p > 0.05 for the outcome: aged 60 years older or younger, male or female, grade 3/4 or grade 1/2, stage III/IV or stage I/II, vascular invasion yes or no, alpha-fetoprotein (AFP) 200 ng/ml above or less, the top 50 % of gene expression of FBXL2, FBXL3, FBXL8, LRRC29, KDM2B, KDM2A, FBXL12, FBXL13, FBXL15, FBXL16, FBXL17, FBXL20, FBXL21, and FBXL22.) (Fig. 2A) ]. The significant predictors mentioned above were used to construct a multivariate Cox regression model. The results revealed that pathological staging (hazards ratio [HR] = 2.702, 95 % confidence interval [CI]: 1.897–3.848, p < 0.001), FBXL5 (HR = 0.609, 95 % CI: 0.427–0.869, p = 0.006), and FBXL19 (HR = 1.675, 95 % CI: 1.181–2.375, p = 0.004) could be considered as independent prognostic factors for OS in patients with HCC (Fig. 2B). To predict the prognosis of patients with HCC, we used the X-tile program to determine the optimal cut off values of FBXL5 and FBXL19 mRNA levels, which were found to be 4.6 and 1.9, respectively (Fig. S2). Kaplan–Meier analysis revealed that higher expression of FBXL5 was associated with better prognosis (Fig. 2C), whereas higher expression of FBXL19 was associated with poorer outcomes (Fig. 2D). Considering the high expression of FBXL5 in HCC tissues, there appears to be a contradictory relationship between FBXL5 expression and OS. Thus, we identified FBXL19 as a potential biomarker for predicting the prognosis of patients with HCC.
Fig. 2.
Screening of potential biomarkers among FBXL family members via differential expression, Cox regression, and survival analysis in TCGA-LIHC cohort. The gene expression of 22 FBXLs was screened between normal tissues and HCC tissues. The results (Fig. S1) showed that 20 FBXLs were differentially expressed except for FBXL4 and FBXL14. Thereafter, 20 FBXLs were analysed in the subsequent step. A. Univariate Cox regression analysis was performed in subgroups of important clinicopathological variables and the expression level of 20 FBXLs genes. B. Multivariate Cox regression analysis was further performed, including seven significant factors from univariate Cox regression analysis. C & D. Kaplan–Meier curves of overall survival analysis based on mRNA expression of FBXL5 and FBXL19, respectively.
3.2. Increased FBXL19 expression was linked to the malignant progression of HCC
To better understand the role of FBXL19 in HCC, we examined 371 RNA-seq data samples acquired from TCGA-LIHC database to evaluate FBXL19 expression. Our analysis demonstrated a substantial increase in the expression levels of FBXL19 in cancerous tissues compared to those in normal tissues (Fig. 3A). Moreover, the examination of 50 paired samples confirmed that FBXL19 expression was consistently high in paired cancer tissues (Fig. 3B). We analysed FBXL19 expression in various clinicopathological cohorts and observed a significant increase in FBXL19 expression in patients with large tumours (Fig. 3C), severe clinical stages (Fig. 3D), and high histological grades (Fig. 3E), except for T4 and stage IV. To further validate this association, we performed IHC staining for FBXL19 in tumour and paracancerous tissues from patients with HCC. Interestingly, we observed high expression of FBXL19 in 68.97 % (N = 60) of HCC tissues and only 20.69 % (N = 18) of adjacent normal tissues (Fig. 3F and G). These results strongly reinforce the positive correlation between FBXL19 expression and clinical features of malignancy. Furthermore, analysis of clinical parameters revealed compelling associations between FBXL19 expression and important indicators of HCC progression, such as tumour size, differentiation, and grade. However, we found no significant correlation between sex, age, AFP, hepatitis B surface antigen (HBsAg), and hepatocirrhosis (Table 3). By integrating the expression levels of FBXL19 with either clinical staging or pathological grading, we categorised patients with HCC into four distinct groups. The results indicated that patients with higher FBXL19 expression in stage 3/4 had a significantly shorter median OS (14.4 months) than those with high FBXL19 expression in stage 1/2 (not reached), low FBXL19 expression in stage 3/4 (61.7 months), or low FBXL19 expression in stage 1/2 (70.5 months) (Fig. 3H). The results also indicated that patients exhibiting high FBXL19 expression in grade 3/4 had the shortest median OS of 25.2 months, whereas those with high FBXL19 expression in grade 1/2 had a prolonged OS of 37.8 months. Conversely, patients with HCC with low FBXL19 expression in grade 3/4 demonstrated a prolonged OS of 56.5 months, while those with low FBXL19 expression in grade 1/2 retained the longest OS of 59.7 months (Fig. 3I). Thus, it can be inferred that FBXL19 upregulation is a significant indicator of advanced HCC.
Fig. 3.
FBXL19 overexpression in patients with HCC is associated with poor outcomes. The transcriptome sequencing data and clinical characteristics of HCC were obtained from the TCGA database using the cBioPortal website (http://www.cbioportal.org) to analyse the FBXL19 mRNA expression level. The protein level of FBXL19 was further analysed in 87 clinical samples via immunohistochemical staining. A. The FBXL19 mRNA expression level in HCC tissues was significantly higher than that in normal tissues. B. In the 50 tumour-normal paired cases, most tumour tissues had higher FBXL19 mRNA level than paracancerous normal tissues. C. Compared to normal tissues, FBXL19 expression was confirmed in cancer tissues at different periods according to TNM staging criteria. D & E. the mRNA level of FBXL19 was evaluated in different stages (D) or grades (E) of HCC progression. F & G. The protein level of FBXL19 in HCC was detected using immunohistochemical staining (F) and quantification analysis (G). H. Kaplan–Meier analyses of overall survival in patients with HCC with low FBXL19 in stage 1/2, low FBXL19 in stage 3/4, high FBXL19 in stage 1/2 and high FBXL19 in stage 3/4 (N = 337). I. Kaplan–Meier analyses of overall survival in patients with HCC with low FBXL19 in grade 1/2, low FBXL19 in grade 3/4, high FBXL19 in grade 1/2 and high FBXL19 in grade 3/4 (N = 365). *p < 0.05, **p < 0.01, ***p < 0.001.
Table 3.
Correlation analysis between FBXL19 expression and clinical features of patients with HCC.
| Clinical features | Cases | FBXL19 |
p value | ||
|---|---|---|---|---|---|
| Low | High | ||||
| Gender | 0.129 | ||||
| Female | 12 | 6(50 %) | 6(50 %) | ||
| Male | 75 | 21(28 %) | 54(72 %) | ||
| Age(years) | 0.237 | ||||
| ≤50 | 42 | 10(23.81 %) | 32(76.19 %) | ||
| >50 | 45 | 17(37.78 %) | 28(62.22 %) | ||
| AFP(ng/mL) | 0.542 | ||||
| ≤200 | 59 | 23(38.98 %) | 36(61.02 %) | ||
| >200 | 28 | 9(32.14 %) | 19(67.86 %) | ||
| HBsAg | 0.462 | ||||
| Positive | 70 | 23(32.86 %) | 47(67.14 %) | ||
| Negative | 17 | 4(23.53 %) | 13(76.47 %) | ||
| Hepatocirrhosis | 0.239 | ||||
| Yes | 68 | 23(33.82 %) | 45(66.18 %) | ||
| No | 19 | 4(21.05 %) | 15(78.95 %) | ||
| Tumor size(cm) | <0.001 | ||||
| ≤5 | 35 | 12(34.29 %) | 23(65.71 %) | ||
| >5 | 52 | 15(28.85 %) | 37(71.15 %) | ||
| Differentiation | 0.04 | ||||
| Well to moderate | 61 | 23(37.70 %) | 38(62.30 %) | ||
| Low | 26 | 4(15.38 %) | 22(84.62 %) | ||
| TNM stage | 0.021 | ||||
| I-II | 63 | 24(38.10 %) | 39(61.90 %) | ||
| III-IV | 24 | 3(12.50 %) | 21(87.50 %) | ||
Footnotes: AFP, alpha fetoprotein; HBsAg, hepatitis B surface antigen; TNM, tumor node metastasis.
3.3. Knockdown of FBXL19 inhibited cell proliferation in SNU-449 cells
Owing to the strong correlation between FBXL19 expression and a poor prognosis in patients with HCC, it can be believed that FBXL19 plays an oncogenic role in this disease. To verify this, we performed in vitro functional assays. Prior to conducting these experiments, we selected a suitable HCC cell line for testing. Therefore, we measured the mRNA levels of FBXL19 in five HCC cell lines and found that the SNU-449 cell line had the highest expression of this gene (Fig. 4A). Subsequently, SNU-449 cells were infected with a lentivirus carrying an FBXL19-specific short hairpin RNA (shRNA) to facilitate a loss-of-function study. Comprehensive qRT-PCR and Western blot confirmed that the mRNA level and protein expression of FBXL19 were significantly reduced in SNU-449 cells (Fig. 4B and C). This was achieved through the successful application of two shRNAs to FBXL19. To further investigate the effect of FBXL19 on cell growth, we performed CCK-8 and plate clone formation assays. Our results revealed that, in comparison with control cells, those with FBXL19 downregulation exhibited significantly decreased cell proliferation (Fig. 4D) and produced fewer and smaller colonies (Fig. 4E and F). In addition, we performed flow cytometry-based cell cycle analysis and observed that FBXL19 downregulation resulted in partial cell cycle arrest at the G1/S transition. This was due to an increase in the number of cells in the G0/G1 phase and a decrease in the number of cells in the S and G2/M phases, as shown in Fig. 4G and H. Taken together, it can be concluded that the inhibition of FBXL19 expression effectively suppresses the growth of HCC cells.
Fig. 4.
FBXL19 knockdown in SNU-449 cells inhibits cell proliferation and cell cycle progression. SNU-449 cells were infected with Ctrl, shFBXL19-1 or shFBXL19-2 lentivirus, and FBXL19 knockdown was identified for subsequent functional assays. A. The mRNA expression of FBXL19 was tested among five HCC cell lines and one human normal liver tissue (HNL) using qRT-PCR. B & C. Confirmation of FBXL19 knockdown using qPCR (B) and western blotting (C). D. The proliferation of SNU-449 cells after FBXL19 knockdown was performed using CCK8 assays. E & F. Clonogenic ability was examined via plate cloning assays. The representative images (E) and statistical analysis (F) of colony formation were shown. G & H. Cell cycle was determined via flow cytometric analysis using PI staining. The representative histograms (G) and statistical analysis of the percentage of cells in each phase (H) are presented. All data are presented as means ± SD of three independent repeated experiments. **p < 0.01, ***p < 0.001.
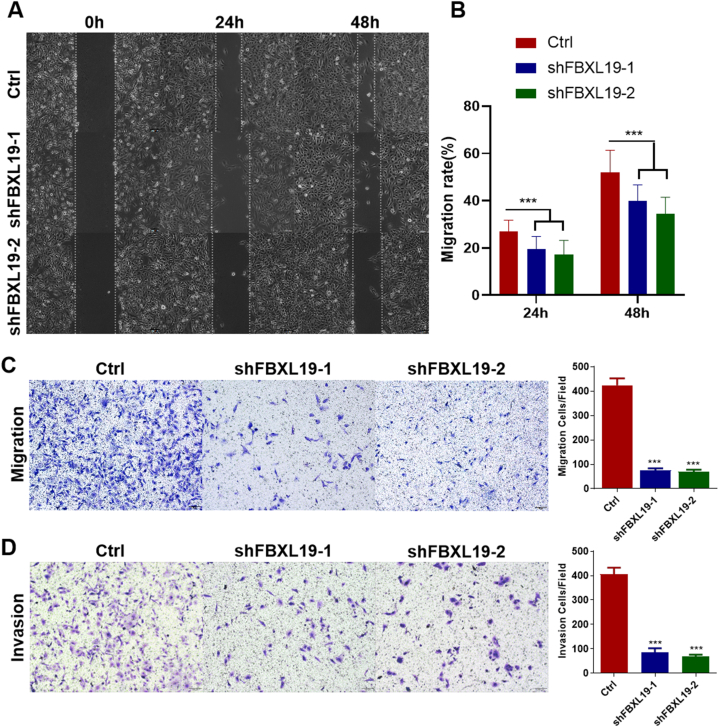
3.4. FBXL19 knockdown inhibited cell migration and invasion in SNU-449 cells
To determine whether suppression of FBXL19 could impede the migration and invasion capabilities of HCC cells, we performed wound healing and transwell assays. Our results indicated that FBXL19 knockdown markedly diminished the migration potential of SNU-449 cells, as evidenced by wound healing (Fig. 5A and B) and transwell assays (Fig. 5C). In addition, compared to the control group, FBXL19 knockdown led to a substantial decrease in cell invasion (Fig. 5D), indicating a significant inhibitory effect on metastasis in HCC. In conclusion, our findings indicated that FBXL19 plays a critical role in suppressing HCC cell metastasis.
Fig. 5.
FBXL19 knockdown in SNU-449 cells suppressed cell motility, migration, and invasion. A & B. Cell motility was tested via scratch assay. Representative images (A) of scratch assay at 0, 24, 48 h and statistical analysis (B) are shown. C. Cell migration was assessed using transwell assay. D. Cell invasion was examined via transwell assay with matrigel coating inserts. Images on the left are representative images, while those on the right are statistical graphs in the panels C and D. For scratch, migration and invasion assays, data are presented as means ± SD of three independent experiments; p-values are calculated using one-way ANOVA with Bonferroni correction. ***p < 0.001.
3.5. FBXL19 knockdown attenuated malignant cell properties by silencing ERK1/2 and p38 pathways
To investigate the signalling pathways responsible for the oncogenic properties of FBXL19, RNA-seq was used to analyse the gene expression profiles of SNU-449 cells that had been transfected with shFBXL19. Additionally, GSEA was performed to gain further insights. Our findings revealed that the DEGs were associated with the activation of MAP3K8-dependent mitogen-activated protein kinase (MAPK) 1/3 (Fig. 6A and B), a component of the MAPK signalling pathway. This pathway is well known for its critical role in controlling cell proliferation, migration, and differentiation. Further analysis confirmed alterations in the levels of phosphorylated proteins associated with the MAPK signalling cascades, namely p-ERK1/2, p-p38, and p-JNK. Our results demonstrated that the shRNA-mediated knockdown of FBXL19 reduced p-ERK1/2 and p-p38 expression, whereas p-JNK exhibited no significant change (Fig. 6C). Furthermore, to identify downstream targets of the ERK1/2 and p38 pathways, we performed qPCR to confirm changes in gene expression related to cell cycle and metastasis markers. Our results demonstrated that FBXL19 knockdown significantly reduced the levels of critical cell cycle proteins for the G1/S phase (CDK4, CDK6) and G2/M phase (Cyclin B) and decreased the expression of metastasis and invasion markers, Vimentin and Twist1, compared with the control group (Fig. 6D–H). Overall, these findings suggest that the downregulation of FBXL19 plays a critical role in inhibiting malignant cell properties by silencing the ERK1/2 and p38 pathways.
Fig. 6.
FBXL19 promotes malignant cell properties via activating ERK1/2 and p38 pathways. A. Heatmap of the signal intensities of differential MAPK1/3 activation-related genes in SNU-449 cells by shFBXL19-mediated knockdown. B. GSEA of MAPK1/3 activation from differentially expressed genes between SNU-449-Ctrl and SNU-449-shFBXL19 cells. C. The protein levels of p-ERK1/2, p-p38 and p-JNK in SNU-449 cells transfected with shFBXL19 were observed via western blotting. D-H. The mRNA expression of cell cycle regulators (CDK4, CDK6, and CyclinB) and metastasis markers (Vimentin and Twist1) were detected via qRT-PCR. **p < 0.01, ***p < 0.001.
4. Discussion
FBXLs are a subfamily of F-box proteins that form an integral part of the SCF-type E3 ubiquitin ligase complex, along with SKP1 and Cullin 1 [26]. These proteins have been implicated in cancer, with many FBXLs serving as oncogenes, tumour suppressors, or regulators depending on their levels of substrate ubiquitination and degradation [27]. We believe that specific FBXL substrates can be used as promising therapeutic targets in cancer therapy.
FBXL19 belongs to the FBXL family and plays critical roles in various cellular processes, including cell proliferation [6], migration [7,8,28], apoptosis [9], and embryonic stem cell differentiation [29]. Several studies have demonstrated the significance of this protein in various cellular functions. FBXL19 is known for its ability to regulate cellular behaviour via ubiquitination and degradation to target small GTPases, such as RAC3, RhoA, RAC1, and CDC42 [7,28,30,31]. These proteins are linked to critical cellular processes, including cell morphology, proliferation, and movement [32]. Notably FBXL19 exerts varying effects on various tumour cells. In oesophageal cancer cells, it promotes the degradation of Rac3, which is required for the downregulation of E-cadherin by TGF-β1 [28]. Conversely, in follicular thyroid carcinoma cells, FBXL19 targets lysine-151 of TTF1 for ubiquitin proteasomal degradation, but does not affect cell proliferation or migration [33]. In glioma cells, FBXL19 is upregulated by SNHG10, leading to a more aggressive phenotype [23]. However, its role in HCC, remains unclear. Here, we present, for the first time, conclusive evidence that FBXL19 exhibits unparalleled prognostic significance within the FBXL family, specifically in relation to HCC. Our study highlights a strong correlation between elevated FBXL19 expression and poor patient outcomes. Furthermore, our findings indicated that FBXL19 suppression significantly impedes the in vitro proliferation and metastasis of HCC cells. These results provide compelling evidence for the putative oncogenic function of FBXL19 in HCC, which is consistent with other cancer types, including breast cancer and gliomas [[22], [23], [24]].
Substrate phosphorylation plays a critical role in the interaction between F-box proteins and their targets by acting as a signal processors [34]. For example, SCFFBXW7α, which mediates the ubiquitination of IRF1, is dependent on IRF1 phosphorylation by GSK3β [35]. Another example is the SCFβTrCP/Slimb E3 ubiquitin ligase, which targets IκB for degradation. This process is based on IκB phosphorylation by IKKs [36]. These findings illustrate that F-box proteins use phosphorylation as a key mechanism to identify and interact with their substrates. The degradation of ST2L via FBXL19 ubiquitination is dependent on the phosphorylation of ST2L at Ser442 by GSK3β [9]. Similarly, degradation of Rac1 and RhoA by FBXL19 is facilitated by the phosphorylation of Rac1 and RhoA by AKT and Erk2, respectively [6,7]. In this study, we demonstrated that FBXL19 plays a critical role in the regulation of ERK1/2 and p38 activation in HCC. ERK1/2 and p38, both serine/threonine kinases, are responsible for the phosphorylation of numerous substrates, thereby regulating a variety of cellular processes, such as cell growth, differentiation, migration, and apoptosis. Our findings provide initial insights into the modulation of ERK1/2 and p38 by FBXL19 in HCC. Therefore, future studies must provide more insights into the specific substrates involved in ERK1/2 and p38 phosphorylation and FBXL19 ubiquitination. This knowledge will be instrumental for understanding the function of FBXL19 in patients with HCC.
However, it is important to note that this study has certain limitations. First, the substrates with which FBXL19 interacts have not yet been identified. Some substrates undergo ubiquitination and degradation following site-specific phosphorylation. While our data showed that FBXL19 could influence the protein levels of phosphorylated ERK1/2 and p38, we have not investigated which specific phosphorylated substrates are targeted by FBXL19 for ubiquitination and degradation. Second, in this study, our experimental validation was limited to only one type of HCC cells in vitro, and there were no in vivo experiments to further validate our conclusions. Third, the findings can only be considered applicable to HCC, further validation is still required to assess the role of FBXL19 in cholangiocellular carcinoma.
In conclusion, our findings demonstrate that elevated FBXL19 expression is associated with a poor prognosis in patients with HCC. Furthermore, FBXL19 knockdown using shRNA restricted tumour cell proliferation and metastasis in vitro. Here, we used a multi-pronged approach to uncover the molecular mechanisms underlying FBXL19-mediated oncogenicity and revealed that both the ERK1/2 and p38 signalling pathways play critical roles in promoting cancer cell growth and metastasis. Taken together, these results suggest that FBXL19 may serve as a potential therapeutic target in combination with other effective treatments for HCC.
Institutional review board statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (2021-KY-Guoji-082 and 2-24-2021).
Data availability statement
Supplemental data for RNA sequencing analysis were stored in Mendeley Data (https://data.mendeley.com), which can easily be accessed at https://data.mendeley.com/datasets/r9v3dcb75r/1.
CRediT authorship contribution statement
Min Xun: Validation, Visualization, Investigation, Writing – original draft. Jiming Wang: Resources, Validation, Investigation. Qiuli Xie: Visualization, Investigation. Bo Peng: Investigation, Resources. Zeyuan Li: Resources, Data curation. Zhengya Guo: Resources, Supervision. Yonglian Zeng: Resources, Supervision. Huizhao Su: Conceptualization, Data curation. Mei Yao: Resources. Lijuan Liao: Resources, Software. Yan Li: Resources, Methodology. Guandou Yuan: Conceptualization, Funding acquisition, Project administration. Shilian Chen: Investigation, Writing – original draft, Writing – review & editing. Songqing He: Funding acquisition, Project administration, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported in part by National Key Research and Development Program (2022YFE0131600), National Natural Science Foundation of China (82160500), The 111 Project (D17011), Special Project of Central Government Guiding Local Science and Technology Development (ZY20198011), Guangxi Science and Technology Base and Talent Project (GuikeAA21220002), Guangxi Key Research and Development Plan (2018AD03001), and Natural Science Foundation of Guangxi (2022GXNSFAA035642).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21771.
Contributor Information
Shilian Chen, Email: chenshilian@stu.gxmu.edu.cn.
Songqing He, Email: dr_hesongqing@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Fig. S1.1.
Gene expression analysis of 22 FBXLs in TCGA-LIHC cohort to identify differentially expressed genes (DEGs). nsp >0.05, *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. S1.2.
Fig. S2.
The X-tile program was utilized to identify the optimal cut-off values for FBXL5 and FBXL19.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71 doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16 doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zucman-Rossi J., Villanueva A., Nault J.C., Llovet J.M. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149 doi: 10.1053/j.gastro.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 4.Katoh M., Katoh M. Identification and characterization of FBXL19 gene in silico. Int. J. Mol. Med. 2004;14 doi: 10.3892/ijmm.14.6.1109. [DOI] [PubMed] [Google Scholar]
- 5.Xu C., Liu K., Lei M., Yang A., Li Y., Hughes T.R., Min J. DNA sequence recognition of human CXXC domains and their structural determinants. Structure. 2018;26 doi: 10.1016/j.str.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Wei J., Mialki R.K., Dong S., Khoo A., Mallampalli R.K., Zhao Y., Zhao J. A new mechanism of RhoA ubiquitination and degradation: roles of SCF(FBXL19) E3 ligase and Erk2. Biochim. Biophys. Acta. 2013;1833:2757–2764. doi: 10.1016/j.bbamcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao J., Mialki R.K., Wei J., Coon T.A., Zou C., Chen B.B., Mallampalli R.K., Zhao Y. SCF E3 ligase F-box protein complex SCFFBXL19 regulates cell migration by mediating Rac1 ubiquitination and degradation. FASEB (Fed. Am. Soc. Exp. Biol.) J. 2013;27 doi: 10.1096/fj.12-223099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong S., Wei J., Bowser R.K., Chen B.B., Mallampalli R.K., Miao J., Ye Q., Tran K.C., Zhao Y., Zhao J. SCF FBXW17 E3 ubiquitin ligase regulates FBXL19 stability and cell migration. J. Cell. Biochem. 2021;122 doi: 10.1002/jcb.29860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J., Wei J., Mialki R.K., Mallampalli D.F., Chen B.B., Coon T., Zou C., Mallampalli R.K., Zhao Y. F-box protein FBXL19-mediated ubiquitination and degradation of the receptor for IL-33 limits pulmonary inflammation. Nat. Immunol. 2012;13:651–658. doi: 10.1038/ni.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postow L., Funabiki H. An SCF complex containing Fbxl12 mediates DNA damage-induced Ku80 ubiquitylation. Cell Cycle. 2013;12 doi: 10.4161/cc.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee B.K., Lee J., Shen W., Rhee C., Chung H., Kim J. Fbxl19 recruitment to CpG islands is required for Rnf20-mediated H2B mono-ubiquitination. Nucleic Acids Res. 2017;45 doi: 10.1093/nar/gkx310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuart P.E., Nair R.P., Ellinghaus E., Ding J., Tejasvi T., Gudjonsson J.E., Li Y., Weidinger S., Eberlein B., Gieger C., Wichmann H.E., Kunz M., Ike R., Krueger G.G., Bowcock A.M., Mrowietz U., Lim H.W., Voorhees J.J., Abecasis G.R., Weichenthal M., Franke A., Rahman P., Gladman D.D., Elder J.T. Genome-wide association analysis identifies three psoriasis susceptibility loci. Nat. Genet. 2010;42:1000–1004. doi: 10.1038/ng.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabaleiro T., Prieto-Pérez R., Navarro R., Solano G., Román M., Ochoa D., Abad-Santos F., Daudén E. Paradoxical psoriasiform reactions to anti-TNFα drugs are associated with genetic polymorphisms in patients with psoriasis. Pharmacogenomics J. 2016;16:336–340. doi: 10.1038/tpj.2015.53. [DOI] [PubMed] [Google Scholar]
- 14.Prieto-Pérez R., Solano-López G., Cabaleiro T., Román M., Ochoa D., Talegón M., Baniandrés O., López-Estebaranz J.L., De La Cueva P., Daudén E., Abad-Santos F. Polymorphisms associated with age at onset in patients with moderate-to-severe plaque psoriasis. J Immunol Res. 2015;2015 doi: 10.1155/2015/101879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman P., Elder J.T. Genetics of psoriasis and psoriatic arthritis: a report from the grappa 2010 annual meeting. J. Rheumatol. 2012 doi: 10.3899/jrheum.111242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandran V. The genetics of psoriasis and psoriatic arthritis. Clin. Rev. Allergy Immunol. 2013;44:149–156. doi: 10.1007/s12016-012-8303-5. [DOI] [PubMed] [Google Scholar]
- 17.Bluett J., Barton A. What have genome-wide studies told us about psoriatic arthritis? Curr. Rheumatol. Rep. 2012;14 doi: 10.1007/s11926-012-0255-5. [DOI] [PubMed] [Google Scholar]
- 18.Jiménez C.M., Ramírez C.P., Martín A.S., Maroun S.V., Santiago S.A.A., del Carmen Ramírez Tortosa M., Morales A.J. Influence of genetic polymorphisms on response to biologics in moderate-to-severe psoriasis. J. Personalized Med. 2021;11 doi: 10.3390/jpm11040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei J., Dong S., Bowser R.K., Khoo A., Zhang L., Jacko A.M., Zhao Y., Zhao J. Regulation of the ubiquitylation and deubiquitylation of CREB-binding protein modulates histone acetylation and lung inflammation. Sci. Signal. 2017;10 doi: 10.1126/scisignal.aak9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhushan R., Rani A., Ali A., Singh V.K., Dubey P.K. Bioinformatics enrichment analysis of genes and pathways related to maternal type 1 diabetes associated with adverse fetal outcomes. J. Diabet. Complicat. 2020;34 doi: 10.1016/j.jdiacomp.2020.107556. [DOI] [PubMed] [Google Scholar]
- 21.Guo P., Gong W., Li Y., Liu L., Yan R., Wang Y., Zhang Y., Yuan Z. Pinpointing novel risk loci for Lewy body dementia and the shared genetic etiology with Alzheimer's disease and Parkinson's disease: a large-scale multi-trait association analysis. BMC Med. 2022;20 doi: 10.1186/s12916-022-02404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.jie Du X., rong Yang X., cai Wang Q., liang Lin G., fei Li P., feng Zhang W. Identification and validation of a five-gene prognostic signature based on bioinformatics analyses in breast cancer. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin L., Huang S., Guan C., Chang S. ETS1-activated SNHG10 exerts oncogenic functions in glioma via targeting miR-532-3p/FBXL19 axis. Cancer Cell Int. 2020;20 doi: 10.1186/s12935-020-01649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong X., Liu L., Xiong J., Li X., Xu J., Xiao Y., Li J., Luo X., Mao D., Liu L. Construction of a prognostic gene signature associated with immune infiltration in glioma: a comprehensive analysis based on the cgga. JAMA Oncol. 2021;2021 doi: 10.1155/2021/6620159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan G., Zhang B., Yang S., Jin L., Datta A., Bae S., Chen X., Datta P.K. Novel role of STRAP in progression and metastasis of colorectal cancer through Wnt/β-catenin signaling. Oncotarget. 2016;7 doi: 10.18632/oncotarget.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin J., Cardozo T., Lovering R.C., Elledge S.J., Pagano M., Harper J.W. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004;18 doi: 10.1101/gad.1255304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tekcham D.S., Chen D., Liu Y., Ling T., Zhang Y., Chen H., Wang W., Otkur W., Qi H., Xia T., Liu X., Piao H.L., Liu H. F-box proteins and cancer: an update from functional and regulatory mechanism to therapeutic clinical prospects. Theranostics. 2020;10 doi: 10.7150/thno.42735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong S., Zhao J., Wei J., Bowser R.K., Khoo A., Liu Z., Luketich J.D., Pennathur A., Ma H., Zhao Y. F-box protein complex FBXL19 regulates TGFβ1-induced E-cadherin down-regulation by mediating Rac3 ubiquitination and degradation. Mol. Cancer. 2014;13 doi: 10.1186/1476-4598-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimitrova E., Kondo T., Feldmann A., Nakayama M., Koseki Y., Konietzny R., Kessler B.M., Koseki H., Klose R.J. FBXl19 recruits CDK-Mediator to CpG islands of developmental genes priming them for activation during lineage commitment. Elife. 2018;7 doi: 10.7554/eLife.37084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khong Z.J., Lai S.K., Koh C.G., Geifman-Shochat S., Li H.Y. A novel function of AAA-ATPase p97/VCP in the regulation of cell motility. Oncotarget. 2020;11 doi: 10.18632/oncotarget.27419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei J., Dong S., Yao K., Martinez M.F.Y.M., Fleisher P.R., Zhao Y., Ma H., Zhao J. Histone acetyltransferase CBP promotes function of SCF FBXL19 ubiquitin E3 ligase by acetylation and stabilization of its F-box protein subunit. FASEB (Fed. Am. Soc. Exp. Biol.) J. 2018;32 doi: 10.1096/fj.201701069R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vega F.M., Ridley A.J. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582 doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 33.Liu J., Dong S., Wang H., Li L., Ye Q., Li Y., Miao J., Jhiang S., Zhao J., Zhao Y. Two distinct E3 ligases, SCFFBXL19 and HECW1, degrade thyroid transcription factor 1 in normal thyroid epithelial and follicular thyroid carcinoma cells, respectively. FASEB (Fed. Am. Soc. Exp. Biol.) J. 2019;33 doi: 10.1096/fj.201900415R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skowyra D., Craig K.L., Tyers M., Elledge S.J., Harper J.W. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91 doi: 10.1016/S0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- 35.Garvin A.J., Khalaf A.H.A., Rettino A., Xicluna J., Butler L., Morris J.R., Heery D.M., Clarke N.M. GSK3-SCFFBXW7 mediated phosphorylation and ubiquitination of IRF1 are required for its transcription-dependent turnover. Nucleic Acids Res. 2019;47 doi: 10.1093/nar/gkz163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spencer E., Jiang J., Chen Z.J. Signal-induced ubiquitination of IkappaBalpha by the F-box protein Slimb/beta-TrCP. Genes Dev. 1999;13:284–294. doi: 10.1101/gad.13.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplemental data for RNA sequencing analysis were stored in Mendeley Data (https://data.mendeley.com), which can easily be accessed at https://data.mendeley.com/datasets/r9v3dcb75r/1.