Abstract
Gibberella fujikuroi is a species complex with at least nine different biological species, termed mating populations (MPs) A to I (MP-A to MP-I), known to produce many different secondary metabolites. So far, gibberellin (GA) production is restricted to Fusarium fujikuroi (G. fujikuroi MP-C), although at least five other MPs contain all biosynthetic genes. Here, we analyze the GA gene cluster and GA pathway in the closest related species, Fusarium proliferatum (MP-D), and demonstrate that the GA genes share a high degree of sequence homology with the corresponding genes of MP-C. The GA production capacity was restored after integration of the entire GA gene cluster from MP-C, indicating the existence of an active regulation system in F. proliferatum. The results further indicate that one reason for the loss of GA production is the accumulation of several mutations in the coding and 5′ noncoding regions of the ent-kaurene oxidase gene, P450-4.
Gibberella fujikuroi has been described as a species complex of at least nine different biological species, frequently termed mating populations (MPs) (17, 47). Recently, these MPs have been reassigned to Gibberella species names parallel to the names of their anamorphs, and additional anamorphs have also been identified (5, 26-28, 47). These Fusarium species have been isolated from different hosts such as maize, rice, sugarcane, pine, mango, and sorghum (20) and cause several serious plant diseases. These species also produce a variety of secondary metabolites such as gibberellins (GAs) (36), moniliformin (21), fumonisins (10, 21), fusaric acid (2), and beauvericin (22) that in some cases may affect human and animal health. Rice pathogens of the G. fujikuroi species complex are described as causative agents of bakanae disease of rice seedlings. The overgrowth symptom in infected plants is due to the secretion of huge amounts of GAs, which function as growth hormones in higher plants, by members of G. fujikuroi MP-C (Fusarium fujikuroi). This fungus is used for the commercial production of GAs, mainly gibberellic acid (GA3) and its precursors, GA4 and GA7, for use as plant growth regulators in agriculture and horticulture (32).
In the last five years, we have cloned and characterized the seven GA biosynthetic genes in MP-C and have shown that they are organized in a gene cluster (36). The regulation of gene expression and enzymatic function of the encoded enzymes have recently been determined (33, 38-40) (Fig. 1A). The seven enzymes catalyze at least 13 biosynthetic steps due to the multifunctional character of the four cytochrome P450 monooxygenases P450-1 to P450-4 (33, 38-40, 43).
FIG. 1.
Gibberellin biosynthesis pathway and gene cluster. (A) Pathway showing genes, enzymes, and final products. The major pathway is denoted by enlarged arrows and letters. GGPP, geranylgeranyl-pyrophosphate; CPP, copalylpyrophosphate. (B) Gene cluster of the GA biosynthesis pathway (about 20 kb). Numbers 1 to 6 indicate the different primer combinations for amplification of the overlapping regions between two cluster genes (1, orf3-Fus1/P450-4-Fus2; 2, P4-Fus1/P1-Fus2; 3, P450-1-Fus3/P450-2-Fus4; 4, P450-2-Fus5/ggs2-Fus6; 5, ggs2-Fus3/cps-Fus5; 6, cps-Fus7/P450-3-Fus8).
Recently, we demonstrated that all genes except for P450-3 are under the control of nitrogen metabolite repression although GAs do not contain nitrogen in their skeleton. The nitrogen metabolite repression is mediated by the general transcription factor AREA-GF. Deletion of the areA-Gf gene resulted in a dramatic reduction of GA production capacity (37). This factor was shown to bind directly to the promoters of the six N-regulated genes (25).
Beside the GA biosynthesis genes, the single-copy cytochrome P450 reductase gene, cpr-Gf, which is probably required for the activity of all cytochrome P450 monooxygenases, also affects GA biosynthesis. Deletion of the cpr-Gf gene resulted in the loss of GA4, GA7, and GA3 production (23).
Previously, we have shown that genomic DNA from members of eight MPs hybridizes at high stringency to all seven (MP-B to MP-G) or some (MP-A and MP-H) of the GA biosynthetic genes of MP-C, suggesting that these genes are present. Genetic relatedness studies of strains belonging to different MPs of the G. fujikuroi species complex revealed the highest degree of similarity between MP-C and MP-D, based on sequence data of one of the GA biosynthetic genes, P450-4, and the cytochrome P450 reductase gene, cpr-Gf (S. Malonek and B. Tudzynski, unpublished data). Despite the high level of sequence homology between corresponding GA pathway genes from different MPs, only F. fujikuroi (MP-C) is able to produce significant amounts of GAs (Malonek and Tudzynski, unpublished).
In this work, we examined the reasons for the loss of GA production capability in Fusarium proliferatum, the species with the highest genetic relatedness to the GA-producing species F. fujikuroi (35). We show that the GA biosynthetic genes in F. proliferatum are organized in a gene cluster similar to that of MP-C. Moreover, we investigated the functionality of two cytochrome P450 monooxygenase genes, P450-1, encoding GA14 synthase, and P450-4, encoding ent-kaurene oxidase, by examining the metabolism of radiolabeled precursors in two MP-D strains and in an MP-C UV mutant (SG139) that had been transformed with both MP-D genes. Strain SG139 has lost the entire gene cluster due to a deletion and is therefore an excellent tool for functional analysis of single GA biosynthetic genes. Furthermore, we compared the expression of P450-1 and P450-4 from both MPs in each genetic background and demonstrate by β-glucuronidase (GUS) reporter assays that single-base-pair differences in the 5′ noncoding regions of both genes resulted in significant reduction of gene expression. We show for the first time that the inability of F. proliferatum to produce GAs results from several mutations in the GA biosynthetic genes rather than in regulatory genes.
MATERIALS AND METHODS
Fungal strains.
Strains IMI58289 (Commonwealth Mycological Institute, Kew, United Kingdom) and m567 (Fungal Culture Collection, Weimar, Germany) are GA-producing wild-type strains of G. fujikuroi MP-C (anamorph F. fujikuroi). Strain SG139 is a UV mutant lacking the whole GA gene cluster (33, 38). MP-D strains D02945 and D00502 were kindly provided by J. F. Leslie (Kansas State University). Strain cTP4GUS-C139 resulted from a transformation of the vector pCP4::GUS (carrying the P450-4 [MP-C] promoter fused to the uidA gene of Escherichia coli) into strain SG139 (25).
Bacterial strains and plasmids.
E. coli strain Top10 (Invitrogen, Groningen, The Netherlands) was used for plasmid propagation. Vector pUCBM20 (Boehringer, Mannheim, Germany) was used to clone DNA fragments carrying the G. fujikuroi cluster genes and gene fragments from MP-C and MP-D. Cosmid pCos1, derived from a cosmid library based on strain m567, contains the entire GA gene cluster including the noncoding 5′ and 3′ regions (about 40 kb). Plasmids pP450-1-GK and pP450-4-GK (38, 39), carrying the corresponding genes from MP-C, were used for complementation of MP-D strains. To obtain plasmid pP450-1GKD, a 3.8-kb HindIII fragment of λ clone DIII-1 was cloned into pAN7.1/HindIII (30). Plasmid pP450-4GKD was constructed by cloning a 4-kb NcoI/SacI fragment of λ clone DIII-1 into pUCBM20/NcoI/SacI. For promoter analysis of the intergenic region between P450-1 and P450-4 in MP-D, plasmids pDP1::GUS and pDP4::GUS were constructed. Both plasmids were derived by amplifying about 900 bp of the promoter by PCR with homologous primers P4/1-GUS1D and P4/1-GUS2D. The PCR product was cloned into pCR2.1TOPO (Invitrogen). After restriction with NcoI, the promoter was cloned into vector pAS1/NcoI containing the hygromycin resistance marker (25). In cotransformation experiments, either pAN7-1 (hygromycin resistance) (30) or pNR1 (nourseothricin resistance) (18, 23) was used for selection of transformants.
Media and culture conditions.
For DNA isolation, fungal strains were grown in 100 ml of liquid CM medium optimized for Fusarium spp. (29). for 3 days at 28°C on a rotary shaker set at 200 rpm. The mycelium was harvested by filtration through a sterile glass filter (G2; Schott, Jena, Germany), washed with sterile distilled water, frozen in liquid nitrogen, and lyophilized for 24 h. The lyophilized mycelia were ground to a fine powder with a mortar and pestle. MP-D strains were cultivated on V8 juice agar for sporulation. For RNA isolation, fungal strains were grown in 100, 20, or 0% ICI medium (13) containing 8% glucose, 0.5% MgSO4, 0.1% KH2PO4, and 5.0, 1.0, or 0 g of NH4NO3/liter, respectively.
For analysis of gene expression and nitrogen regulation, strains were cultivated for 3 days in 10 or 20% ICI medium on a rotary shaker at 28°C. To elucidate nitrogen regulation, mycelium was washed, and 1.5 g (wet weight) each was transferred to 50 ml of 0 or 100% ICI medium. For GA production, the strains were grown for 7 to 10 days on a rotary shaker (200 rpm) at 28°C in 300-ml Erlenmeyer flasks containing 100 ml of either 20% ICI or optimized production medium (OPM) containing 6% sunflower oil, 0.05% (NH4)2SO4, 1.5% corn-steep solids (Sigma-Aldrich, Taufkirchen, Germany), and 0.1% KH2PO4. GUS plate assays were performed as previously described (25).
DNA and RNA isolation.
Genomic DNA was isolated from lyophilized mycelium as described previously by Doyle and Doyle (11). Lambda DNA from positive λ clones was prepared according to the method described by Maniatis et al. (24). Plasmid DNA was extracted by using Genomed (Bad Oeynhausen, Germany) columns according to the manufacturer's protocol. RNA was isolated by using an RNAgents total RNA isolation kit (Promega, Mannheim, Germany).
PCR.
PCRs contained 25 ng of DNA, 10 ng of each primer, 0.2 mM deoxynucleoside triphosphates, and 2 U of Taq polymerase (Red Taq; Sigma-Aldrich, Deisenhofen, Germany) in 50 μl. PCR was carried out at 94°C for 4 min followed by 35 cycles of 94°C for 1 min, 55 to 65°C for 1 to 3 min, and 72°C for 1 min. Annealing and elongation time were applied differently, depending on annealing temperatures of each primer and the amplified fragment.
For analysis of the GA gene cluster organization in F. proliferatum (MP-D) strain D02945, the following primer pairs were synthesized on the basis of sequence data from MP-C genes (Table 1).
TABLE 1.
Primers used in this study
| Primer | Sequence (5′→3′) |
|---|---|
| orf3-Fus1 | TGCTGCCGCTGCTTGATTT |
| P450-4-Fus2 | CCCAGGGCGGTTCTATGC |
| P4-Fus1 | GGCTGGTACTGTTCATGCTGTTGG |
| P1-Fus2 | CGTCAAGACGGTATGAGAGTGGTCC |
| P450-1-Fus3 | GGCGTCATCCTACCCAAGAACA |
| P450-2-Fus4 | TTTTACCGGTGCCGAACAGTGA |
| P450-2-Fus5 | GCTTCGGCCACGGTCAG |
| ggs2-Fus6 | ACAAGGTTCGATCCCCACAG |
| ggs2-Fus3 | GGATACAAGAGCGAAGAATGACGTG |
| cps-Fus5 | TGGCCACCCAAGCAGTGTCATATACC |
| cps-Fus7 | TTCGCTTTCTCCAACTGCCTAATGT |
| P450-3-Fus8 | CCGAACGGACGCTGGGTAAAAA |
For reverse transcription (RT)-PCR analysis of gene P450-1 (MP-D), primers RT1-P450-1-MP-D (5′-TGAGTCCCGACAAGCCATTCC-3′) and RT2-P450-1-MP-D (5′-GGGGGTTTCGACACATCTCCTTAC-3′) were used.
For amplification of the bidirectional promoter between P450-1 and P450-4 in MP-D, primers P4/1-GUS1D (5′-CAAGGCTGGTATTGTCCATGGTGTTAG-3′) and P4/1-GUS2D (5′-ATGGTTGATAGCCCATGGTTATTGCTG-3′) with introduced restriction sites were used (the NcoI sites are underlined). The resulting fragment was about 900 bp.
For analysis of putative pDP1::GUS and pDP4::GUS-transformants, primers LUC-PCR (5′-AGGTGCGCCCCCAGAAGCAATTTCGTGTAG-3′) and GUS (5′-ATTGTTTGCCTCCCTGCTGCG-3′) were applied.
For analysis of the full-length integration of vector pP450-4-GKD after retransformation into strain D02945, transformants were analyzed by using primers REV (5′-GAGCGGATAACAATTTCACACAGG-3′) and P450-4-GD1 (5′-GGTCCAGAGCACTGCCGC-3′).
For analysis of full-length integration of vector pP450-GKD after transformation into strain SG139, transformants were analyzed by using primers P450-4-GD2 (5′-CTTCCTTTCCCATCTGGC-3′) and P4/1-GUS2D.
Screening of the λ DASH II library.
About 35,000 recombinant phages from a λ DASH II library (Stratagene) prepared from genomic DNA of G. fujikuroi strain D02945 were plated and transferred to nylon N+ membranes (Amersham Pharmacia, Freiburg, Germany). Hybridization was performed at high stringency (65°C). The blots were washed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate (SDS) at 65°C followed by 0.1× SSC-0.1% SDS at 65°C. Positive recombinant phages were used for a second round of plaque purification.
Southern and Northern blot analyses.
After digestion with restriction endonucleases and electrophoresis, genomic or λ DNA was transferred onto Hybond N+ filters (Amersham Pharmacia). 32P-labeled probes were prepared by using the random oligomer-primer method (24). Filters were hybridized at 65 or 56°C in 5× Denhardt's solution containing 5% dextran sulfate (24). Filters were washed at the same temperature used for hybridization in 2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7])-0.1% SDS and 1× SSPE-0.1% SDS.
Northern blot hybridizations were accomplished by using a method described previously by Church and Gilbert (9). The G. fujikuroi rRNA gene was used as a control hybridization probe to confirm RNA transfer. For quantification of expression levels, the method of densitometry was performed by using TotalLab1D software, version 1.00 (Amersham Pharmacia). Hybridizing bands were each normalized against the corresponding band of the wild-type (MP-C) gene copy.
Sequencing.
DNA sequencing of recombinant plasmid clones was accomplished with an automatic sequencer (LI-COR 4000; MWG, Munich, Germany). The two strands of overlapping subclones obtained from the genomic DNA clones were sequenced by using the universal and the reverse primers or specific oligonucleotides obtained from MWG Biotech. DNA and protein sequence alignments were done with DNA Star (Madison, Wis.).
Transformation of G. fujikuroi.
The preparation of protoplasts and the transformation procedure were done as previously described (41, 42) with the following modifications for strain D02945. Strain D02945 was precultivated for 7 to 10 days on V8 agar. About 5 × 108 spores/ml were inoculated into 100 ml of CM medium. For complementation experiments, 107 protoplasts (50 μl) of strain SG139, D02945, or Δdes-MP-C were transformed with up to15 μg of the cosmid pCos1, carrying the entire GA gene cluster from MP-C, or circular complementation vectors pP450-1-GK, pP450-1-GKD, pP450-4-GK, pDP1::GUS, and pDP4::GUS. Plasmids pdes-GKD and pP450-4GKD were cotransformed with pNR1 (23) and pAN7.1 (30), carrying the nourseothricin and hygromycin resistance markers, respectively.
Transformed protoplasts were regenerated at 28°C on complete regeneration agar [0.7 M sucrose, 0.05% yeast extract, 0.1% (NH4)2SO4] containing 120 μg of hygromycin B (Calbiochem, Bad Soden, Germany)/ml or 100 μg of nourseothricin (Werner BioAgents, Jena, Germany)/ml for 6 to 7 days. For purification, single conidial cultures were obtained from hygromycin B- or nourseothricin-resistant transformants and used for DNA isolation and Southern blot analysis.
Gibberellin assays.
The complete GA complement produced by the different strains was determined by gas chromatography-mass spectrometry analysis after extraction from the culture fluid as described previously (3, 40). Preparation of microsomal fractions and subsequent high-performance liquid chromatography (HPLC) analysis were performed as previously described (43). For incubations with 14C-labeled substrates, mycelia grown for 3 days in 40% ICI (13) were harvested, washed, and suspended in 10 ml of 0% ICI medium, to which radiolabeled substrates were added (ent-[14C]kaurenoic acid [300,000 disintegrations per min] or [14C]GA12-aldehyde [200,000 disintegrations per min]). The mixture was further incubated at 30°C with shaking for another 2 to 3 days. For time course experiments, 2-ml aliquots were taken from the reaction at different time points. The culture fluid was separated by filtration, and the GAs were extracted as previously described and analyzed by HPLC (33, 38).
Preparation of cell extracts.
Preparation of cell extracts and microsomes was done as described previously by Urrutia et al. (43).
GUS assay.
G. fujikuroi strains SG139, MP-D02945, and pCP1::GUS and transformants carrying vectors pDP1::GUS and pDP4::GUS were analyzed as previously described (25).
GUS plate assay.
Strains were precultivated on 0% ICI agar plates for 3 days and then overlayed with Top-GUS agar (100 mM NaPO4 [pH 7], 0.05% Triton X-100, 1% agar, 5 mg of X-Gluc [5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid] in dimethyl sulfoxide per 5 ml of agar [made fresh for each experiment]). GUS expression could be measured by blue staining in the mycelia after at least 2 days of incubation.
Nucleotide sequence accession number.
The gene sequences for P450-4 (D) and P450-1 (D) of F. proliferatum have been deposited in the GenBank database under accession number AJ628021.
RESULTS
Organization of the GA gene cluster in MP-D.
Recently, we have shown that the seven GA biosynthetic genes from GA-producing strains of F. fujikuroi (MP-C) code for seven enzymes catalyzing at least 16 steps in the GA biosynthesis pathway (Fig. 1A). The seven genes hybridize to the DNA of F. proliferatum strains (MP-D) at high stringency. Although all the genes exist in MP-D strains, they are not able to produce GAs (Malonek and Tudzynski, unpublished). In order to find the reasons for their inability to produce GAs, we first analyzed the organization of the GA gene cluster in MP-D. We performed several PCRs in strain D02945 with primers derived from MP-C gene sequences and overspanning intergenic spacer regions between two genes (Fig. 1B). The fragment size of PCR products and sequence analysis displayed the same cluster organization for MP-D as that of MP-C. Sequence homology of the intergenic noncoding regions between cluster genes from MP-D and MP-C was 85% on average (data not shown).
GA regulatory genes are functional in F. proliferatum.
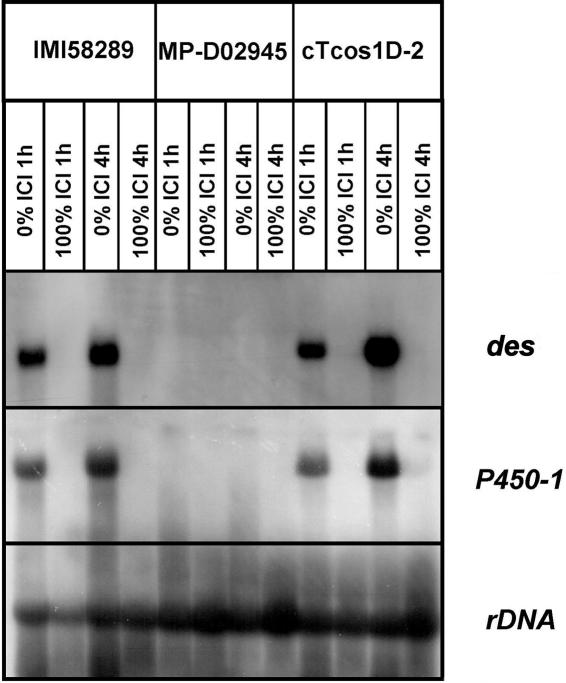
Northern blot analyses of different MP-D strains in several GA production media never revealed the expression of any GA cluster gene (data not shown). In order to exclude the possibility that mutations in genes responsible for regulation of GA biosynthesis are the reason for the loss of GA gene expression and production in MP-D, we transformed the cosmid pCos1 carrying the entire GA gene cluster of MP-C into strain D02945. Southern blot analysis revealed the complete integration of the entire GA gene cluster of MP-C (including the 5′ and 3′ neighborhoods of the GA cluster) in five transformants (data not shown). In contrast to the recipient strain D02945, transformants express all seven GA pathway genes at about the same level as strain F. fujikuroi IMI58289 (MP-C) (shown for P450-1 and des in Fig. 2). Moreover, the genes are regulated in the same way as in MP-C: six of the seven genes are repressed by high amounts of nitrogen (100% ICI) and are highly expressed under conditions of nitrogen starvation (0% ICI) in MP-D, as shown exemplarily for des and P450-1 (Fig. 2). To study GA production, we performed HPLC analysis after incubating D02945, the transformants, and the MP-C control strain IMI58289 in OPM for 7 days. In contrast to the recipient strain D02945, two of the transformants, cTCos1-D4 and cTCos1-D2, produced GA4, GA7, and GA3, although only at about 40 and 8%, respectively, of the amounts produced by the MP-C control strain IMI58289 (Table 2).
FIG. 2.
Comparison of des and P450-1 gene expression for wild-type IMI58289, MP-D02945, and transformant cTcos1D-2. rRNA (rDNA) was used as a loading control. For hybridization, a 1-kb des (C) and a 1.5-kb P450-1 (C) cDNA clone were used.
TABLE 2.
Production of GAs in MP-C and MP-D wild-type strains and different transformants after cultivation of strains for 7 days in OPM
| Strain | Mean GA production (mg/liter) ± SDa
|
||
|---|---|---|---|
| GA3 | GA7 | GA4 | |
| IMI58289 | 313.7 ± 25.4 | 38 ± 17.7 | 54.3 ± 13.9 |
| cTcos1-D1 | − | − | − |
| cTcos1-D2 | 24.3 ± 8.1 | 124.3 ± 12.4 | 66.7 ± 18.5 |
| cTcos1-D4 | 123 ± 19.9 | 90.7 ± 7 | 51.7 ± 16.3 |
| MP-D02945 | − | − | − |
Mean values and standard deviations of three independent cultivations per strain are given. −, less than 1 mg/liter.
For comparison, we also transformed mutant strain SG139 (MP-C), which lacks the entire GA gene cluster due to a deletion (40), with the same cosmid, pCos1. After 8 days of cultivation of three independent transformants, subsequent HPLC analysis revealed the full GA pattern. In contrast to the corresponding complementation transformants in D02945, the tested SG139 transformants (cTcos1-C6, -C8, and -C12) gave up to 86% of the GA content of MP-C wild-type strain IMI58289 (Table 3). These results clearly demonstrate that the known regulatory genes, such as areA-Gf and other as-yet-unknown GA-specific regulator(s), are functional in F. proliferatum and that mutations in the GA biosynthetic gene cluster rather than mutations in regulatory genes or a different chromatin structure are likely to be responsible for the loss of GA production.
TABLE 3.
Production of GAs in the MP-C wild-type strain, mutant SG139, and different transformants after cultivation of strains for 8 days in OPM
| Strain | Mean GA production (mg/liter) ± SDa
|
||
|---|---|---|---|
| GA3 | GA7 | GA4 | |
| IMI58289 | 495 ± 21.2 | 30 ± 8.5 | 61 ± 4.2 |
| cTcos1-C6 | 424 ± 40.8 | 24.3 ± 20.6 | 27.3 ± 22.3 |
| cTcos1-C8 | 359 ± 55.6 | 28.3 ± 27.5 | 20 ± 6.2 |
| cTcos1-C12 | 323.3 ± 51.3 | 25 ± 8.9 | 49.3 ± 40 |
| SG139 | − | − | − |
Mean values and standard deviations of three independent cultivations per strain are given. −, less than 1 mg/liter.
Biochemical analysis of two cytochrome P450 monooxygenases in different strains of MP-D.
In previous studies, the function as well as regulation of gene expression of P450-4 and P450-1, encoding the multifunctional ent-kaurene oxidase and GA14 synthase, respectively, were reported for F. fujikuroi (MP-C) (25, 33, 38). P450-4 catalyzes the conversion of ent-kaurene to ent-kaurenoic acid via ent-kaurenal and ent-kaurenol (Fig. 1A). P450-1 (GA14 synthase) catalyzes four oxidation steps from ent-kaurenoic acid to GA14 via ent-7α-hydroxykaurenoic acid, GA12-aldehyde, and GA14-aldehyde (Fig. 1A) (33).
In order to identify possible enzymatic blocks in MP-D, incubations with 14C-labeled substrates for ent-kaurene oxidase (ent-kaurene) and GA14 synthase (ent-kaurenoic acid and GA12-aldehyde) were carried out with two MP-D strains, D00502 and D02945. ent-[14C]kaurene was added to the cultures under GA production conditions, and radioactive products were analyzed by HPLC after 2 days of incubation. None of the strains were able to metabolize the ent-[14C]kaurene, revealing a block at this point in the GA pathway. In contrast, P450-1 was found to be active in F. proliferatum. In strain D00502, 66% of the ent-[14C]kaurenoic acid was metabolized after 2 days but converted not to [14C]GA3, the end product in MP-C strains, but mainly to [14C]GA1, which was formed with a 19 to 34% yield. Minor amounts of ent-6α,7α-[14C]dihydroxykaurenoic acid (12%) were found in some incubations with this precursor, indicative of a low activity for P450-1 in MP-D. [14C]GA12-aldehyde was almost completely metabolized by D00502 into [14C]GA1 (12%) plus [14C]GA4 (73%). These results show that in contrast to P450-4, the other GA P450 monooxygenases (P450-1, P450-2, and P450-3) are active in F. proliferatum, although they may have lower activities than in MP-C strains. The absence of [14C]GA3 indicates that GA4 desaturase, which catalyzes the synthesis of its precursor, GA7 (Fig. 1A), is inactive or has very low activity in D00502. In strain D02945, we found that [14C]GA12-aldehyde was also metabolized but surprisingly not transformed into any known product of the GA pathway. Instead, the products were identified as 12-hydroxy[14C4]GA12-aldehyde and putative 16,17-dihydro-12,17-dihydroxy[14C4]GA12-aldehyde, which may be formed by nonspecific oxidation by oxidases encoded by genes located outside the GA cluster. These activities would compete with P450-1 if this has only low activity in D02945. Therefore, significant differences exist, even between different strains of MP-D.
In order to detect P450-1 activity in D02945, mycelial microsomal fractions were prepared from this strain and from IMI58289 (MP-C) and assayed with [14C]GA12-aldehyde in the presence of NADPH and flavin adenine dinucleotide. HPLC analysis of the products indicated that D02945 was able to convert the substrate into a polar product with the retention time of [14C]GA14, although overall enzyme activity was very low and 85% of the precursor remained unchanged (data not shown). In contrast, under the same conditions, the microsomal fraction from IMI58289 completely metabolized [14C]GA12-aldehyde into [14C]GA14 (66%) and [14C]GA12 (28%).
To further characterize the activity of ent-kaurene oxidase and GA14 synthase of F. proliferatum strain D02945 in more detail, we cloned and sequenced the corresponding genes P450-4 (D) and P450-1 (D) (where D stands for MP-D) and analyzed their expression and enzyme activity in both MP-C and MP-D backgrounds.
Functional analysis of P450-4 (D) encoding ent-kaurene oxidase.
P450-4 (D) was cloned from the genomic library of strain D02945 using a P450-4 (C) (where C stands for MP-C) cDNA clone as a probe. Sequence analysis revealed 95 and 97% identity at nucleotide and amino acid levels, respectively. Intron positions and heme-binding region were found to be conserved between MP-C and MP-D. Mutations and resulting amino acid substitutions were distributed over the whole protein. Expression of P450-4 (D) was hardly detectable in Northern blot analysis (Fig. 3B). To find out if the low expression level of GA genes in MP-D can be at least partially explained by the location of the GA gene cluster in the genome, P450-4 (D) from strain D02945 was transformed into strain D02945, and transformants were screened for ectopic integrations by Southern analysis (data not shown). Three ectopic transformants revealed much higher levels of expression of P450-4 (D) than that of the recipient strain copy (Fig. 3B), as quantified by densitometry measurement (for details, see Materials and Methods). Transformant dTP4-D3 exhibited a P450-4 expression level of 36% compared to that of MP-C wild-type strain IMI58289, although this transformant has the same copy number (two copies) as transformants dTP4-D4 and dTP4-D11, which show only 12 and 4% of the IMI58289 expression level, respectively. Therefore, the expression is still much lower than that of IMI58289 (MP-C). Additional hybridizing bands (see, e.g., transformant dTP4-D3) may be the result of incorrect integration events in ectopic loci or in the existing homologous P450-4 (D) locus.
FIG. 3.
Northern blot analysis of strains SG139, IMI58289 (MP-C), and D02945 and transformants containing P450-4 (D), P450-4 (C), and P450-1 (C). Strains were grown for 3 days in 20% ICI medium. (A) P450-4 (D) was transformed into strain SG139. A 1.5-kb HindIII fragment of plasmid pP450-4GKD was used for hybridization. (B) P450-4 (D) was transformed into strain D02945. A 1.5-kb HindIII fragment of plasmid pP450-4GKD was used for hybridization. (C) P450-4 (C) was transformed into strain D02945. A 1.5-kb cDNA clone of P450-4 (C) was used for hybridization. (D) P450-1 (C) was transformed into strain D02945. A 1.5-kb cDNA clone of P450-1 (C) was used for hybridization.
To investigate the expression level of P450-4 (D) and functionality of ent-kaurene oxidase from MP-D in the MP-C background, the gene P450-4 (D) was transformed into mutant strain SG139 (MP-C), in which the entire GA gene cluster is deleted (33, 38). This strain has been successfully used as recipient for P450-4 (C) and other genes of the F. fujikuroi GA gene cluster and is a powerful tool for functional analysis of the GA biosynthetic genes in general (33, 38, 40).
Transformants were screened for correct integration of the entire P450-4 (D) gene by PCR with primers P450-4-GD2 and P4/1-GUS2 and by Southern blot analysis (data not shown). Ten transformants, all carrying two or three gene copies, were chosen for Northern blot analysis (Fig. 3A). It was possible to detect transcript of the right size in only four transformants (dTP4-C16, dTP4-C18, dTP4-C26, and dTP4-C27), demonstrating the importance of the integration site in the genome for transcription rate. Transformants dTP4-C26 and dTP4-27 both displayed an expression level of 42% compared to the corresponding P450-4 copy of MP-C wild-type strain IMI58289. However, in incubations with ent-[14C]kaurene, none of the transformants showed any ent-kaurene oxidase (P450-4) activity. In contrast, the control transformant cTP4-C139, resulting from transformation of SG139 with the corresponding gene from MP-C (38), fully converted the substrate into ent-kaurenoic acid.
For comparison of expression levels for gene P450-4 from both MPs in the genetic background of MP-D, we also transformed the gene P450-4 (C) into strain D02945. P450-4 (C) was expressed much more strongly in the MP-D background than was the corresponding gene from MP-D (at an average of 140% normalized to the P450-4 expression level in IMI58289 [Fig. 3C]), confirming the existence of functional GA regulation factors in MP-D.
Functional analysis of P450-1 (D), encoding GA14 synthase.
P450-1 (D) was isolated by screening the λ DASH II genomic library of strain D02945 with a cDNA clone of P450-1 (C) as a probe. Sequence comparison of the coding region revealed 94 and 96% identity at the nucleotide and amino acid levels, respectively. However, in contrast to P450-4 (D), amino acid substitutions were restricted mainly to the N-terminal region but probably resulted in significant reduction of enzymatic activity. The intron positions as well as the heme-binding region were found to be highly conserved. Expression of P450-1 (D) in MP-D is even lower than that of P450-4 (D) and could be detected only by RT-PCR (data not shown). Addition of the substrate ent-kaurenoic acid did not increase the expression level. In order to directly compare the activity of P450-1 from both MPs, P450-1 (D) was transformed into the deletion mutant SG139 (MP-C). Transformants dTP1-C1, dTP1-C4, dTP-C6, and dTP1-C7 revealed a significant transcription level for P450-1 (D) (Fig. 4). However, densitometry measurement clearly showed that the P450-1 expression level was 60% lower than that in MP-C wild-type strain IMI58289, despite the transformants containing two or more gene copies instead of the one gene copy in IMI58289.
FIG. 4.
Northern blot analysis of strains SG139 and IMI58289 and transformants of strain SG139 containing P450-1 (D). Strains were grown for 3 days in 20% ICI medium. A 1.6-kb SphI fragment of plasmid pP450-1GKD was used for hybridization.
Since we could demonstrate the expression of P450-1 (D) in both MP-C and MP-D (RT-PCR), we wanted to determine if the encoded GA14 synthase is functional and to compare the activity of the enzymes in both backgrounds. The utilization of [14C]GA12-aldehyde (Fig. 1A) was measured in a time course experiment with cultures grown in a medium containing no nitrogen source (Fig. 5A). Conversion of the substrate into [14C]GA14 by strain dTP1-C6 (SG139) carrying P450-1 (D) was approximately four times lower than that by strain cTP1-C (SG139) transformed with P450-1 (C). For comparison, the activity of P450-1 from both MPs was also measured in the MP-D background after transforming P450-1 (C) into D00502. Northern blot analysis revealed an even higher expression level for the gene in the MP-D background compared with that of the MP-C wild-type strain, probably due to the higher copy number (Fig. 3D). The same effect was also shown for P450-4 (C) (Fig. 3C). Strain D00502 and transformant cTP1-D2 carrying P450-1 (C) were incubated with [14C]GA12-aldehyde (Fig. 5B). The utilization rate of the substrate in strain D00502 was found to be even lower than that in dTP1-C (SG139), and metabolism was detected only in cultures containing 10-fold-higher amounts of mycelia than those of the experiments shown in Fig. 5A. In cTP1-D2, the conversion rate of [14C]GA12-aldehyde is significantly enhanced in comparison with the MP-D strain after transforming P450-1 (C) (Fig. 5B), demonstrating that the high expression level of P450-1 (C) in MP-D results in higher enzyme activity than what was obtained from P450-1 (D).
FIG. 5.
Enzyme activity test of P450-1 genes from MP-D and MP-C in both genetic backgrounds in a time course experiment after incubation with [14C]GA12-aldehyde. Strains were precultivated as described in Materials and Methods and harvested. Mycelia were then cultivated in 10 ml of 0% ICI medium, and at each time course, a 2-ml aliquot was taken from the culture. Values shown are the results of four independent experiments. Error bars indicate standard deviations. (A) Comparison of P450-1 activity in transformants cTP1-C SG139 [carrying P450-1 (C)] and dTP1-C SG139 [carrying P450-1 (D)]. (B) Comparison of P450-1 activity in F. proliferatum D00502 and transformant cTP1-D2, carrying P450-1 (C).
Thus, P450-1 (D) is functional in both MPs but is expressed at a much higher level in SG139 (MP-C) than in its own genetic background. On the other hand, P450-1 (C) is more highly expressed in both genetic backgrounds than is P450-1 (D), suggesting the presence of mutations in promoter sequence elements of P450-1 (D) which are important for binding GA regulation factors.
Promoter studies for P450-1 (D) and P450-4 (D) using the GUS reporter system.
Genes encoding the multifunctional enzymes P450-1 and P450-4 are transcribed divergently and share a bidirectional promoter in MP-C and MP-D (Fig. 1B). Sequence analysis of the noncoding region between P450-4 and P450-1 in D02945 revealed 79% nucleotide identity compared with the corresponding region in MP-C. Recently, it was shown that two double GATA sequence elements (at positions −304 and −708) are essential for high expression of the corresponding genes in F. fujikuroi (25). As shown in Fig. 6, one of these double GATA elements starting at position −708 to the start codon of P450-1 is mutated in D02945.
FIG. 6.
Sequence alignment of the bidirectional promoter region between genes P450-1 and P450-4 of the GA gene cluster in MP-C and MP-D. Gene directions are marked with arrows, and GATA-binding motifs are indicated as boxes. Mutations in GATA-binding motifs are marked with asterisks above (in the case of MP-C) and below (in the case of MP-D) the GATA boxes. The bidirectional promoter region sequence has been deposited in the GenBank database (accession number AJ628021).
To analyze the impact of this promoter mutation and other base pair differences on expression of P450-1 (D) and P450-4 (D), we fused the MP-D P450-1/P450-4 bidirectional promoter in both directions to the E. coli uidA gene, encoding β-glucuronidase. The plasmids pDP1::GUS and pDP4::GUS, carrying the P450-1 and P450-4 promoters, respectively, were transformed into strains SG139 (MP-C) and D02949, and several transformants were analyzed for GUS activity. For the P450-1 (D) promoter construct, only very low expression of the uidA gene was detected in both genetic backgrounds, as calculated by intensity of blue staining of colonies after 3 to 5 days of incubation on X-Gluc plates and compared with the P450-4 (C) promoter construct pCP4::GUS (Fig. 7A). On the other hand, a much higher expression of the uidA gene was obtained for the promoter of P450-4 (D) in both backgrounds, visually nearly comparable to the expression level of the P450-4 (C) promoter (25).
FIG. 7.
GUS reporter assays. (A) Plate assay with dP1GUS-C transformants of SG139, all carrying more than two gene copies of the promoter construct pDP1::GUS. Plates were incubated for 5 days on X-Gluc agar. (B) Quantitative measurement of GUS activity. The different promoter constructs were fused to the E. coli uidA gene. Relative GUS activity is given on the right of the corresponding promoter construct. Positions of GATA motifs are indicated as ovals, and grey ovals show GATA elements differing from the MP-C promoter (cP4GUS-C).
To avoid the effect of multicopy integrations on the uidA expression level, only transformants with single-copy integrations of the pDP4::GUS and pDP1::GUS promoter constructs were used for a quantitative GUS enzyme assay. GUS activities were calculated from the mean values of three different single-copy transformants obtained with the same vector construct, with three independent measurements per strain, as described previously by Mihlan and coworkers (25). Although, in these transformants, the vector was integrated into different loci of the genome, the variation in uidA expression was rather low, indicating that the locus effect in this case was negligible. The average GUS activity was compared with that of the construct pCP4::GUS [promoter of P450-4 (C) fused to uidA] in strain SG139 (Fig. 7B, cP4GUS-C, 100%). Thus, construct pDP4::GUS shows 64.5% relative GUS activity in SG139 (Fig. 7B, dP4GUS-C), whereas the level for the same construct in the MP-D background (dP4GUS-D) was even lower (31% relative GUS activity).
In preliminary experiments, it has been shown in several Northern analyses that P450-1 and P450-4 of MP-C show an almost-identical high expression level under conditions of nitrogen starvation (33, 38). Therefore, the pCP4::GUS construct was also used as a control for GUS activity of the pDP1::GUS transformants (Fig. 7B, dP1GUS-C and dP1GUS-D). As expected from the sequence data (Fig. 6), the latter transformants showed only 5 to 10% GUS activity compared with that from pCP4::GUS.
These data clearly demonstrate that mutations in this bidirectional promoter differentially affect the expression of P450-4 (D) and P450-1 (D). The double GATA element, which is mutated in the bidirectional promoter in MP-D, is located near the start codon of P450-1 and could be at least one reason for the extremely low expression level of P450-1 (D) compared to that of P450-4 (D). Despite the much higher expression level of P450-4 (D), the gene does not encode a functional protein. On the other hand, although P450-1 (D) encodes a functional enzyme, its expression is hardly detectable.
DISCUSSION
The aim of this study was to determine why F. proliferatum (MP-D), the species with the highest level of phylogenetic relatedness to F. fujikuroi (MP-C), does not produce GAs despite containing a complete GA biosynthetic gene cluster. Similar analyses to determine the ability to produce aflatoxins by different Aspergillus strains were performed. Thus, it has been shown that the polyketide synthase gene, pksA, required for aflatoxin biosynthesis, possesses a nucleotide polymorphism in several Aspergillus flavus strains. This nucleotide change introduces a premature stop codon, thereby preventing toxin production (12). Furthermore, 27% of Aspergillus oryzae and 93% of A. flavus strains lacked either aflR or both aflR-1 and omt-1. Other strains have even larger deletions in the aflatoxin gene cluster and, therefore, have lost the ability to produce aflatoxins (45).
Our results demonstrated that the arrangement of the genes in the GA cluster in F. proliferatum (MP-D) is the same as that in F. fujikuroi (MP-C) (36). Sequence comparison of the MP-C and MP-D genes revealed significantly more differences in the intergenic regions (average of 85% nucleotide identity) than in the coding regions (up to 98% nucleotide identity). A similar observation was made for the trichothecene gene clusters in Fusarium graminearum and Fusarium sporotrichioides strains when trichothecene gene diversity in Fusarium was studied (6). Mutations accumulated to a higher extent in the noncoding regions, whereas the coding regions are more conserved.
We have clearly shown that in F. proliferatum (MP-D), expression of GA biosynthetic genes such as P450-4 and P450-1 was hardly detectable, suggesting that the loss of GA production might be due to defective regulation of the pathway in this MP. However, complementation of strain D02945 with the entire GA gene cluster from MP-C resulted in the restoration of GA production to as high as one-third of that in MP-C strains, indicating that the factors regulating expression of GA cluster genes, such as the general nitrogen regulator AREA (D) and putative unidentified GA-specific regulator(s), are functional in MP-D, although with less efficiency. Expression of P450-4 (D) and P450-1 (D) is significantly higher in SG139 (MP-C) than in MP-D strains. Interestingly, multicopy integrations of both MP-D genes in the MP-D background (D02945) resulted in detectable expression levels in some transformants, indicating that the transcription rate can be affected by the copy number and the integration locus in the genome. Similar results were also described for the aflatoxin biosynthesis gene cluster in Aspergillus parasiticus, where a region of active chromatin seems to be necessary for gene expression and, therefore, aflatoxin production (4). Transformation of individual aflatoxin genes or even a partially duplicated gene cluster never resulted in an expression level comparable to that in the wild type (7).
Recently, we demonstrated that two double GATA sequence elements in the bidirectional promoter for P450-1 and P450-4 are essential for binding of the transcription factor AREA-GF and the resulting high transcription level of P450-4 (C) (25). Similarly, in the avirulence gene Avr9 of Cladosporium fulvum (34), only two of the GATA elements were shown to be important for gene induction, and for the intergenic region between the divergently transcribed niiA and niaD genes of Aspergillus nidulans (31), only some of the AREA and NirA binding sites were used bidirectionally. In F. proliferatum, the double GATA (TATC TCGCA GATA; binding motifs for transcription factor AREA are underlined) sequence element located 708 bp upstream of the translation start of P450-1 (D) is mutated (TATC TCGCA AAT-).
To quantify the effect of this mutation on the expression levels of the P450-4 and P450-1 genes from MP-D, GUS reporter gene assays using the bidirectional promoter region of P450-1/4 (D) in both directions were performed. The results clearly demonstrated that only transformants with the pDP4::GUS construct [P450-4 (D) promoter] showed high expression of the uidA gene, whereas transformants with the pDP1::GUS construct [P450-1 (D) promoter] never reached the expression level of pDP4::GUS transformants in either the MP-C or the MP-D background. These data indicate that the mutation upstream of the P450-1 (D) start codon affected the transcription of this gene much more than that of P450-4 (D). A slight blue staining on agar plates could be observed only for some high-copy transformants of pDP1::GUS after a long incubation period. Thus, the mutation in one of the two essential GATA elements of the F. proliferatum P450-1/P450-4 bidirectional promoter can explain the low expression of these genes, particularly of P450-1.
For some transformants, no expression was detected in plate assays, although the whole promoter-uidA fusion construct was correctly integrated into the genome, again indicating an important role of the integration site for the expression level. In A. parasiticus, similar GUS reporter gene assays with the aflatoxin biosynthetic genes ver1 and nor1 clearly demonstrated that only integration into the homologous site resulted in expression of the fused uidA gene. Single-copy integration into the niaD or pyrG locus led to a 500-fold reduction of the ver1 and nor1 promoter activity compared to that of a single integration at the corresponding homologous loci (8).
In addition to sequence changes in the 5′ noncoding regions, we found several mutations in the coding regions of P450-4 (D) and P450-1 (D). For P450-1 (D), these mutations resulted in amino acid substitutions, mainly at the N terminus of the enzyme, whereas mutations in P450-4 (D) are distributed over the entire protein. For functionality of cytochrome P450 monooxygenases, the hydrophobic N-terminal transmembrane domain is known to be essential for anchoring to the endoplasmic reticulum membrane and interaction with the electron-donating cytochrome P450 reductase (CPR) (44) and also for its interaction with other P450s (1). Mutations in P450-1 (D) resulted in a more hydrophilic N terminus than those in P450-1 (C) and probably resulted in weaker contact to the endoplasmic reticulum membrane and consequently in lower enzyme activity due to less efficient interaction with CPR.
Incubations of radiolabeled ent-kaurenoic acid or GA12-aldehyde with cultures or microsomal fractions of MP-D confirmed that P450-1 (D) was enzymatically functional but with a much lower activity than that of P450-1 (C). The enzymatic activity of P450-1 (D) is higher in the MP-C background, indicating that mutations in an as-yet-unidentified GA-specific regulator could at least be partially involved in reduction of P450-1 enzyme activity in MP-D. The fact that P450-1 (C) is not as active in MP-D as it is in its own background supports this suggestion.
In contrast to P450-1 (D), P450-4 (D) shows no functionality at all in either the MP-D or MP-C background, although the gene is expressed more highly than P450-1 (D). In recent studies, it has been shown that even single amino acid substitutions can be crucial for functionality of the entire enzyme. For the cytochrome P450 monooxygenase P4503 of the GA biosynthesis pathway, a single amino acid substitution from arginine to tryptophan in strain 6314 (MP-C) resulted in a nonfunctional protein and therefore a lack of GA3 production (40). In A. parasiticus, single amino acid substitutions in the cytochrome P450 monooxygenase OrdA, involved in aflatoxin biosynthesis, also resulted in loss of function due either to incorrect folding of the protein or, more likely, to the lost capability of substrate binding (46). For the cytochrome P450 family 2 (cyp2), it was demonstrated that putative substrate recognition sites are dispersively located along the primary structure and constitute about 16% of the total residues (14). In addition, some P450 enzymes were found to form complexes that can be either homo- or heteromeric and can lead to additive, synergistic, or also inhibitory effects (reviewed in reference 1). As amino acid substitutions in P450-4 (D) are distributed over the whole predicted protein sequence, the loss of enzymatic function might be the consequence of disorderd interaction between the enzyme and the substrate, disordered interaction between different P450s, incorrect protein folding, and/or inappropriate P450 complex formation, which subsequently would lead to an inhibitory effect and a nonfunctional enzyme. In addition, a disadvantageous chromosomal location of the GA gene cluster in MP-D and the resulting weaker expression can also not be excluded.
One reason for the loss of GA production could also be a mutated gene copy of cpr-Gf, encoding the cytochrome P450 oxidoreductase (23). Deletion of cpr-Gf in the GA-producing strain IMI58289 resulted in a loss of the ability to produce GA4, GA7, and GA3. However, recent sequence analysis of cpr genes from all MPs revealed no significant sequence differences, indicating a much lower mutation rate in this important gene in comparison to the dispensable GA cluster genes and full functionality in these species (Malonek and Tudzynski, unpublished). Additionally, loss of CPR activity would be likely to affect both P450-1 (D) and P450-4 (D) enzymes and would also affect the growth of the fungus, as was previously shown for MP-C (23).
Therefore, molecular and biochemical analysis of GA biosynthesis in MP-D has revealed that the loss of the ability to produce GAs is the result of multiple mutations in the GA biosynthesis genes, in their 5′ noncoding regions, and, possibly, also in some GA-specific regulators. Interestingly, gene mutations and the resulting loss of enzyme activity differ between single strains of MP-D, demonstrating gene diversity even within one species. Whereas both MP-D strains have a block at the enzymatic level of the ent-kaurene oxidase, we found an additional block at GA4 desaturase only in strain D00502, since [14C]GA4 and [14C]GA1 were produced from ent-[14C]kaurenoic acid or [14C]-GA12-aldehyde rather than [14C]GA3. The same spectrum of products was detected in des (C) gene replacement mutants (40).
Our latest data also imply an enzymatic block at the initial step(s) of the biosynthesis pathway, as no ent-kaurene accumulated in MP-D strains, suggesting mutations in the genes ggs2, encoding the pathway-specific geranylgeranyl diphosphate synthase, and/or cps/ks, encoding the ent-copalyl diposphate/ent-kaurene synthase (Malonek and Tudzynski, unpublished).
As we have shown recently, four MPs of the G. fujikuroi species complex apart from MP-D (MP-B, MP-E, MP-F, and MP-G) contain the entire GA cluster but do not produce any GAs. In two other MPs (MP-A and MP-H), only two and one GA genes, respectively, remain. It would be instructive to undertake a detailed characterization of the GA genes in these MPs also, in order to determine the putative pattern of inheritance of the GA gene cluster during evolution. A previous comparison of gibberellin biosynthesis in plants and F. fujikuroi disproved the hypothesis of horizontal gene transfer between higher plants and fungi and confirmed the suggestion that both organisms have evolved the GA biosynthesis pathways separately early during evolution (15, 16). The general question is why organisms retain such large genetic units, such as gene clusters, that do not have an obvious function in the organism. If such cooperating genes are conditionally dispensable but have adaptive value for colonizing certain ecological niches, the incipient cluster would be maintained by positive selection in those environments (19). However, to answer this question, it would be necessary to investigate the importance of GA production for the fungus itself, e.g., during the infection of rice seedlings. At least for F. fujikuroi (MP-C) strains, it is conceivable that GA production aids pathogenicity on rice plants, whereas GA nonproducers, such as F. proliferatum (MP-D) and Fusarium verticillioides (MP-A), which are also occasionally isolated from infected rice plants, are probably not involved in symptom development of bakanae disease.
This work reflects important steps of a just-started comprehensive investigation of evolutionary aspects in secondary metabolism. The detailed genetic and biochemical analysis of the GA biosynthesis pathway in members of MP-D provides an important and useful basis for the future study of the evolutionary development of GA cluster genes in GA-producing fungi in general. As not only eukaryotes, such as plants and fungi, but also bacteria are capable of producing GAs, it will be interesting to determine whether there is a common origin of GA biosynthesis. Thus, in comparing results from organisms of different kingdoms, it will be interesting to discover if there is any common trend of inheritance of complex gene units such as the secondary metabolism clusters.
Acknowledgments
We thank John Leslie (Kansas State University) for providing us the MP-D tester strains of the G. fujikuroi species complex.
The project was supported by the DFG (Tu 101-9) and Fondo Nacional de Desarrollo Cientifico y Tecnologico (grant 1020140). Rothamstead Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the United Kingdom.
REFERENCES
- 1.Backes, W. L., and R. W. Kelley. 2003. Organization of multiple cytochrome P450s with NADPH-cytochrome P450 reductase in membranes. Pharmacol. Ther. 98:221-233. [DOI] [PubMed] [Google Scholar]
- 2.Bacon, C. W., J. K. Porter, W. P. Norred, and J. F. Leslie. 1996. Production of fusaric acid by Fusarium species. Appl. Environ. Microbiol. 62:4039-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barendse, G. W. M., P. H. van de Werken, and N. Takahashi. 1989. High-performance liquid chromatography of gibberellins. J. Chromatogr. 198:449-455. [Google Scholar]
- 4.Bhatnagar, D., K. C. Ehrlich., and T. E. Cleveland. 2003. Molecular genetic analysis and regulation of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 61:83-93. [DOI] [PubMed] [Google Scholar]
- 5.Britz, H., T. A. Coutinho, M. J. Wingfield, W. F. O. Marasas, T. R. Gordon, and J. F. Leslie. 1999. Fusarium subglutinans f. sp. pini represents a distinct mating population in the Gibberella fujikuroi species complex. Appl. Environ. Microbiol. 65:1198-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, D. W., S. P. McCormick, N. Alexander, R. H. Proctor, and A. E. Desjardins. 2001. A genetic and biochemical approach to study trichothecene diversity in Fusarium sporotrichioides and Fusarium graminearum. Fungal Genet. Biol. 32:121-133. [DOI] [PubMed] [Google Scholar]
- 7.Chang, P.-K., and J. Yu. 2002. Characterization of a partial duplication of the aflatoxin gene cluster in Aspergillus parasiticus ATCC 56775. Appl. Microbiol. Biotechnol. 58:632-636. [DOI] [PubMed] [Google Scholar]
- 8.Chiou, C.-H., M. Miller, D. L. Wilson, F. Trail, and J. E. Linz. 2002. Chromosomal location plays a role in regulation of aflatoxin gene expression in Aspergillus parasiticus. Appl. Environ. Microbiol. 68:306-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Church, G. M., and W. Gilbert. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. USA 81:1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desjardins, A. E., R. D. Plattner, T. C. Nelsen, and J. F. Leslie. 1995. Genetic analysis of fumonisin production and virulence of Gibberella fujikuroi mating population A (Fusarium moniliforme) on maize (Zea mays) seedlings. Appl. Environ. Microbiol. 61:79-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle, J. J., and J. L. Doyle. 1990. Isolation of plant DNA from fresh tissue. Focus 12:13-15. [Google Scholar]
- 12.Ehrlich, K. C., and P. J. Cotty. 2004. An isolate of Aspergillus flavus used to reduce aflatoxin contamination in cottonseed has a defective polyketide synthase gene. Appl. Microbiol. Biotechnol. 65:473-478. [DOI] [PubMed] [Google Scholar]
- 13.Geissman, T. A., A. J. Verbiscar, B. O. Phinney, and G. Cragg. 1966. Studies on the biosynthesis of gibberellins from (−)-kaurenoic acid in cultures of Gibberella fujikuroi. Phytochemistry 5:933-947. [Google Scholar]
- 14.Gotoh, O. 1992. Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J. Biol. Chem. 267:83-90. [PubMed] [Google Scholar]
- 15.Hedden, P., and Y. Kamiya. 1997. Gibberellin biosynthesis: enzymes, genes and their regulation. Annu. Rev. Plant Physiol. Plant Pathol. 48:431-460. [DOI] [PubMed] [Google Scholar]
- 16.Hedden, P., A. L. Phillips, M. C. Rojas, E. Carrera, and B. Tudzynski. 2001. Gibberellin biosynthesis in plants and fungi: a case of convergent evolution? J. Plant Growth Regul. 20:319-331. [DOI] [PubMed] [Google Scholar]
- 17.Kerényi, Z., K. Zeller, L. Hornok, and J. F. Leslie. 1999. Standardization of mating type terminology in the Gibberella fujikuroi species complex. Appl. Environ. Microbiol. 65:4071-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krügel, H., G. Fiedler, C. Smith, and S. Baumberg. 1993. Sequence and transcriptional analysis of the nourseothricin acetyltransferase-encoding gene nat1 from Streptomyces noursei. Gene 127:127-131. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence, J. G. 1997. Selfish operons and speciation by gene transfer. Trends Microbiol. 5:355-359. [DOI] [PubMed] [Google Scholar]
- 20.Leslie, J. F. 1995. Gibberella fujikuroi: available populations and variable traits. Can. J. Bot. 73:S282-S291. [Google Scholar]
- 21.Leslie, J. F., W. F. O. Marasa, G. S. Shephard, E. W. Sydenham, S. Stockenström, and P. G. Thiel. 1996. Duckling toxicity and the production of fumonisin and moniliformin by isolates in the A and F mating populations of Gibberella fujikuroi (Fusarium moniliforme). Appl. Environ. Microbiol. 62:1182-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logrieco, A., A. Moretti, G. Castella, M. Kostecki, P. Golinski, A. Ritieni, and J. Chelkowski. 1998. Beauvericin production by Fusarium species. Appl. Environ. Microbiol. 64:3084-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malonek, S., M. C. Rojas, P. Hedden, P. Gaskin, P. Hopkins, and B. Tudzynski. 2004. The NADPH-cytochrome P450 reductase gene from Gibberella fujikuroi is essential for gibberellin biosynthesis. J. Biol. Chem. 279:25075-25084. [DOI] [PubMed] [Google Scholar]
- 24.Maniatis, T., J. Sambrook, and E. F. Fritsch. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Mihlan, M., V. Homann, T.-W. D. Liu, and B. Tudzynski. 2003. AREA directly mediates nitrogen regulation of gibberellin biosynthesis in Gibberella fujikuroi, but its activity is not affected by NMR. Mol. Microbiol. 47:975-991. [DOI] [PubMed] [Google Scholar]
- 26.Nirenberg, H. I., and K. O'Donnell. 1998. New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia 90:434-458. [Google Scholar]
- 27.Nirenberg, H. I., K. O'Donnell, J. Kroschel, A. P. Andrianaivo, J. M. Frank, and W. Mubatanhema. 1998. Two new species of Fusarium: Fusarium brevicatenulatum from the noxious weed Striga asiatica in Madagascar and Fusarium speudoanthophilum from Zea mays in Zimbabwe. Mycologia 90:459-464. [Google Scholar]
- 28.O'Donnell, K., E. Cigelnik, and H. I. Nirenberg. 1998. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90:465-493. [Google Scholar]
- 29.Pontecorvo, G. V., J. A. Poper, L. M. Hemmonns, K. D. MacDonald, and A. W. J. Buften. 1953. The genetics of Aspergillus nidulans. Adv. Genet. 141:141-238. [DOI] [PubMed] [Google Scholar]
- 30.Punt, P. J., R. P. Oliver, M. A. Dingemanse, P. H. Pouwels, and C. A. M. J. J. van den Hondel. 1987. Transformation of Aspergillus nidulans based on the hygromycin B resistance marker from Escherichia coli. Gene 56:117-124. [DOI] [PubMed] [Google Scholar]
- 31.Punt, P. J., J. Strauss, R. Smit, J. R. Kinghorn, C. A. M. J. J. van den Hondel, and C. Scazzocchio. 1995. The intergenic region between the divergently transcribed niiA and niaD genes of Aspergillus nidulans contains multiple NirA binding sites which act bidirectionally. Mol. Cell. Biol. 15:5688-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rademacher, W. 1997. Gibberellins, p. 193-205. In T. Anke (ed.), Fungal biotechnology. Chapman & Hall, London, United Kingdom.
- 33.Rojas, M. C., P. Hedden, P. Gaskin, and B. Tudzynski. 2001. The P450-1 gene of Gibberella fujikuroi encodes a multifunctional enzyme in gibberellin biosynthesis. Proc. Natl. Acad. Sci. USA 98:5828-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snoeijers, S. S., A. Perez-Garcia, T. Goosen, and P. J. G. M. De Wit. 2003. Promotor analysis of the avirulence gene Avr9 of the fungal tomato pathogen Cladosporium fulvum in the model filamentous fungus Aspergillus nidulans. Curr. Genet. 43:96-102. [DOI] [PubMed] [Google Scholar]
- 35.Steenkamp, E. T., B. D. Wingfield, T. A. Coutinho, M. J. Wingfield, and W. F. O. Marasas. 1999. Differentiation of Fusarium subglutinans f. sp. pini by histone gene sequence data. Appl. Environ. Microbiol. 65:3401-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tudzynski, B., and K. Hölter. 1998. The gibberellin biosynthetic pathway in Gibberella fujikuroi: evidence for a gene cluster. Fungal Genet. Biol. 25:157-170. [DOI] [PubMed] [Google Scholar]
- 37.Tudzynski, B., V. Homann, B. Feng, and G. A. Marzluf. 1999. Isolation, characterization and disruption of the areA nitrogen regulatory gene of Gibberella fujikuroi. Mol. Gen. Genet. 261:106-114. [DOI] [PubMed] [Google Scholar]
- 38.Tudzynski, B., P. Hedden, E. Carrera, and P. Gaskin. 2001. The P450-4 gene of Gibberella fujikuroi encodes ent-kaurene oxidase in the gibberellin biosynthetic pathway. Appl. Environ. Microbiol. 67:3514-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tudzynski, B., M. C. Rojas, P. Gaskin, and P. Hedden. 2002. The Gibberella fujikuroi gibberellin 20-oxidase is a multifunctional monooxygenase. J. Biol. Chem. 277:21246-21253. [DOI] [PubMed] [Google Scholar]
- 40.Tudzynski, B., M. Mihlan, M. C. Rojas, P. Linnemannstöns, P. Gaskin, and P. Hedden. 2003. Characterization of the final two genes of the gibberellin biosynthesis gene cluster of Gibberella fujikuroi: des and P450-3 encode GA4 desaturase and the 13-hydroxylase, respectively. J. Biol. Chem. 278:28635-28643. [DOI] [PubMed] [Google Scholar]
- 41.Tudzynski, P., and B. Tudzynski. 1996. Genetics of phytopathogenic fungi. Prog. Bot. 57:235-252. [Google Scholar]
- 42.Tudzynski, P., and B. Tudzynski. 2000. Genetics of plant pathogenic fungi: genetic aspects of plant-pathogen interaction. Prog. Bot. 61:118-147. [Google Scholar]
- 43.Urrutia, O., P. Hedden, and M. C. Rojas. 2000. Monooxygenases involved in GA12 and GA14 synthesis in Gibberella fujikuroi. Phytochemistry 56:505-511. [DOI] [PubMed] [Google Scholar]
- 44.Van den Brink, H. J. M., R. F. M. van Gorcom, C. A. M. J. J. van den Hondel, and P. J. Punt. 1998. Cytochrome P450 enzyme systems in fungi. Fungal Genet. Biol. 23:1-17. [DOI] [PubMed] [Google Scholar]
- 45.van den Broek, P., A. Pittet, and H. Hajjaj. 2001. Aflatoxin genes and the aflatoxigenic potential of Koji moulds. Appl. Microbiol. Biotechnol. 57:192-199. [DOI] [PubMed] [Google Scholar]
- 46.Yu, J., P.-K. Chang, K. C. Ehrlich, J. W. Cary, B. Montalbano, J. M. Dyer, D. Bhatnagar, and T. E. Cleveland. 1998. Characterization of the critical amino acids of an Aspergillus parasiticus cytochrome P-450 monooxygenase encoded by ordA that is involved in the biosynthesis of aflatoxins B1, G1, B2, and G2. Appl. Environ. Microbiol. 64:4834-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeller, K. A., B. A. Summerell, and J. F. Leslie. 2003. Gibberella konza (Fusarium konzum) sp. nov. from prairie grasses, a new species in the Gibberella fujikuroi species complex. Mycologia 95:943-954. [PubMed] [Google Scholar]