Abstract
The current study presents an overview of heavy metals bioremediation from halo–alkaline conditions by using extremophilic microorganisms. Heavy metal remediation from the extreme environment with high pH and elevated salt concentration is a challenge as mesophilic microorganisms are unable to thrive under these polyextremophilic conditions. Thus, for effective bioremediation of extreme systems, specialized microbes (extremophiles) are projected as potential bioremediating agents, that not only thrive under such extreme conditions but are also capable of remediating heavy metals from these environments. The physiological versatility of extremophiles especially halophiles and alkaliphiles and their enzymes (extremozymes) could conveniently be harnessed to remediate and detoxify heavy metals from the high alkaline saline environment. Bibliometric analysis has shown that research in this direction has found pace in recent years and thus this review is a timely attempt to highlight the importance of halo-alkaliphiles for effective contaminant removal in extreme conditions. Also, this review systematically presents insights on adaptive measures utilized by extremophiles to cope with harsh environments and outlines the role of extremophilic microbes in industrial wastewater treatment and recovery of metals from waste with relevant examples. Further, the major challenges and way forward for the effective applicability of halo-alkaliphilic microbes in heavy metals bioremediation from extremophilic conditions are also highlighted.
Keywords: Alkaliphiles, Biorecovery, Bioremediation, Halophiles, Heavy metals, Wastewater treatment
Introduction
Water is an essential requirement for the survival of organisms. But the pacing industrialization and urbanization has led to the overexploitation of water in terms of irrational usage and untreated/partially treated waste disposal. Industries around the world consume approximately 20–22% of the available freshwater resources, highlighting the tremendous pressure exerted by the industrial processes on available water resources (Boretti and Rosa 2019; Hossain 2019). Moreover, inadequate treatment of industrial effluent renders the ecosystem contaminated (Gayathri et al. 2021). In present scenario, reusing and recycling of water is a prerequisite as it is the most valuable asset and is useful in terms of sustainable management of water resources. The wastewater from domestic and industrial sectors such as electroplating, mining, smelting, electronics, pesticides, tanneries and fertilizers contain different contaminants such as heavy metals, organic compounds-dyes, phenols, nitro compounds, color, pesticides and further their discharge leads to the deterioration of water quality (Chen et al. 2020). These contaminants also possess major threat to the environment including living beings. From the plethora of validated water toxicants/contaminants; heavy metals in particular are pollutants of high concern, as they cannot be remediated easily owing to their bio-accumulative and recalcitrant nature (Bolisetty et al. 2019).
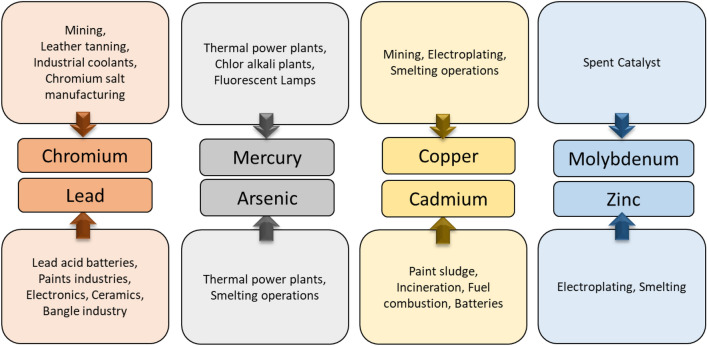
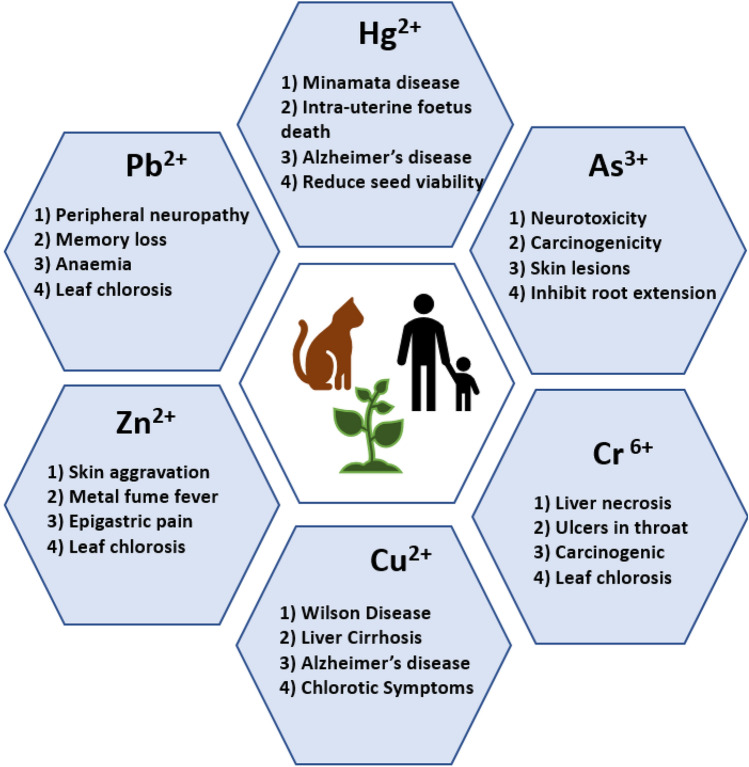
Group of metals and metalloids that possess relatively high atomic density greater than 4–5 g/cm3 are referred to as heavy metals (Masters and Ela 2007; Bhattacharya et al. 2019; Gallo et al. 2021). Natural activities for instance volcanic eruption, rocks weathering, infiltration and anthropogenic activities such as automobile exhaust, fossil fuel burning, mining and industrial wastes result in the contamination of the environment with heavy metals (Sall et al. 2020). Figure 1 enlists various anthropogenic sources of heavy metals. Moreover, availability of heavy metals in various oxidation states and their tendency to bioaccumulate and biomagnify at different trophic levels, further raise their toxic potential. In addition, heavy metals are also known to have teratogenic, mutagenic and carcinogenic properties (Verma and Kuila 2019). Figure 2 shows some of the major diseases caused due to toxicity of heavy metals.
Fig. 1.
Anthropogenic sources of heavy metals contamination in the environment
Fig. 2.
Major health issues associated with heavy metal toxicity/contamination in plants, animals and human beings
Various conventional physico-chemical treatment technologies such as chemical precipitation, membrane filtration and ozonation adopted for the treatment of heavy metal laden industrial effluents are found to be endowed with number of limitations including less efficiency, high operational cost, production of toxic byproducts, limited range of functional capacity and energy intensive nature (Giovanella et al. 2020). Among different remediation options available, bioremediation is considered as a suitable route mainly because of its sustainable nature. Also, bioremediation has proven to be effective and low cost approach lately for heavy metal removal (Pham et al. 2022). However, the problem becomes further aggravated, when heavy metals need to be remediated from extreme environment with high pH and saline conditions. Industrial wastewaters like discharges from textile, tannery and distillery industries are usually characterized by elevated pH and high salt concentrations in addition to the presence of heavy metals such as cadmium, nickel, zinc, hexavalent chromium, copper, lead and cobalt (Ali et al. 2015; Mubashar et al. 2020; Rangasamy et al. 2021). Bioreclamation of such harsh environment is highly challenging as normal/mesophilic metal remediating microbes are not able to function and/or grow effectively in the extreme conditions mentioned above. These conditions make the wastewater treatment extremely tedious and thus necessitate the adoption of remediation methods which are efficient for such environment.
Thereby, specialized microbes which are able to thrive in these extreme situations and dubbed as “extremophiles” are being explored conveniently for remediation of heavy metals. In view of above, the present review is structured to provide an overview on use of extremophiles towards remediation of heavy metals from extreme conditions particularly high pH, and high salt concentration. Taking into consideration the ability of extremophiles to adapt to extremities, this study provides insights into documented literature over utilization of extremophiles for bioremediation on laboratory scale, in real time industrial effluents as well as in the recovery of metals from wastes. Along with these lines, this study could help in giving significant benchmark data to researchers and scientists for determining the optimized conditions for heavy metal remediation in selected high saline–alkaline conditions. Moreover, the review highlights and will benefit researchers, students, industrialists and policy makers in understanding the keynote role of extremophiles for recovering metals from the waste.
Data acquisition and analysis
For understanding scientific communities’ interest along quality, quantity and network dimensions of halo-alkaliphiles for heavy metal removal, literature search was done using Web of Science (WoS). The WoS database was searched on using search query as depicted in Fig. 3a for last 2 decades. After critically analyzing the 111 retrieved results by title and abstract, non-relevant publications were excluded and specifically relevant 42 results from dataset were taken for further analysis. As represented in Fig. 3b publication trend from 2003 to 2022 reflects positively increasing emphasis on engagement of halo-alkaliphiles for heavy metal removal with maximum number of studies published in the year 2021. VOSviewer (version 1.6.19) was used for analyzing basic publication characteristics including keywords co-occurrence contributing as core research power in this regard. Most often co-occurrence of all keywords is shown in Fig. 3c. Keywords with a minimum occurrence of 2 were selected, and therefore out of 356 keywords only 76 met the threshold. Size and distance of the bubble represents the frequency of the keywords and the associations between them, respectively. Five clusters of keywords were generated by VOSviewer and the keyword “bioremediation” has the highest occurrence. Figure 3d illustrates countries’ contribution based on the number of publications from a country. As observed from this data, India’s contribution is highly significant in this area. This analysis might be helpful for academicians in establishing scientific partnerships based on the graphical depiction of active countries for working dynamically while exchanging novel methods and concepts.
Fig. 3.
a Flowchart of search query used in Web of Science database. b Publication trend from 2003 to 2022 based on search query as mentioned in 3 a, c Co-occurrence of all keywords plotted using VOSviewer (version 1.6.19). d Country-wise contribution in terms of number of published articles per year
Heavy metals in saline, hypersaline and alkaline conditions
Heavy metals in the ecosystem can be shipped over significant distances. With time gradient, sometimes the heavy metals can end up to deposit in extreme ecological niches. The chances of heavy metals contamination in the extreme areas of natural ecosystem have increased due to extensive industrialization (Jiao et al. 2021). Presence of the heavy metals has been reported in Greenland snow and ice cores (Boutron et al. 1995), Chaohu Lake, China (Fang et al. 2019), Moshui Lake sediments, Wuhan City, China (Honglei et al. 2008), Jiulong River Estuary, Fujian, China (Zhang et al. 2014), Mai Po Marshes, North-west Hong Kong (Che and Cheung 1998). Though ecological niches are endowed with different types of extremes, however the present study is focused on heavy metals removal from saline and alkaline environments. Such extreme conditions are known to impact the fresh water availability, nutritional imbalance, denaturation of enzymes and toxicity of high Na+ and Cl− level which ultimately results in altered microbial growth (Abdel-Latef et al. 2020; Liu et al. 2021). Hypersaline environments are characterized by the presence of > 60 g/L of total salinity, with NaCl concentration up to 33% (w/v). Alkaline habitat on the other hand, is characterized by dominance of alkali and alkaline earth metals ions, pH values > 9, and the presence of carbonate and bicarbonate ions (Dodia et al. 2006; Uma et al. 2020).
In nature, heavy metal contamination has been reported to be prevalent in some of the saline–alkaline environments such as saline lake located in Beseka, Ethopia (Fuad and Gelaneh 2017), hypersaline lakes of Siwa oasis and Wadi El Natrun, Egypt (Gad et al. 2020), Maharlu Lake, located 23 km southeast of Shiraz, SW Iran (Moore et al. 2009), saline Lakes of the Gobi Desert Region, Western Mongolia (Bayanmunkh et al. 2017), Hamatai alkaline lake of Inner Mongolia plateau (Liu et al. 2021), and alkaline sediments of Lake Fehér at Szeged located in Hungary (Halmos et al. 2015). Additionally, anthropogenic sources like industrial wastewater originating from tannery, textile, distillery and electroplating industries also add heavy metals laden alkaline wastewater to the environment (Gayathri et al. 2021). For instance, Kim et al. (2020) have reported the presence of copper, nickel, zinc and chromium in an alkaline metal plating wastewater sample. Presence of heavy metals namely Iron, Manganese, Zinc, Lead, Copper, Chromium, Nickel, and Cadmium due to electroplating industrial discharge in core sediments of Qi’ao Island, South China has been documented by Gopalakrishnan et al. (2020). Jiang et al. (2017) reported electroplating industries as potential source for the contamination of lead, copper, zinc, arsenic, chromium, cadmium, and nickel in soil samples collected from rural-industrial town in southern Jiangsu, China.
Constraints in remediation of heavy metals from saline and alkaline environments
Salinity impacts the mobility and bioavailability of heavy metals as higher the salinity, more is the concentration of calcium-magnesium salts (Moradi et al. 2019). Increased calcium and magnesium ion levels compete with heavy metal ions, hence prompting their release from complexes, further influencing metal ions mobility (Wang et al. 2019b, a). Similarly, high pH also affects the remediation of heavy metals. Specific pH is required for efficient adsorption, as the adsorption proficiency of heavy metals is dictated and controlled by the underlying pH of the concerned micro environment set-up (Li et al. 2020a, b). pH plays a keynote role in association, dissociation and surface charge of the heavy metals (Soliman and Moustafa 2020). Change in the pH of medium can essentially impact the metal bioavailability and solubility. Extreme low/high pH conditions weakens the surface adsorption henceforth leading towards low remediation rate of heavy metals (Wu et al. 2019).
Along with individual cases of high pH and salinity; scientists and researchers working on remediation also face challenges due to their dual presence in many samples. Conjointly, salinity and alkalinity have been reported in industrial effluents (Merino et al. 2019), soda lakes (Schröder et al. 2020), hypersaline soda lakes (Abo-Alkasem et al. 2022), wetlands (An et al. 2020), lonar lake (Yadav and Patil 2020), sodic soil (Wang et al. 2019b, a). In the above-mentioned conditions salinity–alkalinity together influence heavy metals mobility and availability thus, making the remediation of heavy metals even more challenging in saline–alkaline conditions.
Need of halophilic, alkaliphilic and/or halo-alkaliphilic microbes
Exploration of microbial community for pollution abatement is being looked as a sustainable, efficient, cost effective and environmentally benign approach (Rao et al. 2023). Also, bioremediation offers permanent solution as it generally doesn’t generate any toxic secondary metabolite(s) thus rendering the environment intact (Abdelhafeez et al. 2022). However, success of this technique depends upon microbial survival and working efficiency in the presence of heavy metals as these inorganic pollutants are known to be toxic to different microbes depending upon the concentration and type of heavy metals and nature of microbial strain. Toxicity due to heavy metals is further exemplified manifold in saline and alkaline conditions. Dominance of high pH and high salt concentration makes it challenging for microbes to sustain themselves under such harsh conditions.
Increased salinity impacts the porosity of soil, which in turn disrupts water availability to the microbes. Saline and hypersaline conditions affect fundamental functioning of the microbes due to osmotic and ion effects (Jacob et al. 2018). Increase in soluble salt content leads to negative osmotic potential of soil water, thereby draining microbial cell water and creating a vacuum of water inside the cell. Microbes starve for water and it ultimately leads to plasmolysis causing cell death and thereby reducing the microbial biomass (He et al. 2017). Microbes tend to manage salinity via accumulation of osmolytes (organic and inorganic). However, accumulation of organic osmolytes such as proline is an energy expensive process whereas deposition of inorganic osmolytes like potassium can be toxic and fatal for the microbial cell (Yan and Marschner 2012). Therefore, changes in osmotic levels and concentrated salts render microbes futile in remediating heavy metals in hypersaline and saline ecological niches. Moreover, high pH also influence microbial growth and enzyme activity (Jacob et al. 2018). In alkaline conditions, the microbial growth rate is usually enhanced through replacement of protons by hydroxo-metal complexes, which results in fastest attainment of logarithmic and decline phases owing to lesser encounter time between microbes and heavy metals thereby hampering the metal remediation efficiency (Govarthanan et al. 2016; Jacob et al. 2018). Additionally, mesophiles are incompetent in maintaining enzymatic activity in alkaline conditions. Henceforth, this necessitates recognizing and employing specific microbes which can tolerate salinity and alkalinity, in addition to heavy metals bioremediation.
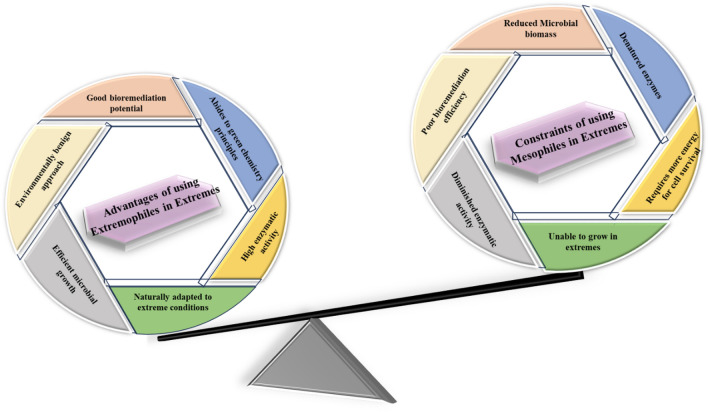
Therefore, identification and exploration of extremophiles that exist at the limits of various life boundaries is new area of interest for scientific community. Bioremediation, using extremophiles presents superior way for detoxification of toxicants attributable to fact that they work proficiently under extreme conditions (Kumar et al. 2018). Extremophilic microbes such as Bacillus sp. A21, Bacillus pumilus, Halomomnas smyrnensis are extensively studied for remediation of heavy metals in presence of alkalinity and salinity. These microbes are laden with specific adaptive features (described in next head) which make them effective to thrive in these extreme conditions and also make them effective in remediation of heavy metals. Some of the advantages associated with extremophiles that make them superior over their counterpart i.e. mesophiles for applying in bioremediation of extreme environment are mentioned in Fig. 4.
Fig. 4.
Advantages of utilizing extremophiles over mesophiles for bioremediation in extreme environment
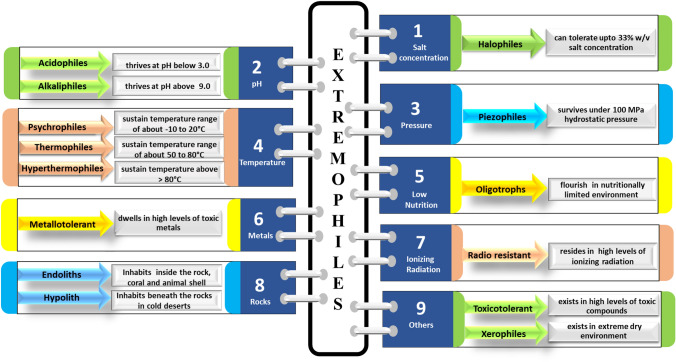
Unless otherwise mentioned, extremophiles are broadly grouped as: extremophilic (which exemplify their preference to dwell in extreme conditions) and extremotolerant (which have attained endurance to sustain in extreme conditions). The detailed classification of extremophiles is shown in Fig. 5. Since the review focuses on treatment of high pH and salt abundant environment; the microorganisms adapted to survive under these conditions are discussed henceforth. Microorganisms that are the occupants of outrageous saline (upto 33%, w/v NaCl) conditions are known as halophilic microorganisms (Dhakar and Pandey 2016) while the inhabitants of alkaline pH above 9 are denoted as alkaliphiles in literature (Zhai et al. 2021). Certain microbes exhibit their capability to flourish in dual presence of salt (upto 33% w/v NaCl) and pH (above 9) conditions and are designated as halo-alkaliphiles (Uma et al. 2020). Since these microbes are endowed with innate ability to survive and work under harsh environment they are being looked as potential bioremediation tool in such environment (Bano et al. 2018).
Fig. 5.
Different classes of extremophiles (Gupta et al. 2014; Shrestha et al. 2018; Gunjal et al. 2021)
Advantages of using extremophiles in heavy metal bioremediation
Extremophilic microbes are valuable in modern remediation methods, as the utilization of extreme microbes for treatment of industrial wastewater (containing high salinity, alkalinity, and heavy metals), minimizes the cost of treatment as there is no extra/separate treatments or equipment requirement for sequential removal of alkalinity, followed by salinity or metals. For instance, the key advantage of halo-alkaliphiles in saline–alkaline environments, in comparison to mesophiles lies in the fact that halo-alkaliphiles can thrive and effectively eliminate contaminants from saline–alkaline wastewater without the necessity for prior treatment, while mesophiles would demand pre-treatment to endure the salinity and alkalinity, incurring additional expenses.
Another important advantage of using extremophiles is lesser chances of contamination or interference of other microbial species during their application since due to the extreme nature of environment; there is less competition/inhibition from competing species, as very few microbes are able to survive in extreme conditions. Henceforth, extremophiles survival in adversities adds to their utilization in such inhospitable conditions where the efficacy and effectiveness of mesophiles is low. Also, microbes possessing polyextremophilic properties viz. heavy metals, salinity and alkalinity tolerant, could easily be applied for the remediation of environment laden with these conditions. These promising characteristics make extremophiles and their biocatalysts a peculiar instrument for remediation of pollutants from environment (Gallo et al. 2021). Also, extremophiles can recuperate metals from wastes, thus not only addressing the contamination issue but in addition offering way to recover metals from the contaminated sites/wastes, thereby adding value to waste (Shrestha et al. 2018; Yadav and Patil 2020; Gallo et al. 2021; Chaudhary et al. 2022).
Adaptive features of microbes towards halo–alkaline and heavy metal-rich environment
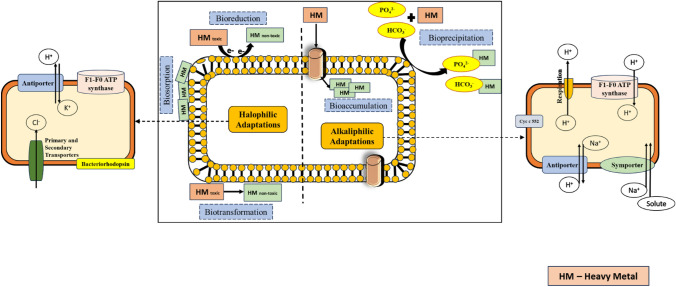
With due course of evolution and in accordance to concerned specific environmental conditions, extremophiles have altered themselves through various adaptations. Adjustment to the extreme conditions is followed by incorporation of requisite unique physiological and metabolic mechanisms. Extremophiles have specialized stable enzymes i.e. extremozymes, unique genomic data governing enhanced rate of transcription and translation, alternative molecular mechanism producing heat shock absorbent or antifreeze proteins, optimized biomolecules maintaining cytoplasmic pH homeostasis acting as osmoprotectants and antiporter system that help their existence in harsh conditions (Gupta et al. 2014; Yin et al. 2019). The major mechanisms adopted by microbes for heavy metal remediation and adaptive features of halophiles and alkaliphiles are presented in Fig. 6.
Fig. 6.
Microbial mechanism for heavy metal bioremediation and alkaliphilic–halophilic adaptations of the microbes for survival in high salt and high pH conditions
Halophilic microorganisms have inherent capacity to endure and prosper under high salt conditions (Gupta et al. 2014). Survival in raised salt niche is ought to be challenging for other forms of life due to varying osmolar concentration. Difference in osmolar concentrations generates metabolic issues such as reduced enzymatic activity, reduced microbial biomass, changes in microbial community structure, drawing of water content from the cell-organelles and ultimately leading to plasmolysis and cell death. Halophiles overcome these metabolic issues via adoption of strategies listed below:
Deposition of inorganic or organic osmoprotectants. Inorganic osmoprotectants such as KCl/NaCl and organic solutes like sucrose, polyols within the cell maintains osmotic balance (Jeong and Choi 2020). Few halophiles maintain osmotic balance using bacteriorhodopsin and a light-determined proton pump to deliver ATP to drive the antiporter (Gunde-Cimerman et al. 2018).
Follow salt-in strategy which involves influx of K+ and efflux of Na+ ions to avoid intracellular water loss (Gunde-Cimerman et al. 2018; Salma et al. 2020).
Proteins of halophilic microbes show following adaptations:
Negative charge on external surface of halophilic proteins gives them rigidness and adaptability which prevents cells dehydration and agglomeration in high salinity (Ortega et al. 2015).
Halophilic enzymes have structural stability owing to H-bond networks and stable salt bridges (Nayek et al. 2014).
Presence of P45 protein prevents the denaturation in hyposaline conditions (Franzetti et al. 2001; Kumar et al. 2018).
Due to TATA-box-restricting protein (TBP), halophiles display expanded collaboration with DNA in high salinity in comparison to mesophilic counterparts (Kumar et al. 2018).
In regions of high alkalinity, production of ATP (energy unit of cell) is challenging for mesophilic forms of life as for efficient ATP synthesis, extracellular pH ought to be less than intracellular pH. Alkaliphiles maintain the cellular functioning by regulating the ATP production in high alkalinity. Their adaptations aid their existence in high ranges of pH (Dhakar and Pandey 2016). Alkaliphiles cope with high alkalinity due to the following adaptations:
Acidic polymers such as galacturonic acid, glutamic acid, aspartic acid, gluconic acid, and phosphoric acid are present in cell walls of the alkaliphiles. Also, alkaliphiles develop an additional secondary cell wall which is acidic in nature and composed of teichuronic acid or teichurono-peptide. It plays a keynote role in restricting the entry of OH− in the cells (Mandeep 2020).
Alkaliphiles have reduced cell membrane permeability and porosity as compared with the mesophilic counterparts. Moreover, lesser number of unsaturated fatty acids has been reported as a strategy against high pH (Dhakar and Pandey 2016).
Presence of cytochrome c in Gram-positive bacteria and cytochrome c-552 in Gram-negative bacteria have been reported to aid the growth of alkaliphiles in high pH ranges. Cytochromes regulate pH homeostasis by proton deposition (Gunjal et al. 2021).
Alkaliphilic enzyme phosphoserine aminotransferase forms dimer with increased hydrogen bonds, more number of negative amino acids and exhibits high hydrophobic interactions providing stability to alkaliphiles (Kumar et al. 2018).
ATP synthase plays a vital role in cellular mechanism of alkaliphiles. Subunit c of ATP synthase has unique combination of amino acid motifs for retention of H+ ions in the cell (Kulkarni et al. 2019).
Heavy metal remediation under saline–alkaline conditions
Numerous mechanisms are utilized by extremophiles for remediation of heavy metals, viz. (i) Microbial cells enable the conversion of the heavy metals from one oxidation state to another, henceforth reducing their toxicity, (ii) In addition, metals and metalloids are utilized by microbes as a source of electron donors or acceptors for the process of energy production, and (iii) Sorption/Adsorption of heavy metals on cell surface.
However, the success rate of bioremediation relies on several factors such as the type of contaminated site, degree of contamination, selected microbial species, accessibility of heavy metal for uptake, heavy metal ion concentration, redox potential, valance and oxidation state of the heavy metal and other physico-chemical factors for instance pH, temperature, salinity and alkalinity (Boopathy 2000). Remediation becomes challenging when contaminated site has more than one parameter in sync such as halo alkaline site along with high concentration of heavy metals or presence of heavy metals in complex state or in coordination compounds. Henceforth, characterization of contaminated site plays a key role in determining the type of bioremediation technique to be engaged for detoxification. It is vital to know about the soil type, quantity and concentration of pollutants as these will affect the bioremediation efficiency (Azubuike et al. 2016). Extremophilic microbes that have been used for remediation of heavy metal in alkaline–saline conditions are enlisted in Table 1 with details of isolation source, optimized salt conditions, target heavy metal(s) and percentage removal.
Table 1.
Remediation of heavy metals using extremophilic microorganisms under high salt and/or alkaline conditions
| Heavy metal | Extremophile | Type | Isolation source | Optimised salt conditions | pH | Initial metal concentration | Metal removal efficiency | References |
|---|---|---|---|---|---|---|---|---|
|
Lead Nickel |
Bacillus sp. A21 | Halophile | Khara Salt Lake in Iran | 10% w/v salt | – | 1 mM |
97.5% 76% |
Diba et al. (2021) |
|
Lead Nickel |
Oceanobacillus sp. A22 | Halophile | Khara Salt Lake in Iran | 10% w/v salt | – | 1 mM |
98.8% 73.5% |
|
|
Lead Nickel |
Salinicoccus sp. A43 | Halophile | Khara Salt Lake in Iran | 10% w/v salt | – | 1 mM |
92% 71.7% |
|
| Lead | Bacillus pumilus | Halophile | Mangrove region of Karnataka, India | – | 6, 8 | 900 ppm | 99.3–99.4% | Sahoo and Goli (2020) |
| Hexavalent chromium | Halomonas smyrnensis KS802 | Halophile | Multi-pond solar salterns of Gujarat, India | 4% galactose, 5%NaCl | 7 | 2 mM | 100% | Biswas et al. (2018) |
| Mercury | Pseudomonas aeruginosa | Halophile | Non-active sanitary landfill leachate, Indonesia | 10% salinity level | – | 10 mg/L | 99.7% | Imron et al. (2019) |
|
Nickel Lead Mercury Copper Zinc Cadmium |
Penibacills sp. strain SEM1 | Halophile |
Enyigba Pb–Zn mining site Ebonyi State, Nigeria |
5–15% NaCl | 5 | 1700 mg/L |
97.62% 96.84% 90% 86.36% 84.85% 70% |
Orji et al. (2021b) |
|
Copper Nickel Lead Mercury Zinc Cadmium |
Morganella sp. strain WEM7 | Halophile |
Enyigba Pb–Zn mining site Ebonyi State, Nigeria |
5–10% NaCl | – | 1700 mg/L |
100% 98.64% 97.48% 93.33% 80.85% 68.42% |
|
| Hexavalent chromium | Brevibacillus laterosporus CrRPSD40 | Halotorelant | Marine sediments of Paradip port, Odisha, India | 3% w/v NaCl | 8 | 100 mg/L | 92% | Mohapatra et al. (2017a) |
| Hexavalent chromium | Exiguobacterium indicum strain MW1 | Halotorelant | Marine water of Paradip Port, Odisha, India | 1% NaCl | 8 | 100 mg/L | 92–91% | Mohapatra et al. (2017b) |
|
Mercury Lead Cadmium Nickel Copper Zinc |
Klebsiella sp. USL2S | Halotorelant |
Uburu salt Lake Southeastern, Nigeria |
5–15% NaCl | – | 1700 mg/Ls |
85% 97.13% 73.33% 88.06% 100% 42.30% |
Orji et al. (2021a) |
|
Mercury Lead Cadmium Nickel Copper Zinc |
Pseudomonas sp. strain USL5W | Halotorelant |
Uburu salt Lake Southeastern, Nigeria |
5–15% NaCl | – | 1700 mg/L |
95% 98.89% 77.42% 99.54% 100% 84.52% |
|
|
Mercury Lead Cadmium Nickel Copper Zinc |
Pseudomonas sp. USL4W | Halotorelant |
Uburu salt Lake Southeastern, Nigeria |
5–15% NaCl | – | 1700 mg/L |
95% 97.55% 69.72% 97.91% 83.62% 98.9% |
|
|
Iron Copper |
Bacillus pumilus | Halophilie | soil and water of Karnataka mangrove, India | 5% NaCl | 10 | 1000 mg/L |
96% 88% |
Divakar et al. (2018) |
| Iron | Desulfovibrio halophilus sp. | Halophilie | Kashmar cotton delinting factory, Iran |
10% NaCI 1%MgCl2.6H20 |
6.9–7.1 | 350 mg/L | 85.3% | Torbaghan and Torghabeh (2019) |
| Cadmium | strain R1 MTCC 3265 | Halophilie | – | 25% NaCl | – | 1 mM | 63% | Bragança and Furtado (2017) |
| Strontium | Bacillus sp. strain TK2d | Halophilie | Fukushima Daiichi Nuclear Power Plant | 3% NaCl | – | 1 × 10–3 mol/L | > 99% | Horiike et al. (2017) |
|
Lead Cadmium Mercury |
Halomonas elongata | Halophilie | – | 10% NaCl | 5 |
1 mg/L 3 mg/L 0.5 mg/L |
97.82–98.47% 74.17–89.16% 69.52–81.11% |
Asksonthong et al. (2018) |
|
Lead Cadmium Mercury |
Tetragenococcus halophilus |
Halophilic lactic acid bacteria |
– | 10% NaCl |
6 7 7 |
1 mg/L 3 mg/L 0.5 mg/L |
91.60–96.54% 93.50–95.12% 8.64–12.69% |
|
| Hexavalent chromium | Parapedobacter sp. ISTM3 | Halophilie + alkaliphile | Mawsmai cave, Meghalaya, India | – | 8 | 20 mg/L | 95.10% | Tyagi et al. (2020) |
| Hexavalent chromium | Paenibacillus pabuli (JX561107) | Alkaliphile | Alkaline industrial contaminated soil, Pondicherry, India | – | 9.5 | 50 mg/L | 98% | Rangasamy et al. (2021) |
| Hexavalent chromium | Bacillus cereus (JX561108) | Alkaliphile | Alkaline industrial contaminated soil, Pondicherry, India | – | 9.5 | 50 mg/L | 74% | |
| Hexavalent chromium | Citricoccus alkali tolerans(CSB1) | Alkaliphile | Sambhar Salt Lake, Rajasthan, India | 10% NaCl | 9 | 120 µg/L | 98% | Chandra et al. (2016) |
| Hexavalent chromium | Microbacterium sp. M5 | Alkaliphile + Halotolerant |
Soil samples from CETP Jamalpur, Ludhiana, India |
– | 9 | 400 mg/L | 100% | Kumar and Saini (2019) |
|
Copper Nickel Zinc |
Micrococcus luteus 2YB-25OH |
Halotolerant |
Wastewater from Oued El Harrach in west of Algiers Algeria |
10% NaCl | 6–8.5 | 25 µg/L |
51.45–83.90% 52.59–78.81% 59.55–78.90% |
Benmalek and Fardeau (2016) |
Halophilic–alkaliphilic isolates from the contaminated site exemplify tremendous potential of bioremediating heavy metals under high salt and elevated pH conditions, thus acting as a potent bioremediating agent on both laboratory scale and in real field applications. The current understanding and applications of halophiles, alkaliphiles and halo-alkaliphiles in detoxification of extremely polluted environment and metals recovery from waste are presented individually in next sub-heads to help researchers and scientists to work proficiently in their area of choice.
Bioremediation potential of microbes isolated from extreme natural environment/industrial wastewater
As the global environmental crisis intensifies, innovative strategies like harnessing the bioremediation potential of native microorganisms hold potential for addressing contamination challenges across diverse ecosystems. Halophilic and alkaliphilic microorganisms isolated from natural polluted environment/industrial wastewater are being tested for their potential to act as micro-bioremediation factories in high alkaline–saline conditions (Jeong and Choi 2020). For instance, Sher et al. (2020) used bacterial strain Micrococcus luteus strain AS2 isolated from industrial wastewater for remediation of arsenic from contaminated water/site and observed 99% of 1000 mM arsenic removal after 10 h of incubation. On the similar note, Mabrouk et al. (2014) used Halomonas sp. isolated from tannery effluent for hexavalent chromium reduction and found that the bacterium could remediate 82% of 50 mg/L of Cr6+ under the saline and alkaline conditions, specified as pH 10 and 5% NaCl. Reduction of 72% of 50 mg/L of Cr6+ by tannery effluent isolated haloalkaliphilic chromate resistant strain designated as Alkalihalobacillus clausii CRA1 in 6 days of incubation was reported by Gautam et al. (2022). Zmorrod et al. (2020) studied the potential of Halomonas alkalicola Ext, an alkaliphilic isolate from tannery industry wastewater for bioaccumulation of 27.3 mg/g Pb2+ from initial concentration of 0.1 mmol/L at pH ranging 9–10.
Zhang et al. (2012) have reported Pseudomonas putida SP1 isolated from marine environment which has resistance to high level mercury. According to this study, the isolate can ingest 100% of total mercury and exhibit the reduction of responsive Hg2+ to Hg0 fume by reductase, which exemplifies its importance in the bioremediation of mercury pollution in marine condition. In another study, an alkaliphile and halotolerant Microbacterium sp. M5 isolated from soil near the vicinity of common effluent treatment plant located in Jamalpur, Ludhiana (Punjab), India (+ 30°54′34,740″, + 75°51′261,972″), which receives industrial as well as the municipal waste, displayed 100% reduction of 200 and 400 mg/L of Cr6+ in 24 and 48 h of incubation at pH 9, respectively. Remediation of the hexavalent chromium by strain M5 makes it a potential microbe for the decontamination and detoxification of the existing polluted sites having alkaline conditions (Kumar and Saini 2019). Ibrahim et al. (2011) stated that the alkaliphile Bacillus sp. strain KSUCr5 isolated from the hypersaline soda lakes located in Wadi Natrun valley extending in northwest by southeast direction between latitudes 30°15´ north and longitude 30°30´ east in northern Egypt showed complete reduction of 80 mg/L of Cr6+ at pH 10 within 24 h of incubation time. Tolerance to other heavy metals namely Cd2+, Mo2+, Mn2+, Cu2+, Ni2+, Pb2+, Co2+ and Zn2+ was also shown by the isolated strain and therefore emphasising the importance of isolation of microbes from extreme environment for bioremediation. Bacillus cereus NWUAB01 isolated from the soil sample of gold mining site at Vryburg, South Africa remediated 83% Pb2+, 60% Cd2+ and 30% Cr6+ at 100 mg/L initial metal concentration individually (Ayangbenro and Babalola 2020). Titah et al. (2020) reported isolation of Burkholderia pseudomallei from the ship breaking area and removal of Fe2+ with efficiency of 57.8% and 58.5% at the salinity of 15 and 20 ppt and pH-7 respectively. Bai et al. (2021) documented the potential of Exiguobacterium sp. JBHLT-3, a halophilic isolate from water sample of Jihuhulangtu Nuur lake (48° 53.214′ N, 118° 5.653′ E) located at the north of the county of Xin Barag Zuoqi, Inner Mongolia, China for removal of 89% Pb2+ under 12% salinity. By understanding the unique genetic, physiological and biochemical traits of the extremophiles employed in the above-mentioned studies, novel avenues for developing sustainable bioremediation technologies can be further explored.
Remediation of heavy metals from industrial wastewater under saline–alkaline conditions
Release of heavy metal laden wastewater from the industries poses health hazards to biota. Hence treatment of effluent is necessary before its discharge in the environment. Owing to the exceptional properties of the halophiles and alkaliphiles, remediation of heavy metals from the wastewater is feasible from saline and alkaline conditions in an environment friendly and cost efficient manner (Leong and Chang 2020). Alkaline leachates discharged from the chromite ore processing unit of a cement industry were bioremediated with the aid of a haloalkaliphile isolated from soda lake belonging to Halomonas genus at pH 10 by Watts et al. (2015). The bacterium showed the reduction of 2.5 mM of hexavalent chromium under anaerobic conditions upto pH 11 (Watts et al. 2015). While, the microbial strains, Microbacterium arborescens and Pseudomonas stutzeri, tolerant to high salt concentration exhibited 54% and 52% reduction of 0.48 mg/L of hexavalent chromium, respectively on 9th day of incubation when utilized for tannery effluent treatment by Ashraf et al. (2018). Accomplishment of 85.3% iron removal from the highly saline synthetic wastewater of cotton delinting factory was obtained using Desulfovibrio halophilus sp., a halophilic isolate by Torbaghan and Torghabeh (2019). Aji (2021) reported removal of chromium from saline tannery effluent with the aid of halophillic strain Pseudomonas aeruginosa sthc002 isolated from Puthalam salt pan Tamil Nadu, India.
Addressing the remediation of heavy metals from industrial wastewater under saline–alkaline conditions presents an essential challenge in ongoing efforts towards environmental sustainability. Through comprehensive research and innovative strategies, it is worthwhile to further refine and develop heavy metal bioremediation techniques, considering factors such as efficiency, cost-effectiveness and environmental impact. By harnessing the potential of halo-alkaliphilic microbes as mentioned by researchers in aforementioned studies, detrimental effects of heavy metal contamination can be reduced along with preserving our precious water resources for generations to come. Further, in order to reduce environmental burden on natural resources, it is important to recover metals from the waste. Thereby, the next section presents the research studies conducted on recovery of metals from waste.
Recovery of metals from wastes (e-waste, landfill wastes, waste streams)
In modern day society, electronic gadgets have become a basic necessity. Improper dismantling and disposal of these gadgets leaves behind heaps of heavy metal enriched e-waste (Arya and Kumar 2020). Along with e-waste, another challenging issue is the management of heavy metals laden fly ash generated by incineration of municipal solid waste and biomedical waste (Wang et al. 2019a, b). Extracting metal from waste presents an essential way of contributing towards sustainability and adding value to the waste. Therefore, utilization of biological identities for metal recovery is budding area of research and exploration due to the enhanced efficiency and eco friendliness (Yu et al. 2020). Bioleaching experiment conducted for biorecovery of metals from e-waste exemplified the potential of an alkali tolerant strain namely Bacillus foraminis for the extraction of Ag-100%, Mo-56.8% and Cu-41.4% from the Active-Matrix Organic Light-Emitting Diode (AMOLED) screens of discarded mobile phones (Golzar-Ahmadi and Mousavi 2021). In another study, alkaliphilic strains namely Agromyces aurantiacus TRTYP3, Alkalibacterium pelagium TRTYP5, Alkalibacterium sp. TRTYP6 and B. foraminis TRTYP17 isolated from the local fly ash landfill site exhibited the metal tolerance ability along with the bioleaching properties for the heavy metals specifically for the Cu. Combination of the aforementioned isolates in the ratio of 1:1:3:1 leached 88, 81, 78, 76, 70, and 55% Cu, correspondingly, after treating 1, 2.5, 5, 10, 15, and 20% (w/v) of fly ash (Ramanathan and Ting 2015). Ramanathan and Ting (2016) documented the employment of Alkalibacterium sp. TRTYP6, an alkaliphilic isolated from fly ash landfill site, Singapore for recovery of 52% copper at alkaline pH 9–10 from fly ash generated via municipal solid waste incineration. As reported by Maes et al. (2016a) recovery of > 98% Pt 2+ and > 97% Pt 4+ was done from liquid streams by using a halophilic consortium comprising of microbes from Bacillaceae, Halomonadaceae and Idiomarinacea families. On similar note, in another study conducted by Maes et al. (2016b), 79–99% platinum recovery could be obtained from refinery process stream under 10–80 g/L salt concentration by employing a halophilic mixed culture. Recovery of silver from waste X-ray sheets was reported by utilizing alkaline protease from moderately halophilic Bacillus sp. EMB9 isolated from seawater, Goa, India (Sinha et al. 2014). Employment of a halophilic Thiobacillus ferroxidans N-11, isolated from hypersaline soils of Kolhapur, Maharashtra was done for 78% copper recovery in 20 days from low grade ore Bornite (Nakade 2013). Extraction and recovery of valuable metals from the waste is both environmentally and financially appealing, as it reduces the burden of waste management and boosts the utilization of recovered metals as raw materials for further processing. Consequently, effective studies in this direction of employing halo-alkaliphiles for metal recovery appears to be one of the most robust, reliable and sustainable waste management solutions. In addition, it also embraces the circular economy notion by adding value to the waste.
Key challenges and way forward
The fact that highly tolerant halo-alkaliphilic microbes offer the contaminant removal potential in the extreme environments has provided an advantage to existing bioremediation methods. Number of studies have reported isolation of various alkaline and/or—saline tolerant microbes from extreme environments and have successfully displayed innate potential of these isolates for heavy metal removal from wastewaters. However, despite their great potential in bioremediations, extremophilic species are not fully explored yet. Being present in extremely harsh conditions, extremophilic species require special enrichment, isolation and culturing conditions (Singh et al. 2022). Moreover, maintenance of such extremophiles needs unique laboratory conditions, which sometimes becomes challenging (Charlesworth and Burns 2016). Also, certain extreme locations which still withhold immense potential of extremophilic microbes are inaccessible for scientists and researchers and are thus, left unattended. Extremophiles encompass a wide range of microorganisms, each with its own genetic makeup and capabilities. Identifying the specific extremophiles with the most effective heavy metal removal capabilities requires extensive genetic analysis and screening. Ccompared to mesophiles, extremophiles exhibit slower growth rates, necessitating efforts to accelerate their growth when applied for bioremediation. This acceleration can be achieved by employing bioaugmentation or through use of microbial consortia (which consist of multiple microbes having tolerance/removal capabilities for a wide range of heavy metals) during large-scale bioremediation processes. Another strategy to enhance the removal process is to increase the pollutant removal efficiency of microorganisms by using acclimatized strains.
Advanced isolation strategies, as well as in depth details of optimized operational conditions are required to fully utilise halo-alkaliphilic extremophiles for improved environmental remediation, societal benefits and resource recovery. As discussed by Giovanella et al. (2020) multi-omics technologies have filled knowledge gaps in the ecological niche, genetic makeup, gene expression, and metabolism of extremophiles. However, research studies incorporating these technologies are limited. In this regard, national and international funding agencies in the discipline of extremophiles should be encouraged and sustained in order to fund and support conditions for sample collection in extreme environments and further research work on extremophiles. Moreover, engineered extremophiles and engineered techniques must be mapped and integrated from the laboratory scale to pilot scale and eventually to real time field level to achieve significant advances in the bioremediation process. Also, utilization of extremophiles for metal recovery/bioremediation faces significant challenges; first, the recovery of low-concentration metals from large volumes of dilute leachates/waste is not supported by a high successful rate and second selective recovery of metals of interest is a tedious task. In addition, a significant concern in the bioremediation of heavy metals is ensuring that the metals that have been remediated do not re-enter the environment in their original or any other form. Further, heavy metals might be present in complex forms in extreme contaminated environments, making them less accessible to microbes. Thus, developing strategies to improve the bioavailability of heavy metals to microbes is crucial for efficient removal. After heavy metal removal, the microbial biomass must be separated from the treated water or soil, and the extracted heavy metals need to be recovered for safe disposal or reuse. Developing cost-effective and efficient methods for biomass recovery and metal extraction is a significant challenge. Therefore, more novel biotechnological advancements for recovery of metals from waste using extremophiles and utilization of recovered metals for environmental applications should be encompassed and focussed. Coordination of researchers from various disciplines, as well as updated technologies and platforms, are critical for developing better environmental management strategies in the future.
Conclusion
Environmental pollution with heavy metals results in toxification of extreme niches including saline, alkaline and hypersaline environment. Discovery of novel microorganisms with site explicit metabolic capacities for concurrent salt, high pH and heavy metal resistance paves way for usage of these innate microorganisms for removal of heavy metals from aforementioned contaminated sites. Wastewater coming from various industrial sources is dynamic in nature with continuous changes in its physico-chemical properties making the bioremediation process even more difficult. However, use of tolerant extremophilic species can sustain harsh conditions which makes them a promising tool for designing future bioremediation strategies. Extremozymes isolated from these microbes can be utilized in novel biocatalytic cycles as they are quicker, precise and pertains to sustainable economy concept. Extremophiles till date are majorly used for heavy metal decontamination under laboratory conditions, but they have yet to demonstrate their efficacy in reducing environmental pollution in practical settings. Concurrent research improvements and harmonized advanced innovations in exploration of extremophiles will result in developing sustainable routes for enhancing the utilization of these microbes and/or their biocatalysts in industrial applications such as wastewater treatment, and for biorecovery of metals from wastes.
Acknowledgements
The authors acknowledge University Grants Commission (UGC) for providing Senior Research Fellowship (SRF) to author SV.
Author contributions
SV: Conceptualization, Methodology, Formal analysis, Writing-original draft. AB: Conceptualization, Validation, Writing-review and editing. AG: Conceptualization, Resources, Supervision, Writing-review and editing.
Funding
This study was funded by University Grants Commission (UGC) (Grant No. 200510110893) under Senior Research Fellowship (SRF).
Availability of data and materials
The datasets analysed during the current study are included in this article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Informed consent
Not applicable.
Research involving human participants or animals
Not applicable.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. They have no conflict of interest in the publication.
References
- Abdel Latef AAH, Abu Alhmad MF, Kordrostami M, Abo-Baker ABAE, Zakir A. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum reinforces maize growth by improving physiological activities under saline conditions. J Plant Growth Regul. 2020;39:1293–1306. doi: 10.1007/s00344-020-10065-9. [DOI] [Google Scholar]
- Abdelhafeez IA, El-Tohamy SA, ul-Malik MAA, Abdel-Raheem SAA, El-Dar FMS. A review on green remediation techniques for hydrocarbons and heavy metals contaminated soil. Curr Chem Lett. 2022;11:43–62. doi: 10.5267/j.ccl.2021.9.006. [DOI] [Google Scholar]
- Abo-Alkasem MI, Maany DA, El-Abd MA, Ibrahim ASS. Bioreduction of hexavalent chromium by a novel haloalkaliphilic Salipaludibacillus agaradhaerens strain NRC-R isolated from hypersaline soda lakes. 3 Biotech. 2022;12:1–15. doi: 10.1007/s13205-021-03082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aji R. GCMS analysis of tannery effluent by using halophilic bacterial strain Pseudomonas aeruginosa sthc002 and Keratinase enzyme. Ann Rom Soc Cell Biol. 2021;25:1448–1467. [Google Scholar]
- Ali Z, Malik RN, Shinwari ZK. Enrichment, risk assessment, and statistical apportionment of heavy metals in tannery-affected areas. Int J Environ Sci Technol. 2015;12:537–550. doi: 10.1007/s13762-013-0428-4. [DOI] [Google Scholar]
- An S, Liu X, Wen B, Li X, Qi P, Zhang K. Comparison of the photosynthetic capacity of Phragmites australis in five habitats in saline–alkaline wetlands. Plants. 2020;9:1–17. doi: 10.3390/plants9101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya S, Kumar S. E-waste in India at a glance: current trends, regulations, challenges and management strategies. J Clean Prod. 2020;271:122707. doi: 10.1016/j.jclepro.2020.122707. [DOI] [Google Scholar]
- Ashraf S, Naveed M, Afzal M, Ashraf S, Rehman K, Zahir HA, ZA, Bioremediation of tannery effluent by Cr- and salt-tolerant bacterial strains. Environ Monit Assess. 2018 doi: 10.1007/s10661-018-7098-0. [DOI] [PubMed] [Google Scholar]
- Asksonthong R, Siripongvutikorn S, Usawakesmanee W. Heavy metal removal ability of Halomonas elongata and Tetragenococcus halophilus in a media model system as affected by pH and incubation time. Int Food Res J. 2018;25:234–240. [Google Scholar]
- Ayangbenro AS, Babalola OO. Genomic analysis of Bacillus cereus NWUAB01 and its heavy metal removal from polluted soil. Sci Rep. 2020;10:1–12. doi: 10.1038/s41598-020-75170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azubuike CC, Chikere CB, Okpokwasili GC. Bioremediation techniques–classification based on site of application: principles, advantages, limitations and prospects. World J Microbiol Biotechnol. 2016;32:1–18. doi: 10.1007/s11274-016-2137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Liu D, Zheng W, Ma L, Yang S, Cao J, Lu X, Wang H, Mehta N. Microbially-induced calcium carbonate precipitation by a halophilic ureolytic bacterium and its potential for remediation of heavy metal-contaminated saline environments. Int Biodeterior Biodegrad. 2021;165:105311. doi: 10.1016/j.ibiod.2021.105311. [DOI] [Google Scholar]
- Bano A, Hussain J, Akbar A, Mehmood K, Anwar M, Hasni MS, Ullah S, Sajid S, Ali I. Biosorption of heavy metals by obligate halophilic fungi. Chemosphere. 2018;199:218–222. doi: 10.1016/j.chemosphere.2018.02.043. [DOI] [PubMed] [Google Scholar]
- Bayanmunkh B, Sen-Lin T, Narangarvuu D, Ochirkhuyag B, Bolormaa O. Physico-chemical composition of saline lakes of the Gobi desert region, western Mongolia. J Earth Sci Clim Chang. 2017;8:1–7. doi: 10.4172/2157-7617.1000388. [DOI] [Google Scholar]
- Benmalek Y, Fardeau ML. Isolation and characterization of metal-resistant bacterial strain from wastewater and evaluation of its capacity in metal-ions removal using living and dry bacterial cells. Int J Environ Sci Technol. 2016;13:2153–2162. doi: 10.1007/s13762-016-1048-6. [DOI] [Google Scholar]
- Bhattacharya A, Gupta A, Kaur A, Malik D. Alleviation of hexavalent chromium by using microorganisms: insight into the strategies and complications. Water Sci Technol Water Supply. 2019;79(3):411–424. doi: 10.2166/wst.2019.060. [DOI] [PubMed] [Google Scholar]
- Biswas J, Bose P, Mandal S, Paul AK. Reduction of hexavalent chromium by a moderately halophilic bacterium, Halomonas smyrnensis KS802 under saline environment. Environ Sustain. 2018;1:411–423. doi: 10.1007/s42398-018-00037-x. [DOI] [Google Scholar]
- Bolisetty S, Peydayesh M, Mezzenga R. Sustainable technologies for water purification from heavy metals: review and analysis. Chem Soc Rev. 2019;48:463–487. doi: 10.1039/c8cs00493e. [DOI] [PubMed] [Google Scholar]
- Boopathy R. Factors limiting bioremediation technologies. Bioresour Technol. 2000;74:63–67. doi: 10.1016/S0960-8524(99)00144-3. [DOI] [Google Scholar]
- Boretti A, Rosa L. Reassessing the projections of the world water development report. NPJ Clean Water. 2019;2(1):15. doi: 10.1038/s41545-019-0039-9. [DOI] [Google Scholar]
- Boutron CF, Candelone JP, Hong S. Greenland snow and ice cores: unique archives of large-scale pollution of the troposphere of the northern hemisphere by lead and other heavy metals. Sci Total Environ. 1995;160:233–241. doi: 10.1016/0048-9697(95)04359-9. [DOI] [Google Scholar]
- Bragança JM, Furtado I. Removal of cadmium by halobacterium strain R1 MTCC 3265 from saline and non-saline econiches. Indian J Geomar Sci. 2017;46:2215–2219. [Google Scholar]
- Chandra P, Bhimrao B, Prakash A, Bhimrao RB, Singh DP. Isolation of alkaliphilic bacterium Citricoccus alkalitolerans CSB1: an efficient biosorbent for bioremediation of tannery waste water arsenic adsorption and its mobilization in the soil of arsenic-affected areas. Cell Mol Biol. 2016;62(3):135. doi: 10.4172/1165-158X.1000135. [DOI] [Google Scholar]
- Charlesworth J, Burns BP. Extremophilic adaptations and biotechnological applications in diverse environments. AIMS Microbiol. 2016;2:251–261. doi: 10.3934/microbiol.2016.3.251. [DOI] [Google Scholar]
- Chaudhary S, Yadav S, Singh R, Sadhotra C, Patil SA. Extremophilic electroactive microorganisms: promising biocatalysts for bioprocessing applications. Bioresour Technol. 2022;347:126663. doi: 10.1016/j.biortech.2021.126663. [DOI] [PubMed] [Google Scholar]
- Che RO, Cheung SG. Heavy metals in Metapenaeus ensis, Eriocheir sinensis and sediment from the Mai Po marshes. Hong Kong Sci Total Environ. 1998;214(1–3):87–97. [Google Scholar]
- Chen H, Chen A, Xu L, Xie H, Qiao H, Lin Q, Cai K. A deep learning CNN architecture applied in smart near-infrared analysis of water pollution for agricultural irrigation resources. Agric Water Manag. 2020;240:106303. doi: 10.1016/j.agwat.2020.106303. [DOI] [Google Scholar]
- Dhakar K, Pandey A. Wide pH range tolerance in extremophiles: towards understanding an important phenomenon for future biotechnology. Appl Microbiol Biotechnol. 2016;100:2499–2510. doi: 10.1007/s00253-016-7285-2. [DOI] [PubMed] [Google Scholar]
- Diba H, Cohan RA, Salimian M, Mirjani R, Soleimani M, Khodabakhsh F. Isolation and characterization of halophilic bacteria with the ability of heavy metal bioremediation and nanoparticle synthesis from Khara salt lake in Iran. Arch Microbiol. 2021;203:3893–3903. doi: 10.1007/s00203-021-02380-w. [DOI] [PubMed] [Google Scholar]
- Divakar G, Sameer RS, Bapuji M. Screening of multi-metal tolerant halophilic bacteria for heavy metal remediation. Int J Curr Microbiol Appl Sci. 2018;7:2062–2076. doi: 10.20546/ijcmas.2018.710.238. [DOI] [Google Scholar]
- Dodia MS, Joshi RH, Patel RK, Singh SP. Characterization and stability of extracellular alkaline proteases from halophilic and alkaliphilic bacteria isolated from saline habitat of coastal Gujarat, India. Braz J Microbiol. 2006;37:276–282. doi: 10.1590/S1517-83822006000300015. [DOI] [Google Scholar]
- Fang T, Yang K, Lu W, Cui K, Li J, Liang Y, Hou G, Zhao X, Li H. An overview of heavy metal pollution in Chaohu lake, China: enrichment, distribution, speciation, and associated risk under natural and anthropogenic changes. Environ Sci Pollut Res. 2019;26:29585–29596. doi: 10.1007/s11356-019-06210-x. [DOI] [PubMed] [Google Scholar]
- Franzetti B, Schoehn G, Ebel C, Gagnon J, Ruigrok RWH, Zaccai G. Characterization of a novel complex from halophilic archaebacteria, which displays chaperone-like activities in vitro. J Biol Chem. 2001;276:29906–29914. doi: 10.1074/jbc.M102098200. [DOI] [PubMed] [Google Scholar]
- Fuad A, Gelaneh W. Determination of heavy metals concentrations within the ever growing lake Baseka, Ethiopia using spectrophotometric technique. African J Environ Sci Technol. 2017;11:146–150. doi: 10.5897/ajest2016.2225. [DOI] [Google Scholar]
- Gad A, Abd El Bakey SM, Sakr S. Concentrations of heavy metals and associated human health risk in unrefined salts of inland hypersaline lakes, Egypt. Int J Environ Anal Chem. 2020;00:1–14. doi: 10.1080/03067319.2020.1736056. [DOI] [Google Scholar]
- Gallo G, Puopolo R, Carbonaro M, Maresca E, Fiorentino G. Extremophiles, a nifty tool to face environmental pollution: from exploitation of metabolism to genome engineering. Int J Environ Res Public Health. 2021 doi: 10.3390/ijerph18105228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A, Kushwaha A, Rani R. Reduction of hexavalent chromium [Cr(VI)] by heavy metal tolerant bacterium Alkalihalobacillus clausii CRA1 and its toxicity assessment through flow cytometry. Curr Microbiol. 2022;79:1–12. doi: 10.1007/s00284-021-02734-z. [DOI] [PubMed] [Google Scholar]
- Gayathri R, Gopinath KP, Kumar PS. Adsorptive separation of toxic metals from aquatic environment using agro waste biochar: application in electroplating industrial wastewater. Chemosphere. 2021;262:128031. doi: 10.1016/j.chemosphere.2020.128031. [DOI] [PubMed] [Google Scholar]
- Giovanella P, Vieira GAL, Ramos Otero IV, Pais Pellizzer E, de Jesus FB, Sette LD. Metal and organic pollutants bioremediation by extremophile microorganisms. J Hazard Mater. 2020;382:121024. doi: 10.1016/j.jhazmat.2019.121024. [DOI] [PubMed] [Google Scholar]
- Golzar-Ahmadi M, Mousavi SM. Extraction of valuable metals from discarded AMOLED displays in smartphones using Bacillus foraminis as an alkali-tolerant strain. Waste Manag. 2021;131:226–236. doi: 10.1016/j.wasman.2021.06.006. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan G, Wang S, Mo L, Zou J, Zhou Y. Distribution determination, risk assessment, and source identification of heavy metals in mangrove wetland sediments from Qi’ao Island, south China. Reg Stud Mar Sci. 2020;33:100961. [Google Scholar]
- Govarthanan M, Mythili R, Selvankumar T, Kamala-Kannan S, Rajasekar A, Chang YC. Bioremediation of heavy metals using an endophytic bacterium Paenibacillus sp. RM isolated from the roots of Tridax procumbens. 3 Biotech. 2016;6:1–7. doi: 10.1007/s13205-016-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunde-Cimerman N, Plemenitaš A, Oren A. Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiol Rev. 2018;42:353–375. doi: 10.1093/femsre/fuy009. [DOI] [PubMed] [Google Scholar]
- Gunjal AB, Waghmode MS, Patil NN. Role of extremozymes in bioremediation. Res J Biotechnol. 2021;16:240–252. [Google Scholar]
- Gupta GN, Srivastava S, Khare SK, Prakash V. Extremophiles: an overview of microorganism from extreme environment. Int J Agric Environ Biotechnol. 2014;7:371. doi: 10.5958/2230-732x.2014.00258. [DOI] [Google Scholar]
- Halmos L, Bozsó G, Pál-Molnár E. Adsorption properties of Ni, Cu, and Zn in young alkaline lake sediments in south Hungary (Lake Fehér, Szeged) Soil Water Res. 2015;10(4):244–251. doi: 10.17221/165/2014-SWR. [DOI] [Google Scholar]
- He H, Chen Y, Li X, Cheng Y, Yang C, Zeng G. Influence of salinity on microorganisms in activated sludge processes: a review. Int Biodeterior Biodegrad. 2017;119:520–527. doi: 10.1016/j.ibiod.2016.10.007. [DOI] [Google Scholar]
- Honglei L, Liqing LI, Chengqing YIN, Baoqing SHAN. Fraction distribution and risk assessment of heavy metals in sediments of Moshui lake. J Environ Sci. 2008;20(4):390–397. doi: 10.1016/S1001-0742(08)62069-0. [DOI] [PubMed] [Google Scholar]
- Horiike T, Dotsuta Y, Nakano Y, Ochiai A, Utsunomiya S, Ohnuki T, Yamashita M. Removal of soluble strontium via incorporation into biogenic carbonate minerals by halophilic bacterium Bacillus sp. strain TK2d in a highly saline solution. Appl Environ Microbiol. 2017;83(20):00855–17. doi: 10.1128/AEM.00855-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MF. Water. In: Hossain MF, editor. Sustainable design and build. London: Butterworth-Heinemann; 2019. pp. 301–418. [Google Scholar]
- Ibrahim ASS, El-Tayeb MA, Elbadawi YB, Al-Salamah AA. Bioreduction of cr (vi) by potent novel chromate resistant alkaliphilic Bacillus sp. strain ksucr5 isolated from hypersaline soda lakes. Afr J Biotechnol. 2011;10:7207–7218. doi: 10.4314/ajb.v10i37. [DOI] [Google Scholar]
- Imron MF, Kurniawan SB, Soegianto A. Characterization of mercury-reducing potential bacteria isolated from Keputih non-active sanitary landfill leachate, Surabaya, Indonesia under different saline conditions. J Environ Manag. 2019;241:113–122. doi: 10.1016/j.jenvman.2019.04.017. [DOI] [PubMed] [Google Scholar]
- Jacob JM, Karthik C, Saratale RG, Kumar SS, Prabakar D, Kadirvelu K, Pugazhendhi A. Biological approaches to tackle heavy metal pollution: a survey of literature. J Environ Manag. 2018;217:56–70. doi: 10.1016/j.jenvman.2018.03.077. [DOI] [PubMed] [Google Scholar]
- Jeong SW, Choi YJ. Extremophilic microorganisms for the treatment of toxic pollutants in the environment. Molecules. 2020;25:13–15. doi: 10.3390/molecules25214916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Chao S, Liu J, Yang Y, Chen Y, Zhang A, Cao H. Source apportionment and health risk assessment of heavy metals in soil for a township in Jiangsu Province, China. Chemosphere. 2017;168:1658–1668. doi: 10.1016/j.chemosphere.2016.11.088. [DOI] [PubMed] [Google Scholar]
- Jiao X, Dong Z, Kang S, Li Y, Jiang C, Rostami M. New insights into heavy metal elements deposition in the snowpacks of mountain glaciers in the eastern Tibetan plateau. Ecotoxicol Environ Saf. 2021;207:111228. doi: 10.1016/j.ecoenv.2020.111228. [DOI] [PubMed] [Google Scholar]
- Kim T, Kim TK, Zoh KD. Removal mechanism of heavy metal (Cu, Ni, Zn, and Cr) in the presence of cyanide during electrocoagulation using Fe and Al electrodes. J Water Process Eng. 2020;33:101109. doi: 10.1016/j.jwpe.2019.101109. [DOI] [Google Scholar]
- Kulkarni S, Dhakar K, Joshi A. Alkaliphiles: diversity and bioprospection. In: Das S, Dash HR, editors. Microbial diversity in the genomic era. London: Elsevier; 2019. pp. 239–263. [Google Scholar]
- Kumar M, Saini HS. Reduction of hexavalent chromium (VI) by indigenous alkaliphilic and halotolerant Microbacterium sp. M5: comparative studies under growth and nongrowth conditions. J Appl Microbiol. 2019;127:1057–1068. doi: 10.1111/jam.14366. [DOI] [PubMed] [Google Scholar]
- Kumar A, Alam A, Tripathi D, Rani M, Khatoon H, Pandey S, Ehtesham NZ, Hasnain SE. Protein adaptations in extremophiles: an insight into extremophilic connection of mycobacterial proteome. Semin Cell Dev Biol. 2018;84:147–157. doi: 10.1016/j.semcdb.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Leong YK, Chang JS. Bioremediation of heavy metals using microalgae: recent advances and mechanisms. Bioresour Technol. 2020;303:122886. doi: 10.1016/j.biortech.2020.122886. [DOI] [PubMed] [Google Scholar]
- Li D, Jiang J, Yan C, Zhang M, Zhao Y, Xiang Y, Ma W, Jia H, Zhao X. Ecological heavy metals risk of saline lake sediments in northwestern China. Pol J Environ Stud. 2020;29(4):1. doi: 10.15244/pjoes/112206. [DOI] [Google Scholar]
- Li H, Watson J, Zhang Y, Lu H, Liu Z. Environment-enhancing process for algal wastewater treatment, heavy metal control and hydrothermal biofuel production: a critical review. Bioresour Technol. 2020;298:122421. doi: 10.1016/j.biortech.2019.122421. [DOI] [PubMed] [Google Scholar]
- Liu HQ, Bin LuX, Li ZH, Tian CY, Song J. The role of root-associated microbes in growth stimulation of plants under saline conditions. Land Degrad Dev. 2021;32(13):3471–3486. doi: 10.1002/ldr.3955. [DOI] [Google Scholar]
- Mabrouk MEM, Arayes MA, Sabry SA. Hexavalent chromium reduction by chromate-resistant haloalkaliphilic halomonas sp. M-Cr newly isolated from tannery effluent. Biotechnol Biotechnol Equip. 2014;28:659–667. doi: 10.1080/13102818.2014.937092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes S, Claus M, Verbeken K, Wallaert E, De Smet R, Vanhaecke F, Boon N, Hennebel T. Platinum recovery from industrial process streams by halophilic bacteria: influence of salt species and platinum speciation. Water Res. 2016;105:436–443. doi: 10.1016/j.watres.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Maes S, Props R, Fitts JP, De Smet R, Vilchez-Vargas R, Vital M, Pieper DH, Vanhaecke F, Boon N, Hennebel T. Platinum recovery from synthetic extreme environments by halophilic bacteria. Environ Sci Technol. 2016;50:2619–2626. doi: 10.1021/acs.est.5b05355. [DOI] [PubMed] [Google Scholar]
- Mandeep SP. Microbial nanotechnology for bioremediation of industrial wastewater. Front Microbiol. 2020 doi: 10.3389/fmicb.2020.590631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters GM, Ela WP. Water pollution. In: Masters GM, Ela WP, editors. Introduction to environmental engineering and science. 3. London: Pearson; 2007. pp. 173–280. [Google Scholar]
- Merino N, Aronson HS, Bojanova DP, Feyhl-Buska J, Wong ML, Zhang S, Giovannelli D. Living at the extremes: extremophiles and the limits of life in a planetary context. Front Microbiol. 2019 doi: 10.3389/fmicb.2019.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra RK, Pandey S, Thatoi H, Panda CR. Reduction of chromium(VI) by marine bacterium Brevibacillus laterosporus under varying saline and pH conditions. Environ Eng Sci. 2017;34:617–626. doi: 10.1089/ees.2016.0627. [DOI] [Google Scholar]
- Mohapatra RK, Parhi PK, Thatoi H, Panda CR. Bioreduction of hexavalent chromium by Exiguobacterium indicum strain MW1 isolated from marine water of Paradip port, Odisha, India. Chem Ecol. 2017;33:114–130. doi: 10.1080/02757540.2016.1275586. [DOI] [Google Scholar]
- Moore F, Forghani G, Qishlaqi A. Assessment of heavy metal contamination in water and surface sediments of the Maharlu saline lake, sw Iran. Iran j Sci Technol. 2009;33(1):43–55. [Google Scholar]
- Moradi S, Rasouli-Sadaghiani MH, Sepehr E, Khodaverdiloo H, Barin M. Soil nutrients status affected by simple and enriched biochar application under salinity conditions. Environ Monit Assess. 2019 doi: 10.1007/s10661-019-7393-4. [DOI] [PubMed] [Google Scholar]
- Mubashar M, Naveed M, Mustafa A, Ashraf S, Shehzad Baig K, Alamri S, Siddiqui MH, Zabochnicka-Swiatek M, Szota M, Kalaji HM. Experimental investigation of Chlorella vulgaris and Enterobacter sp. MN17 for decolorization and removal of heavy metals from textile wastewater. Water. 2020;12(11):3034. doi: 10.3390/w12113034. [DOI] [Google Scholar]
- Nakade DB. Bioleaching of copper from low grade ore bornite using halophilic Thiobacillus ferroxidans, N-11. Res J Recent Sci. 2013;2:162–166. [Google Scholar]
- Nayek A, Gupta PS, Banerjee SS, Mondal B, Bandyopadhyay AK. Salt-bridge energetics in halophilic proteins. PLoS ONE. 2014;9:1–11. doi: 10.1371/journal.pone.0093862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orji OU, Awoke JN, Aja PM, Aloke C, Obasi OD, Alum EU, Udu-Ibiam OE, Oka GO. Halotolerant and metalotolerant bacteria strains with heavy metals biorestoration possibilities isolated from Uburu salt lake, southeastern. Nigeria Heliyon. 2021;7(7):e07512. doi: 10.1016/j.heliyon.2021.e07512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orji OU, Awoke JN, Aloke C, Obasi OD, Oke B, Njoku M, Ezeani NN. Toxic metals bioremediation potentials of Paenibacillus sp. strain SEM1 and Morganella sp. strain WEM7 isolated from Enyigba Pb–Zn mining site. Ebonyi State Nigeria Bioremediat J. 2021;25:285–296. [Google Scholar]
- Ortega G, Diercks T, Millet O. Halophilic protein adaptation results from synergistic residue-ion interactions in the folded and unfolded states. Chem Biol. 2015;22:1597–1607. doi: 10.1016/j.chembiol.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Pham VHT, Kim J, Chang S, Chung W. Bacterial biosorbents, an efficient heavy metals green clean-up strategy: prospects, challenges, and opportunities. Microorganisms. 2022;10:1–16. doi: 10.3390/microorganisms10030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan T, Ting YP. Selective copper bioleaching by pure and mixed cultures of alkaliphilic bacteria isolated from a fly ash landfill site. Water Air Soil Pollut. 2015 doi: 10.1007/s11270-015-2641-x. [DOI] [Google Scholar]
- Ramanathan T, Ting YP. Alkaline bioleaching of municipal solid waste incineration fly ash by autochthonous extremophiles. Chemosphere. 2016;160:54–61. doi: 10.1016/j.chemosphere.2016.06.055. [DOI] [PubMed] [Google Scholar]
- Rangasamy A, Gandhi S, Tamilchelvan V. Biosorption of hexavalent chromium by Paenibacillus pabuli and Bacillus cereus isolated from alkaline industrial contaminated soil in Puducherry, India. Nat Environ Pollut Technol. 2021;20:729–735. doi: 10.46488/NEPT.2021.v20i02.032. [DOI] [Google Scholar]
- Rao A, Varshney S, Bhadra S, Kaushik A, Gupta A, Sevda S. Use of biofilm bacteria to enhance overall microbial fuel cell performance. In: Das S, Kungwani NA, editors. Understanding microbial biofilms fundamental to applications. London: Elseiver; 2023. pp. 699–712. [Google Scholar]
- Sahoo S, Goli D. Bioremediation of lead by a halophilic bacteria Bacillus pumilus isolated from the mangrove regions of Karnataka. Int J Sci Res. 2020;9:1337–1343. doi: 10.21275/ART20204172. [DOI] [Google Scholar]
- Sall ML, Diaw AKD, Gningue-Sall D, Efremova Aaron S, Aaron JJ. Toxic heavy metals: impact on the environment and human health, and treatment with conducting organic polymers, a review. Environ Sci Pollut Res. 2020;27:29927–29942. doi: 10.1007/s11356-020-09354-3. [DOI] [PubMed] [Google Scholar]
- Salma M, Abdulla MK, Samina M. Osmoadaptation in halophilic bacteria and archaea. Res J Biotechnol. 2020;15:154–161. [Google Scholar]
- Schröder C, Burkhardt C, Antranikian G. What we learn from extremophiles. Chemtexts. 2020;6:1–6. doi: 10.1007/s40828-020-0103-6. [DOI] [Google Scholar]
- Sher S, Hussain SZ, Rehman A. Phenotypic and genomic analysis of multiple heavy metal–resistant Micrococcus luteus strain AS2 isolated from industrial waste water and its potential use in arsenic bioremediation. Appl Microbiol Biotechnol. 2020;104:2243–2254. doi: 10.1007/s00253-020-10351-2. [DOI] [PubMed] [Google Scholar]
- Shrestha N, Chilkoor G, Vemuri B, Rathinam N, Sani RK, Gadhamshetty V. Extremophiles for microbial-electrochemistry applications: a critical review. Bioresour Technol. 2018;255:318–330. doi: 10.1016/j.biortech.2018.01.151. [DOI] [PubMed] [Google Scholar]
- Singh R, Chaudhary S, Yadav S, Patil SA. Protocol for bioelectrochemical enrichment, cultivation, and characterization of extreme electroactive microorganisms. STAR Protoc. 2022;3:101114. doi: 10.1016/j.xpro.2021.101114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Srivastava AK, Khare SK. Efficient proteolysis and application of an alkaline protease from halophilic Bacillus sp EMB9. Prep Biochem Biotechnol. 2014;44:680–696. doi: 10.1080/10826068.2013.844711. [DOI] [PubMed] [Google Scholar]
- Soliman NK, Moustafa AF. Industrial solid waste for heavy metals adsorption features and challenges; a review. J Mate Res Technol. 2020;9:10235–10253. doi: 10.1016/j.jmrt.2020.07.045. [DOI] [Google Scholar]
- Titah HS, Putera RI, Pratikno H, Moesriati A. Removal of iron(II) by Burkholderia pseudomallei in brackish environment. Environment Asia. 2020;13:75–85. doi: 10.14456/ea.2020.7. [DOI] [Google Scholar]
- Torbaghan ME, Torghabeh GHK. Biological removal of iron and sulfate from synthetic wastewater of cotton delinting factory by using halophilic sulfate-reducing bacteria. Heliyon. 2019;5:e02948. doi: 10.1016/j.heliyon.2019.e02948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi B, Gupta B, Thakur IS. Biosorption of Cr(VI) from aqueous solution by extracellular polymeric substances (EPS) produced by Parapedobacter sp. ISTM3 strain isolated from Mawsmai cave, Meghalaya, India. Environ Res. 2020 doi: 10.1016/j.envres.2020.110064. [DOI] [PubMed] [Google Scholar]
- Uma G, Babu MM, Prakash VSG, Nisha SJ, Citarasu T. Nature and bioprospecting of haloalkaliphilics: a review. World J Microbiol Biotechnol. 2020;36:1–13. doi: 10.1007/s11274-020-02841-2. [DOI] [PubMed] [Google Scholar]
- Verma S, Kuila A. Bioremediation of heavy metals by microbial process. Environ Technol Innov. 2019;14:100369. doi: 10.1016/j.eti.2019.100369. [DOI] [Google Scholar]
- Wang M, Chen S, Chen L, Wang D. Responses of soil microbial communities and their network interactions to saline–alkaline stress in cd-contaminated soils. Environ Pollut. 2019;252:1609–1621. doi: 10.1016/j.envpol.2019.06.082. [DOI] [PubMed] [Google Scholar]
- Wang P, Hu Y, Cheng H. Municipal solid waste (MSW) incineration fly ash as an important source of heavy metal pollution in China. Environ Pollut. 2019;252:461–475. doi: 10.1016/j.envpol.2019.04.082. [DOI] [PubMed] [Google Scholar]
- Watts MP, Khijniak TV, Boothman C, Lloyd JR. Treatment of alkaline Cr(VI)-contaminated leachate with an alkaliphilic metal-reducing bacterium. Appl Environ Microbiol. 2015;81:5511–5518. doi: 10.1128/AEM.00853-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Pang H, Liu Y, Wang X, Yu S, Fu D, Chen J, Wang X. Environmental remediation of heavy metal ions by novel-nanomaterials: a review. Environ Pollut. 2019;246:608–620. doi: 10.1016/j.envpol.2018.12.076. [DOI] [PubMed] [Google Scholar]
- Yadav S, Patil SA. Microbial electroactive biofilms dominated by Geoalkalibacter spp. from a highly saline–alkaline environment. NPJ Biofilms Microbiomes. 2020;6:1–10. doi: 10.1038/s41522-020-00147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N, Marschner P. Response of microbial activity and biomass to increasing salinity depends on the final salinity, not the original salinity. Soil Biol Biochem. 2012;53:50–55. doi: 10.1016/j.soilbio.2012.04.028. [DOI] [Google Scholar]
- Yin K, Wang Q, Lv M, Chen L. Microorganism remediation strategies towards heavy metals. Chem Eng J. 2019;360:1553–1563. doi: 10.1016/j.cej.2018.10.226. [DOI] [Google Scholar]
- Yu Z, Han H, Feng P, Zhao S, Zhou T, Kakade A, Kulshrestha S, Majeed S, Li X. Recent advances in the recovery of metals from waste through biological processes. Bioresour Technol. 2020;297:122416. doi: 10.1016/j.biortech.2019.122416. [DOI] [PubMed] [Google Scholar]
- Zhai L, Xie J, Feng H, Sun S, Cheng K, Yao S. Mechanism of TonB-dependent transport system in Halomonas alkalicola CICC 11012s in response to alkaline stress. Extremophiles. 2021;25:39–49. doi: 10.1007/s00792-020-01209-6. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chen L, Liu D. Characterization of a marine-isolated mercury-resistant Pseudomonas putida strain SP1 and its potential application in marine mercury reduction. Appl Microbiol Biotechnol. 2012;93:1305–1314. doi: 10.1007/s00253-011-3454-5. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Xu XR, Sun YX, Yu S, Chen YS, Peng JX. Heavy metal and organic contaminants in mangrove ecosystems of China: a review. Environ Sci Pollut Res. 2014;21:11938–11950. doi: 10.1007/s11356-014-3100-8. [DOI] [PubMed] [Google Scholar]
- Zmorrod N, Moudallal HS, Yusef H, Amer RA, Hakawati NAL. Removal of lead(I) ions from aqueous solutions by alkaliphilic bacteria Halomonas alkalicola ext. Asian J Microbiol Biotech Environ Sci. 2020;22:448–457. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are included in this article.