Abstract
Solanum anguivi Lam. fruits (SALF) possess bioactive compounds, such as phenolics, alkaloids, saponins, flavonoids, and vitamin C, that are beneficial for preventing oxidative stress-related diseases. It has been documented that ripeness stage influences the nutritional quality of fruits. However, there is limited information on the effect of the ripeness stages (unripe, yellow, orange and red) on the bioactive compounds' contents (BCC) and antioxidant activity (AA) of SALF. We investigated the effect of ripening on the BCC and AA of different SALF accessions. Spectrophotometry was used to determine SALF's total contents of phenolics, flavonoids, saponins, vitamin C, and AA and gravimetry for total alkaloids. The AA was determined as free radical scavenging activity (FRSC) and total antioxidant capacity (TAC). The total phenolics (7.6–22.6 mg gallic acid equivalent/g DW), flavonoids (1.3–4.1 mg quercetin equivalent (QE)/g DW), saponins (44.8–152.5 mg diosgenin equivalent/g DW), vitamin C (2.2–6.4 mg ascorbic acid equivalent/g DW), alkaloids (141.2–296.9 mg/g DW), FRSC (1.5–66.2 %) and TAC (0.1–14.2 mg QE/g DW) significantly differed among the ripeness stages. Fruits in the unripe stage were rich in phenolics, flavonoids, and AA; in the red stage in alkaloids and vitamin C; and in the orange stage, in saponins and flavonoids. The AA had strong positive correlations with total flavonoids and phenolics (r = 0.72 and 0.81, respectively) and a moderate negative correlation with total alkaloids (r = −0.67). Overall, unripe stage fruits had the highest AA and total phenolics and thus may have the highest health-promoting properties. Botanists and farmers may, therefore, focus on harvesting and trading SALF to markets/consumers while still unripe.
Keywords: Solanum anguivi, Bioactive compounds, Antioxidant capacity, Free radical scavenging capacity, Accession, Ripeness stage, Maturity stage, Bitter berries
1. Introduction
Fruits and vegetables are among the most important sources of bioactive compounds for the human diet [1]. Bioactive compounds possess many health benefits, such as antioxidative, antibacterial, antifungal, antiviral, cholesterol‐lowering, antithrombotic, and anti‐inflammatory effects [2]. Consumption of fruits and vegetables has, therefore, been associated with the prevention of chronic and degenerative diseases [3]. Fruits from Solanaceae plants are important as they contain various bioactive compounds, which are suggested to have medicinal properties [4]. Solanum, a widespread plant genus of the family Solanaceae, has over 1000 species worldwide, with at least 100 indigenous species in Africa and adjacent islands, which include several valuable crop plants and some poisonous ones [5]. The fruits of Solanum anguivi Lam. have been reported to treat various diseases, including hypertension, atherosclerosis, and diabetes [6,7].
The bioactive compounds’ contents (BCC) in fruits of the same species may vary with the genotype and ripeness stage [8]. Different varieties or cultivars amongst plants of the same species, grown under the same conditions, have been previously reported to differ in the BCC and antioxidant activity (AA) due to genetic differences [[9], [10], [11]]. Various studies have similarly shown that ripening may boost or diminish the BCC of fruits. For example, total phenolics significantly differed at the different ripeness stages of apples [12] and tomatoes [13]. Both Silva et al. [12] and Bhandari & Lee [13] further showed that the differences during ripening were influenced by the variety for apples and cultivars for tomatoes. On the contrary, Amira et al. [14] reported that although total phenolics for date fruits differed significantly with the ripeness stage, the effect was not significantly different among the cultivars. In addition to the genotype and ripeness stage, environmental factors such as climate, soil properties, and agricultural practices may affect the BCC and AA of fruits and vegetables [8,15,16]. The BCC and AA of fruits for the same species, as reported by different authors, may vary due to variations in the abovementioned factors. To analyze the effect of the ripeness stage on fruits of the same plant species, factors that may confound the results, such as location and agricultural practices, should be similar for the plants under investigation.
Solanum anguivi Lam. fruits (SALF) have been reported to possess phenolics, flavonoids, saponins, alkaloids, vitamin C, and tannins [[17], [18], [19], [20]]. However, the effect of the ripeness stage has only been investigated on the total contents of phenolics, flavonoids, tannins, and vitamin C [17,18]. However, data on the effect of ripening on SALF total saponin and alkaloid contents is scarce. Furthermore, there is a dearth of information on the effect of ripening on the antioxidant activity and bioactive compounds’ contents among different accessions of SALF. This study, therefore, investigated the influence of the ripeness stage on the BCC (total contents of flavonoids, phenolics, vitamin C, saponins, and alkaloids) and AA (free radical scavenging capacity and total antioxidant capacity) of different accessions of SALF. This would establish the ripeness stage at which SALF may be consumed for potentially high health benefits. The study also determined the associations between the BCC and AA of SALF.
2. Materials and methods
2.1. Reagents and chemicals
The reference standards (gallic acid, quercetin, diosgenin, l-ascorbic acid), HPLC grade ethanol, acetic acid, ammonium hydroxide, sulphuric acid, methanol; and analytical grade 1-diphenyl-2-picrylhydrazyl (DPPH), trichloroacetic acid, vanillin, hydrated copper (II) sulphate, potassium acetate, thiourea, sodium bicarbonate, dinitrophenyl hydrazine aluminium chloride and Folin-Ciocalteu reagent, were all purchased from Sigma-Aldrich (Munich, Germany). The reference standards (gallic acid, quercetin, diosgenin and ascorbic acid) for quantification of total phenolics, flavonoids, saponins and vitamin C were obtained from Sigma-Aldrich, Steinheim, Germany.
2.2. Sample collection
All the SALF samples in this study were obtained from the same geographical location to eliminate or minimize the effects of other variables. The samples were collected from Nabiyagi village (GPS 0.472336, 32.802484), Mukono district (Uganda), in March 2019. The fruits at four ripeness stages (unripe, yellow, orange, and red) [17,18] were collected from four Solanum anguivi Lam. accessions coded in this study as GP1, WP1, CP1, and GC1. The fruits from the four accessions and four ripeness stages were all available in the stated location and month of collection. The colors of SALF at the unripe stage differed among the accessions. Unripe fruits of GP1 were light greenish-cream, WP1 and CP1 were white, while GC1 was light green with white venations, as illustrated in Fig. 1 [21]. The SALF colors for the rest of the ripeness stages under investigation were similar among the accessions, as illustrated in Fig. 2.
Fig. 1.
Photographs of the four of Solanum anguivi Lam. fruit accessions at the unripe stage. Photographs are not to scale. Adapted from Nakitto et al. [21].
Fig. 2.
Photographs illustrating the yellow, orange and red stages of ripeness of Solanum anguivi Lam. fruits. Photographs are not to scale.
2.3. Experimental design
Fifty fruits per ripeness stage were randomly collected from 12 plants per accession and prepared on the same day as described in the “sample preparation” section. This process was replicated the second time by obtaining fruit samples from each accession two weeks after the first collection. The samples were prepared to obtain flours, which were analyzed for total contents of flavonoids, phenolics, vitamin C, saponins, alkaloids, and AA (free radical scavenging capacity and total antioxidant capacity).
2.4. Sample preparation
Procedurally, the fruits were sorted to remove those with damaged pericarps. Stalks were plucked off the fruits, the latter washed with distilled water and then patted dry with a cotton cloth. The fruits were cut into four parts, and those with pests were discarded. The cut fruits were dried in an oven (Infrared Food Oven GL-2A, Guangzhou Itop Kitchen Equipment Co, Ltd. Guangdong, China) at 40 °C for 16 h. The samples were then milled (Wonder Mill, Pocatello, Idaho) at a “pastry” setting to obtain fine flours that were then stored in sealed plastic bottles at −20 °C until further analysis. All samples had two flours obtained independently.
2.5. Extraction of bioactive compounds for total quantification
Using 80 % methanol [21,22], the bioactive compounds were extracted from the SALF powders as described by FAO/IAEA [23] with slight modifications. Briefly, SALF powder (0.2 g) was weighed into a falcon tube, and 80 % methanol (20 ml) was added. The falcon tube was then put into an ultrasonic water bath (Bransonic series, M 2800‐E; Branson Ultrasonics Co, Danbury, CT) and subjected to ultrasonic treatment at room temperature for 10 min. The water in the ultrasonic water bath was prevented from getting warm by replacing half of it every 2 min (to maintain it at room temperature 24–25 °C). The contents of the falcon tubes were cooled on ice and then centrifuged (Fisher Scientific 225, Pittsburg, PA, USA) at 3000×g for 10 min. The supernatant was collected into another falcon tube and kept on ice. An equal volume of 80 % methanol was added to the remaining pellet, and this was also subjected to ultrasonic treatment for the same duration as the first extraction. The contents were centrifuged, and the supernatant was collected as described above. This procedure was repeated for the third time. All three extracts were pooled together and stored at −20 °C until further analysis. Each sample had two extracts from the two independent flour samples.
2.6. Quantification of bioactive compounds and antioxidant activity
The extracts of the SALF samples were analyzed for total contents of phenolics, flavonoids, saponins, vitamin C, alkaloids, and AA. The analysis of total phenolics was done by the Folin-Ciocalteu reagent method [24] and total flavonoids as described by R. S. Kumar et al. [25]. The total saponins were determined by the method of Hiai et al. [26], vitamin C content was determined using the 2,4-Dinitrophenylhydrazine method as described by Omaye et al. [27] and Roe [28], while total alkaloids were determined by the method of Harborne [29]. The total contents of phenolics, flavonoids, saponins, vitamin C, and alkaloids were expressed as gallic acid equivalent (GAE), quercetin equivalent (QE), diosgenin equivalent (DE), ascorbic acid equivalent (AAE), and mg/g, respectively.
The AA was analyzed by determining the free radical scavenging capacity (FRSC) of the samples, using the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay according to Brand-Williams et al. [30]. A quercetin standard curve was prepared with the FRSC (%) against the quercetin concentration (1–10 μg/ml), and the total antioxidant capacity (TAC) of the samples was subsequently estimated from the quercetin standard curve and expressed as mg QE/g. The FRSC was calculated as shown in Equation (1) (Eq. (1)):
| (1) |
All the absorbances in this study were measured using an ultraviolet spectrophotometer (PerkinElmer 3100, Artisan Technology Group 101E Mercury Drive, Champaign, IL, USA). To obtain the blank readings, the same procedures were conducted by replacing the sample extract with 80 % methanol. The moisture contents of the flour samples were determined using AOAC method # 24.003. Subsequently, the dry matter of the samples was computed, and the results for the bioactive compounds’ contents and total antioxidant capacity were expressed on a dry weight (DW) basis.
3. Statistical data analysis
Statistical analyses and graph presentations were carried out using Statistical Package for the Social Sciences (SPSS) software (Version 21, IBM, Armonk, NY, USA) and GraphPad Prism (Version 8.4.1, GraphPad Software, San Diego, CA, USA). The BCC and AA experiments had two independent extracts per sample, and the analyses were done in triplicates. The BCC and AA data were proven for normality of distribution (Kolmogorov–Smirnov and Shapiro–Wilk) or transformed if not normally distributed using a two-step approach described by Templeton [31]. Briefly, step 1 involves transforming the variable into a percentile rank that results in uniformly distributed probabilities, and step 2 applies the inverse-normal transformation to the results of step 1 to form a variable consisting of normally distributed z-scores. The means were compared using ANOVA to determine statistically significant differences in sample means of BCC and TAC data. The post-hoc Duncan test was used to separate the means at p < 0.05. The results were expressed as mean ± standard error of the mean (SEM). A generalized linear model (GLM) was used to determine the effect size (partial eta squared) for accession, ripeness stage, and interaction between accession and ripeness stage on the BCC and TAC. Pearson correlation analysis was carried out to determine linear relationships between the BCC (independent variables) and AA (dependent variables). Principal component analysis (PCA) was applied for the BCC, AA, and ripeness stage to determine the contribution of different variables to the variance of data and to further determine the association between the BCC, AA, and ripeness stage. The PCA rotation method used was Varimax, with correlations below 0.3 excluded. Multilinear regression by the step-wise method was carried out to derive models for AA, and all the data used were normalized, as described above. To investigate the effect size of the variables on the BCC and AA of SALF, Partial eta squared was carried out. Significance was accepted at p < 0.05 for all statistical analyses.
4. Results
4.1. Bioactive compounds’ contents and antioxidant activities of SALF accessions as affected by ripening
The total phenolics of SALF in this study significantly differed among the ripeness stages and accessions (7.6–22.6 mg GAE/g DW) (Table 1).
Table 1.
Bioactive compounds’ contents of SALF accessions at different stages of ripeness.
| Accession code | Ripeness stage | TPC mg GAE/g | TFC mg QE/g | TSC mg DE/g | TAL mg/g | VCC mg AAE/g |
|---|---|---|---|---|---|---|
| GC1 | Unripe | 8.4 ± 0.23A,Bf,g | 1.73 ± 0.14Af,g | 86.97 ± 1.99Af | 165.85 ± 2.5De | 3.42 ± 0.09Cg |
| Yellow | 7.63 ± 0.23Bg | 1.28 ± 0.01Ci,j | 80.71 ± 1.8Bg | 189.9 ± 4.01Cc,d | 4.6 ± 0.16Bd,e,f | |
| Orange | 8.57 ± 0.3Af,g | 1.64 ± 0.02Ag,h | 69.11 ± 1.48Ch | 216.8 ± 3.77Bb | 4.8 ± 0.24Bc,d,e,f | |
| Red | 9.15 ± 0.4Ae,f | 1.47 ± 0.03Bh,i | 87.66 ± 2.64Af,g | 296.94 ± 5.78Aa | 5.35 ± 0.12Ab,c,d,e | |
| CP1 | Unripe | 14.62 ± 0.49Ac | 1.32 ± 0.05Bi,j | 134.08 ± 2.55Bc | 156.29 ± 3.88Be,f,g | 6.02 ± 0.17Aa,b |
| Yellow | 9.68 ± 0.13Be,f | 1.26 ± 0.05Cj | 144.51 ± 1.93Ab | 190.88 ± 3.78Ac,d | 4.55 ± 0.67Be,f | |
| Orange | 8.67 ± 0.32Cf,g | 2.79 ± 0.08Ad | 148.59 ± 1.9Aa,b | 192.86 ± 4.33Ac,d | 4.51 ± 0.21Be,f | |
| Red | 8.88 ± 0.17Ce,f,g | 1.44 ± 0.04Bi,j | 126.9 ± 2.52Bd | 199.79 ± 3.82Ac | 3.95 ± 0.39Bf,g | |
| WP1 | Unripe | 12.88 ± 0.44Ad | 1.89 ± 0.03Af | 58.28 ± 0.77Ai | 161.04 ± 4.07Be,f | 2.19 ± 0.33Dh |
| Yellow | 9.77 ± 0.3Be,f | 1.7 ± 0.04Bg | 52.1 ± 1.36Bj | 149.88 ± 4.53Bf,g,h | 3.2 ± 0.24Cg | |
| Orange | 9.46 ± 0.38Be,f | 2.09 ± 0.05Ae | 56.47 ± 0.69Ai,j | 190.17 ± 3.67Ac,d | 4.02 ± 0.23Bf,g | |
| Red | 10.18 ± 0.18A,Be | 2.08 ± 0.13Ae | 44.75 ± 2.13Ck | 188.7 ± 4.87Ac,d | 5.44 ± 0.11Ab,c,d | |
| GP1 | Unripe | 22.57 ± 0.64Aa | 4.09 ± 0.04Aa | 83.03 ± 1.31Cf,g | 146.69 ± 4.05Bg,h | 5.52 ± 0.15Ba,b,c |
| Yellow | 13.83 ± 1.19Cc,d | 3.76 ± 0.06Bb | 111.05 ± 2.46Be | 141.23 ± 3.7Bh | 5.68 ± 0.24Ba,b | |
| Orange | 16.01 ± 0.26Bb | 4.16 ± 0.08Aa | 152.51 ± 2.07Aa | 182.23 ± 8.21Ad | 6.39 ± 0.23Aa | |
| Red | 16.77 ± 0.46Bb | 3.49 ± 0.07Bc | 122.23 ± 1.44A,Bd | 191.37 ± 5.54Ac,d | 5.9 ± 0.1Ba,b |
Values are the mean ± SEM of two independent experiments measured in triplicates. The fruits in each independent experiment were obtained from 12 plants per accession per ripeness stage. Results refer to the corresponding dry weights of SALF. Significant differences between all samples for each compound are shown by different lowercase letters while those per accession are shown in uppercase letters. TFC = total flavonoid content, QE = quercetin equivalent, TPC = total phenolic content, GAE = gallic acid equivalent, TSC = total saponin content, DE = diosgenin equivalent, TAL = total alkaloid content, VCC = Vitamin C content, AAE = ascorbic acid equivalent.
The total phenolics of SALF were highest at the unripe stage for all accessions. The total phenolics significantly decreased with ripening progression among three SALF accessions (WP1, CP1, and GP1), while accession GC1 was unaffected by ripening. For the accessions affected by ripening, there was a difference in the total phenolics trend from the unripe to the red stage. Similar to the total phenolics, total flavonoids significantly differed (1.3–4.2 mg QE/g DW) among ripeness stages for all the SALF accessions (Table 1). Evidently, total flavonoids in all accessions decreased from unripe to the yellow stage, increased at the orange stage, and then decreased (GP1, CP1, GC1) or remained constant (WP1) at the red stage. The total flavonoids were highest at the unripe and/or orange stage(s) and least at the yellow stage.
The effect of ripening on total saponins (44.8–152.5 mg DE/g DW) and total alkaloids (146.7–296.9 mg/g DW) of SALF (Table 1) significantly differed among the accessions. The total saponins were highest at the orange stage for accessions WP1, CP1, and GP1, and the unripe and red stages for GC1, while the total alkaloids significantly increased with ripening for all SALF accessions. Vitamin C of most SALF accessions (GP1, GC1, and WP1) in this study significantly increased with ripening, from 2.2 to 5.5 mg AAE/g DW at the unripe stage to 6.4 mg AAE/g DW at the orange stage (GP1) or 5.4 mg AAE/g DW at the red stage (GC1 and WP1) (Table 1). However, the vitamin C of accession CP1 decreased with ripening, from 6.0 to 3.9 mg AAE/g DW, for the unripe to red stage.
Ripening significantly decreased the antioxidant activity of SALF accessions (Table 2). At the unripe stage, the FRSC and TAC reduced from a range of 6.39–66.17 % and 1.12–14.2 mg QE/g DW to 1.47–26.22 % and 0.06–5.38 mg QE/g DW at the red stage.
Table 2.
Free radical scavenging capacity and total antioxidant capacity of SALF accessions at different stages of ripeness.
| Accession | Ripeness stage | FRSC (%) | TAC (mg QE/g) |
|---|---|---|---|
| GC1 | Unripe | 6.39 ± 1.85Af | 1.12 ± 0.39Af |
| Yellow | 2.08 ± 0.24A,Bg | 0.19 ± 0.05A,Bg | |
| Orange | 2.29 ± 0.63Bg | 0.24 ± 0.14A,Bg | |
| Red | 1.47 ± 0.12Bg | 0.06 ± 0.03Bg | |
| CP1 | Unripe | 22.72 ± 1.63Ac,d | 4.65 ± 0.36Ac,d |
| Yellow | 9.4 ± 1.63Bf | 1.77 ± 0.35Bf | |
| Orange | 8.19 ± 0.48Bf | 1.49 ± 0.1Bf | |
| Red | 8.4 ± 0.96Bf | 1.58 ± 0.21Bf | |
| WP1 | Unripe | 40.59 ± 1.48Ab | 8.63 ± 0.33Ab |
| Yellow | 20.11 ± 0.65Bd,e | 4.12 ± 0.14Bd,e | |
| Orange | 17.09 ± 0.7Ce | 3.48 ± 0.16Ce | |
| Red | 21.38 ± 0.48Bd | 4.37 ± 0.1Bd | |
| GP1 | Unripe | 66.17 ± 3.15Aa | 14.18 ± 0.7Aa |
| Yellow | 37.41 ± 0.49Bb | 7.92 ± 0.12Bb | |
| Orange | 37.9 ± 0.95Bb | 7.96 ± 0.22Bb | |
| Red | 26.22 ± 1.83Cc | 5.38 ± 0.37Cc |
Values are the mean ± SEM of two independent experiments measured in triplicates. The fruits in each independent experiment were obtained from 12 plants per accession per ripeness stage. Results refer to the corresponding dry weights of SALF. FRSC = free radical scavenging capacity, TAC = total antioxidant capacity, QE = quercetin equivalent.
4.2. Effect of accession and ripeness stage on the bioactive compounds’ contents and antioxidant activity of SALF
The partial eta square analysis showed that both accession and ripeness stage significantly influenced the bioactive compounds’ contents and antioxidant activity of SALF (Table 3). However, there was a significant interaction between the accession and ripeness stage (Table 3); thus, the influence of the ripeness stage on the BCC and AA of SALF was also significantly dependent on the accession. The partial eta square analysis further showed that the models had very high adjusted r2, which may thus explain the variance of 84 %, 91 %, 94 %, 83 %, 71 %, and 92 % for total contents of phenolics, flavonoids, saponins, alkaloids, vitamin C and antioxidant activity, respectively, in SALF.
Table 3.
Effect sizes of the variables (ripeness stage and accession) on the bioactive compounds’ contents and antioxidant activity of SALF.
| Chemical composition | Accession | Ripeness stage | Accession*ripeness stage | Adjusted r2 |
|---|---|---|---|---|
| TFC | 0.90 | 0.67 | 0.58 | 0.91 |
| TPC | 0.82 | 0.47 | 0.44 | 0.84 |
| TSC | 0.94 | 0.52 | 0.76 | 0.94 |
| TAL | 0.69 | 0.79 | 0.46 | 0.83 |
| VCC | 0.62 | 0.17 | 0.56 | 0.71 |
| FRSC | 0.92 | 0.73 | 0.29 | 0.92 |
| TAC | 0.92 | 0.70 | 0.26 | 0.92 |
Partial eta squared was carried out to assess the effect sizes of the variables. All the displayed effect sizes of the variables and variable interactions in the table were significant at p < 0.05. TFC = total flavonoid content, TPC = total phenolic content, TSC = total saponin content, TAL = total alkaloid content, VCC = Vitamin C content, FRSC = free radical scavenging capacity, TAC = total antioxidant capacity, * = represents an interaction.
4.3. Relation between antioxidant activity and bioactive compounds’ content in SALF
4.3.1. Correlation and principal component analysis
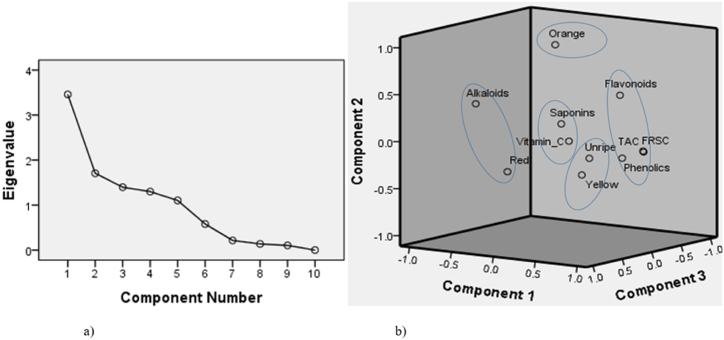
Computations revealed that AA positively correlated with total flavonoids (r = 0.612) and total phenolics (r = 0.820) and negatively with total alkaloids (r = −0.578). The principal component analysis of BCC, AA, and the ripeness stage generated five components based on (i) Kaiser's criterion of Eigenvalues >1 and (ii) Catell's scree graph spot of abrupt level out [32] (Fig. 3a). The components accounted for a total variance of 88.7 % (Fig. 3b). Components 1, 2, 3, 4, and 5 explained 31.9, 15.7, 15.6, 12.8, and 12.7 % variance of the data.
Fig. 3.
A scree graph and components formed from the principal component analysis. a) A scree graph showing the number of principal components derived with an Eigenvalue greater than 1 (5 components). b) A representation of the five components derived from the principal component analysis on a 3-component axis. Component 1 = TAC, FRSC, phenolics, and flavonoids; component 2 = orange; component 3 = red stage and alkaloids; component 4 = saponins and vitamin_C; and component 5 = unripe and yellow stages. TAC = total antioxidant capacity, FRSC = free radical scavenging capacity, phenolics = total phenolic content, flavonoids = total flavonoid content, saponins = total saponin content, alkaloids = total alkaloid content, vitamin_C = vitamin C content; unripe, yellow, orange and red = stages of ripeness.
4.3.2. Regression analysis
Regression models for AA were derived in the study. Three factors, that is, the statistical significance of the independent variables (statistical significance of F-ratio in the derived ANOVA table), the significance of the unstandardized coefficients of the independent variables in the model, and the significance and strength of the adjusted r2 (to determine how well the regression model fits the data) were evaluated. The regression models were based on the formula in Equation (2) (Eq. (2)).
| (2) |
Where YA=Predicted mean AA, b0 = constant, and b1, b2, and bnth = regression unstandardized coefficients for independent variables X1, X2, and Xnth, respectively.
The overall regression models for TAC and FRSC (irrespective of accession and ripeness stage) (Equations (3) and (4), respectively) had high adjusted r2 (0.791 and 0.792, respectively), which showed that they both explained 79 % variance for SALF AA.
| (3) |
| (4) |
Since the adjusted r2 for the TAC and FRSC models were not significantly different, only the TAC regression models at different ripeness stages were derived, as shown below (Equations (5), (6), (7), (8))).
-
a)
Unripe stage
| (5) |
-
b)
Yellow stage
| (6) |
-
c)
Orange stage
| (7) |
-
d)
Red stage
| (8) |
The models had high adjusted r2 values at the unripe, yellow, orange, and red stages, which showed 87 %, 89 %, 78 %, and 75 % variance in TAC of SALF at different stages of ripeness, respectively, may be explained by the models.
5. Discussion
Phenolics have been reported to have antidiabetic [33] and free radical scavenging properties [34]. The total phenolic content of SALF in this study is comparable to that of black chokeberries (19.54–52.9 mg GAE/g DW) [35], which are recorded as fruits with the highest total phenolics [36]. The findings by Abbe et al. [17] and Dan et al. [18] conform with the present results, where total phenolics decreased from unripe to red stage (11.6–4.5 and 9.6 to 5.5 mg GAE/g DW, respectively. The decline in total phenolics during ripening may have been due to the oxidation of phenolics by polyphenol oxidase, which has been reported to increase with fruit ripening [14,37]. Polyphenols in plants are generally involved in defence against ultraviolet radiation or aggression by pathogens [38]. The integration of phenolic esters into cell walls is an essential mechanism by which plants strengthen their cell walls, defend themselves against pathogens, and protect the cells against membrane damage by oxidative stress [14]. The decrease in the total phenolics is possibly attributed to the presence of free esters of phenolic acids at the unripe stage, which may aid the progressive binding of the phenolic acids to the cell walls during ripening [14], thus resulting in lower free phenolic compounds at the red stage. Flavonoids, a sub-group of phenolics, may prevent coronary heart disease and possess antioxidative, hepatoprotective, anti-inflammatory, antiviral, and anticancer activities [39]. The total flavonoids findings in the present study were agreeable with the results by Bhandari & Lee [13], who reported that total flavonoids in Solanum lycopersicum L. cultivars significantly differed at the different stages of ripening.
SALF saponins have been documented to have hypoglycemic, anti-peroxidative, and anti-hyperlipidemic effects [7], as well as antioxidant properties [40]. The soybeans' total saponins (5.6 % or 56 mg/g DW) [41], which have been reported as a very rich source of saponins [42,43], fall within this study's total saponins range. Thus, SALF may be considered a rich source of saponins. The differences in the trends for total saponins among the accessions in this study may be attributed to genetic differences. A phytochemical screening by Dan et al. [18] showed that ripening increased the saponin content of SALF. This is similar to the trends exhibited by accessions CP1 and GP1 of the present study from the unripe to the orange stage (total saponins decreased at the red stage). Similarly, the saponin content of Solanum gilo and Solanum aethiopicum fruits was observed to increase during ripening [44]. There is scarce information regarding the effect of ripening on saponin contents of Solanaceae fruits and vegetables. The enormous health benefits of saponins justify future studies related to factors that may influence their total saponins contents.
Alkaloids have health-promoting properties, including antidiabetic [45], antibacterial, antihypertensive, and anticancer effects [46]. The total alkaloids of SALF in the current study were higher than documented for tea leaves (47.6 mg/g DW [47]), which have been defined as rich sources of alkaloids [48]. Contradictory to our findings, a phytochemical screening of SALF by Dan et al. [18] showed an absence of alkaloids at all the ripeness stages. The increased total alkaloids during the ripening of all the SALF accessions in the present study may have resulted from an increase in the glycoalkaloid contents such as solamargine [49] as reported in S. sodomaeum, S. aethiopicum, S. integrifolium, and ten eggplant lines [50]. However, little is known about the biosynthetic pathway of glycoalkaloids and the factors that regulate their levels in plants [51]. Agreeable with the current findings, Bagheri et al. [52] reported increased glycoalkaloid (solasonine) content of eggplants with ripening; however, the reason for this increase was also unknown to the researchers. On the contrary, the alkaloid contents in Solanum lycocarpum [53] and Solanum lycopersicum [54] decreased with ripening. Alkaloids are produced by plants for resistance to challenges such as insects and pests [51], and it has been previously hypothesized that their reduction during ripening was to attract seed dispersers at the ripe/red stage [55] This hypothesis was possibly based on the bitterness of the alkaloids [56], which were suggested to be less in the ripe fruits, given their preference by animals compared to the unripe fruits. Since the total alkaloids in eggplants and SALF increased from the unripe to red stage, this hypothesis may only explain why they are consumed unripe rather than when red by humans (less bitter when unripe) [6,50]. However, the hypothesis does not explain the increase of alkaloids during the ripening of SALF, as well as eggplants. More studies are, therefore, necessary to elucidate the factors that influence the increase or decrease of alkaloids in Solanaceae fruits, including SALF.
Vitamin C has various health benefits. It may protect DNA, proteins, and lipids from oxidative damage by scavenging free radicals [57]. It also increases iron bioavailability by reducing non-heme iron from the ferric (Fe3+) to the ferrous (Fe2+) form, which is more easily absorbed in the intestine. Thus, vitamin C indirectly protects against anaemia [58]. The vitamin C of SALF at the red stage in this study was similar to the content in ripe tomatoes (5.1–6.4 mg/g DW) [56], which have been defined as rich vitamin C sources [59]. The increased vitamin C of SALF accessions (GP1, GC1, and WP1) in this study may largely stem from its increased biosynthesis through the Smirnoff-Wheeler pathway, as documented for tomatoes [60]. Similar to our findings, increased vitamin C with ripening has been reported for tomato cultivars [13,61]. In contrast, Chaudhary et al. [62] showed a higher vitamin C in unripe tomatoes than in red ones, which is similar to SALF accession CP1 of the present study. A similar trend as accession CP1 was also observed by Abbe et al. [17] and Dan et al. [18], who reported a decline in SALF vitamin C from unripe to ripe as 0.3 to 0.1 mg/g fresh weight and 0.3 to 0.1 mg/g DW, respectively; with also significant differences between ripeness stage. However, the decreased vitamin C for accession CP1 may have ensued from the degradation of ascorbate, culminating in the formation of oxalic acid, threonic acid, and oxalyl threonic acid [60]. Genetic differences may dictate the variations in the vitamin C content trends exhibited among the accessions in the current study.
Antioxidant activity was highest at the unripe stage for all accessions, which was similarly observed for total phenolics. In agreement with our findings, Abbe et al. [17] reported a decrease in SALF FRSC from the unripe (87 %) to the red stage (71 %), although only one accession was studied. Additionally, the FRSC among the eggplant cultivars was significantly different and reduced from the unripe to the ripe stage [63].
The BCC and AA of SALF were significantly influenced by the ripeness stage and accession. In agreement with our findings, a significant effect of cultivar and ripeness stage on the total phenolics of tomatoes has been reported [64]. Although the effect of the ripeness stage on the total phenolics of SALF in the present study was significantly influenced by accession, Anton et al. [64] reported no significant interaction between the two variables in tomatoes. In the present study, the strong positive correlations of total phenolics with AA suggest that phenolics contributed the most to the AA of SALF. The red stage, which had the highest total alkaloids, had the lowest AA. Comparable to this study, a significant positive correlation (r = 0.68) between total phenolics and FRSC was reported for tomato cultivars at different ripeness stages [64] and also in ten eggplant lines, S. sodomaeum, S. aethiopicum, and S. integrifolium [50].
The PCA results are comparable to those from the correlation and bioactive compounds’ contents analyses. Component 1 showed that an increase in total flavonoids and total phenolics led to an increased TAC and FRSC, while component 3 showed that total alkaloids were highest at the red stage. The regression models (Equations (3) and (4)) showed that total contents of phenolics, flavonoids, alkaloids, and vitamin C were the most significant in the quantities of the TAC and FRSC in SALF and also the BCC that mostly affected the TAC of SALF at the different stages of ripeness (Equations (5), (6), (7), (8))). The regression models agree with the correlation results, which showed that an increase in total phenolics and flavonoids resulted in increased AA and, similar to our previous findings, which showed that vitamin C had a negative correlation with AA at the unripe stage [21]. The regression results, however, show that AA is negatively correlated with total saponins at the orange stage and total alkaloids at the red stage. These findings agree with Milugo et al. [65], who showed that the co-occurrence of saponins and alkaloids resulted in an antagonistic effect on AA in the quinine tree (Rauvolfia caffra sond.). Thus, the low AA at the orange and red stages may have been due to the high antagonistic effect of the high contents of saponins and alkaloids versus those at the unripe and yellow stages. The lower AA at the orange and red stages may also partially be due to the lower total phenolics compared to the unripe stage.
6. Conclusion
The total flavonoids, phenolics, saponins, vitamin C, alkaloids and AA significantly varied with the SALF ripeness stage and accession. All the BCC and AA of SALF at the different ripeness stages were significantly influenced by the accessions. The unripe stage was the richest in total phenolics, flavonoids, and AA, the orange stage in total flavonoids and saponins, and the red stage in vitamin C (for most accession) and total alkaloids. Furthermore, very good regression models were derived in the study, which showed the BCC that mostly affected the AA of SALF at the different stages of ripeness. The study findings provide information on the ripeness stage that may give the highest nutraceutical benefits and should be of most interest to botanists. More studies are recommended to elucidate the factors that affect the saponin and alkaloid contents of fruits, including SALF, as the available information is limited.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Funding
The study was financed by the German Academic Exchange Service (DAAD), the Food Technology and Business Incubation Centre (FTBIC) (Makerere University) and Justus-Liebig University Giessen (JLU) Open Access Publication Fund. The funding sources were not involved in conducting the research and/or preparing this article, and we are very grateful for their immense support.
Data Availability Statement
The data will be made available on request.
CRediT authorship contribution statement
Aisha Musaazi Sebunya Nakitto: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. Yusuf B. Byaruhanga: Methodology, Resources, Supervision, Validation, Writing – review & editing. Anika E. Wagner: Methodology, Resources, Supervision, Validation, Funding acquisition, Writing - review & editing. John H. Muyonga: Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are very thankful to the DAAD (Germany), the FTBIC (Uganda) and the JLU Open Access Publication Fund (Germany) for their financial support towards this study.
References
- 1.Oz A.T., Ebru K. Superfood and Functional Food - an Overview of Their Processing and Utilization. 2017. Phytochemicals in fruits and vegetables; pp. 175–184. [DOI] [Google Scholar]
- 2.Schreiner M., Huyskens-Keil S. Phytochemicals in fruit and vegetables: health promotion and postharvest elicitors. CRC Crit. Rev. Plant Sci. May 2006;25(3):267–278. doi: 10.1080/07352680600671661. [DOI] [Google Scholar]
- 3.Yimer A., Fikereyesus Forsido S., Addis G., Ayelign A. Phytochemical profile and antioxidant capacity of some wild edible plants consumed in Southwest Ethiopia. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonne A.H., Nunes C.N., Brecht K.J. In: Health-promoting Properties of Fruits and Vegetables. Terry L.A., editor. CAB International; 2011. Tomato and other Solanaceous fruits; pp. 321–351.www.cabi.org (Oxfordshire, U.K.). [Online]. Available: [Google Scholar]
- 5.Jaeger P.M.L., Hepper F.N. In: Solanaceae: Biology and Systematics. D'Arcy W.G., editor. Columbia University Press; New York: 1986. A review of the genus Solanum in Africa; pp. 41–55. [Google Scholar]
- 6.Bukenya Z.R., Carasco J.F. Solanum (Solanaceae) in Uganda. Bothalia. 1995;25(1):43–59. doi: 10.4102/abc.v25i1.711. [DOI] [Google Scholar]
- 7.Elekofehinti O.O., Kamdem J.P., Kade I.J., Rocha J.B.T., Adanlawo I.G. Hypoglycemic, antiperoxidative and antihyperlipidemic effects of saponins from Solanum anguivi Lam. fruits in alloxan-induced diabetic rats. South Afr. J. Bot. 2013;88:56–61. doi: 10.1016/j.sajb.2013.04.010. [DOI] [Google Scholar]
- 8.Lee S.K., Kader A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000;20(2000):207–220. www.elsevier.com/locate/postharvbio [Online]. Available: [Google Scholar]
- 9.Benvenuti S., Pellati F., Melegari M., Bertelli D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004;69(3):FCT164–FCT169. doi: 10.1111/j.1365-2621.2004.tb13352.x. [DOI] [Google Scholar]
- 10.Milivojević J., Nikolić M., Pristov J.B. Physical, chemical and antioxidant properties of cultivars and wild species of Fragaria and Rubus genera. Journal of Pomology. 2010;44:55–64. 169–170. [Google Scholar]
- 11.Okmen B., Sigva H.O., Mutlu S., Doganlar S., Yemenicioglu A., Frary A. Total antioxidant activity and total phenolic contents in different Turkish eggplant (Solanum Melongena L.) cultivars. Int. J. Food Prop. 2009;12(3):616–624. doi: 10.1080/10942910801992942. [DOI] [Google Scholar]
- 12.da Silva K.M., et al. Effect of fruit ripening on bioactive compounds and antioxidant capacity of apple beverages. Food Science and Technology. 2018;39:1–7. doi: 10.1590/fst.25317. [DOI] [Google Scholar]
- 13.Bhandari S.R., Lee J.G. Ripening-dependent changes in antioxidants, color attributes, and antioxidant activity of seven tomato (Solanum lycopersicum L.) cultivars. J Anal Methods Chem. 2016;2016:1–13. doi: 10.1155/2016/5498618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amira E., et al. Effects of the ripening stage on phenolic profile, phytochemical composition and antioxidant activity of date palm fruit. J. Agric. Food Chem. Nov. 2012;60(44):10896–10902. doi: 10.1021/jf302602v. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S., Yadav A., Yadav M., Yadav J.P. Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm.f. BMC Res. Notes. 2017;10(1):1–12. doi: 10.1186/s13104-017-2385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leskovar D.I., Crosby K., Jifon J.L. Impact of agronomic practices on phytochemicals and quality of vegetable crops. Acta Hortic. 2009;841:317–322. doi: 10.17660/ActaHortic.2009.841.38. [DOI] [Google Scholar]
- 17.Abbe C.Y., Aboa N., Ahi P.A., Dan G.C. Antioxidant content in Solanum anguivi Lam berries as affected by cooking at different stages of ripening. Asian Food Science Journal. 2019;13(2):1–10. [Google Scholar]
- 18.Dan C., Kouassi K., Ban K., Nemlin G., Kouame P. Influence of maturity stage on nutritional and therapeutic potentialities of Solanum anguivi Lam berries (Gnagnan) cultivated in Côte d ’ Ivoire. Int. J. Nutr. Food Sci. 2014;3(5–1):1–5. doi: 10.11648/j.ijnfs.s.2014030501.11. [DOI] [Google Scholar]
- 19.Elekofehinti O.O., et al. African eggplant (Solanum anguivi Lam.) fruit with bioactive polyphenolic compounds exerts in vitro antioxidant properties and inhibits Ca2+-induced mitochondrial swelling. Asian Pac. J. Trop. Biomed. 2013;3(10):757–766. doi: 10.1016/S2221-1691(13)60152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oyeyemi S.D., Ayeni M.J., Adebiyi A.O., Ademiluyi B.O., Tedela P.O., Osuji I.B. Nutritional quality and phytochemical studies of Solanum anguivi (Lam.) fruits. J. Nat. Sci. Res. 2015;5(4):99–105. https://www.iiste.org/Journals/index.php/JNSR/article/view/20175/20834 [Google Scholar]
- 21.Nakitto A.M.S., Byaruhanga Y.B., Wagner A.E., Muyonga J.H. Morphological characteristics, bioactive compounds content, and antioxidant activity of different accessions of African eggplant (Solanum anguivi Lam.) J. Appl. Bot. Food Qual. Dec. 2021;94:220–228. doi: 10.5073/JABFQ.2021.094.027. [DOI] [Google Scholar]
- 22.Kim D., Chun O.K., Kim Y.J., Moon H., Lee C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003;51(22):6509–6515. doi: 10.1021/jf0343074. [DOI] [PubMed] [Google Scholar]
- 23.FAO/IAEA Quantification of tannins in tree foliage. Viena. 2000 doi: 10.1007/978-94-017-0273-7. [DOI] [Google Scholar]
- 24.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1998;299(1974):152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 25.Kumar R.S., Rajkapoor B., Perumal P. Antioxidant activities of Indigofera cassioides Rottl. Ex. DC. using various in vitro assay models. Asian Pac. J. Trop. Biomed. 2012;2(4):256–261. doi: 10.1016/S2221-1691(12)60019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiai S., Oura H., Nakajima T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Med. Mar. 1976;29(2):116–122. doi: 10.1055/s-0028-1097639. [DOI] [PubMed] [Google Scholar]
- 27.Omaye S.T., Turnbull D.J., Sauberlich H.E. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. 1979;62:3–11. doi: 10.1016/0076-6879(79)62181-X. [DOI] [PubMed] [Google Scholar]
- 28.Roe J.H. Comparative analyses for ascorbic acid by the 2,4-dinitrophenylhydrazine method with the coupling reaction at different temperatures: a procedure for determining specificity. J. Biol. Chem. 1961;236(5):1611–1613. http://www.ncbi.nlm.nih.gov/pubmed/13742654 [Online]. Available: [PubMed] [Google Scholar]
- 29.Harborne J.B. first ed. Chapman and Hall; London, UK: 1973. Phytochemical Methods; A Guide to Modern Techniques of Plant Analysis. [DOI] [Google Scholar]
- 30.Brand-Williams W., Cuvelier M.E., Berset C. vol. 28. Lebensmittel-Wissenschaft und -Technologie; 1995. pp. 25–30. (Use of a Free Radical Method to Evaluate Antioxidant Activity). [DOI] [Google Scholar]
- 31.Templeton G.F. A two-step approach for transforming continuous variables to normal: implications and recommendations for IS research. Commun. Assoc. Inf. Syst. Feb. 2011;28(1):41–58. doi: 10.17705/1cais.02804. [DOI] [Google Scholar]
- 32.Grané A., Jach A. Mathematical and Statistical Methods in Food Science and Technology. John Wiley & Sons, Ltd; Chichester, UK: 2013. Applications of principal component analysis (PCA) in food science and technology; pp. 55–86. [DOI] [Google Scholar]
- 33.Tresserra-rimbau A., Castro-barquero S., Vitelli-storelli F. Associations between dietary polyphenols and type 2 diabetes in a cross-sectional analysis of the PREDIMED-Plus trial: role of body mass index and sex. Antioxidants. 2019;8:537. doi: 10.3390/antiox8110537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudonné S., Vitrac X., Coutiére P., Woillez M., Mérillon J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. Mar. 2009;57(5):1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 35.Tolić M.T., Jurčević I.L., Krbavčić I.P., Marković K., Vahčić N. Phenolic content, antioxidant capacity and quality of chokeberry (Aronia melanocarpa) products. Food Technol. Biotechnol. 2015;53(2):171–179. doi: 10.17113/ftb.53.02.15.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pérez-Jiménez J., Neveu V., Vos F., Scalbert A. Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010;64:112–120. doi: 10.1038/ejcn.2010.221. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez J.C., et al. Effects of the fruit ripening stage on antioxidant capacity, total phenolics, and polyphenolic composition of crude palm oil from interspecific hybrid Elaeis oleifera×Elaeis guineensis. J. Agric. Food Chem. 2016;64(4):852–859. doi: 10.1021/acs.jafc.5b04990. [DOI] [PubMed] [Google Scholar]
- 38.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. Nov. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: an overview. Sci. World J. 2013;2013 doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elekofehinti O.O., Kamdem J.P., Kade I.J., Adanlawo I.G., Rocha J.B.T. Saponins from Solanum anguivi lam. Fruit exhibit in vitro and in vivo antioxidant activities in alloxan-induced oxidative stress. Asian J. Pharmaceut. Clin. Res. 2013;6(2):249–254. https://www.researchgate.net/publication/312449941_Saponins_from_Solanum_anguivi_Lam_fruit_exhibit_in_vitro_and_in_vivo_antioxidant_activities_in_alloxan-induced_oxidative_stress [Online]. Available: [Google Scholar]
- 41.Shi J., Arunasalam K., Yeung D., Kakuda Y., Mittal G., Jiang Y. Saponins from edible legumes: Chemistry, processing, and health benefits. J. Med. Food. Mar. 2004;7(1):67–78. doi: 10.1089/109662004322984734. [DOI] [PubMed] [Google Scholar]
- 42.Kregiel D., et al. In: Application and Characterization of Surfactants. Najjar R., editor. InTech; 2017. Saponin-based, biological-active surfactants from plants; pp. 183–205. [DOI] [Google Scholar]
- 43.Lásztity R., Hidvégi M., Bata Á. Saponins in food. Food Rev. Int. 1998;14(4):371–390. doi: 10.1080/87559129809541169. [DOI] [Google Scholar]
- 44.Agoreyo B.O., Ayaruja F., Okhihie O., Omorogie O.A., Obayiuwana O. 2nd International Conference and Exhibition (OWSD-FUTA), Akure, Nigeria. 2015. Effect of ripening on the phytochemical constituents and antioxidant activity of the various solvent fractions of Solanum gilo and Solanum aethiopicum.https://www.researchgate.net/publication/311546086_Effect_of_ripening_on_the_phytochemical_constituents_and_antioxidant_activity_of_the_various_solvent_fractions_of_Solanum_gilo_and_Solanum_aethiopicum [Online]. Available: [Google Scholar]
- 45.Sharma B., Salunke R., Balomajumder C., Daniel S., Roy P. Anti-diabetic potential of alkaloid rich fraction from Capparis decidua on diabetic mice. J. Ethnopharmacol. Feb. 2010;127(2):457–462. doi: 10.1016/j.jep.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Kuete V. Toxicological Survey of African Medicinal Plants. Elsevier Inc.; 2014. Health effects of alkaloids from African medicinal plants; pp. 611–633. [DOI] [Google Scholar]
- 47.Wong N.K., Chin F.-S., Chong K.-P., Markus A. Tea polyphenols and alkaloids content using soxhlet and direct extraction methods. World J. Agric. Sci. 2013;9(3):266–270. doi: 10.5829/idosi.wjas.2013.9.3.1737. [DOI] [Google Scholar]
- 48.Kurek J. Alkaloids - Their Importance in Nature and Human Life. IntechOpen; 2019. Introductory chapter: alkaloids - Their importance in nature and for human life; pp. 1–7. [DOI] [Google Scholar]
- 49.Ripperger H., Himmelreich U. Anguivine and isoanguivine steroid alkaloid glycosides from Solanum anguivi. Phytochemistry. 1994;37(6):1725–1727. doi: 10.1016/S0031-9422(00)89600-4. [DOI] [PubMed] [Google Scholar]
- 50.Mennella G., et al. Chemical and bioactive quality traits during fruit ripening in eggplant (S. melongena L.) and allied species. J. Agric. Food Chem. Nov. 2012;60(47):11821–11831. doi: 10.1021/jf3037424. [DOI] [PubMed] [Google Scholar]
- 51.Siddique M.A.B., Brunton N. In: Alkaloids - Their Importance in Nature and Human Life. Kurek J., editor. IntechOpen; 2019. Food glycoalkaloids: distribution, structure, cytotoxicity, extraction, and biological activity; pp. 1–26. [DOI] [Google Scholar]
- 52.Bagheri M., Shahnejat Bushehri A.A., Hassandokht M.R., Naghavi M.R. Evaluation of Solasonine content and expression patterns of SGT1 gene in different tissues of two Iranian eggplant (Solanum melongena L.) genotypes. Food Technol. Biotechnol. 2017;55(2):236–242. doi: 10.17113/ftb.55.02.17.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pereira J.D., Fraga V.G., Santos A.L.M., Carvalho M., Caramelli P., Gomes K.B. Alzheimer's disease and type 2 diabetes mellitus: a systematic review of proteomic studies. J. Neurochem. Mar. 2021;156(6):753–776. doi: 10.1111/jnc.15166. [DOI] [PubMed] [Google Scholar]
- 54.Friedman M. Tomato glycoalkaloids: role in the plant and in the diet. J. Agric. Food Chem. 2002;50(21):5751–5780. doi: 10.1021/jf020560c. [DOI] [PubMed] [Google Scholar]
- 55.Cipollini M.L., Levey D.J. Secondary metabolites of fleshy vertebrate‐dispersed fruits: adaptive hypotheses and implications for seed dispersal. Am. Nat. Sep. 1997;150(3):346–372. doi: 10.1086/286069. [DOI] [PubMed] [Google Scholar]
- 56.Borguini R.G., Bastos D.H.M., Moita-Neto J.M., Capasso F.S., Da Silva Torres E.A.F. Antioxidant potential of tomatoes cultivated in organic and conventional systems. Braz. Arch. Biol. Technol. 2013;56(4):521–529. doi: 10.1590/S1516-89132013000400001. [DOI] [Google Scholar]
- 57.Turck D., et al. Vitamin C and protection of DNA, proteins and lipids from oxidative damage: evaluation of a health claim pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. Jan. 2017;15(1):4685. doi: 10.2903/j.efsa.2017.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lane D.J.R., Richardson D.R. The active role of vitamin C in mammalian iron metabolism: Much more than just enhanced iron absorption. Free Radic. Biol. Med. 2014;75:69–83. doi: 10.1016/j.freeradbiomed.2014.07.007. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- 59.Lykkesfeldt J., Michels A.J., Frei B. Vitamin C. Adv. Nutr. 2014;5(1):16–18. doi: 10.3945/an.113.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laing W., Davey M., Botella M.A., Fenech M., Amaya I., Valpuesta V. Vitamin C content in fruits: biosynthesis and regulation. Front. Plant Sci. 2019;9:2006. doi: 10.3389/fpls.2018.02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valšíková-Frey M., Komár P., Rehuš M. The effect of varieties and degree of ripeness to vitamin C content in tomato fruits. Acta Horticulturae et Regiotecturae. Jan. 2018;20(2):44–48. doi: 10.1515/ahr-2017-0010. [DOI] [Google Scholar]
- 62.Chaudhary P., Sharma A., Singh B., Nagpal A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. Aug. 2018;55(8):2833–2849. doi: 10.1007/s13197-018-3221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fategbe M.A., Ibukun E.O., Kade I.J., Rocha J.B.T. A comparative study on ripe and unripe eggplant (Solanum melongena) as dietary antioxidant sources. J. Med. Plants Res. 2013;7(6):209–218. doi: 10.5897/JMPR09.086. [DOI] [Google Scholar]
- 64.Anton D., et al. Changes in polyphenols contents and antioxidant capacities of organically and conventionally cultivated tomato (Solanum lycopersicum L.) fruits during ripening. Int J Anal Chem. May 2017;2017:1–10. doi: 10.1155/2017/2367453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Milugo T.K., et al. Antagonistic effect of alkaloids and saponins on bioactivity in the quinine tree (Rauvolfia caffra sond.): further evidence to support biotechnology in traditional medicinal plants. BMC Complement Altern Med. Oct. 2013;13:285. doi: 10.1186/1472-6882-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
The data will be made available on request.