Abstract
Pharmacogenomics (PGx) implementation into clinical care is rapidly increasing in China. However, the extent to which the public understands PGx testing and important knowledge domains requiring patient education or counseling remains unclear. To address this, we created and validated the Chinese version of the Minnesota Assessment of Pharmacogenomic Literacy (MAPL‐CTM). The MAPL‐C was developed by translating the English MAPL to Chinese following cross‐cultural translation guidelines. An online survey validated the MAPL‐C and assessed Chinese individuals' PGx literacy. Validation analyses were performed and associations of PGx literacy with participants' characteristics were quantified. Of 959 high‐quality responses, the majority of respondents were Han Chinese (96.3%), men (54.5%), aged 18–29 years (70.9%), residing in China (97.3%), and had received college or higher education (95.0%). Out of 15 starting items developed to query specific predefined knowledge domains, two uninformative items were excluded, resulting in a 13‐item MAPL‐C. Chinese participants' MAPL‐C performance was best explained by a three‐factor model, encompassing PGx concepts and function, testing limitations, and privacy. Higher MAPL‐C performance was associated with younger age, higher education, and previous genetic testing experience. Correct response rates for questions related to testing limitations were lower than those in other domains. The creation and validation of the MAPL‐C fills a gap in determining PGx knowledge among Chinese speakers, quantifying PGx literacy within a Chinese cohort, and identifying response patterns and knowledge gaps. The MAPL‐C can be useful in clinical practice to guide patient counseling, assess PGx education interventions, and quantify PGx knowledge in relation to outcomes in research studies involving Chinese participants.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The Minnesota Assessment of Pharmacogenomic Literacy (MAPL) was recently developed as the first instrument to quantitativly assess of pharmacogenomic (PGx) literacy within clinical and research environments. Initially created in English, its generalizability to other languages and cultural context needs to be established.
WHAT QUESTION DID THIS STUDY ADDRESS?
To develop and validate the Chinese version of the MAPL (MAPL‐C) and examine PGx literacy in China.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The successful creation and validation of MAPL‐C fills a crucial gap in determining PGx knowledge among Chinese speakers. It quantifies PGx literacy within a Chinese cohort, characterized response patterns, and knowledge gaps.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The MAPL‐C is a valuable tool to evaluate PGx knowledge in patients or research study participants. Responses to the MAPL‐C may be used to guide patient counseling, develop educational tools, or quantify PGx knowledge in the context of research studies in the Chinese population.
INTRODUCTION
Pharmacogenomics (PGx) is a rapidly advancing field that investigates how an individual's genetics affect medication response and tolerability. 1 By tailoring treatment regimens based on this information, PGx may help clinicians optimize clinical outcomes and improve patient care. The growing application of PGx in clinical care is supported by more than 30 years of translational research. This progress is evident in several pioneering healthcare institutions from North America and Europe where PGx has been successfully integrated into routine clinical practice. 2 , 3 These initiatives are strongly backed by the accumulating evidence‐based PGx clinical guidelines and supporting resources developed by professional organizations and consortia, such as the Clinical Pharmacogenetics Implementation Consortium in the United States, 4 the Dutch Pharmacogenetics Working Group, 5 the Canadian Pharmacogenomics Network for Drug Safety, 6 and the French Network of Pharmacogenetics. 7
In the past decade, there has been a notable surge in PGx research specifically targeting non‐European populations. This emphasis has significantly expanded the clinical application of PGx in countries with predominantly non‐European populations, aiming to better serve diverse populations. 8 Converging findings highlight significant differences in the frequency and distribution of genetic variants affecting drug response across different ancestral groups. 9 One striking example is the Han Chinese population, which represents the world's largest ancestral group native to China. Studies have revealed that this population exhibits significantly higher frequencies of actionable genetic variants and altered phenotypes in certain clinically important pharmacogenes compared to non‐Asian populations. 10 , 11 , 12 Prominent examples include CYP2C19, HLA‐B, and VKORC1. Overlooking these differences can lead to suboptimal treatment outcomes and elevated risks of adverse reactions. Some implicated medications are commonly prescribed among the Chinese, such as clopidogrel, warfarin, carbamazepine, and allopurinol. 11 , 13 , 14 , 15 , 16
The clinical significance of actionable drug‐gene pairs has recently emerged as a catalyst for the implementation of PGx testing into healthcare systems in China. 17 , 18 , 19 This trend is further compounded by the increasing availability of commercial PGx testing products offered by genetic testing companies. 20 A nationwide survey conducted in 2019 revealed that ~10% of hospitals across the country provide PGx testing services. 21 The majority of these hospitals conduct tests for specific medications, with warfarin and clopidogrel being the most commonly tested. 14 , 17 The extent of clinical implementation, however, varies significantly based on the types of institution and geographic locations, with tertiary hospitals in municipalities and provincial capitals exhibiting higher programmatic utilization of PGx. 18 , 19 , 22 Healthcare professionals in China have increasingly demonstrated a positive attitude toward the clinical application of PGx. 17 , 23 Unfortunately, despite the enthusiasm and potential surrounding PGx, several barriers to its widespread adoption in China still require attention. These obstacles include cost, infrastructural support, awareness, and education, all of which have previously hindered the broader implementation of PGx in other countries and regions, regardless of their current stages of adoption. 23 , 24 Addressing these barriers is crucial for advancing the clinical uptake of PGx in China.
To promote the effective adoption of PGx, it is important to raise awareness and provide education to various stakeholders. 25 Research assessing knowledge of PGx is emerging worldwide, including in countries like China, using diverse methodologies ranging from self‐report assessments to customized instruments. 17 , 23 , 26 , 27 , 28 However, these efforts have predominantly focused on evaluating the readiness of health professionals to offer PGx services, rather than assessing the understanding of PGx among patients or test recipients. Shared decision making and counseling about medical tests and treatments are essential components of clinical care and personalizing treatment. In the context of PGx testing, effective patient counseling necessitates that patients have sufficient understanding to actively participate in the decision‐making process of whether to obtain a test and to appreciate potential benefits and limitations. 29 , 30 Recognizing the need for a PGx‐specific instrument to assess patients' knowledge of key PGx concepts, our group recently developed the Minnesota Assessment of Pharmacogenomic Literacy (MAPL). 26 This validated instrument is the first to quantitatively measure the knowledge that test recipients have about PGx in clinical and research settings. It is available for clinical, academic, and research use. Based on findings from a scoping review to identify areas important for patient education 31 followed by a confirmatory focus group assessment, the foundation of the MAPL consists of 15 items designed to assess four primary knowledge domains: underlying concepts, limitations, benefits, and privacy, which are crucial for test recipients to comprehend their results (Table 1). 26 Initially developed in English, the instrument was validated among 646 participants in the United States, with subsequent efforts underway to confirm the generalizability of the MAPL and expanding its accessibility across different populations.
TABLE 1.
Fifteen items of the original version of MAPL in English. 26
| Item | Question | Answer key | Knowledge domain |
|---|---|---|---|
| 1 a | Different people will respond to medications differently | True | Concepts |
| 2 | Genes are made of DNA | True | Concepts |
| 3 | If a medication works for your family member, it will work for you too | False | Limitations |
| 4 | Genes are one of many different things that can affect how you respond to a medication | True | Limitations |
| 5 | Pharmacogenomic test results will tell you how you will respond to every medication | False | Limitations |
| 6 a | Your genes are inherited from your parents | True | Concept |
| 7 | Genes can affect how much medication is in your body after you take a pill | True | Benefits |
| 8 | Pharmacogenomic test results may tell you that a medication is likely to cause side effects | True | Benefits |
| 9 | Your body breaks down medications to get rid of them | True | Concepts |
| 10 | Pharmacogenomic testing will tell you the best medication to treat your condition | False | Limitations |
| 11 | When deciding what medication is best for you, your genetic makeup is more important than age, weight, or other medications you are taking | False | Limitations |
| 12 | Pharmacogenomic testing will help determine your diagnosis | False | Limitations |
| 13 | Health insurance companies can use your pharmacogenomic test results to deny coverage | False | Privacy |
| 14 | Pharmacogenomic testing companies have the right to use your data however they want without your consent | False | Privacy |
| 15 | Pharmacogenomic testing can tell you that you may need a different dose of a medication | True | Benefits |
In the validation of the original MAPL with the Minnesota cohort, Items 1 and 6 with >95% correct response rate were determined uninformative and removed, resulting in the 13‐item MAPL.
Abbreviation: MAPL, Minnesota Assessment of Pharmacogenomic Literacy.
In this study, we translated and adapted the MAPL into Chinese, considering that Chinese is one of the most widely spoken languages globally. With over 1.3 billion native speakers, representing over 16% of the global population, 32 Chinese holds significant linguistic importance. The translated version of the MAPLTM, named MAPL‐CTM, was validated using a study sample of native Mandarin Chinese speakers. By validating the MAPL‐C, our aim was to bridge the knowledge gap and assess the overall level of PGx literacy among the Chinese public. Additionally, we investigated the potential influences of sociodemographic and clinical characteristics on PGx knowledge.
METHODS
Survey development
The approaches that the original MAPL applied to assess patient literacy have been used in assessing medical genetics literacy. 33 The conversion and cultural adaption of all original 15 MAPL 26 items from English to Chinese followed established cross‐cultural translation guidelines. 34 Fifteen items were integrated into a survey for subsequent validation in Chinese. Additional revisions were made based on feedback from a pilot survey prior to the full‐scale administration. These detailed procedures are available in the Supplemental Methods.
The validation survey for MAPL‐C was conducted online using the Research Electronic Data Capture (REDCap) platform. The survey included a total of 42 questions including the 15 prevalidated MAPL‐C items with response options of “Yes,” “No,” and “I don't know.” Twenty‐seven other questions were included to gather participants' sociodemographic and healthcare‐related characteristics, as well as their health literacy assessed with the All Aspects of Health Literacy Scale 35 (see Supplemental Methods for details).
Survey administration
Eligibility and recruitment
The full‐scale survey administration took place from January 20 to February 24, 2023, utilizing a combination of simple random sampling and snowball sampling techniques through major Chinese social media platforms. The target population consisted of adults who self‐identified as being of Chinese descent and possessed native proficiency in Chinese. To participate in the survey, individuals self‐identified by responding to posters and social media advertisements. A questionnaire to verify eligibility was administered prior to obtaining consent. An introductory screen was presented on their personal devices through a common uniform resource locator, providing an overview of the study, including the inclusion/exclusion criteria and relevant consent information. Exclusion criteria for participation included being less than 18 years of age, not of Chinese descent, lacking native proficiency in Chinese, and being unable or unwilling to provide informed consent in Chinese. After providing consent, participants were instructed to proceed with the survey.
Data collection
To ensure comprehensive data collection, all questions in the survey were configured as mandatory, requiring participants to answer all fields. All participant responses were collected and securely stored in the REDCap database. To ensure data accuracy and quality, a research staff member verified the collected data. This research project was reviewed and approved by the University of Minnesota Institutional Review Board #00017889 on December 21, 2022.
Data analyses
Data cleaning
Completed survey responses were exported from REDCap and reviewed by research team members. Responses that raised doubts or uncertainties were flagged for further discussion. The final decision regarding inclusion was made based on agreement between the first and senior authors. Responses that exhibited straight‐line patterns (consistently selecting the same response option, such as all “True,” all “False,” or all “I don't know”) across MAPL‐C items were excluded from analyses. Additionally, duplicate survey entries were identified, confirmed, and excluded from the analyses. Please refer to Figure S1 for more information about the data cleaning process.
Statistical analyses
Participants' characteristics were summarized using descriptive statistics. The proportion of correct responses was calculated to assess the difficulty level and performance of the MAPL‐C items. Each item was scored as 1 for a correct response and 0 for an incorrect or a “do not know” response. Similar to the approach employed with the original MAPL, items with a correct or incorrect rate exceeding 95% were re‐evaluated for inclusion in the final version of the MAPL‐C. This was because they were deemed either “too easy” or “too hard” and lacked the discriminatory capacity to effectively assess a respondent's real knowledge level. A MAPL‐C total score was calculated by summing the scores across individual items, resulting in a composite measure of PGx literacy. The relationships between participants' characteristics and MAPL‐C total scores were examined using paired t‐tests, analysis of variance, Spearman correlation, and linear regression analyses, depending on the nature of the variables under investigation.
Psychometric analysis
Tetrachoric correlation was used to assess the correlation across the correctness of individual MAPL‐C items. To explore the latent structure of participants' performance on the MAPL‐C, we initially conducted exploratory factor analysis (EFA) with varimax rotation. This allowed us to identify the underlying relationships between individual MAPL‐C items and empirically determine the number of factors that explain the correlation pattern within the item set. The resulting latent factor structure was subsequently validated using confirmatory factor analysis (CFA) with weighted least square mean and variance adjusted estimation. The fit of the CFA model was evaluated using the root mean square error of approximation (RMSEA; good fit if <0.05, acceptable fit if <0.08), comparative fit index 36 (CFI; good fit if >0.9 and adequate fit if >0.8), and Tucker‐Lewis index 37 (TLI; good fit if >0.9 and mediocre fit if >0.8). The determination of dimensionality (i.e., the optimal number of factors) was obtained through EFA and CFA, which further guided interpretations of internal consistency. The alpha values ranged from 0 to 1 with the value between 0.7 and 0.9 indicating good internal consistency of the instrument. The CFA was performed in Mplus 8 38 and other analyses were performed in R version 4.2.1 39 using the following packages (“tidyverse,” “psych,” and “stats”).
RESULTS
Characteristics of survey respondents
Out of the 1043 completed surveys, high‐quality responses from 959 adult respondents were included in the analyses (Figure S1). All respondents were born in China with 96.3% self‐identifying as Han Chinese (Table 2). The proportion of men (54.5%) slightly exceeded that of women. The median age was 25 years (interquartile range = 10) and ~71% of participants were less than 30 years of age. Over 97% of participants resided in China, representing all seven geographic regions. Ninety‐five percent of respondents had attained at least some college education, and 22% had received or were pursuing graduate degrees. Approximately 43% identified as students or trainees. Over 30% were involved in healthcare‐related professions. A significant majority (94.6%) reported having health insurance. Excluding medications taken for short‐term use, such as those for treating symptoms of coronavirus disease 2019 (COVID‐19) infection, 45.7% of participants reported taking at least one prescription medication, and 57.8% reported taking at least one non‐prescription medication in the past 30 days (refer to Table S3 for more details). Regarding prior experience with genetic testing, nearly 91% of participants had heard about it, and ~17% had undergone previous genetic testing.
TABLE 2.
Sociodemographic and healthcare‐related characteristics of participants (n = 959).
| n | % | |
|---|---|---|
| Gender | ||
| Men | 523 | 54.54 |
| Women | 434 | 45.26 |
| Other | 2 | 0.21 |
| Age, years a | ||
| 18–29 | 680 | 70.91 |
| 30–44 | 172 | 17.94 |
| 45–59 | 78 | 8.13 |
| 60+ | 29 | 3.02 |
| Ethnic group (Minzu) b | ||
| Han | 923 | 96.25 |
| Other | 36 | 3.75 |
| Geographic region of birth | ||
| Central China | 126 | 13.14 |
| East China | 274 | 28.57 |
| North China | 141 | 14.70 |
| Northeast China | 61 | 6.36 |
| Northwest China | 150 | 15.64 |
| South China | 140 | 14.60 |
| Southwest China | 67 | 6.99 |
| Geographic region of current residence | ||
| Central China | 92 | 9.59 |
| East China | 264 | 27.53 |
| North China | 146 | 15.22 |
| Northeast China | 40 | 4.17 |
| Northwest China | 133 | 13.87 |
| South China | 206 | 21.48 |
| Southwest China | 52 | 5.42 |
| Overseas | 26 | 2.71 |
| Education | ||
| No college | 48 | 5.01 |
| Junior college | 119 | 12.41 |
| Pursuing or Holding Bachelor's Degree | 580 | 60.48 |
| Pursuing or Holding Master's/Doctoral Degree | 212 | 22.11 |
| Students/trainees | ||
| Yes | 410 | 42.75 |
| No | 549 | 52.25 |
| Healthcare‐related professions | ||
| Yes | 288 | 30.03 |
| No | 671 | 69.97 |
| Having health insurance | ||
| Yes | 907 | 94.58 |
| No | 52 | 5.42 |
| Previous experience with genetic testing | ||
| Received it in the past | 167 | 17.41 |
| Heard about it, never received it | 704 | 73.41 |
| Never heard about it | 88 | 9.18 |
| Median | IQR | |
|---|---|---|
| AAHLS total score c | 16 | 6 |
Abbreviations: AAHLS, All Aspects of Health Literacy Scale; IQR, interquartile range.
Age information wi originally collected as integer values in years and then classified into four groups. The median and interquartile range of age among participants were 25 and 10, respectively.
Minzu (or mínzú) in Chinese refers to the 56 ethnic groups (nationalities) in China. Han constitutes the largest ethnic group making up approximately 92% of the total population in China.
AAHLS total score ranges from 0 to 20, higher score suggesting higher health literacy.
Comparisons of MAPL‐C with the original MAPL
Fifteen MAPL‐C items are summarized in Table S1, along with their corresponding English MAPL items. Both manual and computerized‐based backward translations showed a strong similarity to the original MAPL, indicating that the meaning and intent of each item were well‐preserved in the MAPL‐C (Table S2).
Figure 1a presents the distribution of participants' responses across 15 MAPL‐C items. The difficulty level of each item was determined by the correct answer rate, with a lower rate indicating higher difficulty. Chinese participants' responses to 13 out of 15 MAPL‐C items closely matched the difficulty ranking of the corresponding MAPL items among US participants (i.e., within ±1 ranking difference) across the entire assessment (Figure 1a,b). Consistent with the MAPL validation, responses to Items 1 and 6 on the MAPL‐C received correct responses from over 95% of respondents. Consequently, they were determined to be uninformative and excluded from the assessment. This resulted in a 13‐item version of the MAPL‐C in direct alignment with the original MAPL.
FIGURE 1.
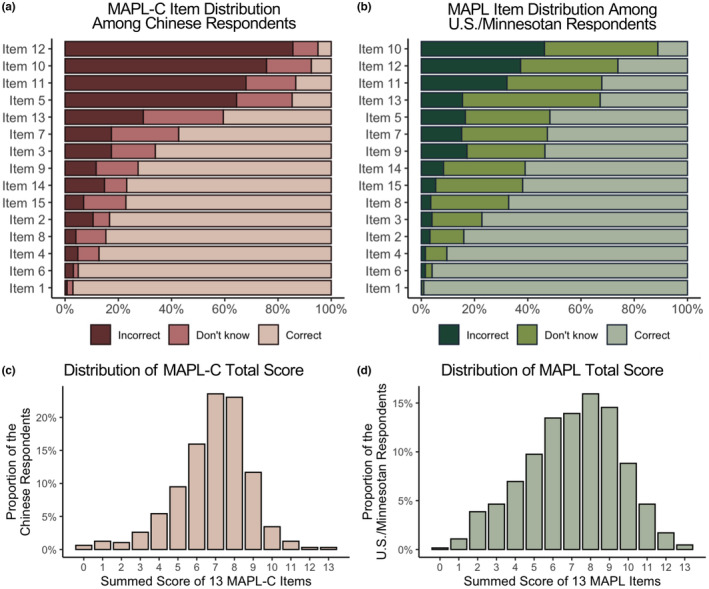
Distribution of MAPL‐C responses by Chinese participants alongside the distribution of original MAPL responses previously obtained from US/Minnesota participants. MAPL, Minnesota Assessment of Pharmacogenomic Literacy; MAPL‐C, Chinese version of the MAPL.
Similar to previous MAPL validations, the distribution of MAPL‐C total scores exhibited a unimodal symmetric pattern (Figure 1c,d). The MAPL‐C total scores shared the same median score of 7 with the MAPL total scores but exhibited higher kurtosis (MAPL‐C: 4.176 vs. MAPL: 2.593). There was no statistically significant difference (t(1160) = −1.334, p = 0.183) in the average performance between Chinese respondents on the MAPL‐C (total score mean = 6.864, SD = 1.961) and US respondents on the MAPL (total score mean = 7.020, SD = 2.492; Figure S2).
The MAPL‐C total score exhibited significant correlations with all individual items except for Item 12—“Pharmacogenomic testing will help determine your diagnosis” (Table S4). This may be attributed to its low correct response rate (5.03%), which is over five‐fold lower than that of the original MAPL (26.2%).
Factor structure of Chinese participants' performance on the MAPL‐C
Figure 2a presents a correlation matrix quantifying pairwise relationships across individual MAPL‐C item scores. Based on the similarities of statistical correlations and domain knowledge, three distinct groups were identified, which was further confirmed by a three‐factor EFA (see Table S5 for factor loadings). All items, except for Item 2 and Item 3, exhibited loadings above 0.45 on their respective factors, signifying at least moderate correlations between individual items and factors. They were retained in the first factor considering their semantic meaning, intent, and prespecified knowledge domains, along with five other items (4, 7, 8, 9, and 15) to represent performance on the concepts and function of PGx. The second factor comprised four items (5, 10, 11, and 12), whereas the third factor included two items (13 and 14), representing participants' performance on limitations and privacy of PGx testing, respectively. To validate the three‐factor solution, CFA was conducted, demonstrating good model performance (RMSEA = 0.044, CFI = 0.888, and TLI = 0.859). Figure 2b illustrates the structural model, with all items showing positive and statistically significant loadings on their respective factors (p < 0.05). Participants' knowledge of privacy in PGx testing was significantly correlated with their knowledge of concepts and function of PGx (effect size = 2.994, p = 0.003) as well as limitations of PGx testing (effect size = 2.701, p = 0.003). There were no significant correlations between understanding the limitations of PGx testing and knowledge of concepts and function of PGx among Chinese participants. Due to the three‐factor solution and the small number of items within each factor, assessing internal consistency using Cronbach's alpha was not applicable in this study. 40
FIGURE 2.
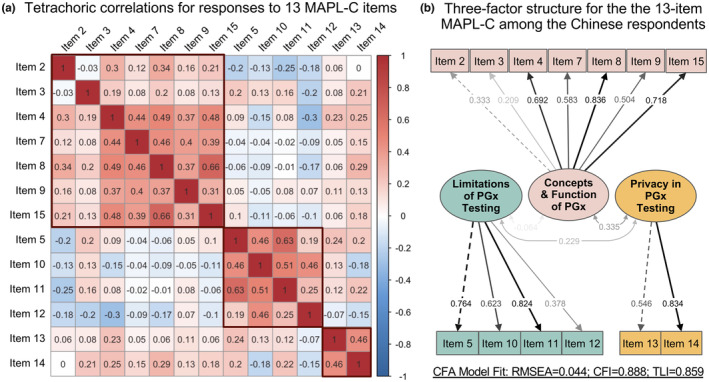
Chinese participants' performance across 13 MAPL‐C items is explained by a three‐factor model. (a) Tetrachoric correlation analysis revealing pairwise relationships across individual MAPL‐C item scores. The color scale indicates the direction of correlation: red for positive and blue for negative correlation. The correlation coefficient (r T) for each item pair indicates the strength of the relationship, ranging from −1 to 1. (b) Confirmatory factor analysis (CFA) using structural equation model for the 13‐item MAPL‐C. The values on the paths between items and the corresponding factors indicate standardized factor loadings. The values between factors indicate standardized covariance. CFI, comparative fit index; MAPL‐C, Chinese version of the Minnesota Assessment of Pharmacogenomic Literacy; RMSEA, root mean square error of approximation; TLI, Tucker‐Lewis index.
Assessment of PGx literacy in the Chinese
There were no significant differences in PGx literacy based on gender, ethnic groups, health insurance status, or number of medications used in the past month among the participants (Figures S3 and S4). Age, education attainment, current student or trainee status, involvement in a healthcare‐related profession, and previous experience with genetic testing were each found to be associated with PGx literacy. In a multivariable regression analysis considering these factors together, younger age (under 45 years), pursuing or holding a bachelor's degree or higher, and prior knowledge of genetic testing were all significantly associated with higher levels of PGx literacy among the Chinese individuals assessed here. These associations remained independent of the positive association between overall health literacy and PGx literacy (Table 3).
TABLE 3.
Multivariable regression models of the MAPL‐C total scores on Chinese participants' characteristics. a
| Independent variables | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Coefficient | SE | p value | Coefficient | SE | p value | |
| Age (reference: 18–29 years) | ||||||
| 30–44 | 0.093 | 0.178 | 0.602 | 0.077 | 0.177 | 0.664 |
| 45–59 | −1.113 | 0.250 | <0.001 | −1.141 | 0.248 | <0.001 |
| 60+ | −0.616 | 0.374 | 0.100 | −0.678 | 0.371 | 0.068 |
| Education (reference: no college) | ||||||
| Junior college | 0.288 | 0.323 | 0.373 | 0.339 | 0.320 | 0.291 |
| Pursuing or holding Bachelor's Degree | 0.679 | 0.296 | 0.022 | 0.657 | 0.294 | 0.025 |
| Pursuing or holding Master's/Doctoral Degree | 1.381 | 0.318 | <0.001 | 1.306 | 0.316 | <0.001 |
| Students/trainees (reference: No) | 0.125 | 0.098 | 0.205 | 0.128 | 0.098 | 0.192 |
| Healthcare‐related occupation (reference: No) | 0.200 | 0.099 | 0.044 | 0.167 | 0.098 | 0.090 |
| Ever heard of genetic testing (reference: No) | 0.728 | 0.211 | <0.001 | 0.686 | 0.210 | 0.001 |
| Ever received genetic testing (reference: No) | 0.168 | 0.165 | 0.307 | 0.203 | 0.163 | 0.215 |
| AAHLS total score | – | – | – | 0.078 | 0.019 | <0.001 |
Abbreviations: AAHLS, All‐Aspect of Health Literacy Scale; MAPL‐C, Minnesota Assessment of Pharmacogenomic Literacy – Chinese version.
A total of two multivariable linear regression models were built with the MAPL‐C total score as the dependent variable. Independent variables for model 1 consist of four categorical variables of sociodemographic and health‐related characteristics selected based on their relationship with the MAPL‐C total score. The AAHLS total score was included as an additional independent variable in model 2.
Bold font denotes statistically significant associations <0.05 significance level (two‐sided).
DISCUSSION
In this study, we successfully adapted and validated the MAPL‐C, derived from the original English MAPL, by using rigorous linguistic translation, cultural adaptation, and psychometric validation processes that are widely recognized and utilized in this type of assessment tools to measure human genetic knowledge and health literacy. 33 , 35 Through this process we confirmed the reliability and cultural appropriateness for assessing knowledge of PGx in native Chinese speakers. Performance on the MAPL‐C was represented by a three‐factor structure, indicating varying levels of understanding of the concepts and function of PGx, limitations of PGx testing, and privacy of PGx testing among the Chinese. Furthermore, we identified several sociodemographic factors, such as age, education, healthcare‐related occupation, and prior experience with genetic testing, that were associated with PGx literacy. The MAPL‐C, therefore, has potential significance in facilitating the development of targeted interventions and educational programs to address the diversity of PGx knowledge gaps and enhance PGx literacy among the Chinese population.
Multidimensional PGx literacy in the Chinese population: Variations, knowledge gaps, and cultural considerations
The present findings suggest that PGx literacy in the Chinese participants assessed in this study is multidimensional, showing notable variations in knowledge levels across various aspects of PGx. This stands in contrast to the unidimensional structure observed in the performance pattern of US participants on the MAPL. For example, participants demonstrated relatively low performance on average when answering questions related to the limitations of PGx testing, compared to their understanding of underlying concepts and function of PGx, as well as privacy considerations. The correct answer rates for the four items concerning limitations (i.e., 5, 10, 11, and 12) ranged from 5.0% to 14.8%, indicating notable knowledge gaps in this domain. These gaps were also observed among US participants using the English MAPL, 26 but they appear to be more pronounced among the Chinese participants surveyed herein. These findings underscore how PGx knowledge and response patterns may differ across populations. By understanding a patient's misunderstanding and potential confusion, healthcare providers can tailor their education to facilitate informed decision‐making regarding testing and its potential benefits and limitations. This information enables healthcare providers to proactively address any misconceptions through the provision of educational materials and discussions.
Low medication literacy among the public may contribute to confusion and misunderstandings regarding certain aspects of PGx literacy among the Chinese population. Recent studies highlight a concerning pattern of insufficient medication literacy among patients in China who receive pharmacotherapy for chronic medical conditions. 41 , 42 , 43 These studies across different disease states consistently reveal that only one‐third of patients possess adequate knowledge about their medications. This percentage is likely to be even lower among the general population, particularly those with less experience with medications. Understanding the complexities of PGx testing and its limitations requires familiarity with medication‐related concepts and terminology. Individuals who have limited knowledge about medications, including the mechanisms of action and the biological factors that can impact effectiveness and tolerability, may encounter difficulties in accurately comprehending the scope and limitations of PGx testing. Furthermore, traditional Chinese medicine (TCM) holds deep cultural significance in China and is practiced alongside with contemporary Western medicine. 44 TCM emphasizes a holistic approach to understanding body function and disease processes, which conceptualizes disease diagnoses and treatment distinctly compared to Western medicine. 45 Pharmacokinetics (PK), the branch of pharmacology that explores how biological factors impact the absorption, distribution, metabolism, and excretion of drugs in the body, is not traditionally emphasized within the framework of TCM. This cultural context may create inherent barriers for some Chinese individuals to recognize and appreciate the role of genetic factors in influencing these PK processes, where PGx has substantial clinical relevance.
Enhancing overall medication literacy can be accomplished through direct patient education and medication counseling. In the United States, medication therapy management (MTM) has emerged as a crucial healthcare service where pharmacists provide education and counseling to patients who are on multiple medications to optimize therapeutic outcomes. 46 The increased availability and incorporation of PGx information in MTM enables clinicians to further refine patients' treatment regimens by considering drug‐gene interactions. 47 In China, both MTM and PGx implementation are still in their early stages but experiencing rapid growth. 17 , 48 The exploration of an integrated model that combines these approaches in a culturally sensitive fashion holds great potential to expedite their development and generate synergistic effects, ultimately leading to more optimized and personalized treatment outcomes for patients.
Sociodemographic and clinical relationships with PGx literacy
When examining the potential influences of sociodemographic and clinical characteristics, we observed that younger age and higher education level exhibited the strongest associations with higher PGx literacy among our Chinese study cohort. These results are consistent with the findings from the US cohort as well as other studies that highlight the relationships between these factors and health literacy across different population groups in various regions of China. 49 , 50 , 51 , 52 In the present study, a statistically significant positive correlation was observed between overall health literacy and PGx literacy among the participants. However, the small effect size (Spearman rho = 0.140, p < 0.001) suggests that this correlation may have limited clinical significance. Although individuals with higher health literacy may have a better grasp of general health information, this does not necessarily translate into adequate knowledge about PGx testing. PGx involves specific knowledge concerning genetics and its impact on medication outcomes, which extends beyond the concepts of general health. On the other hand, a higher level of PGx literacy was observed among participants who have prior knowledge or experience with genetic testing, suggesting that familiarity with genetic testing concepts can contribute to a better understanding of PGx.
Tailoring patient educational to PGx literacy
In clinical practice, it is important to recognize that having higher health literacy alone does not automatically guarantee sufficient knowledge about all components of PGx testing. Clinicians should address knowledge gaps and misunderstandings surrounding specific PGx knowledge domains through tailored education and counseling. When using the MAPL‐C to assess PGx knowledge in practice, it is conceivable that an individual may lack understanding in certain domains while having good comprehension in others. Therefore, a case‐specific educational approach is necessary. Our population‐level findings reveal that Chinese individuals, in particular, may have a higher tendency to misunderstand the limitations of PGx testing compared to other aspects. Therefore, emphasizing what is within and outside the scope of PGx testing during the counseling section becomes particularly crucial. This approach ensures test recipients acquire necessary knowledge to make informed decisions, accurately interpret the test results, and actively participate in their personalized healthcare plans.
Variations of PGx literacy among healthcare professionals in China
In addition to age, education, and prior experience with genetic testing, our study also suggests that healthcare‐related occupation is independently associated with higher performance on the MAPL‐C (Table 3). However, the difference in overall PGx literacy between healthcare professionals and non‐healthcare individuals was relatively small (mean difference = 0.69, same median score of 7) and unlikely to be clinically relevant (Figure S5). It is also important to note that there was a wide range of MAPL‐C scores among the health professionals included in this study. The slightly better PGx literacy observed among healthcare professionals compared to other participants in our study may be attributed to their higher overall health literacy acquired through healthcare education and professional experience (Table 3). However, this does not necessarily guarantee they all possess comprehensive PGx knowledge. Recent studies have consistently highlighted that healthcare workers across various disciplines in China perceive and demonstrate their knowledge of PGx to be inadequate. 17 , 23 These findings are in line with our study results, confirming the consistency of self‐assessed and actual knowledge gaps of PGx testing among Chinese health professionals. Therefore, significant efforts should be made to enhance PGx knowledge and competency among Chinese health professionals through ongoing education, training, and professional development programs. This will ensure that health professionals are equipped with the necessary skills and understanding to effectively integrate PGx into routine clinical practice. 23
Study design considerations, limitations, and implications for future endeavors
Study design considerations and limitations are important for interpreting and generalizing the findings. Participant enrollment and survey delivery through social media platforms might introduce selection bias, as those who chose to engage with this type of survey delivery may have different characteristics or motivations compared to those who did not participate. The study sample showed an over‐representation of relatively younger individuals and those with higher levels of education, which may limit the generalizability of the findings to the older population. Additionally, the use and engagement with social media can vary across different geographic locations and between urban and rural populations in China, further influencing the representativeness of the study sample. Another limitation is the lack of stratified sampling, which could result in certain geographic regions being over‐represented or under‐represented in the study sample. Although all seven geographic regions in China were included, the sample may not equally represent the actual population distribution. Last, the study primarily relied on cross‐sectional data, making it difficult to establish causality or determine changes in PGx literacy over time.
Despite these limitations, this study represents a significant first step in understanding PGx literacy in the Chinese population. Precision medicine has been an integral part of the national agenda, specifically the “Healthy China 2030” initiative, which seeks to address major healthcare challenges in China through the application of precision medicine. 53 This study is in line with that component of the national blueprint, offering valuable insights and serving as a notable example for future research in assessing PGx literacy and implementing community educational programs to promote precision medicine. Moving forward, it will be important for future studies to explore opportunities to engage older populations and expand outreach efforts to more rural geographic locations. By doing so, a more comprehensive understanding of PGx literacy can be achieved, leading to improved healthcare outcomes and better implementation of PGx in clinical practice.
In conclusion, we successfully validated the MAPL‐C, a tool to assess knowledge in various aspects of PGx testing among Chinese speakers. The MAPL‐C fills a notable gap in evaluating PGx knowledge within this population, demonstrating effectiveness in determining the PGx literacy of this Chinese cohort, while also identifying response patterns and knowledge gaps. The MAPL‐C holds great promise as a tool for future clinical practice and research, enabling the assessment of PGx literacy and guiding tailored educational interventions to enhance understanding and utilization of PGx testing among Chinese individuals.
AUTHOR CONTRIBUTIONS
All authors wrote the manuscript. L.Z. and J.R.B. designed the research. L.Z., J.R.B., S.Z., and F.W. performed the research. L.Z. analyzed the data. J.A., J.R.B., and A.L.P. developed the original MAPL questions. Information on licensing and terms of use of the MAPL and MAPL‐C instruments may be accessed at https://license.umn.edu/product/minnesota‐assessment‐of‐pharmacogenomic‐literacy‐mapl.
FUNDING INFORMATION
This work was supported by the University of Minnesota's College of Pharmacy Hadsall‐Uden Award for Pharmacy Advancement (L.Z.).
CONFLICT OF INTEREST STATEMENT
J.D.A. has served as a consultant to Tempus Labs, Inc., which offers pharmacogenomics testing. J.R.B. has served as a consultant to OptumRx for drug information activities unrelated to pharmacogenomics. All other authors declared no competing interests for this work.
Supporting information
Data S1
ACKNOWLEDGMENTS
REDCap utilized in this work was supported by Grant Number UL1TR002494 from the National Institutes of Health's National Center for Advancing Translational Sciences.
Zhang L, Zhou S, Allen JD, Wang F, Pittenger AL, Bishop JR. Assessing pharmacogenomic literacy in China through validation of the Chinese version of the Minnesota Assessment of Pharmacogenomic Literacy. Clin Transl Sci. 2023;16:2356‐2368. doi: 10.1111/cts.13637
Contributor Information
Lusi Zhang, Email: zhan6021@umn.edu.
Jeffrey R. Bishop, Email: jrbishop@umn.edu.
REFERENCES
- 1. Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature. 2015;526:343‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blagec K, Swen JJ, Koopmann R, et al. Pharmacogenomics decision support in the U‐PGx project: results and advice from clinical implementation across seven European countries. PloS One. 2022;17:e0268534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dunnenberger HM, Crews KR, Hoffman JM, et al. Preemptive clinical pharmacogenetics implementation: current programs in five United States medical centers. Annu Rev Pharmacol Toxicol. 2015;55:89‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Relling MV, Klein TE, Gammal RS, Whirl‐Carrillo M, Hoffman JM, Caudle KE. The clinical pharmacogenetics implementation consortium: 10 years later. Clin Pharmacol Ther. 2020;107:171‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swen JJ, Wilting I, Goede AL, et al. Pharmacogenetics: from bench to byte. Clin Pharmacol Ther. 2008;83:781‐787. [DOI] [PubMed] [Google Scholar]
- 6. Ross CJD, Visscher H, Sistonen J, et al. The Canadian pharmacogenomics network for drug safety: a model for safety pharmacology. Thyroid. 2010;20:681‐687. [DOI] [PubMed] [Google Scholar]
- 7. Picard N, Boyer JC, Etienne‐Grimaldi MC, et al. Pharmacogenetics‐based personalized therapy: levels of evidence and recommendations from the French network of pharmacogenetics (RNPGx). Therapie. 2017;72:185‐192. [DOI] [PubMed] [Google Scholar]
- 8. Pirmohamed M. Pharmacogenomics: current status and future perspectives. Nat Rev Genet. 2023;24:350‐362. doi: 10.1038/s41576-022-00572-8 [DOI] [PubMed] [Google Scholar]
- 9. Bachtiar M, Lee CGL. Genetics of population differences in drug response. Curr Genet Med Rep. 2013;1:162‐170. [Google Scholar]
- 10. Chung W‐H, Hung SI, Hong HS, et al. A marker for Stevens–Johnson syndrome. Nature. 2004;428:486. [DOI] [PubMed] [Google Scholar]
- 11. Huang Q, Liao Y, Yu T, et al. A retrospective analysis of preemptive pharmacogenomic testing in 22,918 individuals from China. J Clin Lab Anal. 2023;37:e24855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lo C, Nguyen S, Yang C, et al. Pharmacogenomics in Asian subpopulations and impacts on commonly prescribed medications. Clin Transl Sci. 2020;13:861‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng L, Xiong Y, Qin CZ, et al. HLA‐B*58:01 is strongly associated with allopurinol‐induced severe cutaneous adverse reactions in Han Chinese patients: a multicentre retrospective case–control clinical study. Br J Dermatol. 2015;173:555‐558. [DOI] [PubMed] [Google Scholar]
- 14. Guo C, Kuang Y, Zhou H, et al. Genotype‐guided dosing of warfarin in Chinese adults. Circ Genom Precis Med. 2020;13:e002602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen D‐L, Wang B, Bai J, et al. Clinical value of CYP2C19 genetic testing for guiding the antiplatelet therapy in a Chinese population. J Cardiovasc Pharmacol. 2016;67:232‐236. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Wang J, Zhao LM, et al. Strong association between HLA‐B*1502 and carbamazepine‐induced stevens‐Johnson syndrome and toxic epidermal necrolysis in mainland Han Chinese patients. Eur J Clin Pharmacol. 2011;67:885‐887. [DOI] [PubMed] [Google Scholar]
- 17. Guo C, Hu B, Guo C, et al. A survey of pharmacogenomics testing among physicians, pharmacists, and researchers from China. Front Pharmacol. 2021;12:682020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Xiao F, Chen Y, et al. Analytics of the clinical implementation of pharmacogenomics testing in 12 758 individuals. Clin Transl Med. 2021;11:e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang J, Qi G, Han C, et al. The landscape of clinical implementation of pharmacogenetic testing in Central China: a single‐center study. Pharmgenomics Pers Med. 2021;14:1619‐1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qin W, du Z, Xiao J, Duan H, Shu Q, Li H. Evaluation of clinical impact of pharmacogenomics knowledge involved in CPIC guidelines on Chinese pediatric patients. Pharmacogenomics. 2020;21:209‐219. [DOI] [PubMed] [Google Scholar]
- 21. Cai M, Zhou L, Gao D, et al. A national survey of individualized pharmaceutical care practice in Chinese hospitals in 2019. Front Pharmacol. 2023;14:1022134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang C, Lei J, Liu Y, Wang Y, Huang L, Feng Y. Therapeutic drug monitoring and pharmacogenetic testing in northern China. Front Pharmacol. 2021;12:754380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jia T, Wu C, Hu X, et al. Physicians' knowledge, attitude, and experience of pharmacogenomic testing in China. J Pers Med. 2022;12:2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rigter T, Jansen ME, Groot JM, Janssen SWJ, Rodenburg W, Cornel MC. Implementation of pharmacogenetics in primary care: a multi‐stakeholder perspective. Front Genet. 2020;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koufaki M‐I, Karamperis K, Vitsa P, Vasileiou K, Patrinos GP, Mitropoulou C. Adoption of pharmacogenomic testing: a marketing perspective. Front Pharmacol. 2021;12:724311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Allen JD, Zhang L, Johnson ANK, et al. Development and validation of the Minnesota assessment of pharmacogenomic literacy (MAPL). J Pers Med. 2022;12:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee AJ, Hui AC, Walker AD, Peshkin BN, Swain SM, Smith DM. Evaluation of a longitudinal pharmacogenomics education on pharmacist knowledge in a multicampus healthcare system. Pharmacogenomics. 2022;23:173‐182. [DOI] [PubMed] [Google Scholar]
- 28. Wen Y‐F, Jacobson PA, Oetting WS, Pereira C, Brown JT. Knowledge and attitudes of incoming pharmacy students toward pharmacogenomics and survey reliability. Pharmacogenomics. 2022;23:873‐885. [DOI] [PubMed] [Google Scholar]
- 29. Lipschultz E, Danahey K, Truong TM, et al. Creation of a pharmacogenomics patient portal complementary to an existing institutional provider‐facing clinical decision support system. JAMIA Open. 2021;4:ooab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zierhut HA, Campbell CA, Mitchell AG, Lemke AA, Mills R, Bishop JR. Collaborative counseling considerations for pharmacogenomic tests. Pharmacotherapy. 2017;37:990‐999. [DOI] [PubMed] [Google Scholar]
- 31. Allen JD, Pittenger AL, Bishop JR. A scoping review of attitudes and experiences with pharmacogenomic testing among patients and the general public: implications for patient counseling. J Pers Med. 2022;12:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ethnologue Languages of the World . Languages of the World . 2023. https://www.ethnologue.com/
- 33. Langer MM, Roche MI, Brewer NT, et al. Development and validation of a genomic knowledge scale to advance informed decision making research in genomic sequencing. MDM Policy Pract. 2017;2:2381468317692582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient‐reported outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health. 2005;8:94‐104. [DOI] [PubMed] [Google Scholar]
- 35. Wu Q, Ye X, Wu Y, Zhao L, Wu J. The Chinesization of all aspects of health LIteracy scale and its reliability and validity. Chin Gen Pract. 2017;20:1229. [Google Scholar]
- 36. Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238‐246. [DOI] [PubMed] [Google Scholar]
- 37. Tucker LR, Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38:1‐10. [Google Scholar]
- 38. Muthen LK, Muthen BO. Mplus: Statistical Analysis with Latent Variables: User's Guide (Version 8). Los Angeles, CA 2017.
- 39. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. https://www.R‐project.org/; 2022. [Google Scholar]
- 40. Lavrakas PJ. Encyclopedia of Survey Research Methods. SAGE Publications, Inc; 2008. [Google Scholar]
- 41. Chang X, Wang K, Wang Y, Tu H, Gong G, Zhang H. Medication literacy in Chinese patients with stroke and associated factors: a cross‐sectional study. Int J Environ Res Public Health. 2022;20:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zheng F, Ding S, Luo A, Zhong Z, Duan Y, Shen Z. Medication literacy status of outpatients in ambulatory care settings in Changsha, China. J Int Med Res. 2017;45:303‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhong Z, Ma G, Zheng F, Duan Y, Ding S, Luo A. Medication literacy in a cohort of Chinese patients discharged with essential hypertension. Front Public Health. 2020;7:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nestler G. Traditional Chinese medicine. Med Clin. 2002;86:63‐73. [DOI] [PubMed] [Google Scholar]
- 45. Keji C, Hao X. The integration of traditional Chinese medicine and Western medicine. Eur Rev. 2003;11:225‐235. [Google Scholar]
- 46. Bluml BM. Definition of medication therapy management: development of professionwide consensus. J Am Pharm Assoc. 2005;45:566‐572. [DOI] [PubMed] [Google Scholar]
- 47. Reiss SM, American Pharmacists Association . Integrating pharmacogenomics into pharmacy practice via medication therapy management. J Am Pharm Assoc. 2011;51:e64‐e74. [DOI] [PubMed] [Google Scholar]
- 48. Meng Q, Sun L, Ma Y, et al. The impact of pharmacist practice of medication therapy management in ambulatory care: an experience from a comprehensive Chinese hospital. BMC Health Serv Res. 2023;23:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shen M, Hu M, Liu S, Chang Y, Sun Z. Assessment of the Chinese resident health literacy scale in a population‐based sample in South China. BMC Public Health. 2015;15:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shi J, Qi L, Li Y, Liu X. Investigation of health literacy status in Beijing, China. Health Lit Res Pract. 2020;4:e174‐e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yu Y, Liu ZW, Hu M, et al. Assessment of mental health literacy using a multifaceted measure among a Chinese rural population. BMJ Open. 2015;5:e009054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yuan F, Qian D, Huang C, et al. Analysis of awareness of health knowledge among rural residents in Western China. BMC Public Health. 2015;15:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ye Y. Unleashing the power of big data to guide precision medicine in China. Nature. 2022;606:S49‐S51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


