Abstract
Covert infection with Spodoptera frugiperda multiple nucleopolyhedrovirus, detected by reverse transcription-PCR of virus gene transcripts (ie-0 and polh), was not significantly affected by the presence of an optical brightener (Tinopal UNPA-GX), indicating no change in virus virulence. Detection of the covert infection was dependent on insect life stage and the viral mRNA used for diagnosis.
Baculoviruses are virulent pathogens of insects, especially Lepidoptera, that form the basis for several biological insecticides (16). Baculovirus infections usually kill the host but may also survive as sublethal covert infections (17) and transmit themselves vertically, from parent to offspring (3, 10, 11, 14). Stilbene optical brighteners increase the probability of virus infection by inhibiting chitin synthesis (1, 7), thus increasing the permeability of the midgut peritrophic membrane (21), and by reducing the rate of turnover of infected gut cells (22).
We hypothesized that the optical brightener Tinopal UNPA-GX (Sigma, St. Louis, Mo.), administered in mixtures with occlusion bodies (OBs) of Spodoptera frugiperda multiple nucleopolyhedrovirus (SfMNPV; Baculoviridae), would result in a higher prevalence of covert infection in larvae and adults of the fall armyworm, S. frugiperda (Lepidoptera: Noctuidae). For this experiment, we employed reverse transcription (RT)-PCR with primers specific for two SfMNPV genes, ie-0 and polh. The ie-0 gene is a trans-activator capable of stimulating the transcription of genes required for the replication of viral DNA and is essential for initiating virus replication (9). The polyhedrin (polh) gene encodes a major structural protein that forms the matrix of the viral OB late in the infection cycle (15). polh is not essential for virus replication but is required for horizontal transmission (2).
OBs of SfMNPV, originally isolated in Nicaragua (8), were produced in S. frugiperda from a laboratory colony, purified by centrifugation, and quantified in a bacterial counting chamber (18). Groups of 500 to 600 newly molted second instars were fed OB suspensions (with or without Tinopal UNPA-GX) by the droplet feeding technique (13) that were estimated to cause ∼90% mortality in experimental insects (6.2 × 105 OBs/ml of SfMNPV alone or 4.4 × 104 OBs/ml of SfMNPV and 0.1% [wt/vol] Tinopal UNPA-GX). The procedure was repeated with groups of 75 to 100 newly molted fifth instars treated with 1.3 × 109 OBs/ml of SfMNPV alone or 1.2 × 105 OBs/ml of SfMNPV and 0.1% Tinopal UNPA-GX. Inoculated insects were reared individually on diet plugs and weighed 2 days after pupation. Control larvae were treated identically with solutions not containing virus. The experiment was performed three times with second instars and four times with fifth instars. To confirm the suitability of target genes (ie-0 and polh) as indicators of covert infection, an initial sample of 22 insects inoculated with SfMNPV alone in the fifth instar, which showed no signs of NPV disease, were randomly selected as sixth instars and subjected to RT-PCR. Subsequently, between 20 and 27 insects inoculated in the second or fifth instars were randomly selected in the fifth or adult stage, respectively, and used in RT-PCR analysis.
Total RNA was extracted from the posterior part of the larval body of fifth- and sixth-instar larvae and from the dissected abdomen of adult S. frugiperda as described elsewhere (19). RT was performed on total RNA (up to 1 μg) by using an ImProm-II kit (Promega) following the manufacturer's recommendations. PCR amplification of cDNA was performed by using 5 μl of the RT reaction in a volume of 50 μl. Primers were designed by using SfMNPV sequences of the immediately early gene ie-0 (5′ TACGCTCGAGATGAGTATTAATCATAGATT 3′ [forward] and 5′ CGTACTCGAGTCTGGCAAATGTTACACT 3′ [reverse]) (O. Simón, unpublished data) and very-late polyhedrin gene polh (12) (5′ TCGAGGAGAGGACTTTGGAC 3′ [forward] and 5′ CACGGTTGATGAACTCTTCG 3′ [reverse]). The primers were designed to give amplifications of 783 bp for ie-0 and 770 bp for polh. All positive RT-PCR amplifications were checked for the presence of DNA contamination by PCR using the original samples. Positive and negative controls were also included in each RT-PCR amplification. PCR products were observed by electrophoresis in 0.7% agarose gels stained with ethidium bromide and photographed. Each gel was transferred to nylon membrane (Hybond-N+; Amersham), hybridized with digoxigenin-labeled probes (Boehringer Mannheim), washed twice, and subjected to autoradiography.
The genes ie-0 and polh proved suitable as indicators of covert infection. RT-PCR analysis from sixth instars that survived treatment with SfMNPV alone in the fifth instar showed that 9 of 22 (41%) and 2 of 22 (9%) samples tested positive for ie-0 and polh transcripts, respectively. No virus mortality was observed with the mock-infected control insects.
Larvae of S. frugiperda suffered 91 or 89% mortality when inoculated in the second instar with SfMNPV alone or with SfMNPV and Tinopal UNPA-GX, respectively. When inoculated in the fifth instar, insects suffered 83 or 85% mortality, respectively.
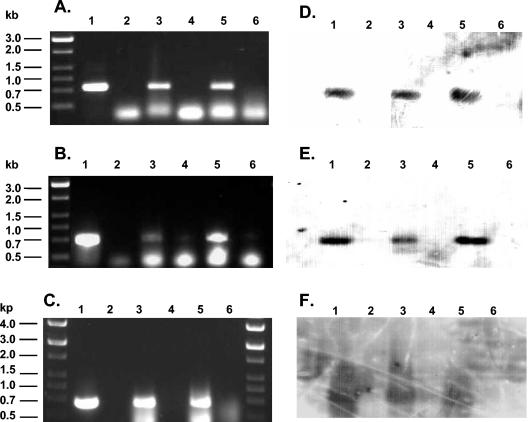
The transcriptional activity of early and late genes differed markedly between the larval and adult life stages. Of the fifth instar insects that survived treatment with SfMNPV alone in the second instar, 4 of 21 (19%) gave a positive result for ie-0 transcripts whereas a single positive individual was detected among insects treated with SfMNPV and Tinopal UNPA-GX (Table 1; Fig. 1A and D). In the case of very late polh gene transcripts, the number of positive insect larvae was extremely low (1 of 21) for both treatments (Fig. 1B and E).
TABLE 1.
Prevalence of different life stages of S. frugiperda positive for SfMNPV-specific transcripts of the immediate-early ie-0 gene and very late polh gene detected by RT-PCRa
| Target gene | Life stage at:
|
No. of insects positive for transcript/No. tested (%)
|
||
|---|---|---|---|---|
| Inoculation | Analysis | SfMNPV | SfMNPV and Tinopal UNPA-GX | |
| ie-0 | Second instar | Fifth instar | 4/21 (19) | 1/21 (5) |
| Adult | 0/21 (0) | 0/20 (0) | ||
| polh | Second instar | Fifth instar | 1/21 (5) | 1/21 (5) |
| Adult | 7/27 (26) | 3/27 (11) | ||
| Fifth instar | Adult | 1/21 (5) | 1/21 (5) | |
Insects were inoculated in the second and fifth instar with SfMNPV alone or in mixtures with Tinopal UPNA-GX. Virus-specific RNA was not detected in any of the untreated control insects, and no PCR products were amplified in any of the RNA controls. In all cases, Southern hybridization analysis confirmed the identity of all the amplification products (Fig. 1D to F).
FIG. 1.
(A) RT-PCR fragments amplified with SfMNPV-specific primers for ie-0 from fifth-instar S. frugiperda that survived inoculation with SfMNPV alone (lane 3) or with SfMNPV and Tinopal UNPA-GX (lane 5) in the second instar. (B) RT-PCR fragments amplified with SfMNPV polh primers from fifth-instar S. frugiperda that survived inoculation with SfMNPV alone (lane 3) or with SfMNPV and Tinopal UNPA-GX (lane 5) in the second instar. (C) RT-PCR fragments amplified with SfMNPV polh primers from adults of S. frugiperda that survived inoculation with SfMNPV alone (lane 3) or with SfMNPV and Tinopal UNPA-GX (lane 5) in the second instar. Positive controls (lethally infected larvae) for ie-0 (A, lane 1) and polh (B and C, lanes 1), negative controls (uninfected larvae) for ie-0 (A, lane 2) and polh (B and C, lanes 2), and RNA controls (PCR on total RNA) (A to C, lanes 4 and 6) are shown. Southern blot analyses of the agarose gels shown in panels A to C used labeled SfMNPV ie-0 (D) or polh (E and F) genes as probes. The molecular marker used was 12-kb DNA ladder (Stratagene); fragment sizes are given on the left.
No ie-0 transcripts were detected in any of the adult insects derived from larvae inoculated in the second instar. In contrast, transcription of the polh gene was detected in 7 of 27 (26%) or 3 of 27 (11%) of adult insects treated with SfMNPV or with SfMNPV and Tinopal UNPA-GX in the second instar, respectively (Table 1; Fig. 1C and F). No difference in the prevalence of polh transcripts was detected between treatments with virus alone and virus with optical brightener for adult insects that had been inoculated in the fifth instar (1 of 21 insects tested positive for each treatment).
The mean weights (± standard errors) of pupae derived from larvae inoculated in the second or fifth instar did not differ significantly in the treatments involving SfMNPV alone (second instar, 186.2 ± 3.5 mg [n = 124]; fifth instar, 157.7 ± 3.5 mg [n = 46]) or SfMNPV and Tinopal UNPA-GX (second instar, 187.2 ± 3.7 mg [n = 64]; fifth instar, 179.8 ± 4.0 mg [n = 67]) compared to their respective controls (second instar, 195.2 ± 3.0 mg [n = 74]; fifth instar, 175.1 ± 4.4 mg [n = 43]) (t test or Kruskal-Wallis test, α = 0.05).
Contrary to our initial hypothesis, the presence of an optical brightener in inocula did not greatly affect the prevalence of covert NPV infections in S. frugiperda larvae or adults. We conclude that the optical brightener does not alter the virulence of the virus, i.e., the severity of disease, in infected hosts (20). Infected cells may struggle to control viral replication or limit the spread of the disease by apoptosis (6). Indeed, baculoviruses contain a number of apoptosis-inhibiting genes designed to reduce the probability of cell suicide (5). The interaction between optical brighteners and baculovirus apoptosis suppressors appears to merit further study.
The persistence of baculoviruses in covertly infected hosts is an effective mechanism that ensures virus survival when the density of the host population is low and opportunities for horizontal transmission are limited (4). The ability to determine accurately the prevalence of covert infection in insect populations depends on the life stages at sampling and the nature of the viral gene mRNA used to detect baculovirus infection.
Acknowledgments
We thank Oihane Simón and Arantza Rico for laboratory assistance.
The study was funded by CICYT project AGL2002-04320-C02-01. Ana-Mabel Martínez received financial support from the Universidad Pública de Navarra and the Agencia Española de Cooperación Internacional.
REFERENCES
- 1.Bartnicki, G. S., J. Persson, and H. Chanzy. 1994. An electron microscope and electron diffraction study of the effect of calcofluor and Congo red on the biosynthesis of chitin in vitro. Arch. Biochem. Biophys. 310:6-15. [DOI] [PubMed] [Google Scholar]
- 2.Blissard, G. W., and G. F. Rohrmann. 1990. Baculovirus diversity and molecular biology. Annu. Rev. Entomol. 35:127-155. [DOI] [PubMed] [Google Scholar]
- 3.Burden, J. P., C. M. Griffiths, J. S. Cory, P. Smith, and S. M. Sait. 2002. Vertical transmission of sublethal granulovirus infection in the Indian meal moth, Plodia interpunctella. Mol. Ecol. 11:547-555. [DOI] [PubMed] [Google Scholar]
- 4.Burden, J. P., C. P. Nixon, A. E. Hodgkinson, R. D. Possee, S. M. Sait, L. A. King, and R. S. Hails. 2003. Covert infections as a mechanism for long-term persistence of baculoviruses. Ecol. Lett. 6:524-531. [Google Scholar]
- 5.Clem, R. J. 2001. Baculoviruses and apoptosis: the good, the bad, and the ugly. Cell Death Differ. 8:137-143. [DOI] [PubMed] [Google Scholar]
- 6.Clem, R. J., and L. K. Miller. 1993. Apoptosis reduces both the in vitro replication and the in vivo infectivity of a baculovirus. J. Virol. 67:3730-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elorza, M. V., H. Rico, and R. Sentandreu. 1983. Calcofluor white alters the assembly of chitin fibrils in Saccharomyces cerevisiae and Candida albicans cells. J. Gen. Microbiol. 129:1577-1582. [DOI] [PubMed] [Google Scholar]
- 8.Escribano, A., T. Williams, D. Goulson, R. D. Cave, J. W. Chapman, and P. Caballero. 1999. Selection of a nucleopolyhedrovirus for control of Spodoptera frugiperda (Lepidoptera: Noctuidae): structural, genetic, and biological comparison of four isolates from the Americas. J. Econ. Entomol. 92:1079-1085. [DOI] [PubMed] [Google Scholar]
- 9.Friesen, P. D. 1997. Regulation of baculovirus early gene expression, p. 141-170. In L. K. Miller (ed.), The baculoviruses. Plenum Press, New York, N.Y.
- 10.Fuxa, J. R. 1991. Insect control with baculoviruses. Biotech. Adv. 9:425-442. [DOI] [PubMed] [Google Scholar]
- 11.Fuxa, J. R., E. H. Weidner, and A. R. Richter. 1992. Polyhedra without virions in a vertically transmitted nuclear polyhedrosis virus. J. Invertebr. Pathol. 60:53-58. [Google Scholar]
- 12.Gonzalez, M. A., G. E. Smith, and M. D. Summers. 1989. Insertion of the SfMNPV polyhedrin gene into an AcMNPV polyhedrin deletion mutant during viral infection. Virology 170:160-175. [DOI] [PubMed] [Google Scholar]
- 13.Hughes, P. R., N. A. M. van Beek, and H. A. Wood. 1986. A modified droplet feeding method for rapid assay of Bacillus thuringiensis and baculoviruses in noctuid larvae. J. Invertebr. Pathol. 48:187-192. [Google Scholar]
- 14.Kukan, B. 1999. Vertical transmission of nucleopolyhedrovirus in insects. J. Invertebr. Pathol. 74:103-111. [DOI] [PubMed] [Google Scholar]
- 15.Lu, A., and L. K. Miller. 1997. Regulation of baculoviruses late and very late gene expression, p. 193-216. In L. K. Miller (ed.), The baculoviruses. Plenum Press, New York, N.Y.
- 16.Moscardi, F. 1999. Assessment of the application of baculoviruses for control of Lepidoptera. Annu. Rev. Entomol. 44:257-289. [DOI] [PubMed] [Google Scholar]
- 17.Rothman, L. D., and J. M. Myers. 1996. Debilitating effects of viral diseases on host Lepidoptera. J. Invertebr. Pathol. 67:1-10. [Google Scholar]
- 18.Simón, O., T. Williams, M. López Ferber, and P. Caballero. 2004. Genetic structure of a Spodoptera frugiperda nucleopolyhedrovirus population: high prevalence of deletion genotypes. Appl. Environ. Microbiol. 70:5579-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simón, O., T. Williams, M. López-Ferber, and P. Caballero. 2004. Virus entry or the primary infection cycle are not the principal determinants of host specificity of Spodoptera spp. nucleopolyhedroviruses. J. Gen. Virol. 45:2845-2855. [DOI] [PubMed] [Google Scholar]
- 20.Thomas, S. R., and J. S. Elkington. 2004. Pathogenicity and virulence. J. Invertebr. Pathol. 85:146-151. [DOI] [PubMed] [Google Scholar]
- 21.Wang, P., and R. R. Granados. 2000. Calcofluor disrupts the midgut defense system in insects. Insect Biochem. Mol. Biol. 30:135-143. [DOI] [PubMed] [Google Scholar]
- 22.Washburn, J. O., B. A. Kirkpatrick, E. J. Haas-Stapleton, and L. E. Volkman. 1998. Evidence that the stilbene-derived optical brightener M2R enhances Autographa californica M nucleopolyhedrovirus infection in Trichoplusia ni and Heliothis virescens by preventing sloughing of infected epithelial cells. Biol. Control 11:58-69. [Google Scholar]