Abstract
The prevalence of older persons with HIV (PWH) disease has increased considerably in the last 20 years, but our understanding of biological factors of aging and their clinical correlates among PWH remains limited. Study participants were 149 persons aged 50 and older, including 107 PWH and 42 seronegatives. All participants completed a blood draw, research medical evaluation, structured psychiatric interview, neurocognitive assessment, questionnaires, and measures of health literacy. Four epigenetic clocks were generated from stored blood samples using standardized laboratory methods. In regression models adjusting for sex and smoking status, PWH had significantly higher epigenetic aging acceleration values than seronegatives on all four indicators. Within the PWH sample, higher levels of epigenetic aging acceleration were moderately associated with lower current CD4 count, AIDS diagnoses, higher scores on the Veterans Aging Cohort Study Index, and lower telomere values. Higher epigenetic aging acceleration indices were also associated with lower health literacy among PWH. PWH experience accelerated aging as measured by a series of epigenetic clocks, which may be linked to immune compromise and risk of all-cause mortality. Health literacy may be a modifiable target for mitigating the risk of accelerated aging among older PWH.
Keywords: Immunovirology, Biological factors, Aging, Infectious disease, Chronic illness, Numeracy
Introduction
People with HIV (PWH) are living longer, as HIV can be effectively managed as a chronic disease among persons who are able to stay engaged in care and adherent to antiretroviral therapies [1]. As such, the prevalence of older PWH has risen steadily over the past 20 years [1]. Older PWH are at risk for accelerated aging, clinically expressed by faster development of geriatric syndromes, including neurocognitive impairment [2], frailty [2], and age-related chronic disorders (e.g., cardiovascular disease, osteoporosis, and diabetes mellitus) [3]. Several biological mechanisms have been proposed to explain the accelerated aging in PWH, most notably immune dysregulation, immune senescence, and chronic inflammation [3, 4]. Alterations in epigenetic clocks, emerging biomarkers of aging, are another possible mechanism that warrants careful examination.
Accelerated epigenetic aging has been described in several chronic diseases, including obesity [5], diabetes mellitus [6], and cardiovascular diseases [7]. This measure is estimated by calculating the deviation of the epigenetic age (based on epigenetic clocks) from the chronological age of the persons, providing an interesting and potentially clinically relevant measure of how faster the biological age is advancing [8, 9]. Epigenetic clocks are calculated according to DNA methylation (DNAm) values of cytosine-phosphate-guanine (CpGs) specific sites regulated by the epigenetic maintenance system [8, 9]. While the first-generation epigenetic clocks (i.e., Horvath’s DNAm age [9] and Hannum’s DNAm age [10]) were developed to predict chronological age, the second-generation clocks were developed to estimate biological age and, thus, better estimate lifespan and health span [11, 12]. The two most commonly used second-generation epigenetic clocks are the DNAm PhenoAge [13] and the DNAm GrimAge [12].
The phenomenon of epigenetic aging acceleration was first described by Horvath and Levine (2015), who showed that PWH have accelerated aging of 7.4 years in brain tissue and 5.2 years in peripheral blood mononuclear cells (PBMC) compared to HIV-seronegative controls [14]. Subsequent studies of epigenetic aging acceleration in PWH have been conducted in adolescents [15, 16], middle-aged adults [17–19], and autopsy tissue samples [7, 20]. Across these studies, we see reliable adverse effects of HIV on accelerated aging, ranging from 5 to 7 years [14, 17]. Evidence of accelerated biological aging in PWH has also been reported across different organ tissues, including the brain [7, 20]. Accelerated aging has been associated with lower neurocognitive functioning [16, 20], multiple neuroimaging indicators of the integrity of both white and grey matter [15], biomarkers of inflammation [19], and medical comorbidities, especially cardiovascular disease [7]. Conversely, potential associations between accelerated aging and aspects of HIV disease and treatment have been less consistent, with only a few studies showing the expected relationships with immunovirological markers (e.g., CD4 count; [16]). Other studies focused on the effects of antiretroviral therapy on epigenetic aging patterns and involved adults with HIV [18, 21, 22]. Only a single study involved older adults (a group of people that may have been exposed to HIV for a longer period) and evaluated associations between epigenetic aging and cognitive domains in Black/African American PWH [23]. This study reported a negative association between intrinsic epigenetic age acceleration (IEAA) and executive function, attention, and working memory and between DNAm PhenoAge and attention [23].
The current study extends the literature on accelerated aging in PWH in several important ways. First, we examined the effects of HIV on accelerated epigenetic aging in a well-characterized sample of adults aged 50 and older using both first- and second-generation clocks. Second, we combined different epigenetic clocks into a single composite score and confirmed its association with telomere length, a well-established marker of aging [24]. Third, we examined whether accelerated aging was associated with multiple HIV-related clinical and virological parameters. Finally, we investigated the potential relationship between accelerated aging and health literacy, which describes the degree to which individuals can access, understand, and apply health information [25]. Lower levels of health literacy are common among persons living with HIV disease and can interfere with health behaviors (e.g., medication adherence) that may worsen age-related health outcomes [26]. As such, it is hypothesized that lower health literacy will be associated with greater accelerated aging among older PWH. We are unaware of any prior studies in HIV or other conditions that have linked epigenetic markers of aging to health literacy, which is an important and modifiable construct in the setting of chronic diseases.
Methods and materials
Participants
The current study participants were 107 PWH and 42 seronegative adults who had stored blood samples available from an NIH-funded study on memory in persons ages 50 and older [27]. All participants provided informed consent for the parent study and the subsequent use of stored samples. Participants were recruited from HIV clinics, local organizations, the community, and by word-of-mouth in San Diego County. HIV serostatus was confirmed with Medmira rapid tests. Exclusion criteria for the parent study were history of severe psychiatric disorders (e.g., psychosis), non-HIV-related neurological conditions (e.g., seizure disorders, dementia, major head injury), estimated verbal IQ <70, and substance dependence within the past 30 days as determined by Composite International Diagnostic Interview (CIDI; [28]). Current smokers were defined according to the methods detailed in Bollepalli et al. (2019) [29].
Procedure
Genome-wide methylation analysis
We isolated DNA from human PBMC using the DNeasy Blood & Tissue Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. Isolated DNA samples were quantified on NanoDrop (Thermo, Waltham, MA, USA) and 500 ng of DNA were bisulfite-converted using the EZ DNA Methylation™ Kit (Zymo Research, Irvine, CA, USA). The bisulfite-converted DNA was hybridized on the Infinium MethylationEPIC BeadChip Kit (Illumina, San Diego, CA, USA). Beadchips were scanned in an iScan microarray reader (Illumina).
Epigenetic clock
Raw DNAm data was preprocessed using the minfi R package [30], including filtering out poor-quality probes (detection p-values < 0.01) and calculating beta-values. Epigenetic aging measures were calculated using the New DNA Methylation Age Calculator, available at https://dnamage.genetics.ucla.edu/. We obtained the following measures for further statistical analyses: Horvath DNAm age [9], Hannum DNAm age [10], PhenoAge [13], and GrimAge [12]. Measures of aging acceleration were estimated by regressing the predicted epigenetic ages on chronological ages and using the residuals as “acceleration indices” (AgeAccelerationResidual, AgeAccelerationResidualHannum, PhenoAgeAccel, and GrimAgeAccel). We also obtained estimates of blood cell count (CD8+ T-lymphocytes (CD8T), CD4+ T-lymphocytes (CD4T), natural killer (NK), B-lymphocytes (Bcell), granulocytes (Gran), and monocytes), and the Smoking Score (SSc) [31] based on DNAm data using the Houseman method [32] and the EpiSmokEr R package [29], respectively.
Telomere length
To validate our results on accelerated epigenetic aging, we evaluated telomere length, a well-recognized biomarker of aging and a predictor of mortality [24]. We measured the telomere length by singleplex real-time quantitative PCR, as described elsewhere [33]. Briefly, the relative telomere length (T/S) was estimated by quantifying the levels of telomere (T) and β-globin (HBG), which was taken as a single copy gene (S). We calculated the relative telomere length for each sample by comparison to a 5-point standard curve derived from the amplification, in the same plate, of serial dilutions of a calibrator DNA sample (1:1.68). Primer sequences and PCR cycling conditions have been previously reported [33]. All reactions were run in duplicate in 96-well plates and included 15 mM Tris pH 8.0, 50 mM KCl, 2 mM MgCl2, 0.2 mM deoxynucleotide (dNTP) mix (ThermoFisher Scientific, Waltham, MA, USA), 5 mM dithiothreitol, 1% dimethyl sulfoxide, 0.375 U AmpliTaq Gold DNA polymerase (ThermoFisher Scientific), 150 nM 6-ROX (C6156, ThermoFisher Scientific), 0.2 x SYBR® Green I (ThermoFisher Scientific), and 21 ng genomic DNA. The final primer concentrations were 270 nM for tel1, 900 nM for tel 2, 400 nM for HBG1, and 400 nM for HBG2. Data were acquired in a QuantStudio™ 7 Flex Real-Time PCR System (Life Technologies), and the specificity of the amplifications was confirmed at the end of each run using melting curve analysis.
Clinical characterization
All participants completed a research assessment of basic medical, psychiatric, and neuropsychological parameters. Psychiatric diagnoses of depression, anxiety, and substance use disorders were determined with the CIDI. Neurocognitive impairment was determined with the Cogstate [34] or the NIH Toolbox [35]. Health literacy was measured with the Newest Vital Sign, the Medication Management Test-Revised, and the 3-BRIEF [36]. Fifty-three PWH met criteria for low health literacy using standard cut-scores derived from these measures. A research nurse conducted a semi-structured interview for medical history and review of systems. The Veterans Aging Cohort Study (VACS) index was derived according to standard methods [37] as a measure of all-cause mortality risk and HIV health outcomes. A phlebotomist conducted a blood draw, from which current CD4 cell counts and viral loads were derived.
Data analysis
The epigenetic aging acceleration biomarker variables were all normally distributed (Anderson-Darling test ps>.10). The primary analysis was conducted with a mixed model analysis of variance, whereby HIV serostatus was the between-subjects independent variable and the four epigenetic aging biomarkers were the within-subjects independent variables. The critical alpha was set to .05 for the primary model. A data-driven approach was used to select covariates for the model [38], whereby any variable in Table 1 that was associated with both HIV and the biomarkers at p<.05 was included as a covariate. All secondary analyses within the HIV sample were evaluated using t-tests, chi-square tests, or correlations (or their non-parametric equivalent, if dictated by non-normal distributions of some variables in Table 1). The epigenetic aging acceleration indices were combined into a composite score by averaging their sample-based z-scores (Cronbach’s alpha = .737). The composite approach has a long psychometric history and affords the advantage of a more reliable and robust measurement of the underlying construct [39]. In our case, it also helps to reduce the risk of type I error by allowing us to conduct fewer family-wise analyses. The critical alpha was set to .01 for these secondary analyses. All statistical analyses were conducted in JMP 16.0 (Carey, NC).
Table 1.
Sociodemographic and clinical characteristics of the study samples
| Variable | PWH (n = 107) | HIV (n = 42) | p | ||
|---|---|---|---|---|---|
| Mean/% | SD | Mean/% | SD | ||
| Demographics | |||||
| Age | 56.4 | (5.5) | 60.7 | (8.4) | .0003 |
| Education (years) | 14.2 | (2.7) | 15.2 | (2.6) | .0493 |
| Sex (% women) | 10.2 | — | 33.3 | — | .0007 |
| Handedness (% right) | 89.7 | — | 78.6 | — | .0836 |
| Race/ethnicity (%) | .3364 | ||||
| Asian | 0.0 | — | 2.4 | — | |
| Black/African American | 15.9 | — | 16.7 | — | |
| Hispanic/Latino | 17.8 | — | 14.3 | — | |
| White/Caucasian | 63.6 | — | 59.5 | — | |
| Other | 2.8 | — | 7.1 | — | |
| Neuropsychiatric conditions | |||||
| Generalized anxiety (%a) | 17.8 | — | 9.52 | — | .1926 |
| Major depression (%a) | 66.4 | — | 31.0 | — | <.0001 |
| Substance use disorder (%a) | 72.9 | — | 57.1 | — | .0665 |
| Neurocognitive impairment (%) | 28.4 | — | 23.7 | — | .5743 |
| General health characteristics | |||||
| Medical comorbidities | 2.0 | (1.5) | 1.4 | (1.3) | .0242 |
| Smoking status (% current) | 22.4 | — | 4.8 | — | .0250 |
| Body mass index | 27.6 | (4.5) | 30.4 | (6.9) | .0049 |
| Health literacyb | 53.5 | — | 60.1 | — | .4185 |
| NK | 0.2 | (0.1) | 0.2 | (0.1) | .3220 |
| B cell | 0.0 | (0.1) | 0.1 | (0.1) | .3729 |
| Monocytes | 0.2 | (0.1) | 0.2 | (0.1) | .7573 |
| Granulocytes | 0.0 | (0.0) | 0.0 | (0.0) | .4237 |
| HIV disease characteristics | |||||
| AIDS (%) | 64.5 | — | — | — | — |
| Duration of infection (years) | 21.2 | (8.8) | — | — | — |
| Current CD4 (cells/μL) | 672.7 | (310.4) | — | — | — |
| Nadir CD4 (cells/μL) | 204.9 | (196.6) | — | — | — |
| Plasma RNA (% detectable) | 6.7 | — | — | — | — |
| Antiretroviral therapy (%) | 90.6 | — | — | — | — |
| VACS total | 25.3 | (11.3) | — | — | — |
| Telomere length | 0.9 | (0.3) | 0.9 | (0.3) | .6284 |
Values are sample mean (standard deviation) unless otherwise specified
AIDS, acquired immunodeficiency syndrome; CD4, cluster of differentiation 4; RNA, ribonucleic acid; VACS, Veterans Aging Cohort Study Index
Bold indicates p < .05
aAny lifetime diagnoses
bAs determined by the Newest Vital Sign, 3-BRIEF, and Medication Management Test-Revised
Results
Associations with age
In the full sample, older age was significantly associated with higher DNAm values across all four indices (ps<.0001), with effect sizes ranging from .56 to .72.
HIV serostatus and epigenetic aging biomarkers
Determining covariates
The sociodemographic and clinical characteristics of the study groups are displayed in Table 1. As compared to the seronegative sample, the PWH group was younger, less educated, had greater body mass indices, and higher frequencies of medical comorbidities, current smokers, depression, and men (ps<.05). Of these six potential covariates, only sex and DNAm-based smoking score were reliably related to all the epigenetic markers (ps<.05) and were therefore included in the primary model.
Primary model
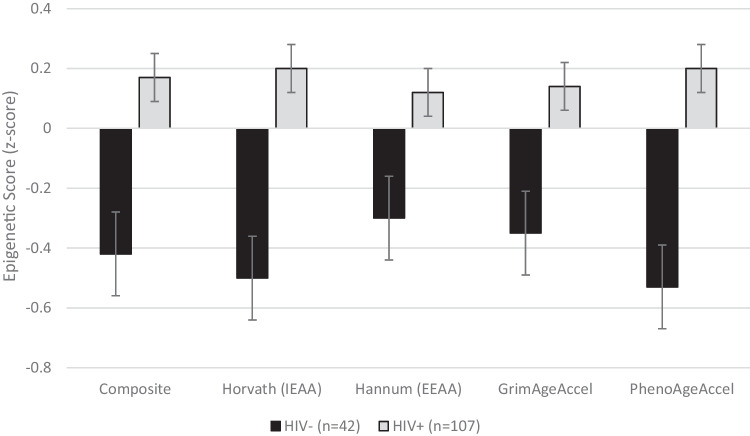
A mixed model was conducted with the four epigenetic aging acceleration markers as the within-subjects factor and HIV, sex, and smoking status as the between-subjects factors. Results showed a significant main effect of HIV status (F (1.142)=10.5, p=.002), such that PWH had significantly higher epigenetic aging acceleration values than seronegatives on all four indicators (see Fig. 1) at broadly medium effect sizes (Cohen’s d values ranged from .42 (Hannum) to .78 (PhenoAgeAccel)). There were also significant main effects of sex (F (1.142)=5.4, p=.022) and DNAm-based smoking score (F (2.142)=3.7, p=.027), such that women and individuals with higher smoking scores had lower epigenetic aging acceleration values. HIV did not interact with sex, smoking status, or epigenetic marker (all ps>.10).
Fig. 1.
Bar chart showing the mean (with standard error) epigenetic aging acceleration biomarkers in persons with (n=107) and without (n=42) HIV disease
Clinical factors and epigenetic aging acceleration biomarkers in PWH
Within the PWH (n=107), higher levels of epigenetic aging acceleration, as determined by the composite score of the four indices, were associated with HIV-specific variables, specifically lower current CD4 count (rs=−.38, p<.0001), AIDS status (p=.008, Cohen’s d =.43), and higher VACS (rs=.36, p=.0002). Higher epigenetic aging acceleration indices were also higher in persons with lower health literacy (p=.0053, Cohen’s d =.51) and lower telomere values (r=−.32, p=.0009) in the PWH sample. No other variables in Table 1 were associated with epigenetic biomarkers in the HIV sample (ps>.01).
Discussion
Our main findings indicate that (i) PWH had significantly higher epigenetic aging acceleration than seronegative individuals; (ii) female sex and smoking status independently affect this population’s aging acceleration; and (iii) higher epigenetic aging acceleration levels are associated with lower current CD4 count, AIDS status, health literacy, and higher morbidity (as assessed by VACS). These findings support the involvement of epigenetic mechanisms in the premature aging-related morbidity and mortality reported in PWH, with an important driving effect of smoking and sex in this population. Moreover, our study examined different epigenetic clocks, including first- and second-generation clocks, and a composite score of these clocks, that correlated with telomere length.
Only a few studies have shown epigenetic aging acceleration in PWH using second-generation clocks [18, 21, 23]. In addition, most prior studies focused on the effects of antiretroviral therapy on epigenetic aging patterns and involved adults with HIV. Second-generation clocks, especially the GrimAgeAccel, differ from prior clocks in having demonstrated a superior predictive ability of lifespan and all-cause mortality, time-to-death, time-to-coronary heart disease, and time-to-cancer, as well as exhibiting a strong relationship with visceral adiposity/fatty liver, a general medical comorbidity index, and general physical functioning levels [12]. In other words, finding higher GrimAgeAccel in older PWH provides a biological measure to explain the high levels of comorbidities and premature mortality observed in this population.
We also examined a new measure to evaluate aging acceleration, a composite score resulting from the combination of the four epigenetic clocks. Defining composite measures allows a more reliable and robust measurement of the underlying construct, besides minimizing statistical errors [39]. One potential caveat is that although the four epigenetic clocks use different combinations of CpGs for their calculation, they do have some overlapping CpGs. As expected, higher levels of epigenetic aging, as determined by the composite, were associated with shorter telomere length, a well-established marker of aging [24]. Previous studies have consistently shown that PWH have telomere length shortening when compared to HIV-seronegative controls [40, 41]. Of note, recent findings have shown that telomere length per se only allows a rough estimate of aging rate and that the links between telomere length and age-related diseases and mortality are less robust than ones provided by epigenetic clocks [42].
One particularly novel aspect of this study was the association between epigenetic aging and health literacy in older PWH. Indeed, we are unaware of any studies on HIV disease or other populations that have identified a linkage between these constructs. In this case, accelerated epigenetic aging was associated with lower health literacy as measured by tests that required numeracy, comprehension/understanding, and application of health-related materials. The association was accompanied by a medium effect size and could not be better explained by other sociodemographic factors. Lower health literacy is common in HIV, particularly in under-served subgroups, and is reliably associated with reduced engagement in healthcare and poorer health outcomes [26]. Therefore, one possibility is that lower health literacy may lead to suboptimal health behaviors (e.g., poorer adherence) that—in turn—increase the risk of age-related physical and mental health difficulties that can accelerate biological aging. The parallel association between epigenetic aging and the VACS index in our older sample of PWH supports this interpretation. The VACS is an all-morbidity index that shows associations with aging-related factors, such as frailty [43] and mortality [44]. The significant VACS association also converges with the recent study by Oursler and colleagues (2022) [45].
Finally, in this study, we did not observe a significant relationship between epigenetic aging and cognition, and the effect sizes were small. One possible reason for this divergence with prior studies [20, 23] was that our neuropsychological index included two separate, brief computerized measures. Although our neurocognitive index has shown evidence of validity in prior studies [27, 36], it nevertheless is not an ideal measure of cognitive functioning. Note that, findings did not differ if we used a continuous rather than dichotomous measure of cognition, nor if we examined the PWH and HIV negative groups separately (all ps>.05). These results indicate the need for further studies involving a mixed older adult population with HIV to evaluate the clinical implications of accelerated epigenetic aging.
Differences in demographic characteristics found between groups are a limitation of the study. However, our analyses were adjusted by these discrepant variables. Of note, despite the conservative age bias (i.e., seronegatives were chronologically older), the HIV group had greater levels of epigenetic aging acceleration. Effect sizes were large and independent of confounds (i.e., sex) and covariates. Another limitation mentioned above was the neurocognitive assessment. Conversely, our study investigated a well-characterized cohort of older adults with HIV, using multiple markers of epigenetic aging, including first- and second-generation clocks, and looking at different clinical factors.
A better understanding of epigenetic aging and related clinical factors in PWH can help identify patients at the highest risk for accelerated aging, eventually contributing to patient stratification for specific interventions or clinical trials of anti-aging drugs, as well as early identification of patients with likely worse prognosis. In this context, health literacy may be a modifiable target for mitigating the risk of accelerated aging among older PWH. The ultimate goal would be the prevention of premature mortality and age-related clinical conditions in this population.
Authors’ contributions
Drs. Teixeira and Woods contributed to the study conception and design. Aspects of material preparation, data collection, and processing were performed by Drs. Martins, Fries, Colpo, and Rocha. Data analysis was conducted by Dr. Woods. The first draft of the manuscript was written collaboratively by Drs. Teixeira and Woods. All authors approved the final manuscript and byline.
Funding
This work was supported by the National Institutes of Health (grant numbers R01-MH073419 and P30-MH062512).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Steven Paul Woods and Antonio L. Teixeira contributed equally to this study.
References
- 1.CDC. HIV in the United States by age. In: Centers for Disease Control and Prevention [Internet]. 11 Jul 2022 [cited 25 Jan 2023]. Available: https://www.cdc.gov/hiv/group/age/index.html
- 2.Allavena C, Blain H, Abulizi X, Slama L, Katlama C, Delobel P, et al. Prevalence and risk factors of frailty among adults living with HIV aged 70 years or older. AIDS. 2023;37:183–189. doi: 10.1097/QAD.0000000000003403. [DOI] [PubMed] [Google Scholar]
- 3.Wing EJ. HIV and aging. Int J Infect Dis. 2016;53:61–68. doi: 10.1016/j.ijid.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Lagathu C, Cossarizza A, Bereziat V, Nasi M, Capeau J, Pinti M. Basic science and pathogenesis of ageing with HIV: potential mechanisms and biomarkers. AIDS. 2017;31(Suppl 2):S105–S119. doi: 10.1097/QAD.0000000000001441. [DOI] [PubMed] [Google Scholar]
- 5.Horvath S, Erhart W, Brosch M, Ammerpohl O, von Schonfels W, Ahrens M, et al. Obesity accelerates epigenetic aging of human liver. Proc Natl Acad Sci U S A. 2014;111:15538–15543. doi: 10.1073/pnas.1412759111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez RF, Fernandez-Morera JL, Romano-Garcia J, Menendez-Torre E, Delgado-Alvarez E, Fraga MF, et al. DNA methylomes and epigenetic age acceleration associations with poor metabolic control in T1D. Biomedicines. 2020;9(1):13. doi: 10.3390/biomedicines9010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horvath S, Lin DTS, Kobor MS, Zoller JA, Said JW, Morgello S, et al. HIV, pathology and epigenetic age acceleration in different human tissues. Geroscience. 2022;44:1609–1620. doi: 10.1007/s11357-022-00560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fahy GM, Brooke RT, Watson JP, Good Z, Vasanawala SS, Maecker H, et al. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell. 2019;18:e13028. doi: 10.1111/acel.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang CX, Schon E, Obeidat M, Kobor MS, McEwen L, MacIsaac J, et al. Occurrence of accelerated epigenetic aging and methylation disruptions in human immunodeficiency virus infection before antiretroviral therapy. J Infect Dis. 2021;223:1681–1689. doi: 10.1093/infdis/jiaa599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11:303–327. doi: 10.18632/aging.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10:573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horvath S, Levine AJ. HIV-1 infection accelerates age according to the epigenetic clock. J Infect Dis. 2015;212:1563–1573. doi: 10.1093/infdis/jiv277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoare J, Stein DJ, Heany SJ, Fouche J-P, Phillips N, Er S, et al. Accelerated epigenetic aging in adolescents living with HIV is associated with altered development of brain structures. J Neurovirol. 2022;28:208–216. doi: 10.1007/s13365-021-00947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horvath S, Stein DJ, Phillips N, Heany SJ, Kobor MS, Lin DTS, et al. Perinatally acquired HIV infection accelerates epigenetic aging in South African adolescents. AIDS. 2018;32:1465–1474. doi: 10.1097/QAD.0000000000001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross AM, Jaeger PA, Kreisberg JF, Licon K, Jepsen KL, Khosroheidari M, et al. Methylome-wide analysis of chronic HIV infection reveals five-year increase in biological age and epigenetic targeting of HLA. Mol Cell. 2016;62:157–168. doi: 10.1016/j.molcel.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esteban-Cantos A, Rodriguez-Centeno J, Barruz P, Alejos B, Saiz-Medrano G, Nevado J, et al. Epigenetic age acceleration changes 2 years after antiretroviral therapy initiation in adults with HIV: a substudy of the NEAT001/ANRS143 randomised trial. Lancet HIV. 2021;8:e197–e205. doi: 10.1016/S2352-3018(21)00006-0. [DOI] [PubMed] [Google Scholar]
- 19.Sundermann EE, Hussain MA, Moore DJ, Horvath S, Lin DTS, Kobor MS, et al. Inflammation-related genes are associated with epigenetic aging in HIV. J Neurovirol. 2019;25:853–865. doi: 10.1007/s13365-019-00777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine AJ, Quach A, Moore DJ, Achim CL, Soontornniyomkij V, Masliah E, et al. Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders. J Neurovirol. 2016;22:366–375. doi: 10.1007/s13365-015-0406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sehl ME, Breen EC, Shih R, Chen L, Wang R, Horvath S, et al. Increased rate of epigenetic aging in men living with HIV prior to treatment. Front Genet. 2021;12:796547. doi: 10.3389/fgene.2021.796547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sehl ME, Rickabaugh TM, Shih R, Martinez-Maza O, Horvath S, Ramirez CM, et al. The effects of anti-retroviral therapy on epigenetic age acceleration observed in HIV-1-infected adults. Pathog Immun. 2020;5:291–311. doi: 10.20411/pai.v5i1.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiau S, Arpadi SM, Shen Y, Cantos A, Ramon CV, Shah J, et al. Epigenetic aging biomarkers associated with cognitive impairment in older African American adults with human immunodeficiency virus (HIV) Clin Infect Dis. 2021;73:1982–1991. doi: 10.1093/cid/ciab563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodes B, Cadinanos J, Esteban-Cantos A, Rodriguez-Centeno J, Arribas JR. Ageing with HIV: challenges and biomarkers. EBioMedicine. 2022;77:103896. doi: 10.1016/j.ebiom.2022.103896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorenson C, Chalkidou K. Reflections on the evolution of health technology assessment in Europe. Health Econ Policy Law. 2012;7:25–45. doi: 10.1017/S1744133111000296. [DOI] [PubMed] [Google Scholar]
- 26.Beltran-Najera A, Woods SP, Evans D, Matchanova A, Mustafa A, Ridgely NC, Thompson JL. In: Health literacy in HIV associated neurocognitive disorders. Hu G, Xiong H, Buch S, HIV-associated Neurocognitive Disorders (HAND), editors. New York: Elsevier; 2023. [Google Scholar]
- 27.Woods SP, Morgan EE, Loft S, Matchanova A, Verduzco M, Cushman C. Supporting strategic processes can improve time-based prospective memory in the laboratory among older adults with HIV disease. Neuropsychology. 2020;34:249–263. doi: 10.1037/neu0000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization (WHO) Composite international diagnostic interview. Genève, Switzerland: World Health Organization; 1998. [Google Scholar]
- 29.Bollepalli S, Korhonen T, Kaprio J, Anders S, Ollikainen M. EpiSmokEr: a robust classifier to determine smoking status from DNA methylation data. Epigenomics. 2019;11:1469–1486. doi: 10.2217/epi-2019-0206. [DOI] [PubMed] [Google Scholar]
- 30.Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, et al. Minfi: a flexible and comprehensive bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30:1363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott HR, Tillin T, McArdle WL, Ho K, Duggirala A, Frayling TM, et al. Differences in smoking associated DNA methylation patterns in South Asians and Europeans. Clin Epigenetics. 2014;6:4. doi: 10.1186/1868-7083-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bloch M, Kamminga J, Jayewardene A, Bailey M, Carberry A, Vincent T, et al. A screening strategy for HIV-associated neurocognitive disorders that accurately identifies patients requiring neurological review. Clin Infect Dis. 2016;63:687–693. doi: 10.1093/cid/ciw399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casaletto KB, Umlauf A, Beaumont J, Gershon R, Slotkin J, Akshoomoff N, et al. Demographically corrected normative standards for the English version of the NIH Toolbox Cognition Battery. J Int Neuropsychol Soc. 2015;21:378–391. doi: 10.1017/S1355617715000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matchanova A, Sheppard DP, Medina LD, Morgan EE, Woods SP. Health literacy mediates the effects of educational attainment on online pharmacy navigation skills in older adults with HIV disease. Psychol Health. 2021:1–21. [DOI] [PMC free article] [PubMed]
- 37.Justice AC, Modur SP, Tate JP, Althoff KN, Jacobson LP, Gebo KA, et al. Predictive accuracy of the Veterans Aging Cohort Study Index for mortality with HIV infection. J Acquir Immune Defic Syndr. 2013;62:149–163. doi: 10.1097/QAI.0b013e31827df36c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edelle [Edee] Field-Fote Mediators and moderators, confounders and covariates: exploring the variables that illuminate or obscure the “active ingredients” in neurorehabilitation. J Neurol Phys Ther. 2019;43:83–84. doi: 10.1097/NPT.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 39.Heaton RK, Akshoomoff N, Tulsky D, Mungas D, Weintraub S, Dikmen S, et al. Reliability and validity of composite scores from the NIH Toolbox Cognition Battery in adults. J Int Neuropsychol Soc. 2014;20:588–598. doi: 10.1017/S1355617714000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pathai S, Lawn SD, Gilbert CE, McGuinness D, McGlynn L, Weiss HA, et al. Accelerated biological ageing in HIV-infected individuals in South Africa: a case-control study. AIDS. 2013;27:2375–2384. doi: 10.1097/QAD.0b013e328363bf7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cobos Jimenez V, Wit FW, Joerink M, Maurer I, Harskamp AM, Schouten J, et al. T-cell activation independently associates with immune senescence in HIV-infected recipients of long-term antiretroviral treatment. J Infect Dis. 2016;214:216–225. doi: 10.1093/infdis/jiw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaiserman A, Krasnienkov D. Telomere length as a marker of biological age: state-of-the-art, open issues, and future perspectives. Front Genet. 2020;11:630186. doi: 10.3389/fgene.2020.630186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Justice AC, Tate JP. Strengths and limitations of the Veterans Aging Cohort Study Index as a measure of physiologic frailty. AIDS Res Hum Retroviruses. 2019;35:1023–1033. doi: 10.1089/aid.2019.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddon H, Grant C, Nosova E, Fairbairn N, Barrios R, Justice AC, et al. The Veterans Aging Cohort Study (VACS) index predicts mortality in a community-recruited cohort of people with human immunodeficiency virus (HIV) who use illicit drugs. Clin Infect Dis. 2021;73:538–541. doi: 10.1093/cid/ciaa1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oursler KK, Marconi VC, Wang Z, Xu K, Montano M, So-Armah K, et al. Epigenetic age acceleration markers are associated with physiologic frailty and all-cause mortality in people with HIV. Clin Infect Dis. 2022;76(3):e638–e644. doi: 10.1093/cid/ciac656. [DOI] [PMC free article] [PubMed] [Google Scholar]