Abstract
The phylogenetic relatedness among 12 agriculturally important species in the order Rhizobiales was estimated by comparative 16S rRNA and dnaK sequence analyses. Two groups of related species were identified by neighbor-joining and maximum-parsimony analysis. One group consisted of Mesorhizobium loti and Mesorhizobium ciceri, and the other group consisted of Agrobacterium rhizogenes, Rhizobium tropici, Rhizobium etli, and Rhizobium leguminosarum. Although bootstrap support for the placement of the remaining six species varied, A. tumefaciens, Agrobacterium rubi, and Agrobacterium vitis were consistently associated in the same subcluster. The three other species included Rhizobium galegae, Sinorhizobium meliloti, and Brucella ovis. Among these, the placement of R. galegae was the least consistent, in that it was placed flanking the A. rhizogenes-Rhizobium cluster in the dnaK nucleotide sequence trees, while it was placed with the other three Agrobacterium species in the 16S rRNA and the DnaK amino acid trees. In an effort to explain the inconsistent placement of R. galegae, we examined polymorphic site distribution patterns among the various species. Localized runs of nucleotide sequence similarity were evident between R. galegae and certain other species, suggesting that the R. galegae genes are chimeric. These results provide a tenable explanation for the weak statistical support often associated with the phylogenetic placement of R. galegae, and they also illustrate a potential pitfall in the use of partial sequences for species identification.
As with most bacteria, evolutionary relationships among the members of the order Rhizobiales are usually estimated through 16S rRNA sequence comparisons. One problem with this approach however, is that these estimates are not always congruent with estimates derived from other loci (8, 23). In this study we obtained 16S rRNA and dnaK sequences for 12 agriculturally important species that were chosen to represent three families (Rhizobiaceae, Phyllobacteriaceae, and Brucellaceae) within the order Rhizobiales. Our goal was to compare estimates of phylogenetic relatedness based on their 16S rRNA sequences with corresponding estimates based on their dnaK sequences.
Within the family Rhizobiaceae, there are two closely related genera of agricultural significance: the nitrogen-fixing mutualists of the genus Rhizobium and the plant pathogens of the genus Agrobacterium. Young et al. (30) recently proposed that the genus Rhizobium be emended to include the species within the genus Agrobacterium. Part of the justification for this proposal was the observation that the 16S rRNA sequences of certain species of Rhizobium (e.g., R. galegae) are more similar to Agrobacterium sequences than they are to Rhizobium sequences. This proposal has been controversial, however, because of the ecological and genomic differences that exist between the two genera (6, 31).
Among the 12 species we examined, 4 were nitrogen-fixing species of the genus Rhizobium (R. galegae, R. leguminosarum, R. tropici, and R. etli) and 4 were phytopathogenic species of the genus Agrobacterium (A. tumefaciens, A. rhizogenes, A. rubi, and A. vitis). The remainder were representatives of three more distantly related genera, including two nitrogen-fixing species of the genus Mesorhizobium, a nitrogen-fixing species of the genus Sinorhizobium, and one mammalian pathogen of the genus Brucella. Since published dnaK sequences (3, 5, 18) were only available for 3 of the 12 species, it was necessary to clone and sequence dnaK genes from the other 9.
The dnaK gene encodes a highly conserved chaperone protein that performs multiple functions in the cell, including the folding of nascent polypeptides, the assembly and disassembly of multimeric protein structures, membrane translocation of secreted proteins, and the degradation of proteins (1, 9, 10). Furthermore, DnaK, the prokaryotic homolog of the eukaryotic Hsp70 protein, has been found in all eubacterial species examined to date. The DnaK proteins of Bacillus subtilis and Escherichia coli have three functionally distinct domains, including an N-terminal ATPase-binding domain, a substrate-binding domain, and a small C-terminal domain that has been associated with the degradation of the σ32 subunit of RNA polymerase (13). Both the ATPase and substrate-binding domains of dnaK are highly conserved and appear to form a species-specific functional unit, while the C-terminal domain of dnaK is less well conserved. Apparently the C-terminal domain can maintain functional activity when exchanged between E. coli and B. subtilis (13).
In this report, we describe the results of a phylogenetic analysis of the 16S and dnaK genes of Brucella ovis, Mesorhizobium ciceri, Mesorhizobium loti, R. etli, R. galegae, R. leguminosarum, A. tumefaciens, A. rhizogenes, A. rubi, R. tropici, A. vitis, and Sinorhizobium meliloti. Because the analysis revealed a lack of congruence in the placement of certain species in the 16S rRNA and dnaK trees, we also examined the sequences for evidence of intragenic recombination.
MATERIALS AND METHODS
Bacterial strains.
Nucleotide sequences for dnaK were obtained from the following nine rhizobial type strains: A. rhizogenes ATCC 11325T, A. rubi ATCC 13335T, A. vitis ATCC 49767T, R. etli ATCC 51251T, R. galegae ATCC 43677T, R. leguminosarum ATCC 10004T, R. tropici ATCC 49672T, M. ciceri ATCC 51585T, and M. loti ATCC 33669T. All strains were grown and maintained in yeast extract-mannitol medium (25).
DNA extraction and PCR amplification.
DNA extractions were performed by standard methods (16). The sequences of the oligonucleotide primers used for PCR amplification are listed in Table 1. These corresponded to conserved regions observed in the published dnaK sequences for S. meliloti and A. tumefaciens. Initially, only 1.4 kb of the 1.9-kb dnaK genes was cloned and sequenced. Building upon these core segments, gene-walking primers were then designed to amplify (and clone) upstream (100 bp) and downstream (400 bp) dnaK segments (Table 2). All primers were synthesized with a Beckman 1000 oligonucleotide synthesizer (Beckman, Fullerton, Calif.). Template DNA for cloning and sequencing was obtained through a 30-cycle amplification series as follows: 94°C for 1 min, 55°C for 45 s, and 72°C for 2 min, with an initial denaturing step of 94°C for 5 min. The annealing temperature was adjusted for increased stringency. Bands of interest were excised from low-melting-point agarose gels (1.5%) and purified with the protocol from the QIAGEN gel extraction kit (QIAGEN, Inc., Valencia, Calif.).
TABLE 1.
Sequences of dnaK primers used in this study
| Primer | Sequence(5′→3′) |
|---|---|
| Forward | |
| KF1 | ATYGGNATYGAYCTNGGNAC |
| KF1modB | ATYGGTATYGACCTKGGMAC |
| HSF1mod | GACCTGGGCACGACCAACTC |
| dnak5F | GACCGAAATCAACCTGCC |
| dnak6F | GACTGAAATCAACCTGCC |
| dnak1F | GGTGAAGACTTCGACAT |
| dnak23F | GAAGATCGAACTGTCCTC |
| dnak23.1F | CAAGATCGAGCTGTCGTC |
| STR-1 | GCTCCTTATATACGCCGCA |
| STR-2 | GGTTCCGACARCTYGCTT |
| STR-3 | CTYGCTTCAAGGAGAGA |
| Reverse | |
| KR2 | TCRAANGTNACYTCDATYTG |
| KR2modB | TCGAASGTSACYTCGATCTG |
| 4.3mod | ATTGGCTTCGGCGTCCTT |
| dnaJ1R | TCGTAGGCYTCGTTGAT |
| dnaJ4R | AAGGCGCTYTTCAGCTCT |
| dnaJ9R | TCGTCCTTGATTTCCTCG |
| HL-2R | TGCTTCACGATCTCCTGG |
TABLE 2.
dnaK sequencing primers used in this study
| Strain | 1,400-bp sequence | 5′ end | 3′ end |
|---|---|---|---|
| M. loti | HSF1mod/4.3mod | STR-3/HL-2R | dnak5F/dnaJ1R |
| R. galegae | KF1modB/KR2modB | STR-1/KR2modB | STR-1/dnaJ9R |
| A. vitis | KF1/KR2 | STR-3/KR2modB | dnak6F/dnaJ1R |
| A. rhizogenes | KF1modB/KR2modB | STR-2/KR2modB | dnak23F/dnaJ9R |
| A. rubi | KF1/KR2 | STR1/KR2modB | dnak5F/dnaJ4R |
| R. etli | KF1modB/KR2modB | STR1/KR2modB | dnak5F/dnaJ1R |
| R. tropici | KF1/KR2 | STR3/KR2modB | dnak1F/dnaJ1R |
| R. leguminosarum | KF1modB/KR2modB | STR1/KR2modB | dnak1F/dnaJ1R |
| M. ciceri | HSF1mod/4.3mod | STR3/HL-2R | dnak23.1F/dnaJ1R |
Cloning and nucleotide sequencing.
Purified PCR products were cloned into the pPCR-ScriptTM Amp SK(+) cloning vector, and transformation was done according to the manufacturer's instructions (Stratagene, La Jolla, Calif.). Transformants were examined for the presence of a recombinant plasmid containing the desired inserts by using the ScreenTest recombinant screening kit (Stratagene). After verification, the selected recombinant plasmids were extracted with the QIAprep miniprep kit (QIAGEN, Inc.), and sequences were obtained with standard sequencing primers supplied by the manufacturer. Both the positive and negative strands of the inserted DNA were sequenced three times to eliminate possible sequencing errors. Sequences were obtained by using a dye-deoxy cycle sequencing kit in combination with an ABI Prism model 373 automated sequencer (Perkin-Elmer Applied Biosystems, Foster City, Calif.). Electropherograms were compared for accuracy of the results, and complete sequences were obtained by assembling overlapping contigs with DNASTAR (DNASTAR, Inc., Madison, Wis.).
Phylogenetic analysis.
The 16S rRNA sequences obtained from the database corresponded to positions 29 through 1491 in the rrnB sequence of E. coli (2). Alignments of the sequences were obtained with the ClustalW (21) program and by inspection. Parameters for the ClustalW program included the slow-accurate alignment parameter, the IUB DNA weight matrix, and (for protein sequences) the PAM 250 protein weight matrix. The dnaK nucleotide sequences corresponded to positions 442 through 2352 in the published sequence of B. ovis (3). These were aligned by a two-step process. In the first step, ClustalW and inspection were used to align the inferred DnaK amino acid sequences. In the second step, the DnaK amino acid sequence alignment was used as input for the CodonAlign 2.0 nucleotide sequence alignment program (http://sinauer.com/hall/). This program generates a nucleotide sequence alignment containing triplet gaps that correspond to the codon gaps in the amino acid alignment. Neighbor-joining phylogenies for the aligned data sets were constructed by using Jukes-Cantor distances. Maximum-parsimony trees for the aligned data sets were generated with a heuristic min-mini tree search option with a search factor of 2. Bootstrap confidence levels were based on 1,000 permutations of the data sets. Software implementations of these programs were available in MEGA, version 2.1 (11). Discordance between 16S rRNA and dnaK phylogenies was tested with the incongruence length difference (ILD) test (7). In this test, the number of steps necessary for minimum-length trees in separate (partitioned) analyses is calculated and then the incongruence between separate data matrices is measured by the additional steps required when the separate matrices are combined into a single analysis. If the summed length of the combined trees is significantly longer than that of the original trees (P < 0.05), more incongruence is present between the two sets of data than can be explained by chance alone.
Statistical tests for recombination.
Two contrasting nucleotide substitution-based methods were used to evaluate the distribution of polymorphic nucleotides in the sequences (17, 20). The Stephens test (20) classifies each polymorphic position (or column) in a sequence alignment according to how the nucleotides at that position partition the sequences into phylogenetic groups. The software for this analysis is available at http://www.shigatox.net/stec/index.html. The Stephens test generates statistics for each possible phylogenic partition, including the following: do, which is the observed number of nucleotides between the most widely spaced sites that support a particular partition, and go, which is the number of nucleotides between consecutive sites that support a particular partition. The program calculates the probability that the distance (d) between a random pair of sites is less than or equal to the observed distance (do) between two sites supporting a particular partition [P (d ≤ do)]. Improbably small do values were taken as evidence of clustering. In contrast, go values that were improbably large [e.g., P (g ≥ go) = 0.01] were taken as evidence of significant gaps between sites supporting a particular partition. In our analysis, partitions that were supported by less than five consecutive polymorphic positions in the alignment were considered to be trivial and were excluded from further analyses. Because the levels of statistical significance associated with recombinant segments could have been influenced by adjacent hypervariable (or nonvariable) regions (20), significant Stephens test statistics were subsequently validated by the stepwise removal of adjacent hypervariable (or nonvariable) regions followed by retesting. The purpose of this additional procedure was to confirm that recombinant segments had not been identified solely on the basis of their proximity to hypervariable (or nonvariable) regions.
Sawyer's Geneconv method (17) also was used to analyze the distribution of polymorphic positions in the sequence alignments. The software for this program was obtained from http://www.math.wustl.edu/∼sawyer/geneconv. This method is used to identify statistically significant runs of sequence similarity through multiple pairwise sequence comparisons. Empirical significance levels for this method are obtained by comparing statistics associated with runs of polymorphic sites with corresponding statistics for 10,000 random permutations of the sites. Unlike the Stephens test, the sum-of-square scores in Sawyer's method are unaffected by mutational hot (or cold) spots (17). The global permutation P values reported are the proportion of run scores in the simulation whose lengths exceeded the observed values for the random permutations. Because segments involved in recombination may be affected by later mutation, the stringent requirements for absolute sequence identity were relaxed in secondary assessments through the implementation of a mismatch scoring penalty.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the dnaK nucleotide sequences included in this study are as follows: A. rhizogenes, AY752737; A. rubi, AY752738; A. vitis, AY752739; A. tumefaciens, X87113; B. ovis, M94063; M. ciceri, AY752740; M. loti, AY752741; R. etli, AY752742; R. galegae, AY752743; R. leguminosarum, AY752744; R. tropici, AY752745; and S. meliloti, L36602. The sequences with an “AY” prefix (above) have not been reported previously. The GenBank accession numbers of the 16S rRNA sequences obtained from the database are as follows: A. rhizogenes, X67224; A. rubi, X67228; A. vitis, X67225; A. tumefaciens, X67223; B. ovis, L26168; M. ciceri, U07934; M. loti, X67229; R. etli, U28916; R. galegae, X67226; R. leguminosarum, X67227; R. tropici, X67233; and S. meliloti, X67222.
RESULTS
DNA sequences.
The nine dnaK sequences determined in this study were aligned with the three published rhizobial dnaK sequences. This alignment resulted in 1,902 bp of dnaK sequence for the analysis. Corresponding 16S rRNA sequences for the same 12 species resulted in a 1,401-bp alignment for analysis.
Phylogenetic tree comparisons and analysis.
Trees generated by neighbor-joining and maximum-parsimony methods had similar topologies (Fig. 1 and 2). A. rhizogenes, R. tropici, R. etli, and R. leguminosarum were all placed in a single group (the Rhizobium clade) that was supported by high bootstrap percentages. With the exception of the two Mesorhizobium species, the placement of the other species varied with respect to each other, as well as with respect to their position relative to the Rhizobium clade. The lack of congruence between the 16S rRNA and dnaK nucleotide sequence trees was evaluated by the ILD test. The incongruence was significant (P = 0.01), indicating that the two nucleotide data sets (16S rRNA and dnaK) provided different phylogenetic signals. Because the ILD test does not accommodate a combination of both nucleotide and amino acid sequence data, the DnaK amino acid sequence data were not included in the ILD analysis.
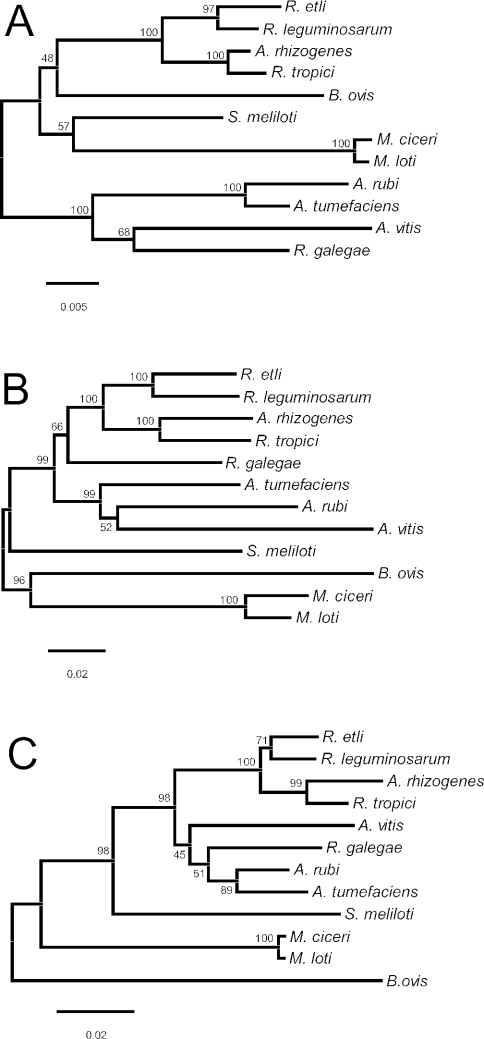
FIG. 1.
Unrooted neighbor-joining trees based on single-gene sequences from 12 species representing five genera (Agrobacterium, Brucella, Mesorhizobium, Rhizobium, and Sinorhizobium) within the order Rhizobiales. Percentage bootstrap support at each internal node is based on 1,000 replicate trees. (A) 16S rRNA nucleotide sequence tree. The total alignment length for the analysis was 1,401 bp. Positions with gaps were omitted, and the Kimura two-parameter distance correction was applied. (B) dnaK nucleotide sequence tree. The total alignment length for the analysis was 1,917 bp. Positions with gaps were omitted, and the Kimura two-parameter distance correction was applied. (C) Inferred DnaK amino acid sequence tree. The total alignment included 642 amino acid residues. Positions with gaps were omitted, and rates of amino acid substitution were assumed to follow a Poisson distribution.
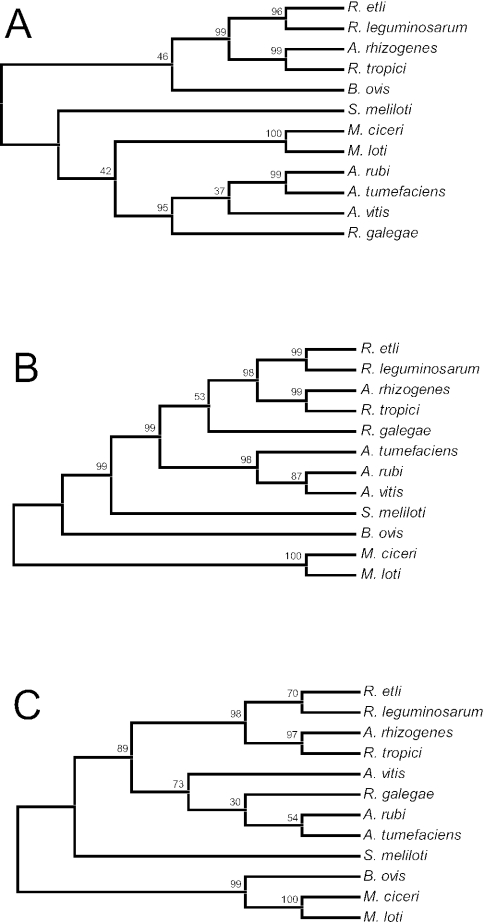
FIG. 2.
Unrooted maximum-parsimony trees based on single-gene sequences from 12 species representing five genera (Agrobacterium, Brucella, Mesorhizobium, Rhizobium, and Sinorhizobium) within the order Rhizobiales. Trees were generated by a heuristic mini-min tree search option with a search factor of 2. The percentage bootstrap support at each internal node is based on 1,000 replicate trees. (A) 16S rRNA nucleotide sequence tree. The total alignment length for the analysis was 1,401 bp. (B) dnaK nucleotide sequence tree. The total alignment length for the analysis was 1,917 bp. (C) Inferred DnaK amino acid sequence tree. The total alignment included 642 amino acid residues.
In both of the dnaK nucleotide sequence trees, R. galegae was placed flanking the Rhizobium clade (Fig. 1B and 2B). In contrast, in both the 16S rRNA and DnaK amino acid trees (Fig. 1A and 2A and 1C and 2C, respectively), R. galegae was placed with the members of the Agrobacterium clade (A. tumefaciens, A. rubi, and A. vitis). In an effort to explain the inconsistent placement of this particular species, as well as the generally low level of statistical support associated with its placement (with the exception of Fig. 2A), the sequences of R. galegae were compared to those of members of the Agrobacterium and Rhizobium clades.
The percent sequence identity between the 16S rRNA genes of R. galegae and those of A. rubi, A. tumefaciens, and A. vitis averaged 95.6%, with values ranging from 95.2 to 96.1%. Correspondingly, the percent sequence identity between the 16S rRNA genes of R. galegae and those of R. etli, R. leguminosarum, R. tropici, and A. rhizogenes was slightly less (95.2%), with values ranging from 94.6 to 95.9%. Particularly noteworthy, however, was the relatively high percentage of sequence identity between the R. galegae 16S rRNA sequence and that of R. leguminosarum (95.9%). Only A. vitis shared a higher level of sequence identity with R. galegae (96.1%).
A similar pattern emerged in parallel comparisons among the DnaK amino acid sequences. The average percent sequence identity between the R. galegae DnaK amino acid sequence and those of the Agrobacterium clade was 93.1%, with values ranging from 91.0 to 94.1%. The average percent sequence identity of the R. galegae DnaK sequence to those of the Rhizobium clade was 91.9%, slightly less than the average for the Agrobacterium clade sequences. As in the 16S rRNA comparisons, the R. galegae DnaK amino acid sequence had a relatively high level of percent sequence identity to the DnaK amino acid sequence of R. leguminosarum (93.1%).
Even though the nucleotide sequence of the dnaK gene in R. galegae was more similar to those of members of the Rhizobium clade, averaging 88.2% sequence identity, it shared a relatively high level of identity with the sequence of A. tumefaciens (88.3%). In contrast, the R. galegae dnaK nucleotide sequence of R. galegae shared only 85.6 and 84.2% sequence identities, respectively, with the dnaK nucleotide sequences of A. rubi and A. vitis.
In an effort to further explain the placement of R. galegae flanking the Rhizobium clade in the dnaK trees, but within the Agrobacterium clades in both the 16S rRNA and DnaK trees, we compared the number of synonymous and nonsynonymous substitution differences between the dnaK genes of R. galegae and those of representatives of the Agrobacterium and Rhizobium clades (A. rubi and R. tropici, respectively). The results supported the differential placement of R. galegae between the dnaK and DnaK trees, in that (over the entire gene) the nucleotide sequence of dnaK in R. galegae has more synonymous site substitution differences from that of A. rubi than it does from that of R. tropici and, conversely, the R. galegae sequence has a slightly larger number of nonsynonymous substitution differences from the sequence of R. tropici than it does from the sequence of A. rubi (Table 3).
TABLE 3.
Number of nucleotide substitution differences between the dnaK genes of R. galegae and representatives of the Agrobacterium and Rhizobium clades
| Sequence for comparison | No. of differences
|
|||
|---|---|---|---|---|
| Entire dnaK gene
|
dnaK segment 862-1178a
|
|||
| Synonymous | Nonsynonymous | Synonymous | Nonsynonymous | |
| A. rubi | 204 | 63 | 37 | 3 |
| R. tropici | 130 | 67 | 13 | 1 |
Segment beginning at nucleotide position 862 and ending at position 1178 and corresponding to amino acid positions 288 through 393 in Fig. 3.
Polymorphic nucleotide position distribution across the 16S rRNA alleles.
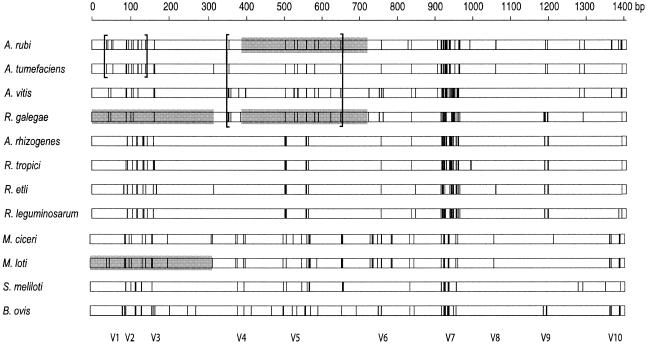
A map of the 182 polymorphic nucleotide positions present in the 16S rRNA sequences revealed some highly variable and some highly conserved regions (Fig. 3). The variable regions were correlated with expansion segments (e.g., V7) described by Raué et al. (15). Some obvious similarities and differences were evident among the polymorphic site patterns. For example, with the exception of the V7 region, the polymorphic site distribution patterns among the members of the Agrobacterium clade and R. galegae were similar (Fig. 3).
FIG. 3.
Linear distribution of 182 polymorphic nucleotide positions in a multiple alignment of 16S rRNA sequences representing five genera (Agrobacterium, Brucella, Mesorhizobium, Rhizobium, and Sinorhizobium) within the order Rhizobiales. Each vertical line represents a deviation from the consensus sequence. The locations of hypervariable expansion segments (V1 through V10) described by Raué et al. (15) are shown at the bottom. Brackets define endpoints of segments that were identified by the Stephens test as containing nonrandom clusters of partition-specific nucleotide sequences. Shaded segments indicate runs of sequence similarity that were identified by Sawyer's Geneconv method.
Analyses of mosaic structure within the 16S rRNA alleles.
Evidence for recombination among the 16S rRNA alleles was obtained by the Stephens test. When applied without regard for specific partitions or groups, significant evidence of polymorphic site clustering was apparent [P (d ≤ do) = 0.001]. Because this method is sensitive to mutational hot (and cold) spots (see Materials and Methods), the highly polymorphic V7 region and the highly conserved region between V7 and V10 were deleted during the analysis. Regardless, significant partition-dependent clustering was observed within the 16S rRNA alleles, indicating that the recombinant segments represented by these clusters were not simply due to the presence of mutational hot (or cold) spots nearby. The locations of the sites supporting these clusters are enclosed by brackets in Fig. 3.
One of the partition-dependent clusters was located at the 5′ end of the 16S rRNA gene and spanned 102 bp in the alleles of A. rubi and A. tumefaciens [P (d ≤ do) = 0.001]. The individual nucleotide positions supporting this partition were numbered 38′, 55′, 96′, 130′, and 140′ in the partial 16S rRNA alignment (Table 4). Only two alternative nucleotides were observed at these five positions, one of which was shared by only A. rubi and A. tumefaciens. Another cluster of partition-dependent polymorphic positions was located in a more central 292-bp segment, where R. galegae and three members of the Agrobacterium clade shared nucleotides at five positions. The nucleotides supporting this partition were located at positions 358, 529, 537, 582, and 650 [P (d ≤ do) = 0.023] in the alignment (Fig. 3). Again, at each of these five positions only two alternative nucleotides were present, one of which was shared by only R. galegae and the three members of the Agrobacterium clade.
TABLE 4.
Polymorphic nucleotide positions in the 16S rRNA genes (V1 through V3 region) of five genera in the order Rhizobiales (Agrobacterium, Brucella, Mesorhizobium, Rhizobium, and Sinorhizobium)
| Species | 16S rRNA nucleotide at positiona:
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 38′ | 42 | 43 | 50 | 51 | 55′ | 83 | 84 | 89 | 90 | 91 | 92 | 93 | 96′ | 102 | 104 | 106 | 108 | 117 | 119 | 120 | 121 | 130′ | 133 | 134 | 136 | 140′ | |
| A. rubi | A | T | C | G | A | T | T | C | C | A | A | C | C | G | T | G | T | T | G | A | A | T | C | A | C | C | A |
| A. tumefaciens | A | C | C | G | G | T | T | C | G | T | G | C | C | G | T | G | T | C | G | A | A | T | C | A | C | C | A |
| R. galegae | G | C | T | A | G | C | T | C | C | A | T | C | C | A | C | A | T | C | G | A | G | C | T | A | C | C | T |
| M. loti | G | C | T | A | G | C | T | C | C | A | T | C | T | A | C | A | T | C | G | A | G | C | T | A | C | T | T |
| A. vitis | G | C | T | A | G | C | T | C | G | T | A | C | C | A | T | G | T | C | G | A | A | T | T | A | C | C | T |
| A. rhizogenes | G | C | C | G | G | C | T | C | C | T | T | T | T | A | T | A | G | A | T | T | G | C | T | G | T | T | T |
| R. tropici | G | C | C | G | G | C | T | C | T | T | T | T | G | A | T | A | G | A | T | T | G | C | T | G | T | T | T |
| R. etli | G | C | C | G | G | C | C | G | C | T | T | T | A | A | T | A | G | A | T | T | G | C | T | G | T | C | T |
| R. leguminosarum | G | C | C | G | G | C | T | C | C | T | T | G | A | A | T | A | G | A | T | T | G | C | T | G | T | T | T |
| M. ciceri | G | C | C | G | G | C | T | C | C | A | T | C | T | A | C | A | T | C | G | A | G | C | T | A | C | T | T |
| S. meliloti | G | C | C | G | G | C | T | C | C | T | T | T | T | A | T | A | G | A | T | T | G | C | T | G | A | C | T |
| B. ovis | G | C | C | G | G | C | C | G | A | T | T | T | G | A | T | A | T | A | T | T | G | C | T | G | T | C | T |
Partition-specific nucleotide positions that were identified by the Stephens test are denoted by primes (38′, 55′, 96′, 130′, and 140′). Nucleotides shared between R. galegae and either A. rubi or M. loti are shown in boldface type.
Supporting evidence for the distinctiveness of the 292-bp central segment (identified by the Stephens' test) was obtained with Sawyer's Geneconv program. A highly significant (P ≤ 0.001) run of 41 identical polymorphic positions was identified in a 333-bp segment (spanning positions 389 through 721) in the sequences of A. rubi and R. galegae. These two identical segments are indicated in Fig. 3 by the shading in the central portion of the A. rubi and R. galegae distributions.
When the parameters in the Geneconv program were adjusted to relax the absolute sequence identity requirement, a second significant run of sequence similarity was identified at the 5′ end of the R. galegae and M. loti 16S rRNA genes—between nucleotide positions 1 and 318 (P ≤ 0.011). Within this segment, there were a total of 43 polymorphic positions across the 12 sequences, 20 of which partitioned the sequence of R. galegae with either M. loti or A. rubi. Among these 20 positions, R. galegae shared specific nucleotides with M. loti at 15, while it shared only 5 with A. rubi. Seventeen of these positions were shown in Table 4. The remaining three were located downstream (not shown).
Because of the relatively high level of similarity between the R. galegae and M. loti sequences at the 5′ end of the gene and the absolute sequence identity between the R. galegae and A. rubi alleles in the more central 333-bp segment (identified by Sawyer's Geneconv), the degree of sequence similarity between the R. galegae and M. loti 16S rRNA alleles in the same 333-bp segment was determined. There were a total of 20 nucleotide mismatches between R. galegae and M. loti within the 333-bp segment, in contrast to the corresponding absolute sequence identity between the A. rubi and R. galegae alleles over the same segment.
Collectively these results indicated that the 5′ half of the R. galegae 16S rRNA gene can be divided into two distinct portions on the basis of clustered nucleotide sequence polymorphisms: one portion at the 5′ end of the gene, where the R. galegae sequence is more similar to that of M. loti, and a second more central portion, where the R. galegae sequence is identical to that of A. rubi. The presence of these two contrasting regions in the 16S rRNA gene of R. galegae could be explained either by independent parallel mutations at multiple sites or perhaps by the intragenic recombination of divergent 16S rRNA alleles.
If these segments of 16S rRNA in R. galegae indeed have independent evolutionary histories, then they would be expected to provide distinct phylogenetic signals. This assumption was examined by using the ILD test to determine whether alignment subsets corresponding to the 5′, the central, or the 3′ segments of the gene (described above) could be separated and then combined without significantly affecting the number of steps required for producing most parsimonious trees. The probabilities of congruence between the 5′ and remaining segments, the central segment and remaining segments, and also the 5′ and the central segment were P = 0.002, P = 0.010, and P = 0.002, respectively. It was therefore concluded that each of these segments produced different phylogenetic signals.
Polymorphic amino acid sequence position differences across the dnaK alleles.
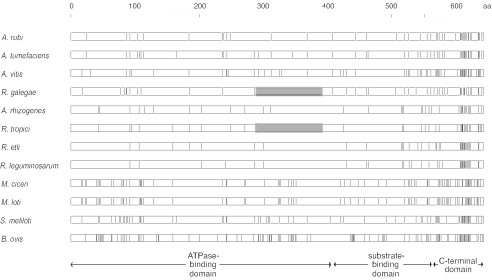
There were a total of 754 polymorphic nucleotide positions among the dnaK sequences (not shown). Because of the very large number of polymorphic positions, the graphical comparison method that was used for the 16S rRNA comparisons was visually uninformative for comparing the dnaK sequences. Consequently, the corresponding 177 polymorphic amino acid positions in the DnaK alignment were presented instead (Fig. 4). The highest degree of amino acid sequence polymorphism was evident at the carboxy-terminal end of the gene. In contrast, there was a strong consensus region at the 3′ end of the ATPase-binding domain, especially among the alleles of the Rhizobium clade (A. rhizogenes, R. tropici, R. etli, and R. leguminosarum). The polymorphic site distribution patterns of two Mesorhizobium species were the most similar, while the patterns of S. meliloti and B. ovis were the most different from each other and from the other species.
FIG. 4.
Linear distribution of 193 polymorphic amino acid positions in a multiple alignment of DnaK protein sequences representing five genera (Agrobacterium, Brucella, Mesorhizobium, Rhizobium, and Sinorhizobium) within the order Rhizobiales. Each vertical line represents a deviation from the consensus sequence. Regions corresponding to the three domains described by Mogk et al. (13) are shown at the bottom. Shaded segments indicate statistically significant runs of nucleotide sequence similarity identified by Sawyer's method.
Analysis of mosaic structure within dnaK alleles.
The Stephens test was used to search for evidence of intragenic recombination among the dnaK sequences. There was no significant evidence of clustering in the absence of specific phylogenetic partitions [P (d ≤ do) = 0.196], thus justifying the use of the entire nucleotide alignment for the analysis of specific partitions. Among the 377 partitions examined, there was significant clustering in only 4; however, none of these could be explained by recombination or gene conversion. When applied to the DnaK amino acid sequence data, the Stephens test revealed no significant evidence of partition-specific clustering.
When Sawyer's Geneconv program was run with the dnaK nucleotide sequence data set, without an allowance for mismatches, no significantly long runs of similarity were detected. However, after relaxing the absolute sequence identity requirement, a 317-bp segment common to both R. galegae and R. tropici (P ≤ 0.039) was revealed. This segment spanned nucleotide positions 862 through 1178 (corresponding to amino acid positions 288 through 393) in the dnaK sequences of R. galegae and R. tropici (Fig. 4). Across this segment, the two species differed at 14 of 121 polymorphic nucleotide positions (data not shown). The localized similarity between the dnaK segments of R. galegae and R. tropici at positions 862 through 1178 was supported by an analysis of the number of synonymous and nonsynonymous substitution differences within this segment (Table 3). The slightly lower number of nonsynonymous site differences between R. galegae and R. tropici in the segment from positions 862 through 1178, relative to the somewhat higher number observed between the corresponding R. galegae and A. rubi segments, did not reflect the pattern of nonsynonymous site differences that was observed between the same species over the entire dnaK gene. Although perhaps not statistically significant, these results support the distinctiveness of the segment from positions 862 through 1178.
DISCUSSION
A two-gene comparative analysis was used to reconstruct phylogenetic relationships among 12 species within the α-subdivision of the Proteobacteria. Nucleotide sequences for two conserved genes (16S rRNA and dnaK) were analyzed, and phylogenetic relatedness estimates were developed with these sequences (Fig. 1 and 2). Although certain groupings were consistent between the trees representing the different loci (for example, the clustering of the two Mesorhizobium species and the clustering of A. rhizogenes, R. tropici, R. etli, and R. leguminosarum), some significant differences between the trees representing the different loci were evident. One of the most noticeable differences was the relative placement of the R. galegae sequences in the different trees, in that this species was placed flanking the A. rhizogenes-Rhizobium cluster in the dnaK nucleotide sequence trees, while it was placed along with the other three Agrobacterium species in both the 16S rRNA and DnaK amino acid trees. It was also noted that the bootstrap support for the placement of this particular species was modest in five of the six trees examined.
The lack of strong statistical support for the placement of R. galegae has been observed in other studies; however, conclusive explanations for this observation were not apparent. For example, in previous studies of rhizobial 16S rRNA genes, bootstrap support for the placement of this species has ranged from 54% (26) to 83% (24). Similarly, in a phylogenetic analysis of the 23S rRNA genes in rhizobia, Pulawska et al. (14) reported 59% bootstrap support for the placement of R. galegae with a representative of the Agrobacterium genus, A. vitis. In an analysis of glutamine synthetase I (GSI) sequences, Turner and Young (23) found no evidence supporting the placement of the R. galegae GSI allele with other GSI alleles of species representing the genus Rhizobium. However, in a parallel analysis of glutamine synthetase II (GSII) sequences, they reported strong support for the placement of the R. galegae allele with these same Rhizobium species. Unfortunately, no Agrobacterium sequences were included in their analyses, so the placement of the R. galegae glutamine synthetase alleles relative to those in Agrobacterium is unknown.
Differences or uncertainties in the phylogenetic estimates obtained through 16S rRNA sequence analyses are of concern because this locus is so widely used in applied studies for the identification of species and also in molecular systematics for the reconstruction of evolutionary relationships among bacteria (12). It is generally assumed that 16S rRNA genes are unlikely to have a history of lateral transfer and recombination because of the adverse effects that this would have on the translational efficiency within the cell. It is not difficult, however, to imagine a situation where recombinant 16S rRNA alleles might actually provide a selective advantage: for example, in the presence of species-specific rRNA-binding antibiotics. Evidence for this possibility was recently reported by Trieber and Taylor (22), who transformed tetracycline-sensitive Helicobacter pylori strains naturally to antibiotic resistance through the introduction of foreign 16S rRNA genes. Of course it is also possible, although probably less likely, that selectively neutral 16S rRNA alleles could be horizontally transferred and stably inherited in natural populations.
Several comparative studies of 16S rRNA gene diversity in bacteria have provided inferential evidence suggesting the existence of recombinant rRNA alleles in natural populations (4, 8, 19, 27, 29, 32). An extensive statistical analysis of rRNA genes in several species of the α-Proteobacteria has recently provided additional evidence suggesting a history of recombination between the 16S rRNA alleles of species of Mesorhizobium and Bradyrhizobium and also between the 16S alleles of Mesorhizobium and Sinorhizobium (24).
To investigate the possibility that a history of recombination might explain the modest levels of bootstrap support observed for certain nodes within the trees in Fig. 1, we examined the sequence data used to generate those trees for patterns of clustered nucleotide sequence substitutions. Perhaps the strongest evidence for the mosaic gene structure that we observed was the presence of clustered substitutions between the 16S rRNA alleles of A. rubi and R. galegae (Fig. 3). While both of these species shared an identical 333-bp segment spanning the hypervariable V4-V5 regions of their 16S rRNA genes, both had distinctly different nucleotide substitution patterns upstream of this segment. In this upstream region (V1 through V3), most of the polymorphic nucleotides in A. rubi matched those of A. tumefaciens, while most of those in R. galegae matched those of M. loti (Table 4). A rational explanation for these results is that different regions of the R. galegae 16S rRNA gene have different evolutionary histories.
There are, however, other plausible explanations for the significant runs of sequence similarity observed among the 16S rRNA and dnaK nucleotide sequences of these species. For example, rates of evolution may differ in different regions of a particular gene (28). Selection for compensatory mutations might also help to stabilize secondary structures of certain gene products: e.g., stem structures in rRNA. This could also influence the relative frequency of compatible positions in the sequence alignments. Regardless of the specific mechanism responsible for the clustered patterns of nucleotide substitutions that we observed, the results of this study are important in that they demonstrate how such patterns can be used to explain topological instabilities that are often encountered in sequence-based phylogenetic trees. The results are also of applied significance in that they expose a potential pitfall in the use of partial gene sequences as a rapid method for species identification.
Acknowledgments
This research was supported by Public Health Service grant AI 22144 (R.K.S.) and a Penn State Berks-Lehigh Valley College Research and Development grant (B.D.E.).
The authors gratefully acknowledge T. S. Whittam for providing software and advice on statistical analyses.
REFERENCES
- 1.Boorstein, W. R., T. Zeigelhoffer, and E. A. Craig. 1994. Molecular evolution of the HSP70 multigene family. J. Mol. Evol. 38:1-17. [DOI] [PubMed] [Google Scholar]
- 2.Brosius, J., T. J. Dull, D. N. Sleeter, and H. F. Noller. 1981. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J. Mol. Biol. 148:107-127. [DOI] [PubMed] [Google Scholar]
- 3.Cellier, M. F. M., J. Teyssier, M. Nicolas, J. P. Liautard, J. Marti, and J. Sri Widada. 1992. Cloning and characterization of the Brucella ovis heat shock protein DnaK functionally expressed in Escherichia coli. J. Bacteriol. 174:8036-8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eardly, B. D., F.-S. Wang, and P. van Berkum. 1996. Corresponding 16S rRNA gene segments in Rhizobiaceae and Aeromonas yield discordant phylogenies. Plant Soil 186:69-74. [Google Scholar]
- 5.Falah, M., and R. S. Gupta. 1994. Cloning of the hsp70 (dnaK) genes from Rhizobium meliloti and Pseudomonas cepacia: phylogenetic analyses of mitochondrial origin based on a highly conserved protein sequence. J. Bacteriol. 176:7748-7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrand, S. K., P. van Berkum, and P. Oger. 2003. Agrobacterium is a definable genus of the family Rhizobiaceae. Int. J. Syst. Evol. Microbiol. 53:1681-1687. [DOI] [PubMed] [Google Scholar]
- 7.Farris, J. S., M. Källersjö, A. G. Kluge, and C. Bult. 1994. Testing significance of incongruence. Cladistics 10:315-319. [Google Scholar]
- 8.Gaunt, M. W., S. L. Turner, L. Rigottier-Gois, S. A. Macgilp, and J. P. W. Young. 2001. Phylogenies of atpD and recA support the small subunit rRNA-based classification of rhizobia. Int. J. Syst. Evol. Microbiol. 51:2037-2048. [DOI] [PubMed] [Google Scholar]
- 9.Gething, M.-J., and J. Sambrook. 1992. Protein folding in the cell. Nature 355:33-45. [DOI] [PubMed] [Google Scholar]
- 10.Hartl, F. U. 1996. Molecular chaperones in cellular protein folding. Nature 381:571-580. [DOI] [PubMed] [Google Scholar]
- 11.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA 2: molecular evolutionary genetic analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 12.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, R. J. Farris, G. M. Garrity, G. J. Olsen, T. M. Schmidt, and J. M. Tiedje. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mogk, A., B. Bukau, R. Lutz, and W. Schumann. 1999. Construction and analysis of hybrid Escherichia coli-Bacillus subtilis dnaK genes. J. Bacteriol. 181:1971-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pulawska, J., M. Maes, A. Willems, and P. Sobiczewski. 2000. Phylogenetic analysis of 23S rRNA gene sequences of Agrobacterium, Rhizobium, and Sinorhizobium strains. Syst. Appl. Microbiol. 23:238-244. [DOI] [PubMed] [Google Scholar]
- 15.Raué, H. A., W. Musters, C. A. Rutgers, J. Van't Riet, and R. J. Planta. 1990. rRNA: from structure to function, p. 217-235, In W. E. Hill, P. B. Moore, A. Dahlberg, D. Schlessinger, R. A. Garret, and J. R. Warner (ed.), The ribosome: structure, function, and evolution. American Society for Microbiology, Washington, D.C.
- 16.Sambrook, J. E., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Sawyer, S. A. 1999. GENECONV: a computer package for the statistical detection of gene conversion., 1.02 ed. Department of Mathematics, Washington University, St. Louis, Mo.
- 18.Segal, G., and E. Z. Ron. 1995. The dnaKJ operon of Agrobacterium tumefaciens: transcriptional analysis and evidence for a new heat shock promoter. J. Bacteriol. 177:5952-5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sneath, P. H. A. 1993. Evidence from Aeromonas for genetic crossing-over in ribosomal sequences. Int. J. Syst. Bacteriol. 43:626-629. (Letter.) [DOI] [PubMed] [Google Scholar]
- 20.Stephens, J. C. 1985. Statistical methods of DNA sequence analysis: detection of intragenic recombination or gene conversion. Mol. Biol. Evol. 2:539-556. [DOI] [PubMed] [Google Scholar]
- 21.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. Clustal-W—improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties, and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trieber, C. A., and D. E. Taylor. 2002. Mutations in the 16S rRNA genes of Helicobacter pylori mediate resistance to tetracycline. J. Bacteriol. 184:2131-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner, S. L., and J. P. W. Young. 2000. The glutamine synthetases of rhizobia: phylogenetics and evolutionary implications. Mol. Biol. Evol. 17:309-319. [DOI] [PubMed] [Google Scholar]
- 24.van Berkum, P., Z. Terefework, L. Paulin, S. Suomalainen, K. Lindström, and B. D. Eardly. 2003. Discordant phylogenies within the rrn loci of rhizobia. J. Bacteriol. 185:2988-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent, J. M. 1970. A manual for the practical study of root nodule bacteria. IBP handbook, no. 15. Blackwell Scientific Publications, Ltd., Oxford, England.
- 26.Wang, E. T., P. van Berkum, D. Beyene, X. H. Sui, O. Dorado, W. X. Chen, and E. Martinez-Romero. 1998. Rhizobium huautlense sp. nov., a symbiont of Sesbania herbacea that has a close phylogenetic relationship with Rhizobium galegae. Int. J. Syst. Bacteriol. 48:687-699. [DOI] [PubMed] [Google Scholar]
- 27.Wang, Y., Z. Zhang, and N. Ramanan. 1997. The actinomycete Thermobispora bispora contains two distinct types of transcriptionally active 16S rRNA genes. J. Bacteriol. 179:3270-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Worobey, M., A. Rambaut, O. G. Pybus, and D. L. Robertson. 2002. Questioning the evidence for genetic recombination in the 1918 “Spanish Flu” virus. Science 296:211-213. [DOI] [PubMed] [Google Scholar]
- 29.Yap, W. H., Z. Zhang, and Y. Wang. 1999. Distinct types of rRNA operons exist in the genome of the actinomycete Thermomonospora chromogena and evidence for horizontal transfer of an entire rRNA operon. J. Bacteriol. 181:5201-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young, J. M., L. D. Kuykendall, E. Martinez-Romero, A. Kerr, and H. Sawada. 2001. A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. Int. J. Syst. Evol. Microbiol. 51:89-103. [DOI] [PubMed] [Google Scholar]
- 31.Young, J. M., L. D. Kuykendall, E. Martinez-Romero, A. Kerr, and H. Sawada. 2003. Classification and nomenclature of Agrobacterium and Rhizobium. Int. J. Syst. Evol. Microbiol. 53:1689-1695. [DOI] [PubMed] [Google Scholar]
- 32.Young, J. P. W., and K. E. Haukka. 1996. Diversity and phylogeny of rhizobia. New Phytol. 133:87-94. [Google Scholar]