Abstract
Proton pump inhibitors (PPIs) are commonly prescribed medications. The existing data suggest that individuals at a high risk of fractures have been exposed to high doses of PPIs for prolonged durations. CYP2C19 plays a pivotal role in metabolism of PPIs and thereby influences their pharmacokinetic profile. Hence, we hypothesize that CYP2C19 genotypes may be associated with fragility fracture among PPIs users due to PPI exposure. This study aimed to investigate the association between CYP2C19 genotypes, bone mineral density (BMD), and osteoporotic fracture in a hospital‐based population. This retrospective cohort study enrolled patients who were prescribed long‐term PPIs at Taichung Veterans General Hospital using data extracted from the Taiwan Precision Medicine Initiative between January 2010 and April 2021. Associations between CYP2C19 phenotypes, comorbidities, and fractures in PPI users were analyzed. We enrolled 1518 long‐term PPI users; 571 (38%), 727 (48%), and 220 (14%) CYP2C19 normal metabolizers (NMs), intermediate metabolizers (IMs), and poor metabolizers (PMs), respectively. Among them, 49 (3.2%) patients developed fractures within the 1‐year follow‐up period; 20 (3.5%) fractures in NMs, 24 (3.3%) in IMs, and 5 (2.3%) in PMs, respectively. No significant difference was observed among CYP2C19 genotypes and fracture. Additionally, BMD measurements during the 1‐year follow‐up period were made available among 75 participants. No significant difference in BMD between CYP2C19 PMs and non‐PMs was found. This real‐world, hospital‐based study concludes that CYP2C19 PMs/IMs are not associated with an increased risk for fractures or reduced BMD in individuals on long‐term PPI therapy.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Proton pump inhibitors (PPIs) are metabolized by the polymorphic CYP2C19 enzyme classified into normal metabolizers (NMs), intermediate metabolizers (IMs), and poor metabolizers (PMs). The association between CYP2C19 genotypes, osteoporotic fractures, and bone mineral density (BMD) remains indeterminate among long‐term PPI users in the Han Chinese population.
WHAT QUESTION DID THIS STUDY ADDRESS?
Is there an association between CYP2C19 genotypes, risk of fractures, and BMD in long‐term PPI users in Taiwan?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
CYP2C19 PMs/IMs do not confer an increased risk for fractures or diminished BMD in individuals who have utilized PPIs for extended periods.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Our findings indicate that long‐term PPI therapy across CYP2C19 metabolic phenotypes appears to be safe for fracture risk and BMD. Additional studies are still required in order to validate our findings.
INTRODUCTION
Proton pump inhibitors (PPIs) are potent acid suppressive agents which are commonly used for peptic ulcer disease, symptomatic gastroesophageal reflux disease, Barrett's esophagus, and the prevention of non‐steroidal anti‐inflammatory drug (NSAID)‐induced ulcers. 1 The US Food and Drug Administration has reported a possible increased risk in fractures of the hip, wrist, and spine through the use of PPIs, which may be associated with dose, duration of use, or both. 2 A retrospective cohort study issued a report claiming that PPI use after stroke may increase the risk of osteoporosis, hip fracture, and vertebral fracture, with the risk being related to the dose. 3 In a nested case–control study, it was reported that individuals who received PPI therapy for more than 1 year had an estimated crude incidence rate of 4.0 hip fractures per 1000 person‐years. In contrast, among those who did not utilize acid suppression medications, the crude incidence rate was 1.8 hip fractures per 1000 person‐years. 4 A meta‐analysis of observational studies revealed that individuals taking a low PPI dosage corresponded to a 17% increased risk of hip fracture, a medium dosage corresponded to a 29% increased risk, and a high dosage corresponded to a 30% increased risk. 5 A population‐based case–control study observed that there was a trend of increasing hip fracture risk in correlation with higher cumulative defined daily doses (DDDs). This study provided evidence that the association between hip fracture and PPI use followed a dose–response pattern. 6 A meta‐analysis reported a reduction in the mean bone mineral density (BMD) of PPI users. 7 In addition, a retrospective cohort study found an increased risk of osteoporosis‐related fracture due to long‐term PPI use. 8 However, the results from another study did not support the connection between long‐term PPI use and bone‐related adverse events. 9
The actions of PPIs are related to variations in pharmacokinetic parameters. The mechanism leading this variability is multifactorial, including genetic and non‐genetic factors which affect the exposure of PPIs, with the former considered to be more important. 10 The non‐genetic factors are mainly related to a patient's medication habits, such as timing and any co‐administration with other anti‐secretory agents. The genetic factors associated with the pharmacokinetic properties of PPIs include cytochrome P450 2C19 (CYP2C19) and cytochrome P450 3A4 (CYP3A4), predominantly the former. 10 PPIs are metabolized via hepatic cytochrome P450 enzymes into inactive metabolites, with most of them being metabolized primarily by CYP2C19. 10 The genotype of CYP2C19 in patients significantly contributes to the interindividual variability in the pharmacokinetics of PPIs. 11 Moreover, the drug response and pharmacokinetic parameters of PPI users are also dependent on the CYP2C19 genotype which can be classified as normal metabolizers (NMs), intermediate metabolizers (IMs), and poor metabolizers (PMs). CYP2C19 IMs and PMs showed 3–14 times higher area under the curve and 2–6 times higher maximal plasma drug concentrations after receiving standard doses of first‐generation PPIs compared to CYP2C19 NMs. 11 , 12 The first‐generation PPIs of omeprazole, lansoprazole, and pantoprazole, as well as the second‐generation PPI dexlansoprazole, are metabolized by CYP2C19 at an approximate 80% rate. Conversely, metabolism of the second‐generation PPIs esomeprazole and rabeprazole are less dependent on CYP2C19. Furthermore, it has been proposed that a reduction in the dosage of first‐generation PPIs, including omeprazole, lansoprazole, pantoprazole, and dexlansoprazole, may be warranted for patients who are identified as CYP2C19 IMs or PMs and require prolonged therapy. This would be done in order to mitigate any potential for adverse events, such as electrolyte imbalances, infections, kidney disease and bone fractures. 10 , 12
Previous investigations have suggested that the correlation between osteoporosis, fractures, and the utilization of PPIs may be contingent upon the dosage administered. 2 , 3 , 8 CYP2C19 IMs and PMs may also exhibit higher drug exposure than NMs. 12 Although Asians experience considerably greater frequencies (ranging from 14% to 20%) of PMs in comparison to individuals of Caucasian, Black, Middle Eastern, Native, and Admixed descent, 13 the presence of a possible correlation between CYP2C19 genotypes and osteoporotic fractures remains indeterminate. Our study aimed to investigate the association between CYP2C19 genotype, risk of fractures, and BMD in long‐term PPI users in Taiwan.
METHODS
Study design
This retrospective cohort study was performed in a medical center in central Taiwan and was approved by the Ethics Committee of Taichung Veterans General Hospital (CE20316A). This study was conducted following the principles of the Declaration of Helsinki.
Study population
The study population was extracted from the Taiwan Precision Medicine Initiative (TPMI), a nationwide project led by the Academia Sinica and a partner hospital. Patients who visited the outpatient department at Taichung Veterans General Hospital were invited to participate in the TPMI. Genetic profiles of the participants were linked to electronic health records held in Taichung Veterans General Hospital.
Participants who were continuously prescribed selected PPIs in the outpatient department over a period of 336 days from January 2010 to April 2021 were enrolled. Within the framework of the Taiwan National Health Insurance system, the prescription cycle for chronic medication adheres to a 28‐day schedule. Consequently, a span of 336 days covers 12 cycles of medication administration. The 336th day of their continuous PPI prescription was defined as the index date. If the PPI prescription was interrupted for more than 28 days, it was considered to be discontinuous, so the initial day of PPI exposure must be restarted. The selected PPIs were omeprazole, pantoprazole, lansoprazole, and dexlansoprazole which metabolized mainly through the CYP2C19 enzyme. In total, 4% participants with undefined CYP2C19 phenotype if the genotyping experiments failed to meet the established quality control standard were excluded. 14
Data collection
PPI exposure during the 336 days period were present as cumulative defined daily dose (DDD). The World Health Organization (WHO) has established the DDD recommendations for PPIs as follows: omeprazole with a DDD of 20 mg, pantoprazole with a DDD of 40 mg, lansoprazole with a DDD of 30 mg, and dexlansoprazole with a DDD of 30 mg. 15
Classification of comorbidities is based on the International Classification of Disease, 9th Revision, Clinical Modification (ICD‐9‐CM) and 10th edition (ICD‐10) diagnostic code. Data, including age at index date, gender, hypertension (401, 402, 403, 404/I10, I11, I12, I13), diabetes (250/E08, E09, E10, E11, E13), and stroke (433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91/I63) were all collected as baseline characteristics. Data regarding any underlying diseases prior to index date which may be correlated with a fracture event were collected, including alcohol abuse (305.00–305.03, 303.90–303.93/F10.1, F10.2), osteoporosis (733/M80‐M81), and rheumatoid arthritis (714/M05, M06). Systemic corticosteroid and therapy for the treatment of osteoporosis prescribed within 1 year before the index date were also collected. This study had a 1‐year follow‐up period after the index date. The fracture events (820–829) which occurred within 1 year after the index date were surveyed. Osteoporotic fractures (805, 806, 812, 813, 820, 821) were fractures related to osteoporosis, and included vertebral fractures, hip fractures, and distal radius fractures. Participants with fractures due to vehicular accidents or high‐impact trauma (E810–E819, E881–E883, E884), pathological fractures (733.14, 733.15/M84), and those with a diagnosis of Paget's disease (731.0/M88) were all excluded.
Dual‐energy X‐ray absorptiometry (DXA) was used to measure the BMD of the lumbar spine (L1‐L4) and bilateral femoral necks, using the Lunar Prodigy (General Electric, Fairfield, CT, USA), with the outcomes expressed in g/cm2. The least significant changes for the lumbar spine (L1‐L4) and femoral neck were ± 0.010 g/cm2 and ± 0.012 g/cm2, respectively. T‐scores were determined in accordance with the manufacturer's reference data. Based on the WHO criteria, osteoporosis is defined as a T‐score ≦ −2.5; low bone mass is defined as a T‐score between −1.0 and −2.5; and normal is defined as a T‐score > −1.0. 16
CYP2C19 genotyping and phenotyping
Genotyping was performed using the Taiwan Biobank version 2 array (TWBv2 array), a single nucleotide polymorphism (SNP) array design for the Taiwanese population which utilized whole‐genome sequencing data from Taiwan Biobank participants to choose SNPs optimized for imputation in Han Chinese samples. Methods for genotyping have been described in previous studies. 14 Rs4244285(*2), rs4986893(*3), and rs72552267(*6) were analyzed and defined as loss‐of‐function variants. Rs11188072 (*17) were analyzed and defined as increased function variants. Patients with two loss‐of‐function alleles were labeled PM, whereas patients with one loss‐of‐function allele were labeled IM. Patients without loss‐of‐function allele but with one increased function alleles were labeled rapid metabolizer (RM) initially. Due to the small patient number of RM and small impact of RM on PPI, we categorized this group of patients as NM in the analysis. Patients without these four variants were labeled as NM. Patients were then divided into PM and non‐PM (IM and NM) groups.
Statistical analysis
Data are presented as numbers (percentages) or mean ± standard deviation. Comparisons were made using the Student's t‐test and ANOVA test. Categorical data are presented as quantities and percentages and compared using either the chi‐square or Fisher's exact test, where appropriate. Multivariate logistic regression was used to investigate the associations of fractures with phenotypes, patient demographics, comorbidities, and medication. Furthermore, the fracture risk of different CYP2C19 phenotypes was analyzed after adjusting for age and gender. To describe the frequency of fracture events according to time since PPI initiation, we constructed Kaplan–Meier curves of cumulative fracture events, stratified according to CYP2C19 phenotype. A p‐value≤0.05 was considered statistically significant. All data were analyzed using the Statistical Package for Social Sciences version 22.0 (SPSS, IBM Corp., Armonk, NY, USA).
RESULTS
Patient characteristics by CYP2C19 phenotypes
From the 58,091 patients enrolled in the TPMI, 1518 participants who had PPI exposure for more than 336 days between January 2010 and April 2021 were enrolled (Figure 1). Among the 1518 long‐term PPI users, 75 participants with a BMD measurement at 1‐year follow‐up were further selected. Among the 1518 long‐term PPI users, there were 571 (38%) NMs, 727 (48%) IMs, and 220 (14%) PMs (Table S1). The distribution of each phenotype was similar to that seen in the general population. The patient characteristics are summarized in Table 1. The average age was 61.3 ± 13.5 years, and 44.9% of PPI users were female. Cumulative defined daily PPI dose was 371 ± 106 DDD in our cohort. Cumulative defined daily PPI dose in NM, IM, and PM were 366 ± 102 DDD, 373 ± 107 DDD, and 376 ± 110 DDD in 336 days, respectively, with no differences among genotypes (p = 0.416). The PPI exposure after the index was 7.9 months on average. No significant differences in age, alcohol consumption, or comorbidities could be observed among the groups.
FIGURE 1.
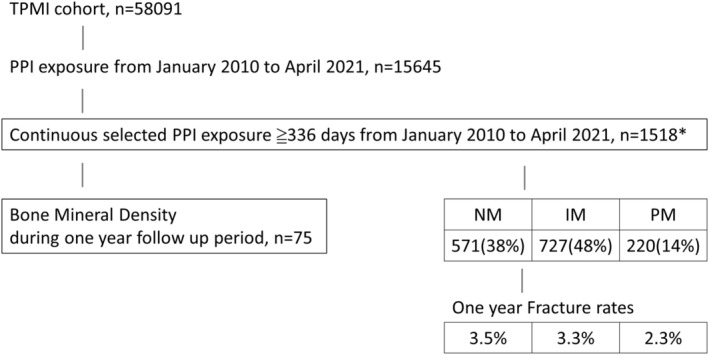
Flow diagram of the participant recruitment process. *Selected PPI: omeprazole, pantoprazole, lansoprazole, and dexlansoprazole. IM, intermediated metabolizer; NM, normal metabolizer; PM, poor metabolizer; PPI, proton pump inhibitor; TPMI, Taiwan Precision Medicine Initiative.
TABLE 1.
Baseline characteristics and 1‐year fracture rates of proton pump inhibitor users by CYP2C19 genotype.
| Characteristic | Total (n = 1518) | Genotype | P value | ||
|---|---|---|---|---|---|
| NM (n = 571) | IM (n = 727) | PM (n = 220) | |||
| Age (years) | 61.3 ± 13.5 | 61.4 ± 14.2 | 61.0 ± 13.0 | 62.1 ± 13.4 | 0.577 |
| Female | 681 (44.9%) | 265 (46.4%) | 317 (43.6%) | 99 (45.0%) | 0.601 |
| Cumulative defined daily PPI dose | 371 ± 106 | 366 ± 102 | 373 ± 107 | 376 ± 110 | 0.416 |
| Osteoporosis | 173 (11.4%) | 61 (10.7%) | 89 (12.2%) | 23 (10.5%) | 0.608 |
| Rheumatoid arthritis | 99 (6.5%) | 32 (5.6%) | 51 (7.0%) | 16 (7.3%) | 0.527 |
| Type 2 diabetes mellitus | 469 (30.9%) | 178 (31.2%) | 219 (30.1%) | 72 (32.7%) | 0.752 |
| Hypertension | 689 (45.4%) | 257 (45.0%) | 327 (45.0%) | 105 (47.7%) | 0.753 |
| Stroke | 70 (4.6%) | 25 (4.4%) | 37 (5.1%) | 8 (3.6%) | 0.630 |
| Medication history | |||||
| Therapy for osteoporosis | 60 (4.0%) | 24 (4.2%) | 28 (3.9%) | 8 (3.6%) | 0.918 |
| Systemic corticosteroid | 336 (22.1%) | 132 (23.1%) | 155 (21.3%) | 49 (22.3%) | 0.740 |
| PPI exposure after index (months) | 7.9 ± 4.1 | 8 ± 4.1 | 7.93 ± 4.1 | 7.85 ± 4.2 | 0.948 |
| Fractures | 49 (3.2%) | 20 (3.5%) | 24 (3.3%) | 5 (2.3%) | 0.673 |
| Osteoporotic fractures | 24 (1.6%) | 8 (1.4%) | 13 (1.8%) | 3 (1.4%) | 0.591 |
Note: Data are presented as number (percentage) and mean ± SD.
Abbreviations: IM, intermediated metabolizer; NM, normal metabolizer; PM, poor metabolizer; PPI, proton pump inhibitor; SD, standard deviation.
CYP2C19 genotypes and risk of fracture in long‐term PPI users
Among the 1518 long‐term PPI users, 49 (3.2%) patients developed fracture within the 1 year follow up period, while 24 of those 49 (49%) were categorized as having experienced osteoporotic fractures. There were 20 (3.5%) fractures in NMs, 24 (3.3%) in IMs, and 5 (2.3%) in PMs (Figure 2). Among those with fracture, 40% of NMs, 54.17% of IMs and 60.00% of PMs experienced osteoporotic fractures. No significant difference between CYP2C19 phenotype and fractures rates was observed (Table 1).
FIGURE 2.

Fracture rate after 1 year of proton pump inhibitor (PPI) exposure. IM, intermediate metabolizer; NM, normal metabolizer; PM, poor metabolizer.
To determine the risk factors for fractures among long‐term PPI users, Cox regression analysis was performed (Table 2). In our cohort, 11.4% of the patients were diagnosed with osteoporosis prior to their index date, while 35% of them were prescribed medication for osteoporosis. Due to the strong correlation between osteoporosis and anti‐osteoporosis medications, only the diagnosis of osteoporosis was incorporated in Cox regression analysis. Age and osteoporosis were associated with fracture risk, while gender, cumulative defined daily PPI dose, comorbidities and therapies for osteoporosis were not correlated with fracture. Additionally, no significant correlation was observed between fracture risk and the presence of CYP2C19 IMs (HR = 0.93; 95% CI, 0.52–1.69; p = 0.822) or PMs (HR = 0.64; 95% CI, 0.24–1.72; p = 0.378) when compared to NMs.
TABLE 2.
Factors associated with fractures in long‐term proton pump inhibitor users.
| Factor | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Phenotype | ||||||
| NM | Reference | |||||
| IM | 0.93 | (0.52–1.69) | 0.822 | |||
| PM | 0.64 | (0.24–1.72) | 0.378 | |||
| Age (years) | 1.05 | (1.02–1.07) | <0.001** | 1.04 | (1.01–1.06) | 0.003** |
| Female | 1.96 | (1.10–3.48) | 0.022* | 1.62 | (0.88–2.97) | 0.119 |
| Cumulative defined daily PPI dose | 1.00 | (1.00–1.00) | 0.357 | |||
| Osteoporosis | 4.27 | (2.37–7.69) | <0.001** | 2.92 | (1.56–5.48) | 0.001** |
| Rheumatoid arthritis | 1.28 | (0.46–3.56) | 0.634 | |||
| Type 2 diabetes mellitus | 1.56 | (0.88–2.75) | 0.128 | |||
| Hypertension | 2.29 | (1.27–4.12) | 0.006** | 1.74 | (0.95–3.18) | 0.072 |
| Stroke | 1.35 | (0.42–4.33) | 0.618 | |||
| Medication history | ||||||
| Therapy for osteoporosis | 3.58 | (1.52–8.41) | 0.003** | |||
| Systemic corticosteroid | 1.57 | (0.85–2.88) | 0.147 | |||
Note: Data are presented as number (percentage) and mean ± SD. *p value<0.05, **p value<0.01.
Abbreviations: CI, confidence interval; HR, hazard ratio; IM, intermediate metabolizer; NM, normal metabolizer; PM, poor metabolizer; SD, standard deviation.
After adjustment for age and gender, Cox regression analysis using CYP2C19 genotypes was conducted in individuals who had been on long‐term PPI therapy (Figure 3). Patients previously diagnosed with osteoporosis (HR = 3.9, 95% CI, 1.4–10.6; p = 0.009) and patients with hypertension (HR = 2.9, 95% CI, 1.0–8.2; p = 0.04) would have higher fracture risk in the NM subgroup. Nevertheless, such associations were not evident among the individuals possessing IM or PM genotypes. The cumulative defined daily PPI dose (HR = 0.96, 95% CI, 0.93–1.00; p = 0.034) was correlated with lower fracture risk in the PM subgroup. However, no significant associations between PPI dose and fracture risk were found among individuals with NM or IM genotypes.
FIGURE 3.

Age‐ and gender‐adjusted risk factors of fractures in different CYP2C19 phenotypes. CI, confidence interval; HR, hazard ratio; IM, intermediate metabolizer; NM, normal metabolizer; PM, poor metabolizer; PPI, proton pump inhibitor. *p value<0.05, **p value<0.01.
CYP2C19 phenotype and BMD
There were 75 patients enrolled with long‐term PPI prescriptions and BMD measurement during the one‐year follow‐up period (Table 3). They were classified as PMs and non‐PMs, with 6 (8%) PMs and 69 (92%) non‐PMs. The average age was 62.2 ± 11.8 years, and 76.0% were female.
TABLE 3.
Demographics and bone mineral density in proton pump inhibitor users by CYP2C19 genotypes.
| Parameter | Total (n = 75) | Genotype | P value | |
|---|---|---|---|---|
| PM (n = 6) | Non‐PM a (n = 69) | |||
| Age (years) | 62.2 ± 11.8 | 57.12 ± 9.7 | 62.6 ± 12.0 | 0.281 |
| Female | 57 (76.0%) | 5 (83.3%) | 52 (75.4%) | 1.000 |
| Cumulative defined daily PPI dose | 362 ± 106 | 383 ± 144 | 360 ± 103 | 0.617 |
| Osteoporosis | 27 (36.0%) | 2 (33.3%) | 25 (36.2%) | 1.000 |
| Rheumatic arthritis | 15 (20.0%) | 1 (16.7%) | 14 (20.3%) | 1.000 |
| Type 2 diabetes mellitus | 25 (33.3%) | 1 (16.7%) | 24 (34.8%) | 0.657 |
| Hypertension | 36 (48.0%) | 2 (33.3%) | 34 (49.3%) | 0.676 |
| Stroke | 1 (1.3%) | 0 (0%) | 1 (1.5%) | 1.000 |
| Medication history | ||||
| Therapy for osteoporosis | 8 (10.7%) | 0 (0%) | 8 (11.6%) | 1.000 |
| Systemic corticosteroid | 32 (42.7%) | 3 (50.0%) | 29 (42.0%) | 1.000 |
| Bone mineral density (g/cm2) | ||||
| Lumbar spine | 0.98 ± 0.21 | 0.87 ± 0.29 | 0.99 ± 0.20 | 0.204 |
| Left femoral neck | 0.73 ± 0.14 | 0.68 ± 0.17 | 0.73 ± 0.14 | 0.394 |
| Right femoral neck | 0.72 ± 0.14 | 0.67 ± 0.15 | 0.72 ± 0.14 | 0.416 |
| T score | 0.846 | |||
| Normal (> − 1) | 8 (10.7%) | 0 (0%) | 8 (11.6%) | |
| Low bone mass (−1 ~ −2.5) | 28 (37.3%) | 3 (50%) | 25 (36.2%) | |
| Osteoporosis (≤−2.5) | 39 (52.0%) | 3 (50%) | 36 (52.2%) | |
Note: Data are presented as number (percentage) and mean ± SD.
Abbreviations: CI, confidence interval; PM, poor metabolizer; SD, standard deviation.
Non‐PM group includes intermediate metabolizers and normal metabolizers.
We found a numerically lower BMD in the PMs when compared with the non‐PM subgroups, in the spine (0.87 g/cm2 ± 0.29 vs 0.99 g/cm2 ± 0.20), left femur (0.68 g/cm2 ± 0.17 vs 0.73 g/cm2 ± 0.14), and right femur (0.67 g/cm2 ± 0.15 vs 0.72 g/cm2 ± 0.14). However, no significant difference in BMD was observed between the CYP2C19 PM and non‐PM groups.
DISCUSSION
To the best of our understanding, this study is the first to investigate the links between CYP2C19 polymorphism, fracture, and BMD among long‐term PPI users in the Han Chinese population. Our results have revealed that the CYP2C19 metabolic phenotype does not appear to confer an increased risk for fractures or a reduction in BMD in individuals who have utilized PPIs for at least 1 year. These findings provide additional evidence supporting the safety of PPI therapy concerning bone health, particularly among individuals possessing PM genotypes.
Precision medicine is an approach which offers the appropriate medicine and dosage to patients through pharmacogenetics, using genotype information to guide treatment decisions and individual treatment plans. 10 Among patients experiencing long‐term PPI use, using CYP2C19 genotype data may potentially predict plasma exposure and minimize the risk of overexposure and toxicity. 12 PPIs are metabolized in the liver, primarily through enzymes such as CYP2C19 and CYP3A4. Among the PPIs, omeprazole, lansoprazole, dexlansoprazole, and pantoprazole are metabolized predominantly by CYP2C19, accounting for over 80% of their metabolism. 10 Factors affecting CYP2C19 activity will influence PPI exposure, including age, 17 medications, and variations in CYP2C19. 10 The polymorphically expressed CYP2C19 is mainly responsible for the metabolic rate. CYP2C19 IMs and PMs showed 3–14 times higher area under the curve and 2–6 times higher maximal plasma drug concentrations after receiving standard doses of first‐generation PPIs compared to CYP2C19 NMs. 12 Hence, PMs and IMs have more pronounced pharmacodynamic responses than NMs, which improves the therapeutic benefits. 12 This interaction leads to a gene–dose effect. For example, when controlling gastric acid secretion, the 24‐h median intragastric pH in PMs is much higher than in NMs, with IMs being in between. Several studies have illustrated that cure rates in Helicobacter pylori infection were approximately 15%–20% higher in PMs and IMs than NMs. In treating gastroesophageal reflux disease, two small trials found the highest healing rates in the PMs group, with the PMs/IMs having about a 20% higher therapeutic gain when compared to NMs, therefore higher dosages of PPIs maybe needed in NMs. 18 , 19 Among different PPIs, omeprazole was shown to have the highest impact caused by interaction with the genotype of CYP2C19. 12
PPIs are commonly prescribed and highly effective medications for many gastrointestinal disorders, particularly among the elderly, who remain a population with a high risk of fractures. 20 A recent meta‐analysis study found that when compared with non‐users, PPI users had a 58%, 26%, and 33% higher risk of spine fracture, hip fracture, and other fractures, respectively. 21 There are certain possible mechanisms by which PPIs may increase fracture risk, but these have not yet been made clear. PPIs inhibit the production and intragastric secretion of hydrochloric acid, leading to hypochlorhydria and malabsorption of calcium or vitamin B12. 8 , 20 , 22 Furthermore, PPI therapy may also lead to gastrin‐induced parathyroid hyperplasia and osteoclastic vacuolar proton pump inhibition. 1 The dose and duration of PPI usage are associated with risks of fracture. 2 High doses of PPIs and/or using a PPI for 1 year or more are associated with hip fracture and other osteoporotic fractures. 8
The potential for adverse events being associated with PPI use may be influenced by the dosage administered and the exhibiting of genetic correlations. Earlier epidemiological research has indicated that an augmented hazard may be associated with greater dosages of PPIs, 23 , 24 although only limited investigations have explored the relationship in the context of CYP2C19 genotypes. Individuals with CYP2C19 IM or PM genotypes may be at a greater risk of PPI‐related unfavorable outcomes as compared to those with NM genotypes. Nevertheless, the correlation between genetic variation and adverse effects is intricate and warrants further investigation. Lima et al. 25 found an association between CYP2C19 genotype and respiratory adverse events which showed a higher frequency of upper respiratory infections in IMs/PMs than those seen in normal metabolizers. Nonetheless, the exact correlation between genetic variation and the potential for fracture risk remains indeterminate, with relatively few studies having investigated this phenomenon. 2 , 26 In our study, the incidence of fractures following a 1‐year course of PPI use was observed to be approximately 2.3% to 3.5%. However, no definitive evidence suggested there was a correlation between CYP2C19 polymorphism and the risk of fractures in individuals who had been on PPI therapy for over a year. Similar findings were reported by Naomi et al. 26 in their study involving an Israeli population. While the author of the previous study recruited patients who had received a minimum of one PPI prescription, 26 our investigation included individuals who underwent a minimum of 336 days of PPI use. However, despite the extension of PPI exposure to 336 days, CYP2C19 polymorphism did not demonstrate any apparent association with an increased risk of fractures. Thus, concerns regarding an increased risk of fractures among individuals with CYP2C19 PM genotypes should not be a reason for withholding PPI treatment within the first year.
In our cohort, the cumulative defined daily PPI dose in 336 days was 371 ± 106 DDD, with a daily DDD of 1.1 ± 0.31 DDD, considered the usual dose of PPIs. Yang et al. reported that the risk of hip fracture increased among patients prescribed PPI doses >1.75/day (OR = 2.65; 95% CI, 1.80–3.90). 4 Pantoprazole concentrations were significantly higher (1.5‐ to 6‐fold) in CYP2C19 PMs compared to NMs in different studies. 27 , 28 , 29 , 30 , 31 , 32 The FDA issued a Drug Safety Communication warning of a possible increased risk of fractures with PPI use, especially with doses higher than over‐the‐counter (OTC) PPI doses (0.5 DDD lansoprazole or 1DDD omeprazole) and/or used a PPI for 1 year or more. Previous reports suggested a correlation between higher PPI dosage and fracture risk. 4 , 5 , 6 Our study hypothesized that CYP2C19 genotypes might be associated with fragility fractures among PPI users but found no significant impact. It is speculated that even individuals with poor CYP2C19 metabolic function might not reach PPI concentrations that increase fracture risk. In addition, potential factors other than PPI prescription might contribute to fractures.
As previously established, the risk of fractures has been found to be linked to advanced age, the female gender, and a history of osteoporosis. 33 These well‐known risk factors carry a substantially greater impact on the potential for fractures relative to genetic variation in drug metabolism. Moreover, the influence of PPI concentrations on fracture risk does not surpass that of other factors. To discern whether varying risk factors were present among different metabolizers, we performed subgroup analyses with adjustments for age and gender. Our findings indicate that a history of osteoporosis increased the risk of fractures (HR:3.9, p = 0.009) in NMs, which is consistent with the results observed across the overall participant population. Nevertheless, this correlation was not apparent in individuals possessing the IM or PM genotypes, indicating there was a diminished effect which previous osteoporosis had on fracture risk in these subgroups. In addition, we found that cumulative PPI dose was associated with reduced risk of osteoporotic fracture in the PM group. Considering the observed number of fractures in the PMs was only five events, we believe that the correlation between cumulative PPI dose and the occurrence of fractures in PMs may not be clinically significant.
It is worth noting that although statistical significance was not achieved, individuals with PM genotypes exhibited lower BMD in the spine (0.87 g/cm2 ± 0.29 vs 0.99 ± 0.20) and bilateral femoral bone (0.68 g/cm2 ± 0.17 vs 0.73 ± 0.14; 0.67 g/cm2 ± 0.15 vs 0.72 ± 0.14) when compared to those without PM genotypes. The mechanism underlying this reduction in bone density may be attributable to PPI‐induced hypochlorhydria, which can increase osteoclastic activity and reduce calcium absorption. 8 , 20 , 22 Previous research has demonstrated that prolonged exposure to PPIs may be associated with lower BMD relative to non‐users, 7 and low BMD is widely acknowledged as a significant contributor to the risk of fractures. 34 , 35 Conversely, in clinical practice it is common to employ the concept of the least significant change (LSC) when evaluating the clinical significance of two distinct BMD values. 36 In our study, LSC ranged from 0.008 to 0.011 g/cm2 at the spine and from 0.006 to 0.016 g/cm2 at the proximal femoral region. We found that the differences in BMD values following PPI treatment surpasses the LSC range. Although the variance in BMD between PMs and non‐PMs may not reach statistical significance in our study, it could still hold clinical significance. It is plausible that PM genotypes may confer an increased tendency towards a lower BMD, which is relevant among individuals who are at a high clinical risk for fractures. Additional studies are still required in order to validate our findings.
While our findings indicate that long‐term PPI therapy across CYP2C19 metabolic phenotypes appears to be safe, it is important to acknowledge that this study was subject to certain limitations. First, it should be noted that our study lacked data pertaining to fracture‐related lifestyle habits, such as exercise and dietary patterns. It remains unclear whether these additional variables may interact with CYP2C19 genotypes. Second, the small case number of participants with BMD data who were available for analysis, combined with the absence of baseline BMD measurements prior to PPI therapy, may have limited our ability to detect any association between CYP2C19 genetic variations and BMD. Post hoc analysis demonstrated 99.7% power in detecting the association between CYP2C19 phenotype and fracture risk, and 46.8% power in identifying a correlation with BMD. Further studies with larger sample size are warranted to better elucidate the impact of CYP2C19 genotypes on BMD outcomes. Third, patient medication habits, including the timing and concurrent administration of other anti‐secretory agents which could impact PPI metabolism, were not accounted for in our study. The potential for drug–drug‐gene interactions should be taken into consideration. Further studies are still required in order to validate our findings. Fourth, not all CYP2C19 non‐functional alleles were analyzed on the TWBv2 array. However, the three alleles we examined represent the majority of the non‐functional alleles in the East Asian population. Lastly, our findings need to be replicated in an independent cohort to confirm them. In conclusion, the findings of our real‐world, hospital‐based investigation suggest that CYP2C19 PMs/IMs do not confer an increased risk for fractures or diminished BMD in individuals who have utilized PPIs for extended periods. Nonetheless, further studies remain warranted in order to better elucidate the impact of CYP2C19 genotypes on fracture occurrence and BMD outcomes among individuals who have been on PPI therapy for prolonged durations.
AUTHOR CONTRIBUTIONS
Y.‐J.L. and Y.‐T.C. designed the research. T.‐H.H. and C.‐H.L. performed the research. C.‐Y.H. and M.‐F.W. analyzed the data. Y.‐M.C. and C.‐S.H. wrote the manuscript.
FUNDING INFORMATION
This study was funded by Academia Sinica 40‐05‐GMM and AS‐GC‐110‐MD02, National Science and Technology Council, Taiwan [NSTC ‐111‐2634‐F‐A49‐014, NSTC‐111‐2218‐E‐039‐001, and NSTC‐111‐2314‐B‐075A‐003‐MY3] and Taichung Veterans General Hospital, Taiwan [TCVGH‐1127301C, TCVGH‐1127302D, and TCVGH‐YM1120110].
CONFLICT OF INTEREST STATEMENT
The authors declared no competing interests for this work.
Supporting information
Table S1
ACKNOWLEDGMENTS
We would like to thank all the participants as well as the investigators from the Taiwan Precision Medicine Initiative. We would also like to thank the Biostatistics Task Force and Clinical Informatics Research & Development Center of Taichung Veterans General Hospital.
Liao Y‐J, Chen Y‐T, Hsiao T‐H, et al. CYP2C19 genotypes and osteoporotic fractures in long‐term users of proton pump inhibitors: A hospital‐based study. Clin Transl Sci. 2023;16:2198‐2208. doi: 10.1111/cts.13620
Yi‐Ju Liao and Yu‐Ting Chen contributed equally to this work.
Yi‐Ming Chen and Chun‐Sheng Hsu also contributed equally to this work.
DATA AVAILABILITY STATEMENT
All data used in this study for this article are available upon reasonable request to the corresponding author.
REFERENCES
- 1. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long‐term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706‐715. doi: 10.1053/j.gastro.2017.01.031 [DOI] [PubMed] [Google Scholar]
- 2. Food and Drug Administration Drug Safety Communication . Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors . 2011. https://www.fda.gov/drugs/postmarket‐drug‐safety‐information‐patients‐and‐providers/fda‐drug‐safety‐communication‐possible‐increased‐risk‐fractures‐hip‐wrist‐and‐spine‐use‐proton‐pump
- 3. Lin SM, Yang SH, Liang CC, Huang HK. Proton pump inhibitor use and the risk of osteoporosis and fracture in stroke patients: a population‐based cohort study. Osteoporos Int. 2018;29:153‐162. doi: 10.1007/s00198-017-4262-2 [DOI] [PubMed] [Google Scholar]
- 4. Yang YX, Lewis JD, Epstein S, Metz DC. Long‐term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947‐2953. doi: 10.1001/jama.296.24.2947 [DOI] [PubMed] [Google Scholar]
- 5. Poly TN, Islam MM, Yang HC, Wu CC, Li YJ. Proton pump inhibitors and risk of hip fracture: a meta‐analysis of observational studies. Osteoporos Int. 2019;30:103‐114. doi: 10.1007/s00198-018-4788-y [DOI] [PubMed] [Google Scholar]
- 6. Chiu HF, Huang YW, Chang CC, Yang CY. Use of proton pump inhibitors increased the risk of hip fracture: a population‐based case‐control study. Pharmacoepidemiol Drug Saf. 2010;19:1131‐1136. doi: 10.1002/pds.2026 [DOI] [PubMed] [Google Scholar]
- 7. Aleraij S, Alhowti S, Ferwana M, Abdulmajeed I, Mutawwam IM. Effect of proton pump inhibitors on bone mineral density: a systematic review and meta‐analysis of observational studies. Bone Reports. 2020;13:100732. doi: 10.1016/j.bonr.2020.100732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis‐related fractures. CMAJ. 2008;179:319‐326. doi: 10.1503/cmaj.071330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Targownik LE, Goertzen AL, Luo Y, Leslie WD. Long‐term proton pump inhibitor use is not associated with changes in bone strength and structure. Am J Gastroenterol. 2017;112:95‐101. doi: 10.1038/ajg.2016.481 [DOI] [PubMed] [Google Scholar]
- 10. El Rouby N, Lima JJ, Johnson JA. Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opin Drug Metab Toxicol. 2018;14:447‐460. doi: 10.1080/17425255.2018.1461835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klotz U, Schwab M, Treiber G. CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol. 2004;95:2‐8. doi: 10.1111/j.1600-0773.2004.pto950102.x [DOI] [PubMed] [Google Scholar]
- 12. Lima JJ, Thomas CD, Barbarino J, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2C19 and proton pump inhibitor dosing. Clin Pharmacol Ther. 2021;109:1417‐1423. doi: 10.1002/cpt.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fricke‐Galindo I, Céspedes‐Garro C, Rodrigues‐Soares F, et al. Interethnic variation of CYP2C19 alleles, ‘predicted’ phenotypes and ‘measured’ metabolic phenotypes across world populations. Pharmacogenomics J. 2016;16:113‐123. doi: 10.1038/tpj.2015.70 [DOI] [PubMed] [Google Scholar]
- 14. Wei CY, Yang JH, Yeh EC, et al. Genetic profiles of 103,106 individuals in the Taiwan biobank provide insights into the health and history of Han Chinese. NPJ Genom Med. 2021;6:10. doi: 10.1038/s41525-021-00178-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization . ATC/DDD Index 2023 . 2023. https://www.whocc.no/atc_ddd_index/
- 16. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569‐2581. doi: 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 17. Ward RM, Kearns GL. Proton pump inhibitors in pediatrics: mechanism of action, pharmacokinetics, pharmacogenetics, and pharmacodynamics. Paediatr Drugs. 2013;15:119‐131. doi: 10.1007/s40272-013-0012-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furuta T, Shirai N, Watanabe F, et al. Effect of cytochrome P4502C19 genotypic differences on cure rates for gastroesophageal reflux disease by lansoprazole. Clin Pharmacol Ther. 2002;72:453‐460. doi: 10.1067/mcp.2002.127637 [DOI] [PubMed] [Google Scholar]
- 19. Kawamura M, Ohara S, Koike T, et al. The effects of lansoprazole on erosive reflux oesophagitis are influenced by CYP2C19 polymorphism. Aliment Pharmacol Ther. 2003;17:965‐973. doi: 10.1046/j.1365-2036.2003.01539.x [DOI] [PubMed] [Google Scholar]
- 20. Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta‐analysis of 11 international studies. Am J Med. 2011;124:519‐526. doi: 10.1016/j.amjmed.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu J, Li X, Fan L, et al. Proton pump inhibitors therapy and risk of bone diseases: an update meta‐analysis. Life Sci. 2019;218:213‐223. doi: 10.1016/j.lfs.2018.12.058 [DOI] [PubMed] [Google Scholar]
- 22. Bo‐Linn GW, Davis GR, Buddrus DJ, Morawski SG, Santa Ana C, Fordtran JS. An evaluation of the importance of gastric acid secretion in the absorption of dietary calcium. J Clin Invest. 1984;73:640‐647. doi: 10.1172/jci111254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238‐246. doi: 10.1001/jamainternmed.2015.7193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors. JAMA Intern Med. 2016;176:172‐174. doi: 10.1001/jamainternmed.2015.7927 [DOI] [PubMed] [Google Scholar]
- 25. Lima JJ, Lang JE, Mougey EB, et al. Association of CYP2C19 polymorphisms and lansoprazole‐associated respiratory adverse effects in children. J Pediatr. 2013;163:686‐691. doi: 10.1016/j.jpeds.2013.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gronich N, Lavi I, Lejbkowicz F, Pinchev M, Rennert G. Association of CYP2C19 polymorphism with proton pump inhibitors effectiveness and with fractures in real‐life: retrospective cohort study. Clin Pharmacol Ther. 2022;111:1084‐1092. doi: 10.1002/cpt.2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deshpande N, Sharanya V, Ravi Kanth VV, et al. Rapid and ultra‐rapid metabolizers with CYP2C19*17 polymorphism do not respond to standard therapy with proton pump inhibitors. Meta Gene. 2016;9:159‐164. doi: 10.1016/j.mgene.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gawrońska‐Szklarz B, Siuda A, Kurzawski M, Bielicki D, Marlicz W, Droździk M. Effects of CYP2C19, MDR1, and interleukin 1‐B gene variants on the eradication rate of Helicobacter pylori infection by triple therapy with pantoprazole, amoxicillin, and metronidazole. Eur J Clin Pharmacol. 2010;66:681‐687. doi: 10.1007/s00228-010-0818-1 [DOI] [PubMed] [Google Scholar]
- 29. Gawrońska‐Szklarz B, Adamiak‐Giera U, Wyska E, et al. CYP2C19 polymorphism affects single‐dose pharmacokinetics of oral pantoprazole in healthy volunteers. Eur J Clin Pharmacol. 2012;68:1267‐1274. doi: 10.1007/s00228-012-1252-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karaca RO, Kalkisim S, Altinbas A, et al. Effects of genetic polymorphisms of cytochrome P450 enzymes and MDR1 transporter on pantoprazole metabolism and Helicobacter pylori eradication. Basic Clin Pharmacol Toxicol. 2017;120:199‐206. doi: 10.1111/bcpt.12667 [DOI] [PubMed] [Google Scholar]
- 31. Román M, Ochoa D, Sánchez‐Rojas SD, et al. Evaluation of the relationship between polymorphisms in CYP2C19 and the pharmacokinetics of omeprazole, pantoprazole and rabeprazole. Pharmacogenomics. 2014;15:1893‐1901. doi: 10.2217/pgs.14.141 [DOI] [PubMed] [Google Scholar]
- 32. Tanaka M, Ohkubo T, Otani K, et al. Metabolic disposition of pantoprazole, a proton pump inhibitor, in relation to S‐mephenytoin 4’‐hydroxylation phenotype and genotype. Clin Pharmacol Ther. 1997;62:619‐628. doi: 10.1016/s0009-9236(97)90081-3 [DOI] [PubMed] [Google Scholar]
- 33. Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194:S3‐S11. doi: 10.1016/j.ajog.2005.08.047 [DOI] [PubMed] [Google Scholar]
- 34. Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185‐1194. doi: 10.1359/JBMR.050304 [DOI] [PubMed] [Google Scholar]
- 35. Marshall D, Johnell O, Wedel H. Meta‐analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ (Clinical Research Ed). 1996;312:1254‐1259. doi: 10.1136/bmj.312.7041.1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nelson L, Gulenchyn KY, Atthey M, Webber CE. Is a fixed value for the least significant change appropriate? J Clin Densitom. 2010;13:18‐23. doi: 10.1016/j.jocd.2009.10.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
All data used in this study for this article are available upon reasonable request to the corresponding author.


